Abstract
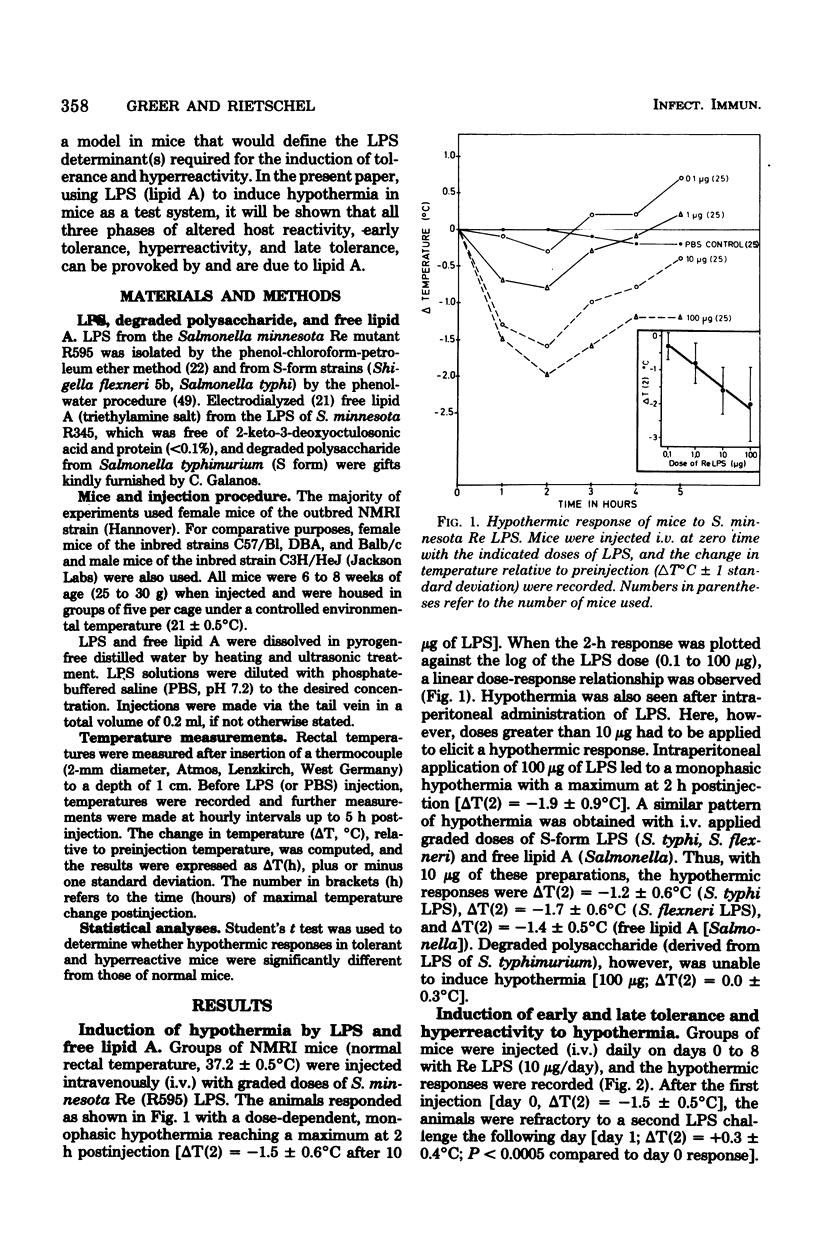
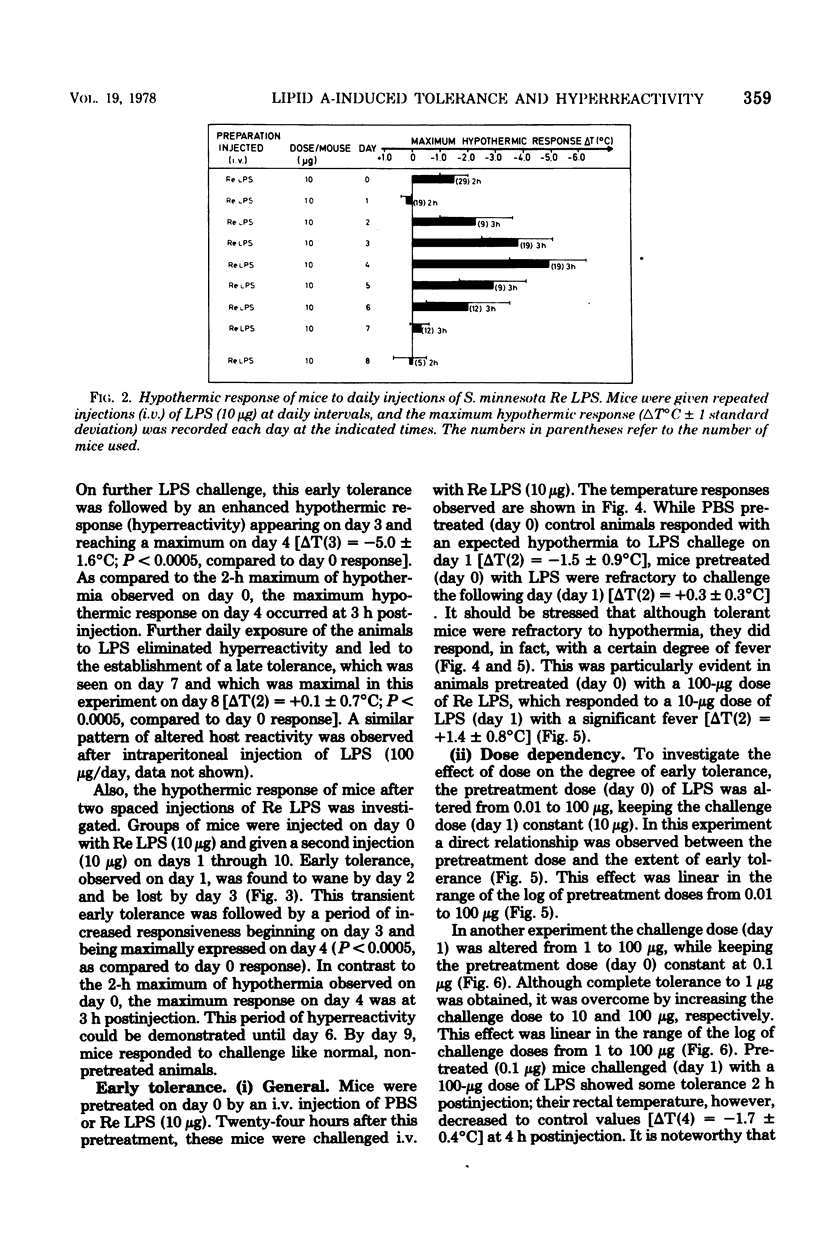
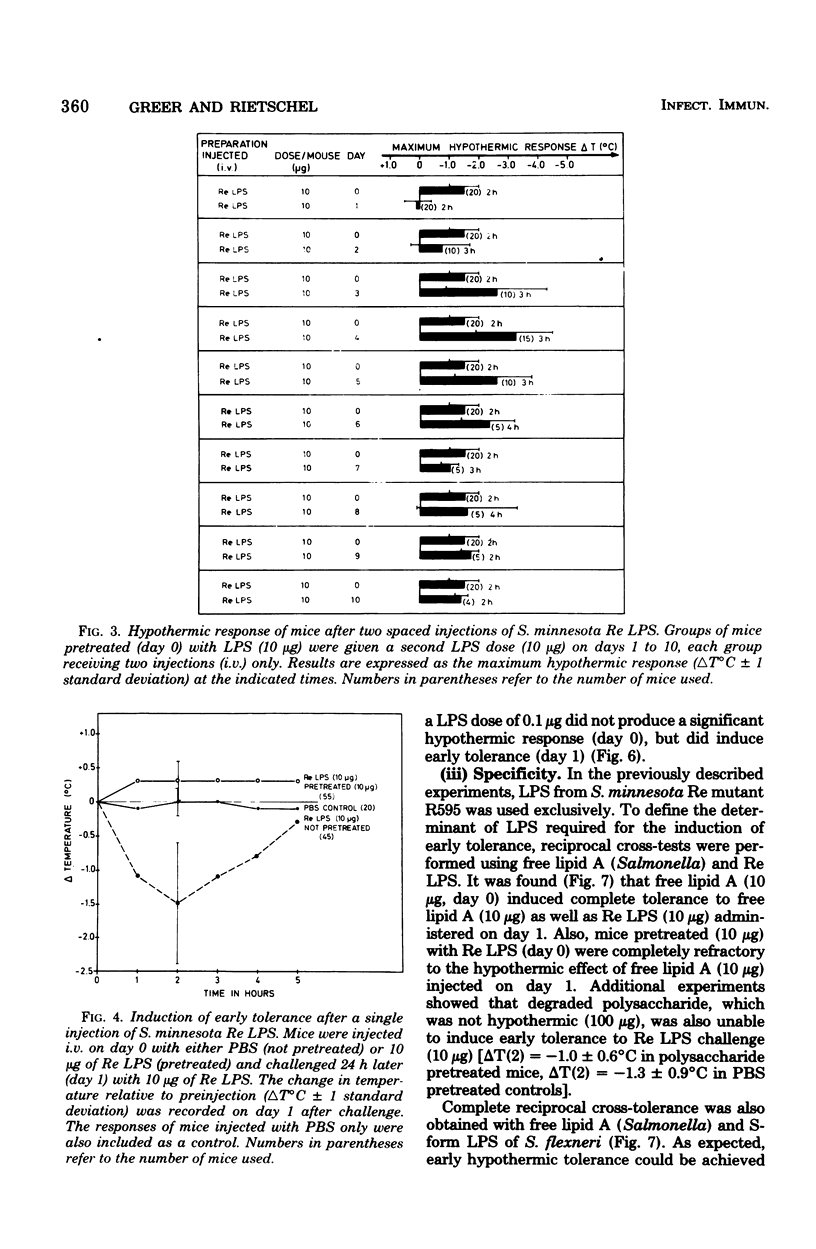
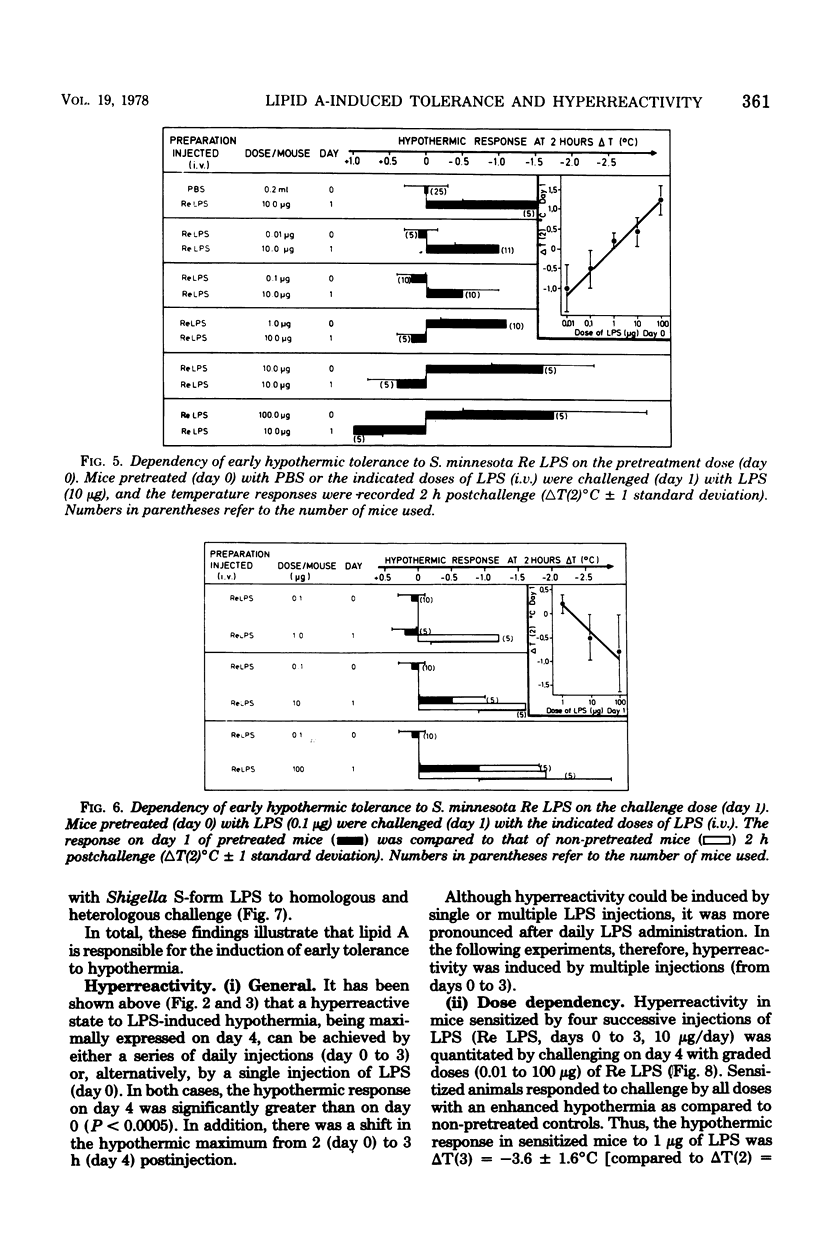
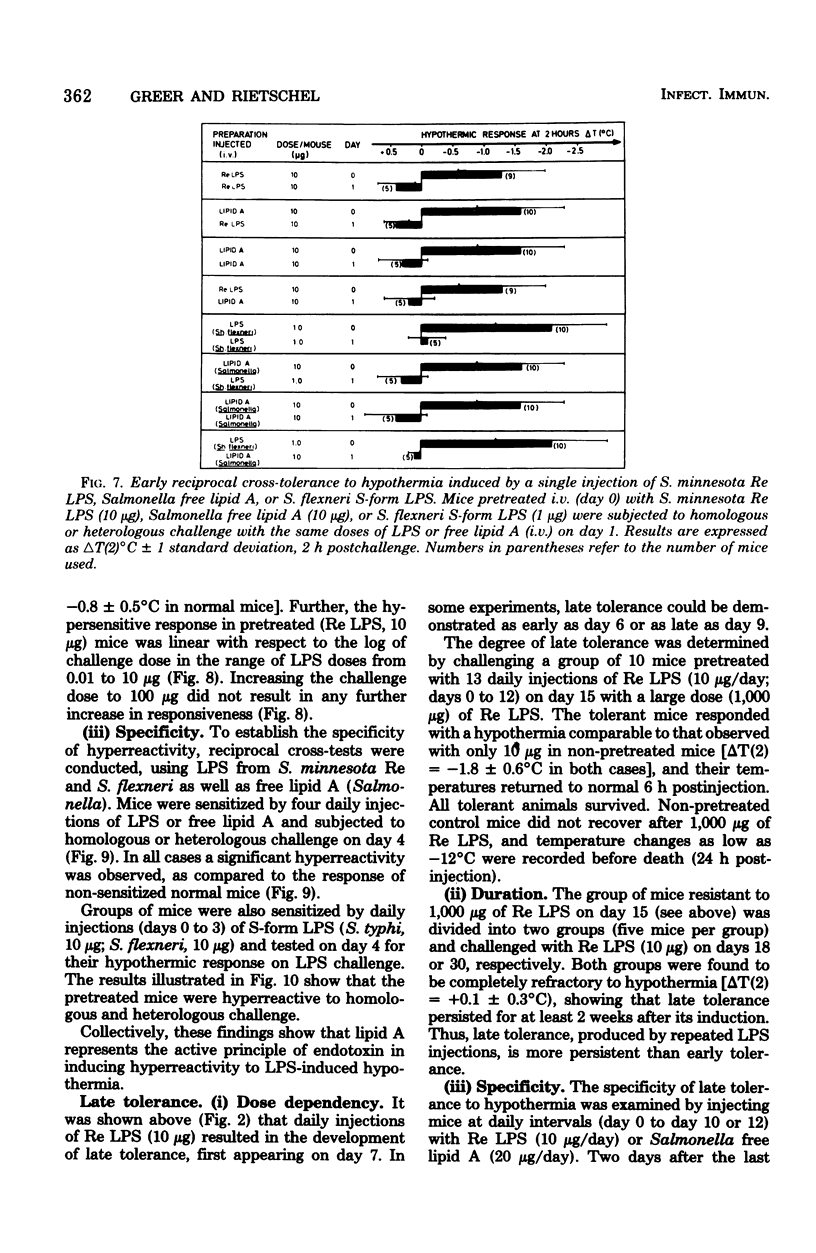
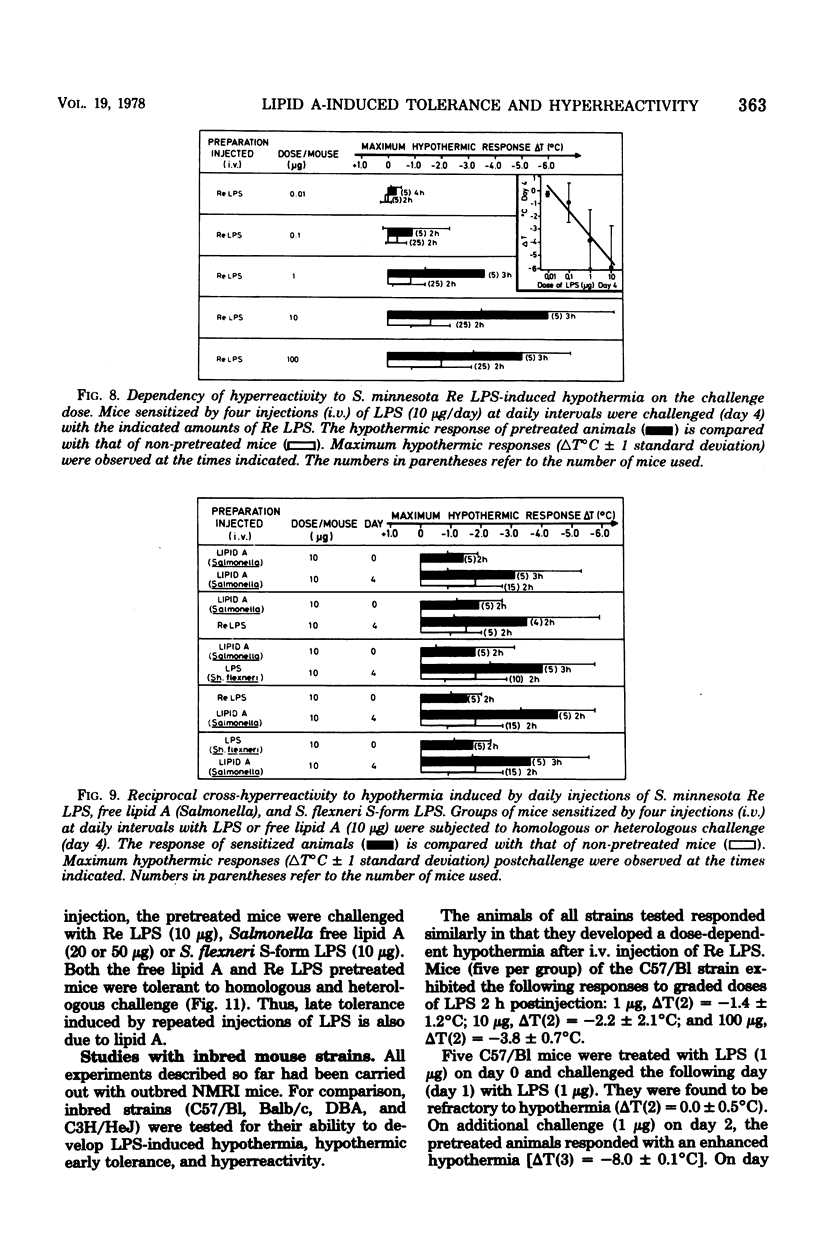
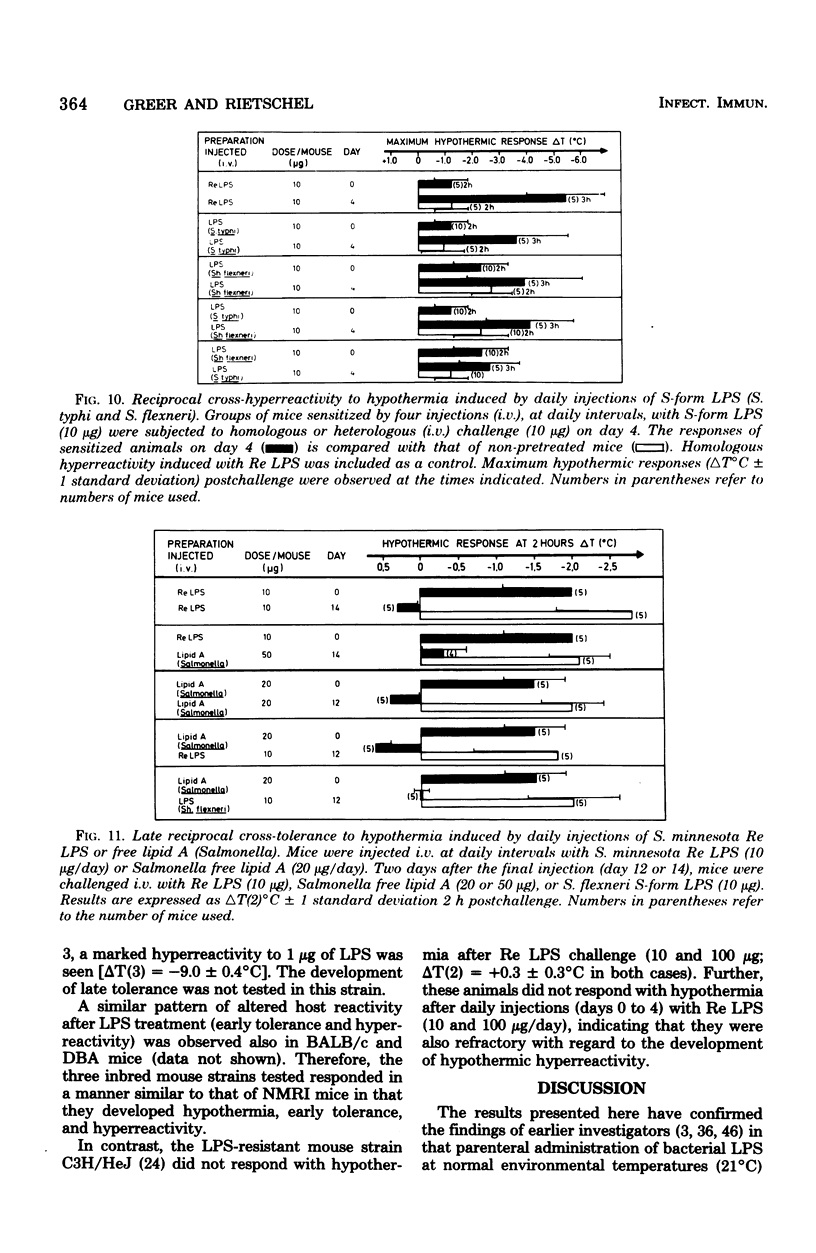
Mice responded to lipopolysaccharide (LPS) with a dose-dependent, monophasic hypothermia reaching a maximum at 2 h postinjection. Degraded polysaccharide was not active; free lipid A, however, induced a similar pattern of hypothermia, indicating that the hypothermic principle of LPS was embedded within the lipid A component. The hypothermic response of mice to LPS was modified by prior exposure of the host to LPS. This altered reactivity was manifested by refractory periods (early and late tolerance), in which animals no longer responded with hypothermia, or a hyperreactive phase (hypersensitivity), in which hypothermic responses were greatly augmented upon LPS challenge. Thus, tolerance observed 24 h after a single injection of LPS (early tolerance) was followed, on further LPS challenge, by an enhanced hypothermic responses reaching a maximum on day 4. Further daily exposure of the animals to LPS eliminated hyperreactivity and led to the establishment of a late tolerance maximally expressed on day 8. Hyperreactivity could also be evoked on day 4 after a single injection of LPS. Mice pretreated with Salmonella S- and R-form LPS or free lipid A (Salmonella) demonstrated tolerance and hyperreactivity to both homologous and heterologous challenge. In addition, complete cross-tolerance was observed with S-form LPS derived from Shigella. It was concluded that the differential effects of LPS on host responses (tolerance and hyperreactivity) were due to lipid A.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABERNATHY R. S. Homologous and heterologous resistance in mice given bacterial endotoxins. J Immunol. 1957 May;78(5):387–394. [PubMed] [Google Scholar]
- Berry L. J. Effect of environmental temperature on lethality of endotoxin and its effect on body temperature in mice. Fed Proc. 1966 Jul-Aug;25(4):1264–1270. [PubMed] [Google Scholar]
- Braude A. I., Douglas H. Passive immunization against the local Shwartzman reaction. J Immunol. 1972 Feb;108(2):505–512. [PubMed] [Google Scholar]
- Braude V. I. Morfologicheskie izmeneniia vne zony spetsificheskogo porazheniia pri tuberkuleze pochek. Probl Tuberk. 1975;(12):69–74. [PubMed] [Google Scholar]
- CLUFF L. E. Studies of the effect bacterial endotoxins on rabbit leucocytes. II. Development of acquired resistance. J Exp Med. 1953 Oct;98(4):349–364. doi: 10.1084/jem.98.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBOS R. J., SCHAEDLER R. W. The effect of bacterial endotoxins on the water intake and body weight of mice. J Exp Med. 1961 May 1;113:921–934. doi: 10.1084/jem.113.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. E., Brown K. R., Douglas H., Tate W. J., 3rd, Braude A. I. Prevention of death from endotoxin with antisera. I. The risk of fatal anaphylaxis to endotoxin. J Immunol. 1969 Mar;102(3):563–572. [PubMed] [Google Scholar]
- FREEDMAN H. H. Further studies on passive transfer of protection against lethality of endotoxin. Proc Soc Exp Biol Med. 1960 Apr;103:867–869. doi: 10.3181/00379727-103-25700. [DOI] [PubMed] [Google Scholar]
- FREEDMAN H. H. Passive transfer of protection against lethality of homologous heterologous endotoxins. Proc Soc Exp Biol Med. 1959 Nov;102:504–506. doi: 10.3181/00379727-102-25296. [DOI] [PubMed] [Google Scholar]
- FREEDMAN H. H. Passive transfer of tolerance to pyrogenicity of bacterial endotoxin. J Exp Med. 1960 Apr 1;111:453–463. doi: 10.1084/jem.111.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filkins J. P., Di Luzio N. R. Endotoxin induced hypothermia and tolerance in the rat. Proc Soc Exp Biol Med. 1968 Dec;129(3):724–726. doi: 10.3181/00379727-129-33409. [DOI] [PubMed] [Google Scholar]
- Freedman H. H., Fox A. E., Willis R. S., Schwartz B. S. Induced sensitization of normal laboratory animals to Brucella abortus endotoxin. J Bacteriol. 1968 Feb;95(2):286–290. doi: 10.1128/jb.95.2.286-290.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman H. H., Fox A. E., Willis R. S., Schwartz B. S. Role of protein component of endotoxin in modification of host reactivity. Proc Soc Exp Biol Med. 1967 Aug-Sep;125(4):1316–1320. doi: 10.3181/00379727-125-32346. [DOI] [PubMed] [Google Scholar]
- Fruhman G. J. Endotoxins and leukocyte mobilization. J Reticuloendothel Soc. 1972 Jul;12(1):62–79. [PubMed] [Google Scholar]
- Galanos C., Lüderitz O. Electrodialysis of lipopolysaccharides and their conversion to uniform salt forms. Eur J Biochem. 1975 Jun;54(2):603–610. doi: 10.1111/j.1432-1033.1975.tb04172.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. Preparation and properties of antisera against the lipid-A component of bacterial lipopolysaccharides. Eur J Biochem. 1971 Dec 22;24(1):116–122. doi: 10.1111/j.1432-1033.1971.tb19661.x. [DOI] [PubMed] [Google Scholar]
- Glode L. M., Scher I., Osborne B., Rosenstreich D. L. Cellular mechanism of endotoxin unresponsiveness in C3H/HeJ mice. J Immunol. 1976 Feb;116(2):454–461. [PubMed] [Google Scholar]
- Greisman S. E., Young E. J., Carozza F. A., Jr Mechanisms of endotoxin tolerance. V. Specificity of the early and late phases of pyrogenic tolerance. J Immunol. 1969 Dec;103(6):1223–1236. [PubMed] [Google Scholar]
- Greisman S. E., Young E. J., DuBuy B. Mechanisms of endotoxin tolerance. 8. Specificity of serum transfer. J Immunol. 1973 Nov;111(5):1349–1360. [PubMed] [Google Scholar]
- Greisman S. E., Young E. J., Woodward W. E. Mechanisms of endotoxin tolerance. IV. Specificity of the pyrogenic refractory state during continuous intravenous infusions of endotoxin. J Exp Med. 1966 Nov 1;124(5):983–1000. doi: 10.1084/jem.124.5.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami M., Hara Y., Osawa N. Experimental salmonellosis: hypersensitivity to cell wall lipopolysaccharide and anti-infectious resistance of mice infected with Salmonella. Infect Immun. 1971 Nov;4(5):519–524. doi: 10.1128/iai.4.5.519-524.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. B., Watson D. W. Biologically active endotoxins from Salmonella mutants deficient in O- and R-polysaccharides and heptose. J Bacteriol. 1967 Nov;94(5):1320–1326. doi: 10.1128/jb.94.5.1320-1326.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. B., Watson D. W. Role of antibodies in reactions to gram-negative bacterial endotoxins. Ann N Y Acad Sci. 1966 Jun 30;133(2):727–745. doi: 10.1111/j.1749-6632.1966.tb52402.x. [DOI] [PubMed] [Google Scholar]
- LANDY M., PILLEMER L. Increased resistance to infection and accompanying alteration in properidin levels following administration of bacterial lipopolysaccharides. J Exp Med. 1956 Sep 1;104(3):383–409. doi: 10.1084/jem.104.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE L., STETSON C. A., Jr Studies on the mechanism of the Shwartzman phenomenon. Accelerated cutaneous reactivity to bacterial endotoxins. J Exp Med. 1960 Jun 1;111:761–772. doi: 10.1084/jem.111.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner K. C. Patterns of tolerance to endotoxin. J Infect Dis. 1973 Jul;128(Suppl):237–245. doi: 10.1093/infdis/128.supplement_1.s237. [DOI] [PubMed] [Google Scholar]
- Morgan H. R. RESISTANCE TO THE ACTION OF THE ENDOTOXINS OF ENTERIC BACILLI IN MAN. J Clin Invest. 1948 Nov;27(6):706–709. doi: 10.1172/JCI102019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prashker D., Wardlaw A. C. Temperature responses of mice to Escherichia coli endotoxin. Br J Exp Pathol. 1971 Feb;52(1):36–46. [PMC free article] [PubMed] [Google Scholar]
- Quesenberry P., Halperin J., Ryan M., Stohlman F., Jr Tolerance to the granulocyte-releasing and colony-stimulating factor elevating effects of endotoxin. Blood. 1975 Jun;45(6):789–800. [PubMed] [Google Scholar]
- Rietschel E. T., Kim Y. B., Watson D. W., Galanos C., Lüderitz O., Westphal O. Pyrogenicity and immunogenicity of lipid A complexed with bovine serum albumin or human serum albumin. Infect Immun. 1973 Aug;8(2):173–177. doi: 10.1128/iai.8.2.173-177.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. M., Snyder I. S. Effect of lipopolysaccharide and lipid A on mouse liver pyruvate kinase activity. Infect Immun. 1975 Nov;12(5):993–998. doi: 10.1128/iai.12.5.993-998.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splawiński J. A., Zacny E., Górka Z. Fever in rats after intravenous E. coli endotoxin administration. Pflugers Arch. 1977 Mar 11;368(1-2):125–128. doi: 10.1007/BF01063464. [DOI] [PubMed] [Google Scholar]
- Tate W. J., 3rd, Douglas H., Braude A. I., Wells W. W. Protection against lethality of E. coli endotoxin with "O" antiserum. Ann N Y Acad Sci. 1966 Jun 30;133(2):746–762. doi: 10.1111/j.1749-6632.1966.tb52403.x. [DOI] [PubMed] [Google Scholar]
- Urbaschek B., Nowotny A. Endotoxin tolerance induced by detoxified endotoxin (endotoxoid). Proc Soc Exp Biol Med. 1968 Feb;127(2):650–652. doi: 10.3181/00379727-127-32765. [DOI] [PubMed] [Google Scholar]
- WATSON D. W., KIM Y. B. MODIFICATION OF HOST RESPONSES TO BACTERIAL ENDOTOXINS. I. SPECIFICITY OF PYROGENIC TOLERANCE AND THE ROLE OF HYPERSENSITIVITY IN PYROGENICITY, LETHALITY, AND SKIN REACTIVITY. J Exp Med. 1963 Sep 1;118:425–446. doi: 10.1084/jem.118.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw A. C., Boorman L., Reid R. Assay of endotoxin by the hypothermic response of mice. Br J Exp Pathol. 1971 Apr;52(2):198–208. [PMC free article] [PubMed] [Google Scholar]



