Background: A host receptor has not yet been identified for glycerol monomycolate (GroMM), an immunostimulatory lipid of mycobacteria.
Results: GroMM recognition occurred in cell transfectants expressing human, but not mouse Mincle. Human Mincle transgenic mice acquired the ability to respond to GroMM.
Conclusion: GroMM is a ligand for human Mincle.
Significance: The molecular basis underlying the innate immune recognition of GroMM has been elucidated.
Keywords: Host-Pathogen Interactions, Innate Immunity, Lipids, Macrophages, Mycobacteria, Transgenic Mice, Glycerol Monomycolate, Mincle
Abstract
An array of lipidic compounds that constitute the cell wall of mycobacteria is recognized by host receptors. Examples include trehalose dimycolate (TDM), which is a major surface-exposed glycolipid of mycobacteria, that interacts with the macrophage inducible C-type lectin, Mincle, and exerts its highly potent adjuvant functions. Recent evidence has suggested that glycerol monomycolate (GroMM), another mycolate-containing lipid species produced by mycobacteria, can stimulate innate immune cells; however, its specific host receptors have yet to be identified. We here demonstrated that cell transfectants expressing human Mincle (hMincle) reacted to both TDM and GroMM, while those expressing mouse Mincle (mMincle) only reacted to TDM and failed to recognize GroMM. Studies using domain swap chimeras confirmed that the ectodomain of hMincle, but not that of mMincle, interacted with GroMM, and site-directed mutagenesis analyses revealed that short stretches of amino acid residues at positions 174–176 and 195–196 were involved in GroMM recognition. To further substantiate the differential recognition of GroMM by hMincle and mMincle, hMincle transgenic/mMincle knock-out mice (i.e. hMincle+ mice) were established and compared with non-transgenic mice (i.e. mMincle+ mice). We showed that macrophages derived from hMincle+ mice were activated by GroMM and produced inflammatory cytokines, whereas those derived from mMincle+ mice did not exhibit any reactivity to GroMM. Furthermore, local inflammatory responses were elicited in the GroMM-injected skin of hMincle+, but not mMincle+ mice. These results demonstrated that GroMM is a unique ligand for hMincle that is not recognized by mMincle.
Introduction
The cell wall of mycobacteria is replete with lipidic components that exhibit biological activities (1). These cell wall lipids and glycolipids, which are located at the host-pathogen interphase, are capable of modulating host immune responses via direct interactions with host receptors. Among these, trehalose dimycolate (TDM)3 has been studied extensively for decades, and its potent adjuvant functions have been demonstrated in in vitro and in vivo studies (2–6). Its receptor has only recently been identified as the macrophage inducible C-type lectin, Mincle, which transmits signals into macrophages through the associated Fc receptor γ (FcRγ) chains (7, 8). The binding of Mincle to TDM has been shown to induce the FcRγ-Syk-Card9-dependent activation of pivotal transcription factors, such as nuclear factor-κB and nuclear factor of activated T cells (NFAT), leading to the up-regulated expression of proinflammatory cytokines (9). Besides its prominent ability to directly activate host innate immune cells, TDM also appears to modulate the magnitude and quality of host acquired immune responses, although TDM per se is not targeted by T cells (10). On the other hand, lipoarabinomannan (LAM), another abundantly expressed cell wall glycolipid, appears to be directly targeted by both the innate and acquired immune systems. Mannose-capped LAM has been shown to modulate dendritic cell functions via its interaction with the dendritic cell-specific intercellular adhesion molecule 3 grabbing nonintegrin (11–13). Furthermore, LAM is also captured by CD1b molecules of the human group 1 CD1 family expressed on dendritic cells and is recognized by clonotypic T cell receptors expressed on T cells (14). Because of the abundant expression of various lipidic compounds with potent immunomodulatory functions, elucidating the molecular basis for their specific interactions with host receptors in more detail is fundamental for our complete understanding of mycobacterial infections.
Glycerol monomycolate (GroMM) has recently been identified as a new CD1b-presented mycobacterial lipid (15). GroMM-specific, CD1b-restricted T cells have been detected in the circulation of patients with latent, but not active tuberculosis. Although it is currently unclear how GroMM-specific T cells are represented preferentially in latently infected individuals, these T cells may efficiently restrict the growth of pathogenic mycobacteria during the subclinical persistent phases of infection, thereby preventing active disease. Similar to TDM and LAM, GroMM also appears to directly stimulate innate immune cells to produce inflammatory cytokines (16). Furthermore, a previous study reported that an injection of GroMM liposome into the skin of naïve guinea pigs resulted in local infiltration by inflammatory cells (17). Nevertheless, host innate immune receptors have not yet been identified for GroMM.
We here demonstrated that GroMM is a ligand for human Mincle. Reporter cell transfectants expressing human Mincle recognized both TDM and GroMM, whereas those expressing mouse Mincle only recognized TDM and failed to recognize GroMM. Consistent with this result, macrophages derived from human Mincle transgenic (Tg) mice, but not those derived from non-Tg mice, responded to GroMM and produced tumor necrosis factor (TNF)-α. Furthermore, human Mincle Tg mice mounted local inflammatory responses in GroMM-injected skin, and this was not observed in non-Tg mice. Finally, human macrophages produced TNF-α in response to GroMM, and the GroMM-specific response was completely blocked by an antibody against human Mincle. These results confirmed that GroMM is a novel mycobacteria-derived ligand for human Mincle, and also emphasized that its recognition occurs differentially in humans and mice.
EXPERIMENTAL PROCEDURES
Chemical Reagents and Lipid Samples
Chemical reagents were purchased from Nacalai Tesque (Kyoto, Japan) unless otherwise indicated. Methods for purifying TDM and GroMM as well as those for producing stearylated octaarginine-containing liposome were described in our previous studies (4, 17, 18). Synthetic glycerol monobehenate (GroMB) was provided by Kao (Tokyo, Japan).
Human Mincle Tg Mice
Animal experiments were performed according to institutional guidelines on animal welfare and the humane treatment of laboratory animals. A human Mincle genomic DNA fragment composed of 1,482 bp of upstream DNA, exon 1, and intron 1 was amplified by PCR and ligated with a polyadenylation signal-attached cDNA fragment derived from exons 2 through 6. The cloned 3.5 kbp DNA fragment was microinjected into fertilized eggs from B57BL/6, and a murine Tg line carrying transgene DNA was established. The human Mincle Tg line was further crossbred with Clec4e−/− mice (19) with a homozygous disruption in the mouse Mincle gene. PCR-based genotyping was performed using genomic DNA from mouse tails as a template. To amplify the human Mincle transgene, samples were subjected to PCR amplification with Biotaq DNA polymerase (Bioline, London, UK) for 35 cycles of 30 s at 94 °C, 30 s at 64 °C, and 1 min at 72 °C, followed by 10 min incubation at 72 °C. The specific primers used were: 5′-CTG GGA TCC CCA TCC TAT TTC TCA GTG C-3′ (sense) and 5′-GCT CCC CTA CAT CCC AGA AGC TCA GAG-3′ (antisense). To amplify the mouse Mincle gene, PCR amplification was carried out with KOD DNA polymerase (Toyobo, Osaka, Japan) for 35 cycles of 10 s at 98 °C, 30 s at 60 °C, and 1 min at 68 °C. The specific primers used were: 5′-CAG AAT GTG GTA CTG AGG CAC A-3′ (sense) and 5′-ACA TAG CCG ACT TGA TCC CAA C-3′ (antisense). PCR products were resolved on 1% agarose gels and visualized by staining with ethidium bromide and UV transillumination.
Cloning of cDNAs Encoding Mincle Mutants
To generate the ectodomain swap mutants of human and mouse Mincle, cDNA fragments encoding the cytoplasmic (CYT)/transmembrane (TM) domains and those encoding the extracellular (EXT) domains with short stretches of antiparallel DNA sequences at their 3′-ends were first obtained by PCR (30 cycles of 10 s at 98 °C, 30 s at 60 °C, and 1 min at 68 °C). Full-length constructs were then obtained by PCR using these fragments as templates (35 cycles of 10 s at 98 °C, 30 s at 60 °C, and 1 min at 68 °C). The primers used were as follows: 5′-GCG GTA CCA TGA ATT CAA CCA AAT CGC CTG-3′ (sense, mouse CYT); 5′-GAA AGA TGC GAA ATG TTA CGA CAC ATC TGG-3′ (antisense, mouse TM); 5′-TGT GTC GTA ACA TTT CGC ATC TTT CAA ACC-3′ (sense, human EXT); 5′-GCG TCT AGA TTA AAG AGA TTT TCC TTT GT-3′ (antisense, human EXT); 5′-GGG GTA CCA TGA ATT CAT CTA AAT CAT CTG-3′ (sense, human CYT); 5′-GAG AGC TGC GAT ATG TCA CAA CAC ATC TGG-3′ (antisense, human TM); 5′-TGT GTT GTG ACA TAT CGC AGC TCT CAA ATT-3′ (sense, mouse EXT); 5′-GCG TCT AGA TTA GTC CAG AGG ACT TAT TTC-3′ (antisense, mouse EXT). These cDNAs were cloned into pEF6, and introduced into NFAT-GFP reporter cells by electroporation. The transfected cells were cultured in the presence of 3 μg/ml of blasticidin (InvivoGen, San Diego, CA), and the surface expression of the swap mutants was confirmed by flow cytometry using an anti-mouse Mincle monoclonal antibody (mAb) (clone 4A9, MBL, Nagoya, Japan) and the anti-human Mincle mAb, 1H2, which was generated in our laboratory (see below).
Site-directed mutagenesis was performed by PCR, using codon-modified primers and the full-length construct of mouse wild-type Mincle cDNA in pEF6 as a template. The primer sets used were: 5′-ATA GCT ACG CTG GAG GAC TGT GCC ACC ATA-3′ (sense) and 5′-TCC AGC GTA GCT ATA TTG TTG GGC TCC CCA-3′ (antisense) for amino acid mutations at positions 174–176; 5′-ATG ATG TAA CCT GTT TCT ACA GTA TGC CTT-3′ (sense) and 5′-ACA GGT TAC ATC ATT CCA GTT CTT CCT GGA-3′ (antisense) for amino acid mutations at positions 195–196. PCR amplification was performed with KOD DNA polymerase for 10 cycles of 20 s at 98 °C, 30 s at 58 °C, and 6.5 min at 68 °C. The amplified PCR products were treated with DpnI (Takara, Otsu, Japan) and used for transformation of DH5α cells (Toyobo). The introduction of these mutations was confirmed by DNA sequencing.
NFAT-GFP Reporter Assays
Purified lipids (1 mg/ml) in chloroform/methanol (2:1, v/v) were serially diluted with isopropanol and added to the wells of 96-well microtiter plates, followed by evaporation of the solvent. 2B4-NFAT-GFP reporter cells (20) expressing either wild-type or mutated Mincle were incubated in the lipid-coated plates, and after 18 h, the cells were harvested, and propidium iodide was added to gate out dead cells. The cell samples were then analyzed for GFP-positive cells by flow cytometry using a BD FACSCanto II flow cytometer (BD Biosciences, San Jose, CA).
Generation of Anti-human Mincle mAb
An anti-human Mincle mAb was generated using the “rat lymph node method” as described previously (21). Briefly, female Wistar rats were immunized with an emulsion containing human Mincle-transfected 293T cells and complete Freund's adjuvant (BD Biosciences), and after 3 weeks, lymph node cells were fused with SP2/0 myeloma cells by a standard polyethylene glycol method. Culture supernatants of the hybridoma clones were tested for their reactivity to human Mincle-transfected 293T cells. A mAb clone, termed 1H2, only reacted to Mincle-transfected, but not mock-transfected cells. To assess blocking activity, NFAT-GFP reporter assays were performed in the presence or absence of the serially diluted hybridoma culture supernatants of 1H2 and control clones.
Macrophage Assays
Mouse bone marrow-derived macrophages and human monocyte-derived macrophages were obtained as described previously (7, 22), except for the use of a recombinant protein (Wako Pure Chemical Industries, Ltd., Osaka, Japan) at 50 ng/ml, instead of culture supernatants, as a source of macrophage colony-stimulating factor. These mouse and human macrophages were harvested and cultured either in lipid-coated plates or in the presence of 100 ng/ml lipopolysaccharide (LPS; Sigma-Aldrich) at a density of 1 × 105 cells/well. After a 24-h incubation at 37 °C, aliquots of the culture supernatants were collected, and the amount of TNF-α released into the medium was measured using mouse and human TNF-α ELISA kits (BD Biosciences), respectively. In some experiments, the macrophage cell culture was performed in the presence of 1H2 ascites at a dilution of 1:400.
Skin Reactions
Liposomes containing 20 μg of GroMM, as well as an equivalent amount of empty liposomes, were injected to elicit skin reactions in mice. After 2 days, the skin was excised, and skin samples were fixed for 16 h with 4% paraformaldehyde, dehydrated, and embedded in paraffin. The tissue sections were stained with hematoxylin and eosin solution (Merck, Whitehouse Station, NJ) and observed under a microscope. The numbers of lymphocytes, macrophages, neutrophils, eosinophils, and basophils in 10 randomly selected high power fields were counted.
RESULTS
GroMM Was Recognized by Reporter Cell Transfectants Expressing Human Mincle
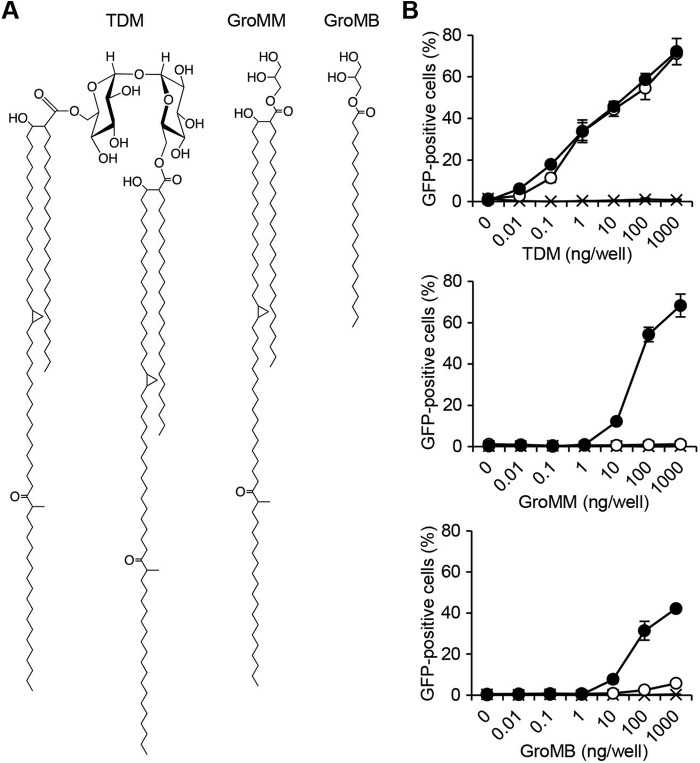
Previous studies have demonstrated the ability of GroMM to elicit innate immune responses (16, 17, 23); however, none have elucidated the molecular basis for its recognition by the host cells. Alternatively, a crystallographic study of human Mincle revealed the presence of shallow hydrophobic grooves that may potentially interact with a meromycolate chain and its alkyl branch of TDM (24). Given the partially overlapping structure of TDM and GroMM (Fig. 1A), we attempted to determine whether GroMM may be recognized by human Mincle. We used a convenient NFAT-GFP reporter assay, that has been used successfully to identify Mincle ligands in previous studies (20, 25, 26). NFAT-GFP reporter cells transfected with FcRγ alone or together with human Mincle were stimulated with plate-coated TDM, and the NFAT-dependent activation of cells was monitored by the flow cytometric detection of GFP-positive cells. As shown in Fig. 1B, a dose-dependent increase in the percentage of GFP-positive cells was observed for human Mincle/FcRγ-expressing cells (top panel, indicated with closed circles), whereas the cells expressing FcRγ alone did not show any reactivity to TDM (indicated with x). We also confirmed that mouse Mincle/FcRγ-expressing cells (open circles) recognized TDM as efficiently as human Mincle/FcRγ-expressing cells.
FIGURE 1.
Recognition of GroMM by human Mincle in NFAT-GFP reporter assays. A, chemical structures of TDM, GroMM, and GroMB are shown. B, NFAT-GFP reporter cells expressing either human Mincle/FcRγ (closed circles) or mouse Mincle/FcRγ (open circles) as well as those expressing FcRγ alone (indicated with x) were tested for their reactivity to TDM (top panel), GroMM (middle panel), and GroMB (bottom panel). Assays were performed in triplicate, and the mean values and standard deviations (S.D.) are shown.
The responses of these cell transfectants to plate-coated GroMM were also assessed to establish whether Mincle could recognize GroMM. We found that human Mincle/FcRγ-expressing cells were able to react to GroMM in a dose-dependent manner (Fig. 1B, middle panel, indicated with closed circles), although the recognition of GroMM did not appear to be as efficient as that of TDM. On the other hand, mouse Mincle/FcRγ-expressing cells failed to recognize GroMM (open circles). The GroMM preparation used in these assays was a natural compound purified from cultured mycobacteria, and there were no indications of any contaminants (17); however, we wanted to confirm that synthetic analogues may also be recognized by human Mincle. Therefore, synthetic glycerol monobehenate (GroMB) (Fig. 1A) was also included in the NFAT-GFP reporter assay. We found that cells expressing human Mincle responded to GroMB (Fig. 1B, bottom panel, indicated with closed circles), whereas only marginal responses were observed in those expressing mouse Mincle (open circles). Thus, these results suggested that human Mincle could interact with monoacylated glycerol species more efficiently than mouse Mincle, and that GroMM was only recognized by human Mincle.
The Ectodomain of Human Mincle Was Responsible for Its Interaction with GroMM
The results obtained above were unexpected because such differential recognition by human and mouse Mincle had not been reported for any of the known Mincle ligands. Therefore, we generated ectodomain swap chimeras, namely the “mouse-human” chimera, in which the extracellular domain of mouse Mincle was swapped with that of human Mincle, and the “human-mouse” chimera, in which the extracellular of human Mincle was swapped with that of mouse Mincle, and tested for their ability to recognize the ligands in NFAT-GFP reporter assays. As shown in the left panel of Fig. 2A, cell transfectants expressing the mouse-human chimera (closed triangles), and those expressing the human-mouse chimera (open triangles) responded similarly to TDM, and the dose-response curves overlapped with those for cells expressing either human (closed circles) or mouse (open circles) wild-type Mincle. Thus, the ectodomain swap mutations did not affect the recognition of TDM by Mincle. On the other hand, only cells expressing the mouse-human chimera (Fig. 2A, right panel, indicated with closed triangles) recognized GroMM as efficiently as those expressing the human wild-type Mincle (closed circles). In contrast, cells expressing mouse Mincle ectodomain-containing molecules, namely the human-mouse chimera (open triangles) and mouse wild-type Mincle (open circles), failed to recognize GroMM despite their ability to react to TDM. Therefore, these results confirmed that the extracellular domain of human, but not mouse Mincle could potentially mediate the recognition of GroMM. The crystallographic structure of human Mincle (24) predicted that short stretches of amino acid residues located near the N-terminal end of the β6 sheet (Val-Leu-Val in mice and Ala-Thr-Leu in humans at positions 174–176) and the C-terminal end of the β7 sheet (Ile-Pro in mice and Val-Thr in humans at positions 195–196) may be critical for interactions with the glycerol framework of GroMM; thus, we generated mouse Mincle mutants in which either amino acid residues at positions 174–176 or those at positions 195–196 were replaced with the corresponding amino acid residues of human Mincle (Fig. 2B). Reporter cell transfectants expressing the mousehu174–176 mutant (gray-filled diamonds) and those expressing the mousehu195–196 mutant (open diamonds) maintained their reactivity to TDM (left panel). These cells also exhibited a significant response to GroMM (right panel), even though it was not fully comparable to that of the human Mincle-expressing cells (closed circles). The doubly mutated mousehu174–176, hu195–196 mutant appeared to undergo a marked conformational change, which resulted in a failure to recognize TDM and GroMM (data not shown). Taken together, these results demonstrated the differential ability of human and mouse Mincle to recognize GroMM.
FIGURE 2.
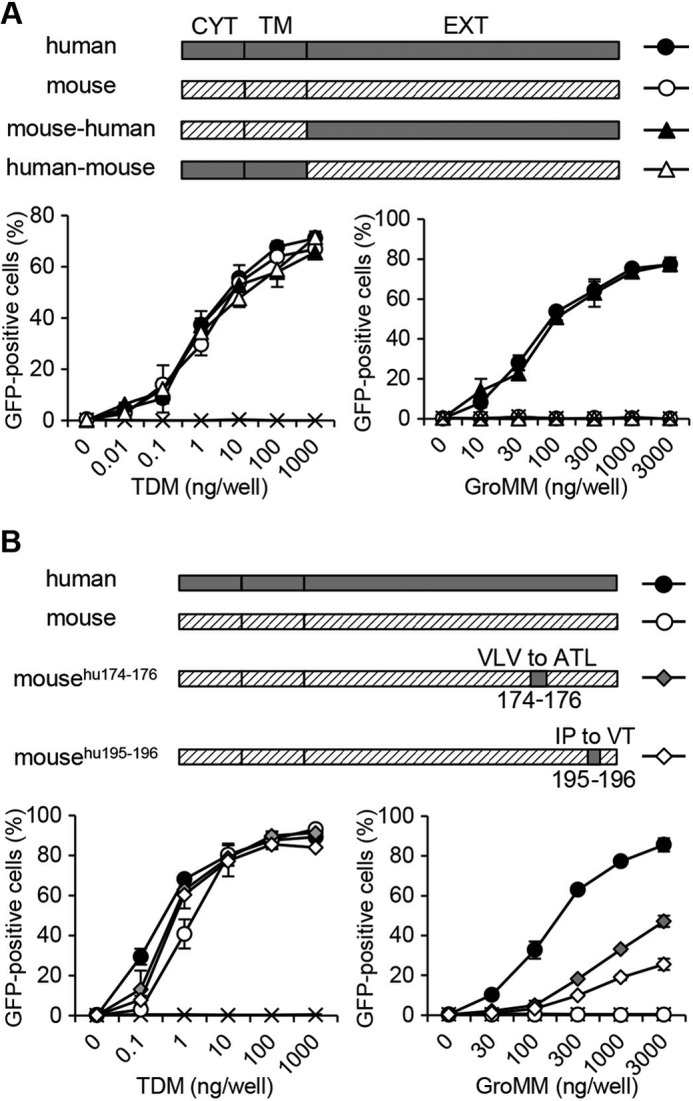
Recognition of GroMM by Mincle mutants. A, mouse-human chimera (closed triangles) containing the cytoplasmic (CYT) and transmembrane (TM) domains of mouse Mincle as well as the extracellular (EXT) domain of human Mincle, and the human-mouse chimera (open triangles) containing the CYT and TM domains of human Mincle as well as the EXT domain of mouse Mincle were expressed in NFAT-GFP reporter cells and tested for their ability to recognize TDM and GroMM. NFAT-GFP reporter cells expressing either human wild-type Mincle (closed circles) or mouse wild-type Mincle (open circles) as well as those expressing FcRγ alone (indicated with x) were also included as controls. B, NFAT-GFP reporter cells expressing either the mousehu174–176 mutant with Val-Leu-Val to Ala-Thr-Leu mutations at positions 174–176 or the mousehu195–196 mutant with Ile-Pro to Val-Thr mutations at positions 195–196 were obtained and analyzed as in A. Assays were performed in triplicate, and the mean values and S.D. are shown.
Responses to GroMM Were Reconstituted in Human Mincle Tg Mice
The NFAT-GFP reporter assay is known to be useful for identifying Mincle ligands; however, the interaction of the receptor with a candidate ligand should be assessed further in more physiological settings. We used the inability of mouse Mincle to react to GroMM, and generated human Mincle Tg mice to reconstitute responses to GroMM in vivo. Tg mice carrying the human Mincle transgene construct, composed of 1,482 bp of upstream DNA, exon 1, and intron 1, followed by cDNA derived from exons 2 through 6 and the polyadenylation signal (Fig. 3A), were established and further crossbred with Mincle knock-out mice. This allowed us to comparatively analyze 3 groups of gene-manipulated mice that were separated in terms of the expression of human and mouse Mincle; namely, endogenous mouse Mincle-expressing (mMincle+) mice, transgenic human Mincle-expressing (hMincle+) mice, and mice without the expression of Mincle of any species (Minclenull) (Fig. 3B).
FIGURE 3.
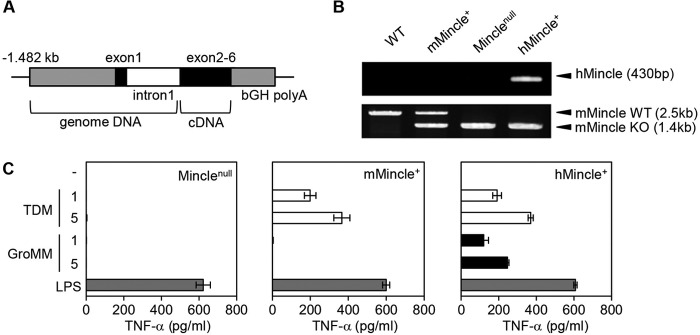
Reconstitution of human Mincle functions in mice. A, transgene construct used is shown. B, genomic DNA samples obtained from wild-type mice (WT), mice with a heterozygous disruption in the mouse Mincle gene (mMincle+), Mincle KO mice (Minclenull), and human Mincle Tg/mouse Mincle KO mice (hMincle+) were subjected to PCR to detect the human Mincle transgene (upper panel) and the mouse Mincle gene (lower panel). The positions (arrowheads) and sizes (in parentheses) of the amplified PCR products corresponding to the human Mincle transgene (hMincle) as well as the wild-type (mMincle WT) and disrupted (mMincle KO) mouse Mincle genes are shown to the right. C, bone marrow-derived macrophages from Minclenull (left panel), mMincle+ (middle panel), and hMincle+ mice (right panel) were stimulated with either plate-coated TDM/GroMM (1 or 5 μg/well) or 100 ng/ml LPS. After 24 h, the culture supernatants were harvested, and the amount of TNF-α released into the medium was measured. Assays were performed in triplicate, and the mean values and S.D. are shown.
The bone marrow-derived macrophages obtained from Minclenull mice produced TNF-α in response to the potent Toll-like receptor 4 ligand, LPS, but failed to react to TDM or GroMM, which confirmed a specific defect in Mincle-dependent signal transduction (Fig. 3C, left panel). In contrast, cells derived from mMincle+ mice responded to TDM, indicating that endogenous mouse Mincle-dependent signaling pathways functioned properly (middle panel). Nevertheless, these cells failed to respond to GroMM, as predicted by the NFAT-GFP reporter assay. Finally, cells derived from hMincle+ mice recognized TDM as efficiently as those derived from mMincle+ mice, which suggested that the function of human Mincle was reconstituted successfully in these cells. In addition, these cells were able to produce TNF-α in response to GroMM. Therefore, these in vitro experiments detected the ability of human, but not mouse Mincle to recognize GroMM.
The recognition of GroMM by human Mincle was further assessed in vivo by injecting either GroMM liposomes or mock liposomes into the skin of Minclenull, mMincle+, and hMincle+ mice. After 2 days, the skin was excised, and local tissue responses in these mice were monitored by microscopically examining hematoxylin and eosin-stained sections. The marked accumulation of infiltrating cells was observed at the GroMM-injected, but not mock-injected skin of hMincle+ mice, and such tissue responses were not apparent in Minclenull or mMincle+ mice (Fig. 4A). The cell types infiltrating the GroMM-injected skin of hMincle+ mice included macrophages and eosinophils (Fig. 4B), as reported previously in guinea pig studies. Thus, in vitro and in vivo analyses of hMincle Tg mice provided compelling evidence for the differential recognition of GroMM by human and mouse Mincle.
FIGURE 4.
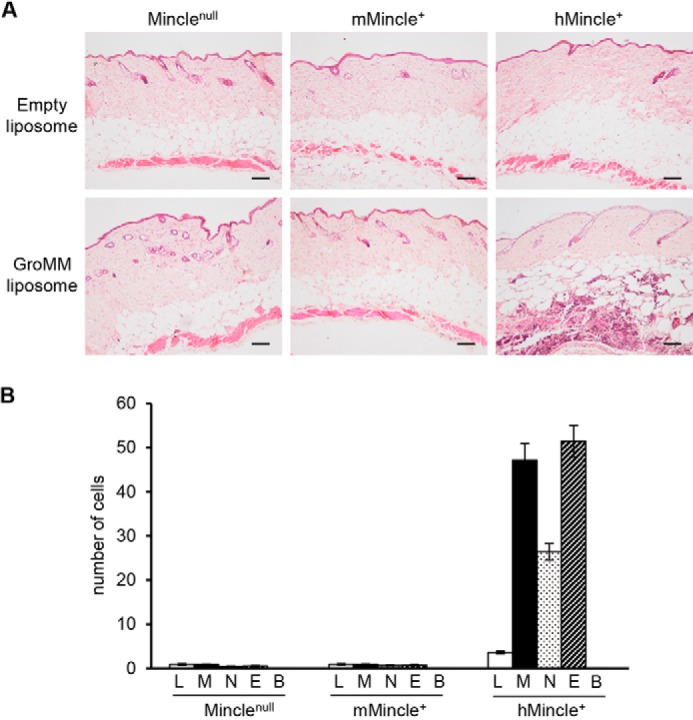
GroMM-elicited skin responses in human Mincle Tg mice. GroMM liposomes and empty liposomes were injected into the skin of the 3 mouse groups, and after 2 days, tissue responses were evaluated by hematoxylin and eosin staining. A, representative micrographs are shown. Scale bars, 100 μm. B, numbers of infiltrating lymphocytes (L), macrophages (M), neutrophils (N), eosinophils (E), and basophils (B) in 10 randomly selected high power fields were counted in 4 animals from each mouse group. The mean values and S.D. are shown.
Human Macrophages Recognized GroMM in a Mincle-dependent Manner
Besides gene manipulation approaches using reporter cell transfectants and Tg mice, we attempted to confirm whether primary human cells were able to react to GroMM in a Mincle-dependent manner. The anti-human Mincle mAb, 1H2, which was capable of blocking the response of NFAT-GFP reporter cell transfectants expressing human Mincle to TDM (Fig. 5A, left panel) and GroMM (right panel), was established and included in assays using human monocyte-derived macrophages. We found that human macrophages produced TNF-α in response to plate-coated GroMM as well as TDM and LPS (Fig. 5B), and the GroMM-elicited response was markedly suppressed by 1H2, but not by a control antibody (Fig. 5C), which suggested a major role for human Mincle in the recognition of GroMM. Thus, the present study demonstrated that GroMM was a novel ligand for human, but not mouse Mincle.
FIGURE 5.
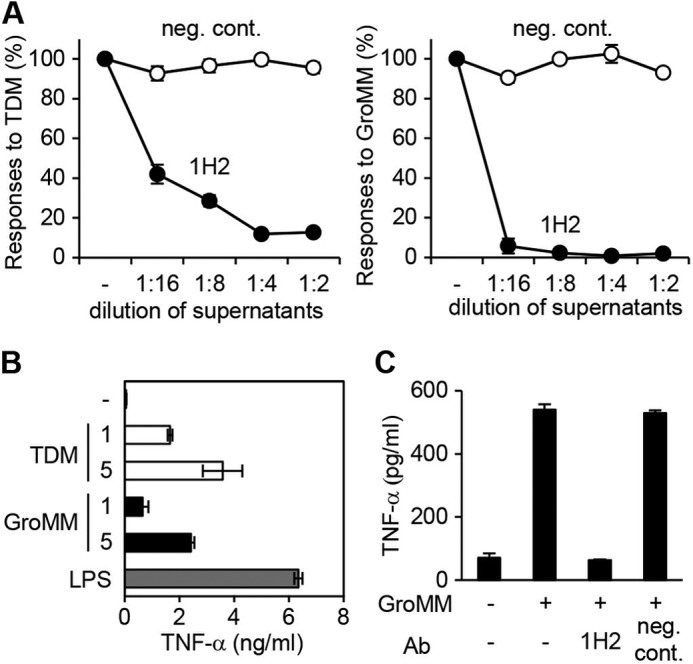
Mincle-dependent recognition of GroMM by human macrophages. A, responses of human Mincle/FcRγ-expressing NFAT-GFP reporter cells to plate-coated TDM (10 ng/well) (left panel) and GroMM (100 ng/well) (right panel) were evaluated in the presence of serially diluted hybridoma cell supernatants of either the 1H2 clone (closed circles) or a negative control clone (open circles). The responses observed without the addition of the supernatants (−) were defined as 100%, and the relative values are shown for each response. B, production of TNF-α by human macrophages in response to plate-coated TDM and GroMM (1 or 5 μg/well) as well as 100 ng/ml LPS was assessed. C, response of human macrophages to plate-coated GroMM (1 μg/well) was assessed in the presence or absence of either 1H2 ascites or negative control ascites (P3). Assays were performed in triplicate, and the mean values and S.D. are shown.
DISCUSSION
The present study provided evidence that GroMM functioned as a novel ligand for human, but not mouse Mincle by utilizing 3 independent, but complementary approaches; namely, NFAT-GFP reporter assays, human Mincle Tg mice, and primary macrophage assays.
The cell wall of mycobacteria contains an array of ligands that interact with Toll-like receptors, C-type lectin receptors, and other host innate immune receptors. These pathways are tightly linked to macrophage activation, and, therefore, could potentially jeopardize the survival of invading microbes. However, pathogenic mycobacteria have evolved evasive maneuvers to hide the ligands from detection by the host innate immune system, replace the ligands with less potent compounds, or digest the ligands. Examples include phthiocerol dimycocerosate, a virulence factor of Mycobacterium tuberculosis, that has been proposed to mask pathogen-associated molecular patterns (PAMPs) and cooperate with the related phenolic glycolipid to selectively recruit permissive macrophages (27). In contrast to these PAMPs for which the expression levels are not markedly reduced during infections, invading pathogenic mycobacteria specifically down-regulate the expression of TDM presumably because of its abundance as well as its highly potent adjuvant functions. A glycolipid exchange from TDM to glucose monomycolate has been shown to occur immediately after infection by utilizing host-derived glucose as a competitive substrate for mycolyltransferases (18). Alternatively, a recent study pointed to a direct degradation pathway for TDM by means of enzymatic hydrolysis, resulting in the accumulation of free mycolates that was associated with the increased influx of nutrients, including glycerol (28). Given that glycerol is ubiquitously present in host cells and mycobacteria can readily utilize exogenously derived glycerol for the production of GroMM (17), GroMM may potentially predominate over TDM during the course of infections. The present study now raises the question of why microbes need to produce another ligand for Mincle while endeavoring to down-regulate the expression of TDM.
This remains an open question that will require further investigations to answer; however, we speculate that TDM and GroMM expression levels may markedly affect the magnitude of the TNF-α response mounted by host macrophages. Evidence obtained from studies with the zebrafish tuberculosis model emphasized that fine-tuning the expression of TNF at “moderate” levels was critical for persistent infections or cohabitation of the host and microbes; suppressed and excess levels of TNF expression have both been associated with severe necroptosis in host macrophages and the exuberant extracellular growth of microbes, rather than the construction of well-organized granulomas (29). Therefore, following the down-regulation of TDM expression, GroMM may function as a surrogate ligand for Mincle that modestly stimulates macrophages, thereby providing a better chance for microbes to cohabitate silently for years in immunocompetent hosts. Intervention by anti-TNF-α therapy may disturb the sustained balance, which could potentially result in reactivation of the microbes (30).
Besides the identification of GroMM as a new ligand for human Mincle, the present study also highlighted the inability of mouse Mincle to recognize GroMM. To the best of our knowledge, such differential recognition of a single mycobacterial ligand has never been noted for any innate immune receptors that are conserved between humans and mice. Nevertheless, several immune pathways, including those mediated by isopentenyl pyrophosphate-reactive γδT cells and by lipid/glycolipid-reactive group 1 CD1-restricted T cells, have been shown to function critically against tuberculosis in humans (31–33), but were totally absent in mice. These differences, as well as the differential recognition of GroMM by human and mouse Mincle, could potentially be related to the distinct histopathologies observed in humans and mice (34, 35). As discussed above, sustained and balanced interactions between the host and microbes result in the formation of well-organized granulomas, which are often constructed in humans, but not in mice. The human Mincle Tg mice established in the present study may be an invaluable tool for assessing a specific role of the GroMM/human Mincle/TNF axis in human tuberculosis.
Acknowledgment
We thank Riki Ishibashi for assistance in generating human Mincle Tg mice.
This work was supported by a grant from the Japan Society for the Promotion of Science (grant-in-aid for Scientific Research (B); grant number 24390255). This work was also supported by a grant from the Ministry of Health, Labor and Welfare, and by Joint Usage/Research Center program of Institute for Virus Research, Kyoto University.
- TDM
- trehalose dimycolate
- FcRγ
- Fc receptor γ chain
- NFAT
- nuclear factor of activated T cells
- LAM
- lipoarabinomannan
- GroMM
- glycerol monomycolate
- Tg mice
- transgenic mice
- TNF
- tumor necrosis factor
- GroMB
- glycerol monobehenate
- mAb
- monoclonal antibody
- LPS
- lipopolysaccharide
- PAMPs
- pathogen-associated molecular patterns.
REFERENCES
- 1. Brennan P. J. (2003) Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis 83, 91–97 [DOI] [PubMed] [Google Scholar]
- 2. Bowdish D. M., Sakamoto K., Kim M. J., Kroos M., Mukhopadhyay S., Leifer C. A., Tryggvason K., Gordon S., Russell D. G. (2009) MARCO, TLR2, and CD14 are required for macrophage cytokine responses to mycobacterial trehalose dimycolate and Mycobacterium tuberculosis. PLoS Pathog. 5, e1000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hunter R. L., Olsen M., Jagannath C., Actor J. K. (2006) Trehalose 6,6′-dimycolate and lipid in the pathogenesis of caseating granulomas of tuberculosis in mice. Am. J. Pathol. 168, 1249–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Otsuka A., Matsunaga I., Komori T., Tomita K., Toda Y., Manabe T., Miyachi Y., Sugita M. (2008) Trehalose dimycolate elicits eosinophilic skin hypersensitivity in mycobacteria-infected guinea pigs. J. Immunol. 181, 8528–8533 [DOI] [PubMed] [Google Scholar]
- 5. Rao V., Fujiwara N., Porcelli S. A., Glickman M. S. (2005) Mycobacterium tuberculosis controls host innate immune activation through cyclopropane modification of a glycolipid effector molecule. J. Exp. Med. 201, 535–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sakamoto K., Kim M. J., Rhoades E. R., Allavena R. E., Ehrt S., Wainwright H. C., Russell D. G., Rohde K. H. (2013) Mycobacterial trehalose dimycolate reprograms macrophage global gene expression and activates matrix metalloproteinases. Infect. Immun. 81, 764–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ishikawa E., Ishikawa T., Morita Y. S., Toyonaga K., Yamada H., Takeuchi O., Kinoshita T., Akira S., Yoshikai Y., Yamasaki S. (2009) Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J. Exp. Med. 206, 2879–2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schoenen H., Bodendorfer B., Hitchens K., Manzanero S., Werninghaus K., Nimmerjahn F., Agger E. M., Stenger S., Andersen P., Ruland J., Brown G. D., Wells C., Lang R. (2010) Cutting edge: Mincle is essential for recognition and adjuvanticity of the mycobacterial cord factor and its synthetic analog trehalose-dibehenate. J. Immunol. 184, 2756–2760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Werninghaus K., Babiak A., Gross O., Hölscher C., Dietrich H., Agger E. M., Mages J., Mocsai A., Schoenen H., Finger K., Nimmerjahn F., Brown G. D., Kirschning C., Heit A., Andersen P., Wagner H., Ruland J., Lang R. (2009) Adjuvanticity of a synthetic cord factor analogue for subunit Mycobacterium tuberculosis vaccination requires FcRgamma-Syk-Card9-dependent innate immune activation. J. Exp. Med. 206, 89–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miyake Y., Toyonaga K., Mori D., Kakuta S., Hoshino Y., Oyamada A., Yamada H., Ono K., Suyama M., Iwakura Y., Yoshikai Y., Yamasaki S. (2013) C-type lectin MCL is an FcRgamma-coupled receptor that mediates the adjuvanticity of mycobacterial cord factor. Immunity 38, 1050–1062 [DOI] [PubMed] [Google Scholar]
- 11. Geijtenbeek T. B., Van Vliet S. J., Koppel E. A., Sanchez-Hernandez M., Vandenbroucke-Grauls C. M., Appelmelk B., Van Kooyk Y. (2003) Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 197, 7–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maeda N., Nigou J., Herrmann J. L., Jackson M., Amara A., Lagrange P. H., Puzo G., Gicquel B., Neyrolles O. (2003) The cell surface receptor DC-SIGN discriminates between Mycobacterium species through selective recognition of the mannose caps on lipoarabinomannan. J. Biol. Chem. 278, 5513–5516 [DOI] [PubMed] [Google Scholar]
- 13. Tailleux L., Schwartz O., Herrmann J. L., Pivert E., Jackson M., Amara A., Legres L., Dreher D., Nicod L. P., Gluckman J. C., Lagrange P. H., Gicquel B., Neyrolles O. (2003) DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J. Exp. Med. 197, 121–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sieling P. A., Chatterjee D., Porcelli S. A., Prigozy T. I., Mazzaccaro R. J., Soriano T., Bloom B. R., Brenner M. B., Kronenberg M., Brennan P. J. (1995) CD1-restricted T cell recognition of microbial lipoglycan antigens. Science 269, 227–230 [DOI] [PubMed] [Google Scholar]
- 15. Layre E., Collmann A., Bastian M., Mariotti S., Czaplicki J., Prandi J., Mori L., Stenger S., De Libero G., Puzo G., Gilleron M. (2009) Mycolic acids constitute a scaffold for mycobacterial lipid antigens stimulating CD1-restricted T cells. Chem. Biol. 16, 82–92 [DOI] [PubMed] [Google Scholar]
- 16. Andersen C. S., Agger E. M., Rosenkrands I., Gomes J. M., Bhowruth V., Gibson K. J., Petersen R. V., Minnikin D. E., Besra G. S., Andersen P. (2009) A simple mycobacterial monomycolated glycerol lipid has potent immunostimulatory activity. J. Immunol. 182, 424–432 [DOI] [PubMed] [Google Scholar]
- 17. Hattori Y., Matsunaga I., Komori T., Urakawa T., Nakamura T., Fujiwara N., Hiromatsu K., Harashima H., Sugita M. (2011) Glycerol monomycolate, a latent tuberculosis-associated mycobacterial lipid, induces eosinophilic hypersensitivity responses in guinea pigs. Biochem. Biophys. Res. Commun. 409, 304–307 [DOI] [PubMed] [Google Scholar]
- 18. Matsunaga I., Naka T., Talekar R. S., McConnell M. J., Katoh K., Nakao H., Otsuka A., Behar S. M., Yano I., Moody D. B., Sugita M. (2008) Mycolyltransferase-mediated glycolipid exchange in Mycobacteria. J. Biol. Chem. 283, 28835–28841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matsumoto M., Tanaka T., Kaisho T., Sanjo H., Copeland N. G., Gilbert D. J., Jenkins N. A., Akira S. (1999) A novel LPS-inducible C-type lectin is a transcriptional target of NF-IL6 in macrophages. J. Immunol. 163, 5039–5048 [PubMed] [Google Scholar]
- 20. Yamasaki S., Ishikawa E., Sakuma M., Hara H., Ogata K., Saito T. (2008) Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat. Immunol. 9, 1179–1188 [DOI] [PubMed] [Google Scholar]
- 21. Kishiro Y., Kagawa M., Naito I., Sado Y. (1995) A novel method of preparing rat-monoclonal antibody-producing hybridomas by using rat medial iliac lymph node cells. Cell Struct. Funct. 20, 151–156 [DOI] [PubMed] [Google Scholar]
- 22. Spada F. M., Borriello F., Sugita M., Watts G. F., Koezuka Y., Porcelli S. A. (2000) Low expression level but potent antigen presenting function of CD1d on monocyte lineage cells. Eur. J. Immunol. 30, 3468–3477 [DOI] [PubMed] [Google Scholar]
- 23. Pirson C., Jones G. J., Steinbach S., Besra G. S., Vordermeier H. M. (2012) Differential effects of Mycobacterium bovis-derived polar and apolar lipid fractions on bovine innate immune cells. Vet. Res. 43, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Furukawa A., Kamishikiryo J., Mori D., Toyonaga K., Okabe Y., Toji A., Kanda R., Miyake Y., Ose T., Yamasaki S., Maenaka K. (2013) Structural analysis for glycolipid recognition by the C-type lectins Mincle and MCL. Proc. Natl. Acad. Sci. U.S.A. 110, 17438–17443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miyake Y., Ishikawa E., Ishikawa T., Yamasaki S. (2010) Self and nonself recognition through C-type lectin receptor, Mincle. Self Nonself 1, 310–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yamasaki S., Matsumoto M., Takeuchi O., Matsuzawa T., Ishikawa E., Sakuma M., Tateno H., Uno J., Hirabayashi J., Mikami Y., Takeda K., Akira S., Saito T. (2009) C-type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia. Proc. Natl. Acad. Sci. U.S.A. 106, 1897–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cambier C. J., Takaki K. K., Larson R. P., Hernandez R. E., Tobin D. M., Urdahl K. B., Cosma C. L., Ramakrishnan L. (2014) Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids. Nature 505, 218–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang Y., Kulka K., Montelaro R. C., Reinhart T. A., Sissons J., Aderem A., Ojha A. K. (2014) A Hydrolase of Trehalose Dimycolate Induces Nutrient Influx and Stress Sensitivity [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roca F. J., Ramakrishnan L. (2013) TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell 153, 521–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Winthrop K. L. (2006) Risk and prevention of tuberculosis and other serious opportunistic infections associated with the inhibition of tumor necrosis factor. Nat. Clin. Pract. Rheumatol. 2, 602–610 [DOI] [PubMed] [Google Scholar]
- 31. Dascher C. C., Hiromatsu K., Xiong X., Morehouse C., Watts G., Liu G., McMurray D. N., LeClair K. P., Porcelli S. A., Brenner M. B. (2003) Immunization with a mycobacterial lipid vaccine improves pulmonary pathology in the guinea pig model of tuberculosis. Int. Immunol. 15, 915–925 [DOI] [PubMed] [Google Scholar]
- 32. Meraviglia S., El Daker S., Dieli F., Martini F., Martino A. (2011) γδ T cells cross-link innate and adaptive immunity in Mycobacterium tuberculosis infection. Clin. Dev. Immunol. 2011, 587315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stenger S., Mazzaccaro R. J., Uyemura K., Cho S., Barnes P. F., Rosat J. P., Sette A., Brenner M. B., Porcelli S. A., Bloom B. R., Modlin R. L. (1997) Differential effects of cytolytic T cell subsets on intracellular infection. Science 276, 1684–1687 [DOI] [PubMed] [Google Scholar]
- 34. Kondratieva E., Logunova N., Majorov K., Averbakh M., Apt A. (2010) Host genetics in granuloma formation: human-like lung pathology in mice with reciprocal genetic susceptibility to M. tuberculosis and M. avium. PLoS One 5, e10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Via L. E., Lin P. L., Ray S. M., Carrillo J., Allen S. S., Eum S. Y., Taylor K., Klein E., Manjunatha U., Gonzales J., Lee E. G., Park S. K., Raleigh J. A., Cho S. N., McMurray D. N., Flynn J. L., Barry C. E., 3rd. (2008) Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect. Immun. 76, 2333–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]