Background: Mutations of α-synuclein (αSyn) can cause early-onset familial Parkinson disease (PD).
Results: The H50Q, H50D, or H50A substitution promotes, whereas the H50R substitution inhibits, αSyn aggregation in vitro.
Conclusion: The recently identified PD-causing αSyn mutant, αSyn(H50Q), accelerates αSyn aggregation.
Significance: The partial positive charge of His-50 at physiological pH likely plays a role in suppressing αSyn aggregation.
Keywords: Mutant, Nuclear Magnetic Resonance, Parkinson Disease, Protein Aggregation, Synuclein
Abstract
α-Synuclein (αSyn) aggregation is involved in the pathogenesis of Parkinson disease (PD). Recently, substitution of histidine 50 in αSyn with a glutamine, H50Q, was identified as a new familial PD mutant. Here, nuclear magnetic resonance (NMR) studies revealed that the H50Q substitution causes an increase of the flexibility of the C-terminal region. This finding provides direct evidence that this PD-causing mutant can mediate long range effects on the sampling of αSyn conformations. In vitro aggregation assays showed that substitution of His-50 with Gln, Asp, or Ala promotes αSyn aggregation, whereas substitution with the positively charged Arg suppresses αSyn aggregation. Histidine carries a partial positive charge at neutral pH, and so our result suggests that positively charged His-50 plays a role in protecting αSyn from aggregation under physiological conditions.
Introduction
Formation of punctate cytoplasmic protein aggregates, known as Lewy bodies in dopaminergic neurons, is a pathological hallmark of PD4 (1, 2). αSyn is a major component of Lewy bodies in which αSyn deposits as β-sheet-rich fibrils (1). Genetically, αSyn gene duplication (3, 4), triplication (5), and missense mutations (6–8) were found to cause early-onset familial PD, which provide a direct genetic link between the αSyn gene and PD pathogenesis. Studies have found that the PD-causing αSyn mutations that lead to single amino acid substitution as A30P, E46K, and A53T accelerate αSyn aggregation into either oligomers or fibrils in vitro (9–13). Moreover, when αSyn or PD-causing αSyn mutants were overexpressed in mice or Drosophila, formation of αSyn aggregates was observed, and the time of αSyn inclusion formation correlates with the progression of PD-like symptoms (14–17). Together, these studies have shown that αSyn aggregation plays a critical role in PD pathogenesis.
αSyn is a 140-amino acid protein that is predominately expressed in the brain (18). αSyn consists of an N-terminal amphipathic domain (amino acids 1–60), a hydrophobic non-Aβ component domain (amino acids 61–95), and an acidic C-terminal region (amino acids 95–140) (19). Aggregation of soluble αSyn monomers into insoluble fibrils can be recapitulated in vitro, in which αSyn aggregation is nucleation and concentration-dependent (20). Analysis of αSyn fibrils revealed that the region encompassing amino acids 32–102 is involved in the formation of the fibril core (21). Although the C-terminal acidic region is not part of the αSyn fibril core, deletion of the C-terminal region significantly promotes αSyn aggregation in vitro and in mice (22–26). Therefore, the C-terminal region plays a role in regulating αSyn aggregation. In support of this, results from several NMR studies have found that the C terminus of αSyn interacts with the N-terminal domain and the non-Aβ component region (27–29). Despite the importance of these long range interactions, their effect on αSyn aggregation had not been defined.
Recently, the H50Q substitution in αSyn was identified as a familial PD-causing mutant by two different groups (30, 31). In the current study NMR experiments demonstrate that the H50Q substitution, but not other examined substitutions, increased the flexibility of the αSyn C-terminal region. Substitution of His-50 with Glu, Asp, or Ala promoted αSyn aggregation in vitro. In striking contrast, the H50R substitution suppressed αSyn aggregation. These results suggest that under physiological pH, a partial positive charge carried by His-50 plays an important role in protecting αSyn from aggregation.
EXPERIMENTAL PROCEDURES
Plasmids
The cDNA of the human SNCA was subcloned into the pET28(a) vector using the NcoI and XhoI sites. All αSyn mutations were generated by site-directed mutagenesis based on the pET28(a)-αSyn plasmid. All plasmids were confirmed by DNA sequencing.
Purification of αSyn
BL21(DE3)-RIPL cells harboring a corresponding αSyn-expressing plasmid were grown at 37 °C in the presence of 40 μg/ml kanamycin and 34 μg/ml chloramphenicol. At A600 = 0.3, cells were cooled to 25 °C. At A600 = 0.6, 0.45 mm isopropyl 1-thio-β-d-galactopyranoside was added into the culture, and protein expression was induced at 25 °C for 16 h. Cells were harvested and resuspended in the cell lysis buffer (20 mm Tris, 100 mm NaCl, and 10% glycerol) supplemented with 1 mm PMSF and 10 μm leupeptin. After sonication the lysate was cleared by centrifugation at 30,000 × g for 30 min at 4 °C. Next, ammonium sulfate powder was slowly added to the supernatant to a final concentration of 1 m. 2 ml of phenyl-Sepharose resins (GE Healthcare) were then added, and the mixtures were rocked at 4 °C for 2 h. The mixtures were then loaded onto a gravity column (46 × 200 mm), and the αSyn-containing flow-through was dialyzed against the cell lysis buffer at 4 °C overnight. The dialyzed mixtures were then applied to a 30-ml Q-Sepharose column on an AKTA FPLC system (GE Healthcare). αSyn eluted between 0.20 and 0.35 m NaCl. The αSyn-containing fractions were pooled and dialyzed against the cell lysis buffer and then applied to a 5-ml Mono Q column (GE Healthcare) on an AKTA FPLC system. αSyn eluted between 0.20 and 0.28 m NaCl. The αSyn-containing fractions were collected, and the final αSyn or αSyn mutant protein purity was >95% based on Coomassie-stained SDS-PAGE analysis. To purify 15N-labeled or 13C- and 15N-labeled αSyn or αSyn mutants, BL21(DE3)-RIPL cells harboring a corresponding αSyn-expressing plasmid were grown in M9 minimal medium containing 1 g/liter [15N]ammonium chloride or 1 g/liter [15N]ammonium chloride with 2 g/liter [13C]glucose, respectively. αSyn and αSyn mutants were purified as described above.
All αSyn proteins were dialyzed into 20 mm NH4HCO3 followed by lyophilization and stored as dry powders. To make αSyn samples for NMR or in vitro aggregation experiments, αSyn powders were dissolved in an appropriate buffer at ∼5 mg/ml and then dialyzed against 2 liters of the experimental buffer. αSyn concentration was determined by using an extinction coefficient of 5120 m−1 cm−1 at 280 nm, except αSyn(DYE/A), which has an extinction coefficient of 3840 m−1 cm−1 at 280 nm.
NMR Spectroscopy
All NMR data were recorded with a Varian 900 MHz NMR spectrometer (Rocky Mountain Regional 900 MHz NMR Facility at University of Colorado Denver Anschutz Medical Campus) at 10 °C in a buffer containing 10 mm sodium phosphate, pH 7.4, 100 mm NaCl, and 6.7% D2O. NMR data were processed by NMRpipe software package (32) and analyzed using CCPNmr (33). Backbone amide assignments of wild type αSyn were completed by using two-dimensional 1H,15N HSQC and three-dimensional HNCACB, CBCA(CO)NH, and NOE experiments together with previously published data (34). Chemical shift changes (Δδ) were processed by using the formula Δδ = [(1/5ΔδN)2 + (ΔδH)2]1/2, where δN and δH represent the difference in nitrogen and proton chemical shifts, respectively. NMR R1 relaxation rate measurements employed relaxation delay times of 10, 100, 200, 300, 400, 500, 600, 700, 800, 900, 1000, and 1100 ms. For determination of R1 (spin-lattice) relaxation rates, resonance amplitudes were extracted and fit as a function of the relaxation delay time.
Thioflavin T Binding Assay
αSyn and αSyn mutants were passed through a YM-100 spin column (Pall Corp.) by centrifugation to remove any potential oligomers or insoluble aggregates before being used for the aggregation assays. The mixtures in the thioflavin T binding assays contained 100 μm αSyn or αSyn mutants, 0.05% NaN3, and 20 μm thioflavin T in PBS, pH 7.4. Quadruple samples were set up for each protein. All samples were kept in a 96-well round-bottom white plate and sealed with an optical adhesive cover (Applied Biosystems). During data acquisition samples were maintained at 37 °C with constant shaking in a Synergy HT microplate reader (BioTek). Shaking was stopped for 1 min for every 15 min because of data acquisition. Thioflavin T fluorescence was recorded using emission/excitation filters at 440/30 and 485/20 nm, respectively. In a kinetic mode the instrument allows 300 maximal readings, and the maximal shaking time is 1000 s before each stop for data acquisition. So we chose to shake the plate for 15 min before each data acquisition in a 72-h kinetic mode. Also, the maximal reading is 100,000 units. With these limitations, some of the aggregation reactions had not completely reached plateaus yet, and the readings for some of the aggregation reactions exceeded the detection limit.
Circular Dichroism (CD) Spectroscopy
Far-UV CD measurements of αSyn proteins were collected using a J815 spectrometer (Jasco). All αSyn samples were prepared at 20 μm in PBS, pH 7.4, and kept in a quartz cuvette with 0.1-cm path length for CD measurements. CD spectra were monitored from 260 to 190 nm with scan speed of 50 nm/min. Six scans were collected for every sample, and average data were shown in the figures. The spectrum of the buffer alone was subtracted as background.
Protease K Digestion Assay
αSyn and αSyn(H50Q) fibrils (3 mg/ml) in 20 mm Tris, pH 7.4, 1 mm EGTA were incubated with proteinase K (3 μg/ml) at 37 °C for 1, 5, 15, and 60 min. At the end of each time point, aliquoted samples were mixed with 5× SDS sample buffer and heated to 90 °C for 10 min to stop the reaction. Proteins were then separated on SDS-PAGE and stained with Coomassie.
RESULTS
αSyn(H50Q) Is a Native Unfolded Protein in Aqueous Buffers
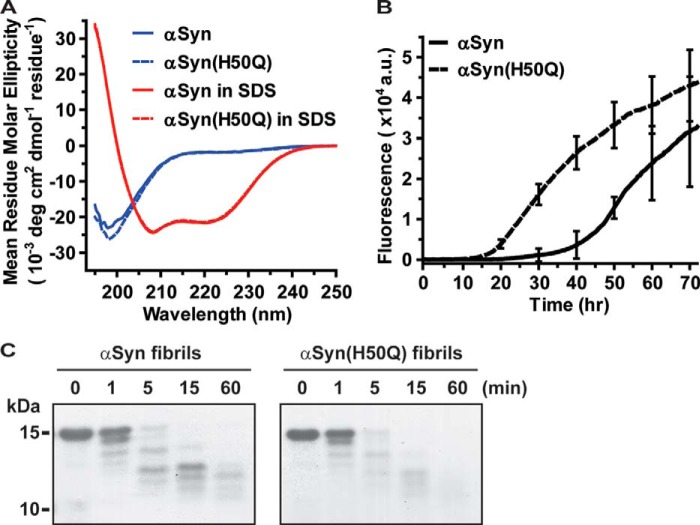
Although αSyn has long been considered as a native unfolded protein in aqueous buffers (35, 36), two recent studies have suggested that αSyn existed as a homotetramer with an α-helical conformation when it was purified using non-denaturing methods (37, 38). In this study recombinant αSyn and αSyn mutants were purified by a non-denaturing method as described under “Experimental Procedures.” The purity of all proteins was >95% as determined by Coomassie-stained SDS-PAGE (data not shown). The secondary structures of αSyn and αSyn(H50Q) were determined by far-UV CD spectroscopy. In PBS buffer both proteins had similar minima at 198 nm (Fig. 1A), which is a characteristic trait of unstructured proteins. Consistent with this result, NMR 1H,15N HSQC spectra of αSyn and αSyn(H50Q) exhibited relatively narrow dispersions in the proton dimension (Fig. 2A), indicative of relatively unstructured proteins as well (39). Together, these results indicate that recombinant αSyn and αSyn(H50Q) are primarily unfolded proteins in aqueous buffers.
FIGURE 1.
The H50Q substitution promotes αSyn aggregation in vitro. A, far-UV CD spectrum of 20 μm αSyn (blue solid line) or αSyn(H50Q) (blue dashed line) in pH 7.4 PBS; αSyn (red solid line) or αSyn(H50Q) (red dashed line) in PBS containing 2 mm SDS. B, thioflavin T binding assays for monitoring 100 μm αSyn (solid line) or αSyn(H50Q) (dashed line) aggregation in pH 7.4 PBS containing 0.05% NaN3 and 20 μm thioflavin T. The data were collected at every 15 min using a microplate reader. Representative error bars at every 10-h time point represent mean ± S.D. of four assays. a.u., arbitrary units. C, αSyn and αSyn(H50Q) fibrils (3 mg/ml) in 20 mm Tris, pH 7.4, 1 mm EGTA were incubated with proteinase K (3 μg/ml) at 37 °C. At each time point, aliquoted samples were mixed with 5× SDS sample buffer and heated at 90 °C for 10 min. Proteins were then separated on SDS-PAGE and stained with Coomassie.
FIGURE 2.
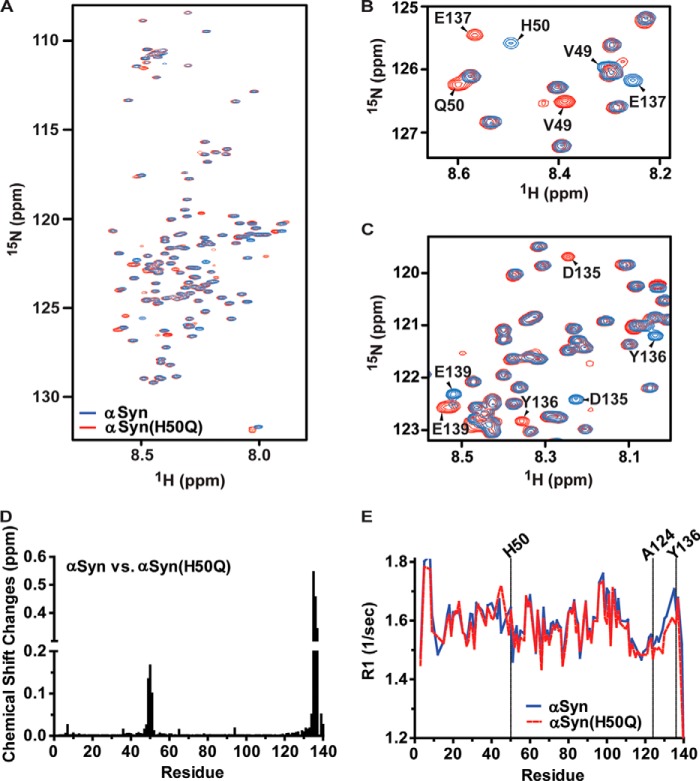
The H50Q substitution increases the flexibility of the αSyn C-terminal region. A, overlapped 1H,15N HSQC spectrum of 100 μm αSyn (blue) with that of αSyn(H50Q) (red). B and C, zoom-in view of HSQC regions that have significant chemical shift changes between αSyn (blue) and αSyn(H50Q) (red). Residues that have chemical shift changes were labeled. D, relative chemical shift changes of αSyn versus αSyn(H50Q) based on the HSQC spectra in Fig. 2A. E, R1 relaxation rates of αSyn (blue) and αSyn(H50Q) (red).
The N-terminal region of αSyn (amino acid 1–95) can bind to the surface of vesicles or micelles and form an α-helical structure (40, 41). As shown in Fig. 1A, the far-UV CD spectra of αSyn and αSyn(H50Q) in 2 mm SDS micelles had very similar minima at 208 and 222 nm, indicating that both are predominantly α-helical when binding to SDS micelles. Thus, the H50Q substitution does not alter αSyn conformations in aqueous buffer and SDS micelles.
αSyn(H50Q) Aggregates More Rapidly Than αSyn in Vitro
Thioflavin T is a fluorescent dye whose fluorescence increases significantly when binding to β-sheet-rich fibrils, and it has been used widely to monitor protein fibrillization in vitro (24, 38, 42, 43). In a typical time-course thioflavin T-based fibrillization assay, the lag phase reflects the nucleation process and shows no change in thioflavin T fluorescence. In the polymerization phase, thioflavin T fluorescence increases significantly, indicative of the formation of β-sheet-rich αSyn fibrils. When αSyn monomer is consumed in the reaction, the thioflavin T fluorescence reaches a plateau, which initiates the final phase of a typical aggregation curve. As shown in Fig. 1B, αSyn(H50Q) exhibited a much shorter lag phase than wild type αSyn in the thioflavin T binding assays, indicating that the H50Q substitution enhances the nucleation process and thus promotes αSyn aggregation in vitro.
To determine whether the H50Q substitution could change the composition of the fibril core, αSyn or αSyn(H50Q) was incubated at 37 °C with constant shaking for 7 days to produce mature fibrils. The resulting fibrils were then subjected to proteinase K digestion. Both αSyn and αSyn(H50Q) fibrils exhibited similar cleavage kinetics and produced similar cleavage products in the proteinase K digestion assays (Fig. 1C), indicating that the structural cores of αSyn and αSyn(H50Q) fibrils are similar.
The H50Q Substitution Increases the Flexibility of the αSyn C-terminal Region
The NMR HSQC experiment is a sensitive tool for probing changes of the microenvironment of backbone amides in proteins and is, therefore, suitable for determining conformational changes that occur as a result of amino acid substitutions. We have recorded the 1H,15N HSQC spectrum of αSyn or αSyn(H50Q). The backbone amides of αSyn were assigned by using two-dimensional 1H,15N HSQC, three-dimensional HNCACB, CBCA(CO)NH, and NOE experiments together with published data (34). Nearly all of the non-proline backbone amides of αSyn in the HSQC experiment were assigned except Asp-2. The chemical shifts of most residues between αSyn and αSyn(H50Q) were unperturbed (Fig. 2A). As expected, chemical shift differences for residues surrounding His-50 were observed as a direct result of the altered local chemical environment from the H50Q substitution (Fig. 2B). Of utmost interest, there were significant chemical shift changes observed for residues at the extreme C terminus of αSyn including Asp-135, Tyr-136, Glu-137, Glu-139, and Ala-140 (Fig. 2, B and C). The new chemical shifts of these residues in the αSyn(H50Q) HSQC spectrum were assigned using an three-dimensional HNCACB experiment with 13C,15N-labeled αSyn(H50Q). By plotting the relative chemical shift change of each residue in αSyn and αSyn(H50Q) HSQC spectra, it is apparent that the H50Q substitution caused chemical shift changes for residues surrounding His-50 but also for residues in the extreme C-terminal region (Fig. 2D).
To determine whether the H50Q substitution alters αSyn conformation, we chose to use amide R1 relaxation rate to monitor αSyn backbone dynamics. Amide R1 relaxation rates are a direct measure of the flexibility of a given amide and thereby can be utilized to monitor the backbone dynamics of proteins. Due to the fact that αSyn is primarily an unfolded protein, its backbone relaxation rates are in the extreme narrowing limit (very fast motion and very short correlation time). In such a regime a higher R1 relaxation rate indicates a less flexible structural conformation. As shown in Fig. 2E, the H50Q substitution caused a substantial decrease of the R1 values for residues from 124–136 in comparison to those in αSyn. Thus, the C-terminal region of αSyn(H50Q) is more flexible than that of αSyn.
We then asked whether other PD-causing αSyn mutants including αSyn(A30P), αSyn(E46K), and αSyn(A53T) affected the conformation of the C-terminal region in the same way as the H50Q substitution. It is somewhat surprising that none of these three αSyn mutants caused significant chemical shifts of the C-terminal residues (data not shown). A previous study has also shown that αSyn(A30P) and αSyn(A53T) did not result in any change in the R1 and R2 relaxation rates in the C-terminal region (44). These results indicate that PD-causing αSyn mutants have different effects on αSyn conformations.
The H50Q Substitution Accelerates αSyn(DYE/A) Aggregation
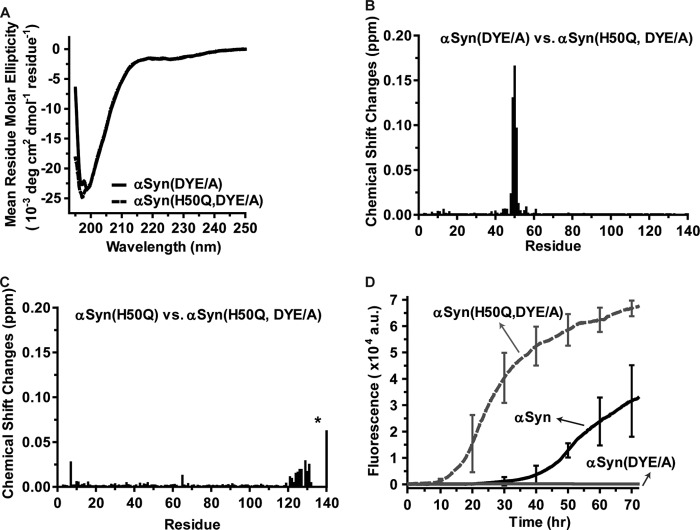
To examine whether mutating the C-terminal residues that experienced the largest chemical shift changes induced by the H50Q substitution could disrupt H50Q-induced conformational changes, we produced a αSyn mutant in which Asp-135, Tyr-136, and Glu-137 were all mutated to an alanine, referred to as αSyn(DYE/A). In addition, we had also made αSyn(DYE/A) bearing the H50Q substitution, referred to as αSyn(H50Q, DYE/A). Both proteins predominantly adopted a random coil conformation in PBS as demonstrated by far-UV CD spectroscopic analyses (Fig. 3A). Comparing HSQC spectra of αSyn(DYE/A) and αSyn(H50Q, DYE/A) revealed that the H50Q substitution did not cause chemical shift changes of the C-terminal residues in αSyn(H50Q, DYE/A) (Fig. 3B). Thus, amino acids 135–137 are important for H50Q-induced chemical shift perturbations in the C-terminal region.
FIGURE 3.
The H50Q substitution promotes aggregation of the αSyn(DYE/A) mutant. A, far-UV CD spectrum of 20 μm αSyn(DYE/A) (solid line) or αSyn(H50Q, DYE/A) (dashed line) in pH 7.4 PBS buffer. B, relative chemical shift changes of αSyn(DYE/A) versus αSyn(H50Q, DYE/A) based on the 1H,15N HSQC spectrum of each protein (spectra not shown). C, relative chemical shift changes of αSyn(H50Q) versus αSyn(H50Q, DYE/A) based on the 1H,15N HSQC spectrum of each protein (spectra not shown) D, thioflavin T binding assays for monitoring αSyn, αSyn(DYE/A), or αSyn(H50Q, DYE/A) aggregation. Representative error bars at every 10-h time point represent mean ± S.D. of four assays. a.u., arbitrary units.
Because the H50Q substitution caused significant chemical shift changes of Asp-135, Tyr-136, and Glu-137 (Fig. 2D), we then examined if mutating these residues alters the chemical environment of Gln-50 by comparing the HSQC spectra of αSyn(Gln-50) and αSyn(Gln-50, DYE/A). As shown in Fig. 3C, the DYE/A substitutions did not induce obvious chemical shift change of Gln-50, indicating that the DYE/A substitutions have no effect on the chemical environment of Gln-50.
We next examined the aggregation propensity of these mutants using the in vitro thioflavin T binding assay. αSyn(DYE/A) significantly inhibited αSyn aggregation (Fig. 3D), similar to previous studies in which the Y136A substitution was found to inhibit αSyn aggregation in vitro (45, 46). Interestingly, the H50Q substitution was able to promote aggregation of αSyn(DYE/A) (Fig. 3D), indicating that the H50Q substitution plays a dominant role in promoting αSyn aggregation.
The H50R Substitution Suppresses αSyn Aggregation
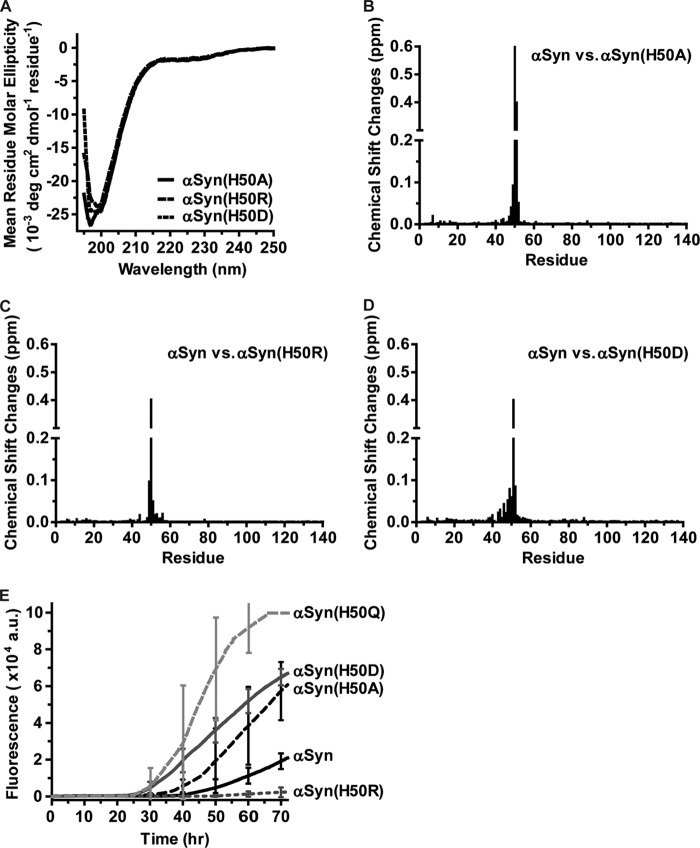
His-50 is the only histidine residue in αSyn. Free histidine has a pKa of ∼6.0, and it carries a partial positive charge at neutral pH. We wondered whether the partial positive charge carried by His-50 has an influence on the structure and aggregation of αSyn. To investigate this we substituted His-50 with Ala, Asp, or Arg in addition to the examined Gln. Far-UV CD spectra show that these three substitutions had a minimal absorption at 198 nm (Fig. 4A), indicating that these proteins primarily adopt a random coil conformation in PBS buffer. We next purified 15N-labeled proteins for HSQC experiments. Unlike the H50Q substitution (Fig. 2D), none of these three mutants caused chemical shift perturbations in the C-terminal region compared with wild type αSyn (Fig. 4, B–D). Together, the C-terminal conformational changes are likely unique to the H50Q substitution.
FIGURE 4.
The H50R substitution suppresses αSyn aggregation in vitro. A, far-UV CD spectrum of 20 μm αSyn(H50A) (solid line), αSyn(H50R) (long dashed line), or αSyn(H50D) (short dashed line) in pH 7.4 PBS buffer. B–D, chemical shift changes of αSyn versus αSyn(H50A) (B), αSyn versus αSyn(H50R) (C), or αSyn versus αSyn(H50D) (D) based on the 1H,15N HSQC spectrum of each protein (spectra not shown). E, thioflavin T binding assays for monitoring αSyn, αSyn(H50Q), αSyn(H50A), αSyn(H50R), or αSyn(H50D) aggregation. Representative error bars at every 10-h time point represent the mean ± S.D. of four assays. a.u., arbitrary units.
We next evaluated the effect of these His-50 substitutions on αSyn aggregation using the in vitro thioflavin T binding assay. Both the H50A and H50D substitutions accelerated αSyn aggregation as their aggregation curves had much shorter lag phases than that of αSyn. In striking contrast, the H50R substitution greatly suppressed αSyn aggregation (Fig. 4E). Thus, substitution of His-50 with a positively charge arginine inhibits αSyn aggregation.
DISCUSSION
In this report we characterized the newly identified PD-causing αSyn mutant bearing the H50Q substitution. Our results show that the H50Q substitution significantly accelerated αSyn aggregation as monitored by in vitro thioflavin T binding assays. Using NMR HSQC experiments, we found that the H50Q substitution caused conformational changes on the extreme C terminus of αSyn and increased the flexibility of this region. In contrast, none of the other examined substitutions on His-50 including H50A, H50D, and H50R or other PD-causing αSyn mutants including A30P, E46K, or A53T had detectable conformational changes on the C-terminal region in HSQC experiments. Because all of these αSyn mutants adopted a random coil conformation in aqueous buffer, our results suggest that the H50Q substitution causes long range tertiary interactions with the C-terminal region. Using NMR paramagnetic relaxation enhancement experiments, several studies have suggested that αSyn has tertiary structures mediated by long range interactions between the C-terminal region and the N-terminal and the non-Aβ component regions (27–29). Although features of these long range tertiary interactions have not been defined, PD-causing αSyn mutants A30P and A53T reduce, whereas E46K enhances these interactions (36, 47). Our results show that the H50Q substitution can directly induce a conformation change on the C-terminal region and increase the flexibility of this region.
While our manuscript was under preparation, Maji and co-workers (48) reported their study of αSyn(H50Q). They also found that the H50Q substitution promotes αSyn aggregation. In contrast to our finding that the H50Q substitution caused significant C-terminal conformational changes, Maji and co-workers (48) observed small chemical shift changes of the C-terminal residues using HSQC experiments. The discrepancy is likely caused by buffer pH. The C-terminal chemical shift changes observed in our HSQC experiments were recorded in pH 7.4 phosphate buffer, whereas in pH 5.8 phosphate buffer, similar to the pH 6.0 buffer used by Maji and co-workers (48), the chemical shift changes of the C-terminal residues induced by the H50Q substitution were completely diminished (data not shown). αSyn is a highly charged protein, and its C-terminal region contains 15 negatively charged amino acids. Compared with neutral pH, a mild acidic pH could cause a profound effect on the physicochemical properties of αSyn, which might diminish the H50Q substitution-induced conformational changes on the C-terminal region.
It is unknown whether Gln-50 directly interacts with the C-terminal residues. In HSQC experiments, substituting Asp-135, Tyr-136, and Glu-137 with alanine abolished the C-terminal conformational changes induced by the H50Q substitution (Fig. 3B). However, mutating these three residues did not cause a substantial chemical shift of Gln-50 (Fig. 3C). One potential interpretation is that Gln-50 does not directly interact with the C-terminal residues. Another possibility is that the C-terminal mutations do not alter the sampling of the folded conformations to the same degree as the H50Q substitution. Elaborating, because αSyn has been shown to exchange rapidly between a primarily unfolded state to a partially folded state (27), the observed chemical shifts are a weighted average between these two states (d = Puδu + Pfδf, where Pu, Pf and δu, δf represent the populations and chemical shifts of the unfolded and folded states, respectively). Thus, αSyn(H50Q) likely induces a larger detectable change than αSyn(DYE/A). Moreover, based on the increased C-terminal flexibility of αSyn(H50Q), as identified via the R1 relaxation studies above, we can predict that the folded state is significantly diminished. Further investigations are necessary to elucidate how the H50Q substitution causes conformational changes on the C-terminal region.
Interestingly, the DYE/A substitutions significantly inhibited αSyn aggregation in vitro. However, the H50Q substitution was still able to accelerate the aggregation of αSyn(DYE/A) despite not being able to induce the C-terminal conformational changes. Taking into account the H50A and H50D substitutions, neither of them caused C-terminal conformational changes in HSQC experiments, yet they promoted αSyn aggregation. Presumably, the C-terminal conformational changes detected in HSQC experiments are not necessary for the H50Q substitution-induced acceleration of αSyn aggregation.
In contrast to the aggregation-promoting effect of the H50A, H50Q, or H50D substitution, the H50R substitution inhibited αSyn aggregation in vitro. These results suggest that a partially positively charged His-50 at physiological pH protects αSyn from aggregation. Certainly, this implication can be strengthened by investigating if the H50K substitution inhibits αSyn aggregation in vitro as well and, more importantly, by determining if the H50R substitution suppresses αSyn aggregation and PD-like symptom-development in animal models when compared with overexpression of wild type αSyn or PD-causing αSyn mutants. How a positively charged His-50 plays a role in suppressing αSyn aggregation is unknown. A potential mechanism is that a positively charged His-50 can enhance the intramolecular interactions between the C-terminal acidic region and the middle region, which could shield the hydrophobic non-Aβ component region to prevent aggregation (27).
Acknowledgments
We thank Dr. Kato for providing the 1H,15N-backbone chemical shift assignments of wild type αSyn. We also thank Dr. Hirsch in the Biophysics Core Facility in the University of Colorado Anschutz Medical Campus for technical support with the instruments.
This work was supported, in whole or in part, by National Institutes of Health Grant 5R01NS72397 (to C.-W. L.).
- PD
- Parkinson disease
- αSyn
- α-synuclein
- HSQC
- heteronuclear single-quantum correlation.
REFERENCES
- 1. Spillantini M. G., Schmidt M. L., Lee V. M., Trojanowski J. Q., Jakes R., Goedert M. (1997) α-Synuclein in Lewy bodies. Nature 388, 839–840 [DOI] [PubMed] [Google Scholar]
- 2. Goedert M., Spillantini M. G., Del Tredici K., Braak H. (2013) 100 years of Lewy pathology. Nat. Rev. Neurol. 9, 13–24 [DOI] [PubMed] [Google Scholar]
- 3. Chartier-Harlin M.-C., Kachergus J., Roumier C., Mouroux V., Douay X., Lincoln S., Levecque C., Larvor L., Andrieux J., Hulihan M., Waucquier N., Defebvre L., Amouyel P., Farrer M., Destée A. (2004) α-Synuclein locus duplication as a cause of familial Parkinson's disease. Lancet 364, 1167–1169 [DOI] [PubMed] [Google Scholar]
- 4. Ibáñez P., Bonnet A.-M., Débarges B., Lohmann E., Tison F., Pollak P., Agid Y., Dürr A., Brice A. (2004) Causal relation between α-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet 364, 1169–1171 [DOI] [PubMed] [Google Scholar]
- 5. Singleton A. B., Farrer M., Johnson J., Singleton A., Hague S., Kachergus J., Hulihan M., Peuralinna T., Dutra A., Nussbaum R., Lincoln S., Crawley A., Hanson M., Maraganore D., Adler C., Cookson M. R., Muenter M., Baptista M., Miller D., Blancato J., Hardy J., Gwinn-Hardy K. (2003) α-Synuclein locus triplication causes Parkinson's disease. Science 302, 841–841 [DOI] [PubMed] [Google Scholar]
- 6. Polymeropoulos M. H., Lavedan C., Leroy E., Ide S. E., Dehejia A., Dutra A., Pike B., Root H., Rubenstein J., Boyer R., Stenroos E. S., Chandrasekharappa S., Athanassiadou A., Papapetropoulos T., Johnson W. G., Lazzarini A. M., Duvoisin R. C., Di Iorio G., Golbe L. I., Nussbaum R. L. (1997) Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science 276, 2045–2047 [DOI] [PubMed] [Google Scholar]
- 7. Krüger R., Kuhn W., Müller T., Woitalla D., Graeber M., Kösel S., Przuntek H., Epplen J. T., Schöls L., Riess O. (1998) A30P mutation in the gene encoding α-synuclein in Parkinson's disease. Nat. Genet. 18, 106–108 [DOI] [PubMed] [Google Scholar]
- 8. Zarranz J. J., Alegre J., Gómez-Esteban J. C., Lezcano E., Ros R., Ampuero I., Vidal L., Hoenicka J., Rodriguez O., Atarés B., Llorens V., Gomez Tortosa E., del Ser T., Muñoz D. G., de Yebenes J. G. (2004) The new mutation, E46K, of α-synuclein causes Parkinson and Lewy body dementia. Ann. Neurol. 55, 164–173 [DOI] [PubMed] [Google Scholar]
- 9. Conway K. A., Harper J. D., Lansbury P. T. (1998) Accelerated in vitro fibril formation by a mutant α-synuclein linked to early-onset Parkinson disease. Nat. Med. 4, 1318–1320 [DOI] [PubMed] [Google Scholar]
- 10. Narhi L., Wood S. J., Steavenson S., Jiang Y., Wu G. M., Anafi D., Kaufman S. A., Martin F., Sitney K., Denis P., Louis J. C., Wypych J., Biere A. L., Citron M. (1999) Both familial Parkinson's disease mutations accelerate α-synuclein aggregation. J. Biol. Chem. 274, 9843–9846 [DOI] [PubMed] [Google Scholar]
- 11. Choi W., Zibaee S., Jakes R., Serpell L. C., Davletov B., Crowther R. A., Goedert M. (2004) Mutation E46K increases phospholipid binding and assembly into filaments of human α-synuclein. FEBS Lett. 576, 363–368 [DOI] [PubMed] [Google Scholar]
- 12. Greenbaum E. A., Graves C. L., Mishizen-Eberz A. J., Lupoli M. A., Lynch D. R., Englander S. W., Axelsen P. H., Giasson B. I. (2005) The E46K mutation in α-synuclein increases amyloid fibril formation. J. Biol. Chem. 280, 7800–7807 [DOI] [PubMed] [Google Scholar]
- 13. Fredenburg R. A., Rospigliosi C., Meray R. K., Kessler J. C., Lashuel H. A., Eliezer D., Lansbury P. T. (2007) The Impact of the E46K Mutation on the properties of α-synuclein in its monomeric and oligomeric states. Biochemistry 46, 7107–7118 [DOI] [PubMed] [Google Scholar]
- 14. Masliah E., Rockenstein E., Veinbergs I., Mallory M., Hashimoto M., Takeda A., Sagara Y., Sisk A., Mucke L. (2000) Dopaminergic loss and inclusion body formation in α-synuclein mice: implications for neurodegenerative disorders. Science 287, 1265–1269 [DOI] [PubMed] [Google Scholar]
- 15. Neumann M., Kahle P. J., Giasson B. I., Ozmen L., Borroni E., Spooren W., Müller V., Odoy S., Fujiwara H., Hasegawa M., Iwatsubo T., Trojanowski J. Q., Kretzschmar H. A., Haass C. (2002) Misfolded proteinase K-resistant hyperphosphorylated α-synuclein in aged transgenic mice with locomotor deterioration and in human α-synucleinopathies. J. Clin. Invest. 110, 1429–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Giasson B. I., Duda J. E., Quinn S. M., Zhang B., Trojanowski J. Q., Lee V. M. (2002) Neuronal α-synucleinopathy with severe movement disorder in mice expressing A53T human α-synuclein. Neuron 34, 521–533 [DOI] [PubMed] [Google Scholar]
- 17. Feany M. B., Bender W. W. (2000) A Drosophila model of Parkinson's disease. Nature 404, 394–398 [DOI] [PubMed] [Google Scholar]
- 18. Jakes R., Spillantini M. G., Goedert M. (1994) Identification of two distinct synucleins from human brain. FEBS Lett. 345, 27–32 [DOI] [PubMed] [Google Scholar]
- 19. Norris E. H., Giasson B. I., Lee V. M. (2004) α-synuclein: normal function and role in neurodegenerative diseases. Curr. Top. Dev. Biol. 60, 17–54 [DOI] [PubMed] [Google Scholar]
- 20. Wood S. J., Wypych J., Steavenson S., Louis J.-C., Citron M., Biere A. L. (1999) α-Synuclein fibrillogenesis is nucleation-dependent: implications for the pathogenesis of Parkinson's disease. J. Biol. Chem. 274, 19509–19512 [DOI] [PubMed] [Google Scholar]
- 21. Qin Z., Hu D., Han S., Hong D.-P., Fink A. L. (2007) Role of different regions of α-synuclein in the assembly of fibrils. Biochemistry 46, 13322–13330 [DOI] [PubMed] [Google Scholar]
- 22. Murray I. V., Giasson B. I., Quinn S. M., Koppaka V., Axelsen P. H., Ischiropoulos H., Trojanowski J. Q., Lee V. M. (2003) Role of α-synuclein carboxyl terminus on fibril formation in vitro. Biochemistry 42, 8530–8540 [DOI] [PubMed] [Google Scholar]
- 23. Hoyer W., Cherny D., Subramaniam V., Jovin T. M. (2004) Impact of the acidic C-terminal region comprising amino acids 109–140 on α-synuclein aggregation in vitro. Biochemistry 43, 16233–16242 [DOI] [PubMed] [Google Scholar]
- 24. Liu C.-W., Giasson B. I., Lewis K. A., Lee V. M., Demartino G. N., Thomas P. J. (2005) A precipitating role for truncated α-synuclein and the proteasome in α-synuclein aggregation: implications for pathogenesis of Parkinson disease. J. Biol. Chem. 280, 22670–22678 [DOI] [PubMed] [Google Scholar]
- 25. Tofaris G. K., Garcia Reitböck P., Humby T., Lambourne S. L., O'Connell M., Ghetti B., Gossage H., Emson P. C., Wilkinson L. S., Goedert M., Spillantini M. G. (2006) Pathological changes in dopaminergic nerve cells of the substantia nigra and olfactory bulb in mice transgenic for truncated human α-synuclein(1–120): implications for Lewy body disorders. J. Neurosci. 26, 3942–3950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wakamatsu M., Ishii A., Iwata S., Sakagami J., Ukai Y., Ono M., Kanbe D., Muramatsu S., Kobayashi K., Iwatsubo T., Yoshimoto M. (2008) Selective loss of nigral dopamine neurons induced by overexpression of truncated human α-synuclein in mice. Neurobiol. Aging 29, 574–585 [DOI] [PubMed] [Google Scholar]
- 27. Dedmon M. M., Lindorff-Larsen K., Christodoulou J., Vendruscolo M., Dobson C. M. (2005) Mapping long-range interactions in α-synuclein using spin-label NMR and Ensemble molecular dynamics simulations. J. Am. Chem. Soc. 127, 476–477 [DOI] [PubMed] [Google Scholar]
- 28. Bertoncini C. W., Jung Y.-S., Fernandez C. O., Hoyer W., Griesinger C., Jovin T. M., Zweckstetter M. (2005) Release of long-range tertiary interactions potentiates aggregation of natively unstructured α-synuclein. Proc. Natl. Acad. Sci. U.S.A. 102, 1430–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bernadó P., Bertoncini C. W., Griesinger C., Zweckstetter M., Blackledge M. (2005) Defining long-range order and local disorder in native α-synuclein using residual dipolar couplings. J. Am. Chem. Soc. 127, 17968–17969 [DOI] [PubMed] [Google Scholar]
- 30. Proukakis C., Dudzik C. G., Brier T., MacKay D. S., Cooper J. M., Millhauser G. L., Houlden H., Schapira A. H. (2013) A novel α-synuclein missense mutation in Parkinson disease. Neurology 80, 1062–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Appel-Cresswell S., Vilarino-Guell C., Encarnacion M., Sherman H., Yu I., Shah B., Weir D., Thompson C., Szu-Tu C., Trinh J., Aasly J. O., Rajput A., Rajput A. H., Jon Stoessl A., Farrer M. J. (2013) α-Synuclein p.H50Q, a novel pathogenic mutation for Parkinson's disease. Mov. Disord. 28, 811–813 [DOI] [PubMed] [Google Scholar]
- 32. Delaglio F., Grzesiek S., Vuister G. W., Zhu G., Pfeifer J., Bax A. (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6, 277–293 [DOI] [PubMed] [Google Scholar]
- 33. Vranken W. F., Boucher W., Stevens T. J., Fogh R. H., Pajon A., Llinas M., Ulrich E. L., Markley J. L., Ionides J., Laue E. D. (2005) The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins 59, 687–696 [DOI] [PubMed] [Google Scholar]
- 34. Sasakawa H., Sakata E., Yamaguchi Y., Masuda M., Mori T., Kurimoto E., Iguchi T., Hisanaga S., Iwatsubo T., Hasegawa M., Kato K. (2007) Ultra-high field NMR studies of antibody binding and site-specific phosphorylation of α-synuclein. Biochem. Biophys. Res. Commun. 363, 795–799 [DOI] [PubMed] [Google Scholar]
- 35. Weinreb P. H., Zhen W., Poon A. W., Conway K. A., Lansbury P. T. (1996) NACP, a protein implicated in Alzheimer's disease and learning, is natively unfolded. Biochemistry 35, 13709–13715 [DOI] [PubMed] [Google Scholar]
- 36. Bertoncini C. W., Fernandez C. O., Griesinger C., Jovin T. M., Zweckstetter M. (2005) Familial mutants of α-synuclein with increased neurotoxicity have a destabilized conformation. J. Biol. Chem. 280, 30649–30652 [DOI] [PubMed] [Google Scholar]
- 37. Bartels T., Choi J. G., Selkoe D. J. (2011) α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature 477, 107–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang W., Perovic I., Chittuluru J., Kaganovich A., Nguyen L. T., Liao J., Auclair J. R., Johnson D., Landeru A., Simorellis A. K., Ju S., Cookson M. R., Asturias F. J., Agar J. N., Webb B. N., Kang C., Ringe D., Petsko G. A., Pochapsky T. C., Hoang Q. Q. (2011) A soluble α-synuclein construct forms a dynamic tetramer. Proc. Natl. Acad. Sci. 108, 17797–17802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chatterjee A., Kumar A., Chugh J., Srivastava S., Bhavesh N. S., Hosur R. V. (2005) NMR of unfolded proteins. J. Chem. Sci. 117, 3–21 [Google Scholar]
- 40. Davidson W. S., Jonas A., Clayton D. F., George J. M. (1998) Stabilization of α-synuclein secondary structure upon binding to synthetic membranes. J. Biol. Chem. 273, 9443–9449 [DOI] [PubMed] [Google Scholar]
- 41. Perrin R. J., Woods W. S., Clayton D. F., George J. M. (2000) Interaction of human α-synuclein and Parkinson's disease variants with phospholipids structural analysis using site-directed mutagenesis. J. Biol. Chem. 275, 34393–34398 [DOI] [PubMed] [Google Scholar]
- 42. Conway K. A., Lee S.-J., Rochet J.-C., Ding T. T., Williamson R. E., Lansbury P. T., Jr. (2000) Acceleration of oligomerization, not fibrillization, is a shared property of both α-synuclein mutations linked to early-onset Parkinson's disease: implications for pathogenesis and therapy. Proc. Natl. Acad. Sci. 97, 571–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Coelho-Cerqueira E., Carmo-Gonçalves P., Pinheiro A. S., Cortines J., Follmer C. (2013) α-Synuclein as an intrinsically disordered monomer: fact or artifact? FEBS J. 280, 4915–4927 [DOI] [PubMed] [Google Scholar]
- 44. Bussell R., Jr., Eliezer D. (2001) Residual structure and dynamics in Parkinson's disease-associated mutants of α-synuclein. J. Biol. Chem. 276, 45996–46003 [DOI] [PubMed] [Google Scholar]
- 45. Ulrih N. P., Barry C. H., Fink A. L. (2008) Impact of Tyr to Ala mutations on α-synuclein fibrillation and structural properties. Biochim. Biophys. Acta 1782, 581–585 [DOI] [PubMed] [Google Scholar]
- 46. Izawa Y., Tateno H., Kameda H., Hirakawa K., Hato K., Yagi H., Hongo K., Mizobata T., Kawata Y. (2012) Role of C-terminal negative charges and tyrosine residues in fibril formation of α-synuclein. Brain Behav. 2, 595–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wise-Scira O., Dunn A., Aloglu A. K., Sakallioglu I. T., Coskuner O. (2013) Structures of the E46K mutant-type α-synuclein protein and impact of E46K mutation on the structures of the wild-type α-synuclein protein. ACS Chem. Neurosci. 4, 498–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ghosh D., Mondal M., Mohite G. M., Singh P. K., Ranjan P., Anoop A., Ghosh S., Jha N. N., Kumar A., Maji S. K. (2013) The Parkinson's disease-associated H50Q mutation accelerates α-synuclein aggregation in vitro. Biochemistry 52, 6925–6927 [DOI] [PubMed] [Google Scholar]