Background: Chronic inflammatory diseases are associated with increased cardiovascular mortality due to accelerated atherosclerosis.
Results: Chronic inflammation in tristetraprolin (TTP)-deficient mice leads to endothelial dysfunction, which is related to enhanced Nox2-dependent reactive oxygen species production but independent from TNF-α.
Conclusion: Inflammation-related oxidative stress is an important mediator in inflammation-driven atherogenesis.
Significance: In inflammatory diseases oxidative stress seems to be a major cause of cardiovascular events.
Keywords: Atherosclerosis, Endothelial Dysfunction, NADPH Oxidase, Reactive Nitrogen Species, Reactive Oxygen Species (ROS), Tumor Necrosis Factor (TNF)
Abstract
Cardiovascular events are important co-morbidities in patients with chronic inflammatory diseases like rheumatoid arthritis. Tristetraprolin (TTP) regulates pro-inflammatory processes through mRNA destabilization and therefore TTP-deficient mice (TTP−/− mice) develop a chronic inflammation resembling human rheumatoid arthritis. We used this mouse model to evaluate molecular signaling pathways contributing to the enhanced atherosclerotic risk in chronic inflammatory diseases. In the aorta of TTP−/− mice we observed elevated mRNA expression of known TTP targets like tumor necrosis factor-α (TNF-α) and macrophage inflammatory protein-1α, as well as of other pro-atherosclerotic mediators, like Calgranulin A, Cathepsin S, and Osteopontin. Independent of cholesterol levels TTP−/− mice showed a significant reduction of acetylcholine-induced, nitric oxide-mediated vasorelaxation. The endothelial dysfunction in TTP−/− mice was associated with increased levels of reactive oxygen and nitrogen species (RONS), indicating an enhanced nitric oxide inactivation by RONS in the TTP−/− animals. The altered RONS generation correlates with increased expression of NADPH oxidase 2 (Nox2) resulting from enhanced Nox2 mRNA stability. Although TNF-α is believed to be a central mediator of inflammation-driven atherosclerosis, genetic inactivation of TNF-α neither improved endothelial function nor normalized Nox2 expression or RONS production in TTP−/− animals. Systemic inflammation caused by TTP deficiency leads to endothelial dysfunction. This process is independent of cholesterol and not mediated by TNF-α solely. Thus, other mediators, which need to be identified, contribute to enhanced cardiovascular risk in chronic inflammatory diseases.
Introduction
Atherosclerosis (AS)3 is a disorder of the medium-sized and large arteries with thrombotic events as its clinical manifestation (1). Alterations in the endothelial function are one of the earliest measurable markers in atherogenesis, which precede the development of morphologic atherosclerotic changes and are predictors of cardiovascular events (2, 3). Endothelial dysfunction is a common feature of patients with different risk factors of atherosclerosis, such as hypercholesterolemia, diabetes, hypertension, and smoking (4).
Today the key role of inflammation during atherogenesis is well accepted (5). Rheumatoid arthritis (RA) is a chronic inflammatory disease associated with enhanced cardiovascular mortality (6–8). The elevated risk for cardiovascular events in RA patients is independent of age, sex, smoking, hypercholesterolemia, or body mass index (9, 10).
Formation of atherosclerotic plaques involves the internalization of lipids into the intima of the vessel wall and endothelial cell activation. This results in enhanced chemokine/cytokine expression leading to increased expression of vascular cell adhesion molecule 1 (VCAM-1) on the surface of endothelial cells (11). The adhesion and infiltration of leukocytes and monocytes into the arterial wall contribute to plaque progression. Activation of the endothelium also leads to an imbalanced secretion of endothelium-derived relaxing and contracting factors and to the development of an endothelial dysfunction. In patients suffering from AS the endothelium-dependent relaxation is impaired and the endothelial dysfunction is accepted as an early atherosclerotic marker (12). Enhanced formation of reactive oxygen and nitrogen species (RONS) is an important factor in this process (13). RONS are able to influence the endothelium via different pathways. Superoxide can directly inhibit soluble guanylyl cyclase, the main target of nitric oxide (NO), and reduce the bioavailability of NO by reaction to peroxynitrite (14). Furthermore, RONS can enhance oxidative stress by the inhibition of superoxide dismutases (14) and uncoupling of NO synthases (15).
In RA patients the molecular mechanisms leading to AS are not well understood, despite the knowledge that both RA and AS are inflammatory diseases. There are several pro-inflammatory mediators known to be elevated in RA patients that are also responsible for the progression of inflammation-induced AS: interleukin-1β and tumor necrosis factor-α (TNF-α) can induce the expression of VCAM-1 and therefore elicit the migration of leukocytes into the intima (16).
The expression of most pro-inflammatory mediators is regulated both transcriptionally and post-transcriptionally. RNA-binding proteins are important for the post-transcriptional regulation of mRNA stability. They bind their target mRNAs predominantly via AU-rich elements (ARE) and lead to either activation or inhibition of ARE-mediated mRNA decay (17). Tristetraprolin (TTP) is an ARE-binding protein and stimulates the deadenylation and breakdown of ARE-containing mRNAs (18). Several genes, for example TNF-α (19) or the macrophage inflammatory protein-1α (MIP-1α) (20), are regulated by TTP.
Mice lacking TTP (TTP−/−) are characterized by a shortened life span due to a complex phenotype of inflammatory arthritis, cachexia, left-sided cardiac valvulitis, and blood cell hyperplasia (21, 22). They have higher levels of TNF-α and their severe phenotype can be improved by an anti-TNF-α therapy (23). As it is known that RA patients also have increased amounts of TNF-α (24), TTP-deficient mice seem to be a suitable model for investigating the relationship between RA and AS mechanistically. Our objective was to identify the mediators for the initiation of AS (endothelial dysfunction) in this chronic inflammatory mouse model, as well as to characterize complex signaling pathways leading from RA to AS.
EXPERIMENTAL PROCEDURES
Animals
All mice were housed in accordance with standard animal care requirements and maintained under specified pathogen-free conditions on a 12/12-h light/dark circle. Water and food were given ad libitum. TTP+/− mice (obtained from the laboratory of Dr. Blackshear) and TNF-α−/− mice (stock 003008; The Jackson Laboratory) had a C57BL/6 background. We further cross-bred TNF-α−/− and TTP+/− animals to obtain TTP−/−/TNF-α−/− mice. Experimental TTP−/−, TTP+/−, and TTP+/+ animals were obtained by mating TTP+/− animals. Experimental TTP−/−/TNF-α−/− animals were obtained by mating TTP+/−/TNF-α−/− animals. Genotyping of the animals was performed by polymerase chain reaction, using primers that span the regions of the wild type genes disrupted by the targeting vectors. The following oligonucleotides (obtained from Sigma) were used for genotyping the Ttp-locus: TTP-wt/ko-for, GAGGGCCGAAGCTGCGGTGGGT; TTP-wt-rev, GGCTGGCCAGGGAGAGCTAGGTC; and TTP-ko-rev, CTGTTGTGCCCAGTCATAGCCG. For genotyping the Tnf-α locus the following oligonucleotides were used: TNF-α-wt-for, GCACAGAAAGCATGATCCG; TNF-α-wt-rev, TCCTTATCTCTCATGCCTCTCTC; TNF-α-ko-for, CTTGGGTGGAGAGGCTATTC; and TNF-α-ko-rev, AGGTGAGATGACAGGAGATC. For the analysis of TTP and Nox2 mRNA expression in the model of collagen-induced arthritis (CIA) DBA/1 mice, expressing a transgenic T cell receptor β-chain (Vβ12) obtained from a collagen type II (CII)-specific T cell clone (25) were used.
The animal studies were approved by the ethical board and were performed in accordance with German animal protection law and the guidelines for the use of experimental animals as stipulated by the Guide of Care and Use of Laboratory Animals of the National Institutes of Health. Mice were euthanized by intraperitoneal injection of 700 μl of pentobarbital solution (1% pentobarbital in PBS).
Induction of CIA and Measurement of mRNA Expression in Paws
CIA induction in Vβ12-DBA/1 mice, RNA isolation, and mRNA expression analyses were performed as described (26). mRNA expression of TTP and Nox2 in paws of PBS- or chicken CII-treated animals was analyzed at day 33 after the first CII treatment.
Analysis of Nox2 mRNA Stability in Peritoneal Cells of TTP−/− or TTP+/+ Animals
To analyze Nox2 mRNA stability in primary cells of TTP−/− or TTP+/+ animals we isolated peritoneal cells as described by Ray and Dittle (27). Adherent cells (mostly monocytes/macrophages) were incubated with LPS (2 μg/ml) and IFN-γ (100 units/ml) to induce Nox2 mRNA expression. After 4 h 6-dichloro-1-ribofuranosylbenzimidazole (DRB) (25 μg/ml) was added to stop RNA Polymerase II-dependent transcription. After 15, 30, 60, 120, and 240 min the expression of Nox2 and GAPDH mRNA were measured. Nox2 mRNA expression was normalized to GAPDH mRNA expression. The relative amount of Nox2 mRNA at 0 h DRB was set to 100%. Curve fittings of the resulting DRB time curves were performed by non-linear regression using Prism 6.0 (GraphPad Software, San Diego, CA).
Organ Bath Experiments
Aortas were isolated from TTP+/+, TTP−/−, or TTP−/−/TNF-α−/− animals, cut into 3-mm rings, and set up in organ bath chambers. After pre-contracting the aortic rings with 100 nmol/liter of norepinephrine, the endothelium-dependent vasodilatation was measured in response to acetylcholine or sodium nitroprusside in the presence or absence of l-NAME (1 mmol/liter) (28).
Cholesterol Measurements
Total and HDL cholesterol as well as triglycerides and blood glucose were measured in mouse serum using the Alere Cholestech LDX® Analyzer and Cholestech LDX® Test Cassettes as described by the manufacturer (Alere, Köln, Germany). The LDL cholesterol was calculated using the method published by Friedewald et al. (29).
Blood Cells Counts
Blood cell counts (leukocytes, erythrocytes and thrombocytes) were performed using the HEMAVET® 950 system as described by the manufacturer.
Blood Pressure Measurement
Blood pressure and heart beat rate were measured by a non-invasive tail-cuff method. A pressure signal from the tail artery was detected by a pulse transducer relayed via a NIBP controller and a Powerlab, and recorded by Chart software (all from AD Instruments, Sydney, Australia). Pressure measurements were performed five times for each mouse to obtain an average value.
RNA Isolation
The organs of TTP+/+, TTP−/−, or TTP−/−/TNF-α−/− animals were homogenized in guanidinium thiocyanate buffer and total RNA was isolated by guanidinium thiocyanate/phenol/chloroform extraction as previously described (30).
Real-time Reverse Transcription Polymerase Chain Reaction Analysis
To analyze the gene expression in mouse samples, two-step real-time RT-PCRs (qRT-PCR) were performed. 500 ng of total RNA was reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Darmstadt, Germany) following the manufacturer's recommendations. Subsequently qRT-PCR (TaqMan or SYBR Green) was performed as described (31, 32) with the following oligonucleotides (obtained from Sigma) as sense and antisense primers, as well as TaqMan hybridization probes (Table 1).
TABLE 1.
Oligonucleotides as sense and antisense primers and TaqMan hybridization probes
| Sequence | |
|---|---|
| CTSS | |
| Sense | CATGGTGTTCTTGTGGTTGG |
| Antisense | CAATAACTAGCAATTCCGCAGTG |
| Probe | TGGCAAGCACGACTTCCGGGTG |
| eNOS | |
| Sense | CCTTCCGCTACCAGCCAGA |
| Antisense | CAGAGATCTTCACTGCATTGGCTA |
| GAPDH | |
| Sense | TTCACCACCATGGAGAAGGC |
| Antisense | GGCATGGACTGTGGTCATGA |
| Probe | TGCATCCTGCACCACCAACTGCTTAG |
| ICAM | |
| Sense | ACCCCGCAGGTCCAATTC |
| Antisense | CCAGAGCGGCAGAGCAAA |
| iNOS | |
| Sense | CAGCTGGGCTGTACAAACCTT |
| Antisense | CATTGGAAGTGAAGCGTTTCG |
| Probe | CGGGCAGCCTGTGAGACCTTTGA |
| Mip-1α | |
| Sense | CTGCAACCAAGTCTTCTCAGC |
| Antisense | CTGCCTCCAAGACTCTCAGG |
| Probe | ACTGCCTGCTGCTTCTCCTACAGCC |
| Nox1 | |
| Sense | GGAGGAATTAGGCAAAATGGATT |
| Antisense | GCTGCATGACCAGCAATGTT |
| Nox2 | |
| Sense | CCAACTGGGATAACGAGTTCA |
| Antisense | GAGAGTTTCAGCCAAGGCTTC |
| Nox 4 | |
| Sense | TGTAACAGAGGGAAAACAGTTGGA |
| Antisense | GTTCCGGTTACTCAAACTATGAAGAGT |
| S100A8 | |
| Sense | CTCCGTCTTCAAGACATCGTTTG |
| Antisense | TCATTCTTGTAGAGGGCATGGTG |
| Probe | CAATGCCGTCTGAACTGGAGAAGGCC |
| SPP1 | |
| Sense | GCTTGGCTTATGGACTGAGG |
| Antisense | CCTCATCTGTGGCATCAGG |
| Probe | TCAAAGTCTAGGAGTTTCCAGGTTTCTGATGA |
| TNF-α | |
| Sense | CATCTTCTCAAAATTCGAGTGACA |
| Antisense | TGGGAGTAGACAAGGTACAACCC |
| Probe | CACGTCGTAGCAAACCACCAAGTGG |
| TTP | |
| Sense | CCATGGATCTCTCTGCCATC |
| Antisense | CAGTCAGGCGAGAGGTGAC |
| Probe | CGGAGGACTTTGGAACATAAACTCGGAC |
| VCAM-1 | |
| Sense | GACTCCATGGCCCTCACTTG |
| Antisense | CGCGTTTAGTGGGCTGTCTATC |
mRNA expression data were normalized to GAPDH mRNA expression. To calculate the relative mRNA expressions, the 2(−ΔΔCt) method (33) was used.
Immunoblot and Dot Blot Experiments
For protein expression analysis, 50–100 μg of protein was separated on SDS-polyacrylamide gels and transferred to nitrocellulose membrane by semi-dry electroblotting. The antibodies for the detection of α-Actinin, β-Actin (both Sigma), CD68, P-VASP (both Merck Millipore), eNOS, iNOS, and Nox2 (all BD Bioscience) were used following the manufacturer's recommendations. Secondary anti-mouse and anti-rabbit HRP antibodies were obtained from Vector Laboratories/Biozol (Eching, Germany). For the determination of peroxynitrite, 20 μg of protein of TTP+/+ and TTP−/− hearts was transferred to nitrocellulose membrane, detected with an anti-nitrotyrosine antibody (Upstate/Millipore) and an anti-mouse antibody (Vector Laboratories/Biozol, Eching, Germany). The immunoreactive proteins on the blots were visualized by the enhanced chemiluminescence detection system (ECL, Thermo Scientific, Braunschweig, Germany).
Whole Blood RONS Formation
The oxidative burst of whole blood samples was measured by L-012 (100 μmol/liter) ECL under PDBu (1 μmol/liter)-stimulated conditions as described (34). Briefly, blood was freshly collected in citrate monovettes and diluted in L-012 containing PBS. PDBu-stimulated RONS formation was determined using a Centro chemiluminescence plate reader from Berthold Technologies (Bad Wildbach, Germany). The ECL signal was expressed as counts (photons) per s.
Membranous Superoxide Formation (NADPH Oxidase Activity)
The NADPH oxidase activity in cardiac membrane fractions was determined by Amplex Red (100 μmol/liter)/horseradish peroxidase (0.1 μmol/liter) ECL in the presence of NADPH (200 μmol/liter) using a Twinkle fluorescence plate reader as described (34, 35). Membranous fractions were generated from glass/glass-homogenized heart tissue in DTT (5 mmol/liter)-containing Tris buffer by differential centrifugation at 2,000, 20,000, and 100,000 × g. The protein amount was determined by Lowry analysis and the final protein content was adjusted to 0.2 mg/ml. NADPH oxidase-derived superoxide formation was induced by addition of NADPH and lucigenin-derived enhanced chemiluminescence was detected using a Lumat LB9507 single vial chemiluminometer from Berthold Technologies. The ECL signal was expressed as counts (photons) per 30 s.
Determination of Vascular RONS Formation
Vascular RONS formation was determined by dihydroethidine (1 μmol/liter)-dependent fluorescence microtopography in aortic cryosections (36, 37).
Statistics
Data represent mean ± S.E. Statistical differences were determined by factorial analysis of variance followed by one-way or two-way ANOVA multiple comparison test. In the case of two means classical t test analyses were used. All statistical analyses were performed using GraphPad Prism 6.0.
RESULTS
Tristetraprolin Deficiency Causes an Increase in Pro-inflammatory and -Atherosclerotic Marker Gene Expression
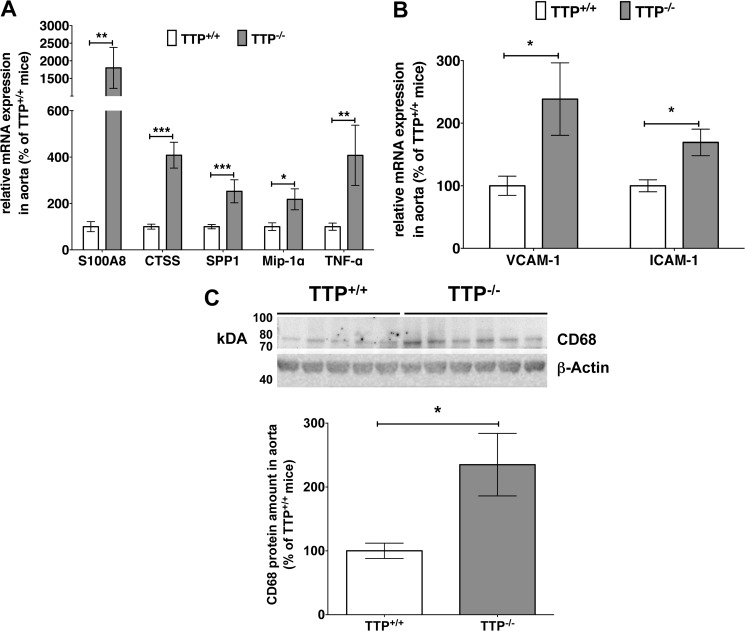
In 3–4-month-old TTP−/− mice aortas we observed a significant increase in the mRNA expression of known TTP target genes (e.g. Tnf-α and Mip-1α, Fig. 1A). These results indicate a highly inflamed status in the aorta of those mice. In addition we detected increased mRNA expression of other pro-atherosclerotic mediators (Fig. 1A) in the aorta of TTP−/− mice: Calgranulin A (S100A8) a biomarker for chronic inflammatory diseases (26), Cathepsin S (CTSS) and Osteopontin (SPP1), which are related to atherogenesis (38), and the recruitment of monocytes/macrophages (39), respectively. Furthermore, vascular inflammation was evident by enhanced mRNA expression of adhesion molecules VCAM-1 and ICAM-1 in the aorta of TTP−/− mice (Fig. 1B). By analyzing monocyte/macrophage infiltration with Western blot experiments using a specific CD68 antibody we observed a clear enhancement of this monocyte/macrophage marker (40) in the aorta of TTP−/− mice (Fig. 1C).
FIGURE 1.
TTP deficiency leads to an up-regulation of pro-inflammatory and -atherosclerotic mediators and to macrophage infiltration into the intima. A and B, RNA isolated from aortas of TTP−/− and TTP+/+ mice was analyzed for S100A8, CTSS, SPP1, Mip-1α, and TNF-α mRNA-expression (A) and for VCAM-1 and ICAM-1 mRNA-expression (B) in qRT-PCR experiments. Data shown are mean ± S.E. of 4–16 mice (*, p < 0.05; **, p < 0.01; ***, p < 0.001 versus TTP+/+ mice; t test). C, CD68 (macrophage marker) and β-actin expression in aortas of TTP+/+ and TTP−/− mice were analyzed using specific anti-CD68 or anti-β-Actin antibodies (for normalization). Each lane represents a different animal. A representative Western blot is shown in the upper panel. The positions of the CD68 and β-Actin proteins are indicated. The lower panel displays the summary (mean ± S.E. of three blots) of densitometric analyses of Western blots as shown in the upper panel (*, p < 0.05 versus TTP+/+ mice; t test).
Tristetraprolin Deficiency Leads to an Increase in the Numbers of Neutrophil Granulocytes, Monocytes, and Thrombocytes
Blood cell counts using whole blood from TTP+/+ and TTP−/− mice revealed slight enhancement of neutrophil granulocyte, monocyte (see Table 2), and thrombocyte (see Table 2) cell numbers in the blood of TTP−/− mice. In contrast, lymphocyte cell numbers were decreased in TTP−/− mice (see Table 2). No changes were seen in the numbers of eosinophil or basophil granulocytes and erythrocytes.
TABLE 2.
TTP deficiency leads to an enhancement in the numbers of neutrophil granulocytes, monocytes, and thrombocytes, whereas lymphocyte numbers were decreased
Blood cell counts of whole blood (20 μl) (leucocytes, thrombocytes, and erythrocytes) from TTP+/+ and TTP−/− mice were performed using the HEMAVET® 950 system as described by the manufacturer. Data shown (mean ± S.E.) are from 8 to 10 animals).
| Cell type | TTP +/+ |
TTP −/− |
p value | ||
|---|---|---|---|---|---|
| Mean | S.E. | Mean | S.E. | ||
| % of white blood cells | |||||
| Neutrophils | 2.34 | 0.54 | 5.18 | 0.85 | 0.0384 |
| Eosinophils | 0.19 | 0.08 | 0.17 | 0.06 | >0.05 |
| Basophils | 0.00 | 0.00 | 0.02 | 0.01 | >0.05 |
| Monocytes | 3.50 | 1.07 | 9.56 | 0.76 | 0.0014 |
| Lymphocytes | 93.97 | 1.36 | 85.07 | 1.45 | 0.0029 |
| Cell counts (×103 cells/μl) | |||||
| Thrombocytes | 657,75 | 50,18 | 939,83 | 97,74 | 0.0297 |
| Erythrocytes | 7.10 | 0.27 | 6.61 | 0.27 | >0.05 |
Tristetraprolin Deficiency Leads to a Cholesterol-independent Endothelial Dysfunction
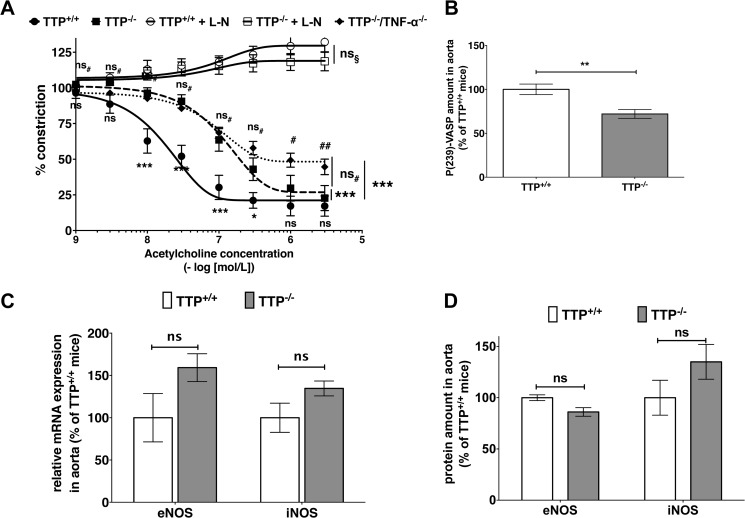
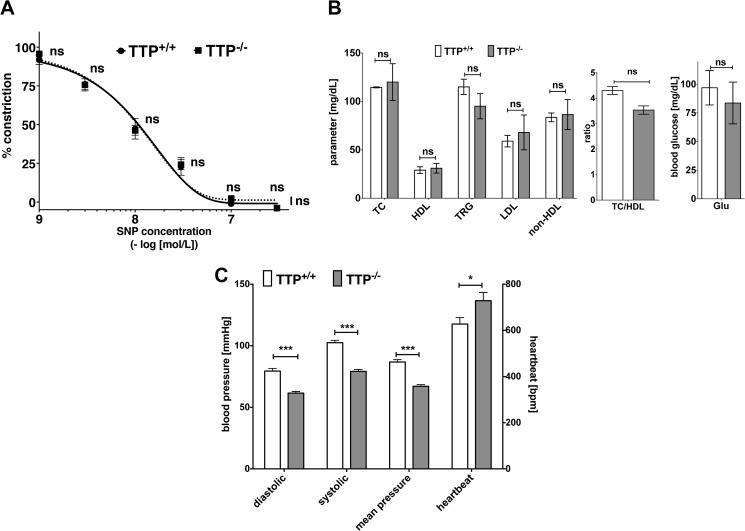
As endothelial dysfunction is one of the earliest markers in AS pathogenesis (2, 3) and a common feature of AS patients with different risk factors (4), we analyzed the endothelial function in TTP−/− mice. In comparison to wild type (TTP+/+) mice, aortas of TTP−/− mice were less responsive to acetylcholine-induced relaxation (Fig. 2A). For both groups the NOS inhibitor l-NAME abolished the acetylcholine-induced relaxation (Fig. 2A), which indicates a NO-dependent mechanism. In addition, aortas of both groups displayed similar relaxation in response to the NO-donor sodium nitroprusside (Fig. 3A), indicating an intact downstream signaling via the soluble guanylyl cyclase in TTP−/− mice. Importantly the development of this endothelial dysfunction is not cholesterol-dependent, as TTP+/+ and TTP−/− mice showed no significant differences in blood cholesterol and triglyceride levels (Fig. 3B). In addition TTP−/− mice displayed normal blood glucose levels (Fig. 3B) and reduced blood pressure (Fig. 3C).
FIGURE 2.
TTP deficiency leads to endothelial dysfunction with unchanged eNOS or iNOS expression in TTP-deficient mice. A, aortic rings from TTP+/+, TTP−/−, and TTP−/−/TNF-α−/− mice were mounted in a wire myograph for isometric tension recording. Rings were pre-contracted with 100 nmol/liter of norepinephrine and relaxed with increasing concentrations of acetylcholine in the presence or absence of the NOS inhibitor l-NAME (L-N, 1 mmol/liter; only TTP+/+ or TTP−/− mice). Data shown are mean ± S.E. of 4–7 independent measurements (***, p < 0.001; ns, not significant versus wild type mice; ##, p < 0.01; #, p < 0.05; ns, not significant versus TTP−/− aortas; ns§, not significant versus l-NAME treated TTP+/+ aortas; two-way ANOVA). B, the NO/cGMP signaling in the aorta of TTP−/− mice in comparison to wild type mice was measured by immunoblot analyses with a specific P(Ser-239)-VASP antibody. The figure displays the summary (mean ± S.E.) of densitometric analysis of Western blots using proteins from 11 mice (**, p < 0.01 versus TTP+/+ mice; t test). C, RNA from aortas of TTP−/− or TTP+/+ mice was analyzed for iNOS and eNOS mRNA expression by qRT-PCR (ns, not significant versus TTP+/+ mice; t test). D, eNOS or iNOS protein expression in aortas from TTP−/− or TTP+/+ mice was analyzed in immunoblots using specific anti-eNOS or -iNOS antibodies. The figure is the summary (mean ± S.E.) of densitometric analyses of Western blots using protein extracts from 10 to 11 mice (ns, not significant versus TTP+/+ mice; t test).
FIGURE 3.
TTP deficiency does not change sodium nitroprusside-mediated effects on constriction/relaxation in aortas, blood lipid, and glucose levels but reduces blood pressure in TTP-deficient mice. A, aortic rings from TTP−/− and TTP+/+ mice were mounted in a wire myograph for isometric tension recording. Rings were pre-contracted with 100 nmol/liter of norepinephrine and relaxed with increasing concentrations of sodium nitroprusside (SNP). Data shown are mean ± S.E. of 4–7 independent measurements (ns, not significant versus wild type mice; two-way ANOVA). B, blood lipid levels (TC, total cholesterol; HDL, high density lipoprotein cholesterol; TRG, triglycerides; LDL, low density lipoprotein cholesterol; non-HDL, non-HDL cholesterol; TC/HDL, ratio total cholesterol and HDL cholesterol) and glucose levels were determined in the blood sera of TTP−/− and TTP+/+ mice. Data shown are mean ± S.E. of 5 independent measurements (ns, not significant versus wild type mice; t test). C, blood pressure and heartbeat rate of TTP−/− and TTP+/+ mice were measured by a non-invasive tail-cuff method. Pressure measurements were analyzed 5 times for each mouse to obtain an average value. Data shown are mean ± S.E. of 5 independent measurements (***, p < 0.001; *, p < 0.05, versus wild type mice; t test).
An accepted marker for NO bioavailability and its downstream signaling is phosphorylation of Ser-239 of the vasodilator-stimulated phosphoprotein (VASP). By analyzing P(Ser-239)-VASP expression in the aortas, we found a 25% decrease in NO/cGMP signaling in TTP−/− mice (Fig. 2B). Again, this result indicates a reduced NO bioavailability.
A reduced NO bioavailability can result from a reduction of NO production by NO synthases or by enhanced inactivation of NO by superoxide. In qRT-PCR (RNA) and Western blot (protein) analyses we observed no reduction in the expression of eNOS or iNOS, the main producers of NO in the vascular system (Fig. 2, C and D). Therefore, the reduced NO bioavailability was more likely the result of enhanced NO breakdown by oxidative stress.
Tristetraprolin Deficiency Leads to Enhanced Burden of Oxidative Stress
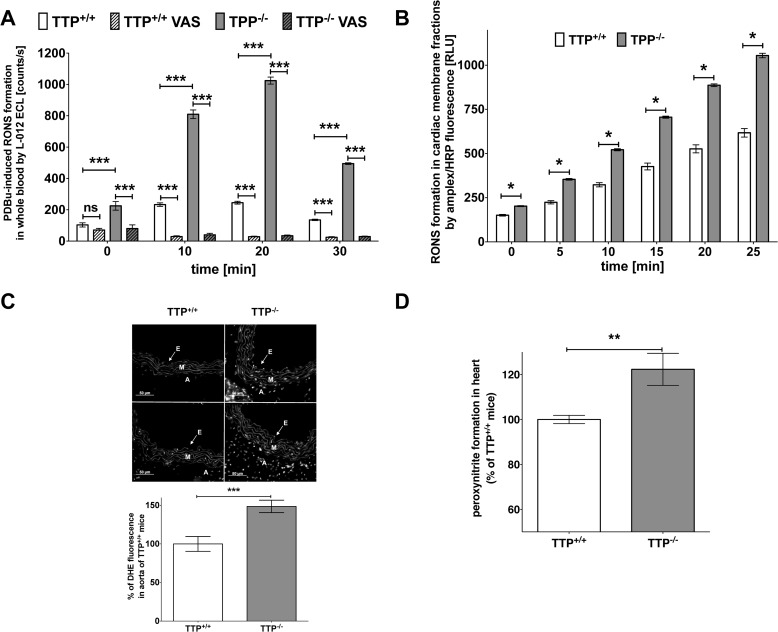
We measured RONS formation either under PDBu-stimulated conditions in whole blood (Fig. 4A) or in the presence of NADPH in cardiac membrane fractions (Fig. 4B) with L-012 ECL or Amplex Red/peroxidase-derived fluorescence. In addition we determined vascular superoxide formation by dihydroethidine-dependent fluorescence microtopography of aortic cryosections (Fig. 4C). In all analyses we observed higher RONS formation in mice lacking TTP (Fig. 4, A–C). Specific inhibition of NAPDH oxidases (Nox) by 3-benzyl-7-(2-benzoxazolyl)thio-1,2,3-triazolo(4,5-d)pyrimidine (VAS-2870, 25 μmol/liter) nearly totally blocked RONS formation in whole blood (Fig. 4A) indicating an involvement of Nox enzymes in the TTP−/−-related enhancement of RONS production. The L-012 signal in the TTP−/− group was also partially suppressed by Cu,Zn-SOD (200 units/ml) indicating formation of superoxide (not shown). Likewise, the Nox-derived H2O2 signal was partially suppressed by VAS-2870 (10 μmol/liter) and PEG-catalase (800 units/ml), underlining the specificity of the Amplex Red/peroxidase assay for H2O2 (not shown).
FIGURE 4.
TTP deficiency leads to enhanced formation of RONS. A, formation of RONS in the blood of TTP−/− and TTP+/+ mice was measured under PDBu (1 μmol/liter)-stimulated conditions in the presence or absence of the NADPH oxidase inhibitor 3-benzyl-7-(2-benzoxazolyl)thio-1,2,3-triazolo(4,5-d)pyrimidine (VAS-2870, 25 μmol/liter) with L-012 (100 μmol/liter) ECL. Data shown are mean ± S.E. of 12 mice (***, p < 0.001; ns, not significant versus wild type; ###, p < 0.001 versus TTP−/− mice; two-way ANOVA). B, formation of RONS in cardiac membrane fractions was measured with Amplex Red/peroxidase-derived fluorescence. Data shown are mean ± S.E. of 16 mice (*, p < 0.05,versus TTP+/+ mice; t test). C, vascular superoxide formation was determined by dihydroethidine-dependent fluorescence microtopography of aortic cryosections. In the upper panel representative dihydroethidine (DHE) stainings with superoxide producing cells are shown. The locations of the endothelium (E), media (M), and adventitia (A) are indicated. In the lower panel a quantitative analysis of several such assays is shown. Data shown are mean ± S.E. of 5–7 mice (***, p < 0.001 versus TTP+/+ mice; t test). D, peroxynitrite formation in the hearts of TTP+/+ and TTP−/− mice was measured by a dot blot using specific anti-nitrotyrosine antibody. Protein expression was analyzed by densitometric analysis of the dot blot membrane. Data shown are mean ± S.E. of 13 mice (**, p < 0.01 versus TTP+/+ mice; t test).
Bioactive NO can react with superoxide to peroxynitrite, a very potent RONS. As a marker for peroxynitrite formation, we checked the nitration of tyrosine residues of proteins in cardiac tissue of TTP+/+ and TTP−/− mice using a specific anti-nitrotyrosine antibody. Hearts from TTP−/− mice displayed higher levels of 3-nitrotyrosine-positive proteins (Fig. 4D).
Increased Nox2 Expression in TTP-deficient Mice Correlates with RONS Formation
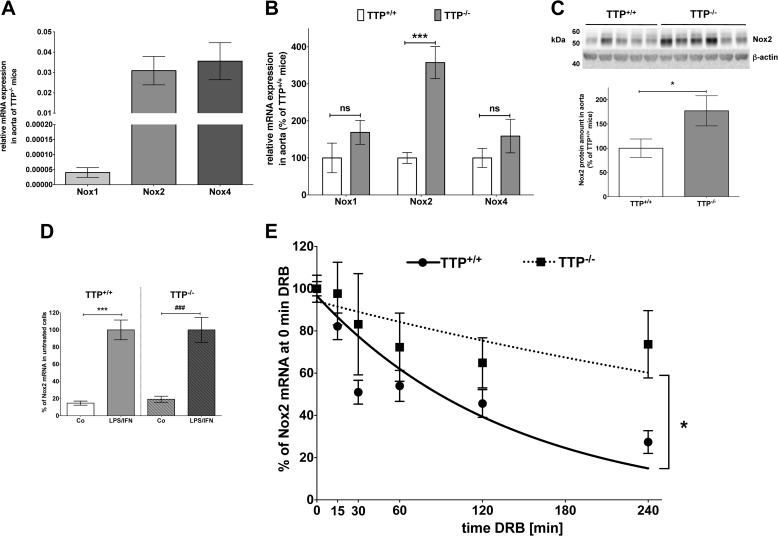
The formation of RONS like superoxide and peroxynitrite is influenced by Nox expression. As Nox1, -2, and -4 had been described to be the major sources of reactive oxygen species in murine aortas (41), we analyzed the mRNA and protein expressions of those enzymes. By analyzing the relative Nox mRNA expression in TTP−/− aortas, we detected much higher expressions of Nox2 and Nox4 than Nox1 mRNA (Fig. 5A). Comparison of the Nox mRNA expression between the aortas of TTP+/+ and TTP−/− mice revealed enhanced Nox2 mRNA expression in TTP−/− aortas (Fig. 5B). Also enhanced Nox2 protein expression was detected (Fig. 5C), whereas no changes in Nox1 or -4 protein expression were seen (data not shown).
FIGURE 5.
Nox2 expression is enhanced in the aorta or peritoneal cells of TTP−/− mice. RNA (A, B, D, and E) or protein (C) from aortas or peritoneal cells of TTP−/− or TTP+/+ mice were analyzed for Nox mRNA or protein expression by qRT-PCR or immunoblot analyses. A, the relative mRNA expression of the Nox1, -2, and -4 genes in aortas of TTP−/− mice is shown. Data presented are mean ± S.E. of 5–13 mice. B, the relative mRNA expression of the Nox1, -2, and -4 genes in the aorta of TTP−/− mice compared with TTP+/+ mice is shown. Expression of Nox mRNAs in TTP+/+ animals was set to 100%. Data shown are mean ± S.E. of 5–13 mice (***, p < 0.001; ns, not significant versus TTP+/+ mice; t test). C, in the upper panel a representative Western blot used for the analysis of Nox2 protein expression is shown. Each lane represents a different animal. The lower panel displays the summary (mean ± S.E. of 11 mice) of densitometric analyses of the Western blot (*, p < 0.05 versus TTP+/+ mice; t test). D, peritoneal cells of TTP+/+ or TTP−/− mice were isolated and adherent cells were treated with LPS (2 μg/ml) and IFN-γ (100 units/ml) for 4 h. RNA was isolated and Nox2 and GAPDH mRNA was measured. Nox2 mRNA expression was normalized to the GAPDH mRNA expression. The relative Nox2 mRNA expression after 4 h LPS/IFN-γ in each genotype was set to 100%. Shown are the mean ± S.E. of n = 10–12 analyses (***, p < 0.001 versus untreated TTP+/+ mice; ###, p < 0.001 versus untreated TTP−/− mice; t test). E, peritoneal cells of TTP+/+ or TTP−/− mice were isolated and adherent cells were treated with LPS (2 μg/ml) and IFN-γ (100 units/ml) to induce Nox2 mRNA expression for 4 h. DRB (25 μg/ml) was added to stop RNA Polymerase II-dependent transcription. After 10, 30, 60, 120, or 240 min RNA was isolated and Nox2 and GAPDH mRNA were measured. Nox2 mRNA expression was normalized to GAPDH mRNA expression. The relative Nox2 mRNA expression after 4 h LPS/IFN-γ was set to 100%. Shown are the mean ± S.E. of n = 10–12 analyses (*, p < 0.05 cells TTP−/− versus TTP+/+ mice; two-way ANOVA). The half-life of the Nox2 mRNA was 97.8 ± 24.1 in TTP+/+ and 402.4 ± 219.3 min in TTP−/− animals, respectively.
TTP Gene Inactivation Enhances Nox2 mRNA Stability
We analyzed the effect of TTP deficiency on Nox2 mRNA stability in primary peritoneal cells isolated from TTP+/+ and TTP−/− mice. Incubation with LPS (2 μg/ml) and IFN-γ (100 units/ml) for 4 h induced Nox2 mRNA expression in peritoneal cells of both genotypes nearly 5-fold (see Fig. 5D). As shown in Fig. 5E inactivation of the Ttp gene resulted in markedly enhanced Nox2 mRNA stability (TTP+/+ t½ = 97.8 ± 24.1 min; TTP−/− t½ = 402.4 ± 219.3 min).
Collagen-induced Chronic Inflammation Results in Reduced TTP and Enhanced Nox2 mRNA Expression
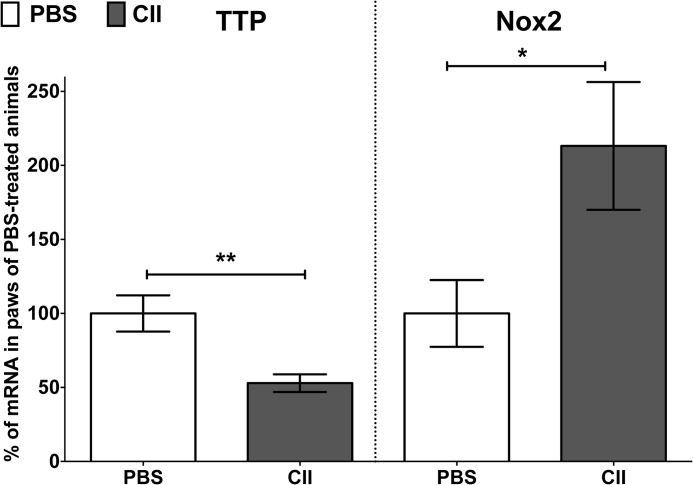
To analyze the correlation of TTP and Nox2 expression in another model of chronic inflammation we performed analyses in mice treated with collagen type II (CII, in CIA) (26). Compared with PBS-treated mice we observed reduced TTP and enhanced Nox2 mRNA expression in the paws of CII-treated mice (Fig. 6).
FIGURE 6.
The TTP mRNA expression is reduced in paws of CII-treated animals, whereas the Nox2 mRNA expression is enhanced. The mRNA expression of TTP and Nox2 was measured in paws of PBS- or CII-treated mice on day 33 after the first immunization by qRT-PCR. TTP or Nox2 mRNA expression was normalized to GAPDH mRNA expression. The TTP or Nox2 mRNA expression in PBS-treated mice was set to 100%. Each treatment group contained seven mice. Data represent the relative TTP or Nox2 mRNA expression (mean ± S.E.) compared with PBS-treated mice (**, p < 0.01; *, p < 0.05 versus PBS-treated mice; t test).
Inactivation of the Tnf-α Gene in TTP−/− Animals Does Not Reverse Endothelial Dysfunction
TTP has been shown to be a major post-transcriptional regulator of TNF-α expression (21) and the pro-inflammatory phenotype of TTP−/− mice can be reversed by TNF-α blockade (23). TNF-α has been described to be an important mediator of atherogenesis (42), as well as to regulate Nox expression in cell culture (43) and mouse models (44). Therefore we expected that genetic inactivation of the Tnf-α gene in TTP−/− mice normalizes the atherogenic phenotype. Surprisingly, inactivation of the Tnf-α gene in TTP−/− mice did not improve the endothelial dysfunction caused by TTP deficiency (Fig. 2A).
Inactivation of the Tnf-α Gene in TTP−/− Animals Only Partially Reduces Enhanced RONS Production
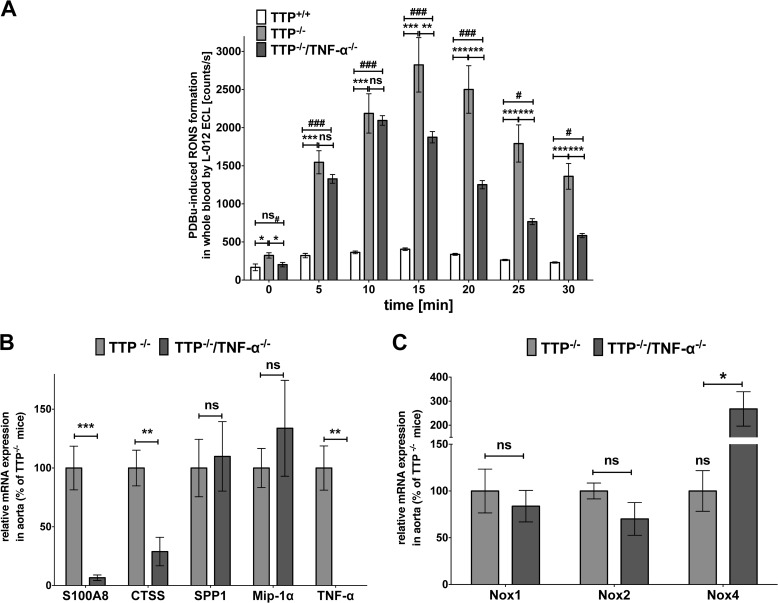
We measured the RONS formation in whole blood of TTP+/+, TTP−/−, and TTP−/−/TNF-α−/− animals under PDBu-stimulated conditions with L-012 ECL chemiluminescence (Fig. 7A) and observed only partial reduction of the enhanced RONS formation in TTP−/−/TNF-α−/− compared with TTP−/− mice. At each time point the RONS production was still significantly higher compared with wild type mice.
FIGURE 7.
Inactivation of the Tnf-α gene in TTP−/− mice only partially reduces TTP-related enhanced RONS formation and does not prevent the TTP−/−-mediated enhancement of SPP1, Mip-1α, or Nox2 mRNA expression. A, RONS formation in the blood was measured under PDBu-stimulated conditions with L-012 ECL substrate. Data shown are mean ± S.E. of 7–8 mice (*, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, not significant versus TTP−/− mice; ##, p < 0.01; ###, p < 0.001; ns#, not significant versus TTP+/+ mice; one-way ANOVA). B and C, RNA from aortas of TTP−/− and TTP−/−/TNF-α−/− mice were analyzed for S100A8, CTSS, SPP1, and Mip-1α mRNA-expression (B) and for Nox1, -2, and -4 mRNA expression (C) in qRT-PCR experiments. Data shown are mean ± S.E. of 4 to 5 independent measurements (*, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, not significant versus TTP−/− mice; t test).
Inactivation of the TNF-α Gene in TTP−/− Animals Has No Influence on TTP−/−-mediated Enhancement of SPP1, Mip-1α, and Nox2 mRNA Expression
We performed qRT-PCR analyses with RNAs from aortas of 3–4-month-old TTP−/− and TTP−/−/TNF-α−/− mice. The inactivation of the Tnf-α gene reduced S100A8 and CTSS mRNA expression. In contrast, the enhanced mRNA expression of SPP1 and Mip-1α was not changed by Tnf-α gene inactivation (Fig. 7B). In addition, compared with TTP−/− animals Nox2 mRNA expression was not reduced in TTP−/−/TNF-α−/− mice (Fig. 7C). Interestingly, Nox4 mRNA expression was significantly enhanced in TTP−/−/TNF-α−/− animals compared with TTP−/− mice.
DISCUSSION
Patients suffering from chronic inflammatory diseases like RA have a 50% increased risk for cardiovascular events, independent of the traditional risk factors (6) and an enhanced cardiovascular disease mortality (45). Up to now the molecular mechanisms leading from RA to AS are not well understood.
Endothelial dysfunction is the earliest maker of atherosclerotic changes in the vessel wall, which precedes plaque development and is a predictor of cardiovascular events (2, 3). Endothelial dysfunction is a common feature of patients with atherosclerosis (4) and has been linked to RA-driven systemic inflammation (46).
The importance of systemic inflammation in atherogenesis is demonstrated by the correlation of increased concentrations of inflammation markers with cardiovascular mortality in RA patients (45, 47). Many pro-inflammatory mediators enhanced in RA, e.g. IL-6 and TNF-α, seem to influence the progression of AS. Several pro-inflammatory mediators are post-transcriptionally regulated by TTP. Therefore this RNA-binding protein is likely to play a central inhibitory role in the inflammation-related pathomechanisms leading to AS. In gene expression analyses enhanced TTP expression could be detected in atherosclerotic plaques from human patients or mice (48, 49). This may be a reflectory response of the organism to down-regulate the pro-inflammatory gene expression.
Even though TTP is an important regulator of pro-inflammatory gene expression, its precise role in the development of RA and AS is not clear (20, 48, 49). For a better understanding of those underlying mechanisms, we used a TTP−/− mouse model that spontaneously develops a pro-inflammatory phenotype (23) mainly due to enhanced TNF-α expression. In the current study we describe the relationship between systemic inflammation in TTP−/− mice and the development of an endothelial dysfunction. Despite no detectable major changes in the NO-cGMP pathway (Fig. 2, C and D, Fig. 3A) TTP−/− mice have an impaired endothelial function (Fig. 2A) in the absence of hypercholesterolemia, normal blood glucose levels, and reduced blood pressure (Fig. 3, B and C). This is similar to RA patients, who develop endothelial dysfunction, in the absence of classical risk factors (50). As endothelial dysfunction is correlated to hypertension (51), the reduced blood pressure measured in TTP−/− mice was not expected. However, in microarray analyses using RNAs from embryonic fibroblasts of TTP+/+ and TTP−/− mice the Ier3 (also named gly96/IEX 1) transcript was characterized as a direct TTP target (52). Mice with disrupted Ier3 gene displayed enhanced blood pressure and cardiac hypertrophy (53). Therefore increased Ier3 expression in TTP−/− mice may explain the reduced blood pressure detected in our experiments. Interestingly, inactivation of the Tnf-α gene in TTP−/− mice did not reverse TTP−/−-dependent endothelial dysfunction (Fig. 2A). Therefore, besides TNF-α-related pro-inflammatory stress, other factors putatively directly regulated by TTP seem to be involved in TTP−/−-induced endothelial dysfunction.
More detailed molecular analyses demonstrated increased expression of several mediators, which are known to trigger either inflammation (TNF-α, Mip-1α, and S100A8) or atherogenesis (SPP1 and CTSS) in the aortas of TTP−/− mice (Fig. 1A). TNF-α and Mip-1α are two well characterized TTP target mRNAs (18–20). Tnf-α gene inactivation in TTP−/− mice normalized the mRNA expression of some (S100A8, CTSS) but not all (SPP1 and Mip-1α) analyzed mediators (Fig. 7B). SPP1 expression has been shown to be increased by TNF-α (54) and post-transcriptional regulation of the SPP1 mRNA by binding of the elongation translation factor-1A1 to the SPP1 5′-UTR has been described (55). Whether TTP directly regulates SPP1 expression, via binding to the SPP1 mRNA, or indirectly, via modulation of other post-transcriptional mechanisms, remains to be determined.
We also observed enhanced VCAM-1 and ICAM-1 expression in the aorta of TTP−/− mice (Fig. 1B). As VCAM-1 has been identified as a putative TTP target in DNA microarray experiments (56), the enhanced VCAM-1 expression may be a direct effect of the TTP deficiency in addition to the well known TNF-α-related increase of ICAM-1 and VCAM-1 expression (57). The enhanced VCAM-1 and ICAM-1 expressions indicate an activation of endothelial cells in the aorta of TTP−/− mice and is able to enhance immune cell migration into the intima (58). Accordingly, we detected enhanced CD68 protein content in the aorta of TTP−/− mice (Fig. 1C).
The increased TNF-α level in TTP−/− mice is involved in the chronic inflammatory phenotype of these animals (23). Also in RA patients TNF-α seems to be one of the main triggers of the disease. The phenotype (e.g. body weight and live span) of TTP−/− mice could be partly reversed with anti-TNF-α antibody treatment, cross-breeding on a TNF-α receptor-deficient strain (59) or on a TNF-α−/− strain (this article). As TNF-α is an important mediator of systemic inflammation, it seems very likely that it plays a major role in atherogenesis in RA patients or TTP−/− mice, but this issue is still under debate. Treatment with TNF-α antagonists, especially in combination with the disease modifying anti-rheumatic drug methotrexate, seems to lower the cardiovascular risk in RA patients (60), favoring a deleterious role of TNF-α in RA-associated AS development. However, contrary studies exist demonstrating no beneficial effect of an anti-TNF-α therapy on cardiovascular risk in RA patients (61). These indifferent results of the clinical data seem to be reflected in our animal model. As shown in Figs. 2A and 7 the endothelial dysfunction, enhanced RONS production, and enhanced pro-inflammatory and pro-oxidative gene expression induced by TTP deficiency are not or only partially reverted by inactivation of the Tnf-α gene in TTP−/− mice. Altogether the data from the human and mouse systems indicate that TNF-α is one important but not the only mediator responsible for AS development in the context of a chronic systemic inflammation.
Our data demonstrate that oxidative stress is one important trigger for the development of an endothelial dysfunction during chronic inflammation. The loss of TTP leads to higher expression levels of the superoxide-producing enzyme Nox2 (Fig. 5, B and C) resulting at least partially from enhanced mRNA stability (Fig. 5E). This higher Nox2 expression leads to enhanced RONS levels (Fig. 4). This results in an imbalanced ratio of vasodilators to vasoconstrictors and impaired NO bioavailability (Fig. 2B) contributing to the observed endothelial dysfunction. Analysis of TTP and Nox2 mRNA expression in the paws of CII-treated mice (CIA) showed reduced TTP and enhanced Nox2 mRNA expression compared with PBS-treated animals (Fig. 6). This indicates a close connection between dysregulated TTP and Nox2 expression in chronic inflammatory diseases in general. Also in synovial fluids obtained from RA patients the levels of RONS including superoxide are significantly raised and positively correlated with higher Nox activity (62). In addition, increased levels of nitrotyrosine, another marker of oxidative stress, were detected in RA patients (63) and also in TTP−/− mice (Fig. 4D). Analyzing CD68 expression (Fig. 1C) we detected an increased infiltration of macrophages into the intima of TTP−/− aorta. In addition as expected from the regulation of GM-CSF mRNA expression by TTP (64), blood cell analyses revealed enhanced neutrophil, granulocyte, and monocyte cell numbers in TTP−/− mice (see Table 2). As Nox2 expression is high in monocytes/macrophages the enhanced RONS production and Nox2 expression in the aorta of TTP−/− mice may be related to the observed macrophage/monocyte infiltration. The enhanced RONS production and Nox2 mRNA expression was not reverted by inactivation of the TNF-α gene in TTP−/− mice (Fig. 7).
The enhanced expression of Nox2 detected in TTP−/− and TTP−/−/TNF-α−/− mice may be a direct consequence of TTP deficiency or an indirect but TNF-α-independent effect, due to systemic inflammatory processes. Reduction of Nox2 expression/activity by p38 MAPK inhibitors has been described (65, 66). p38 MAPK is a major regulator of TTP expression and activity (67). In addition the 3′-UTR of the Nox2 gene contains several AREs, which may be TTP binding sites. In the current study we detected marked enhancement of Nox2 mRNA stability in peritoneal cells isolated from TTP−/− animals (Fig. 5D). Therefore it seems very likely that TTP mediates the decay of Nox2 mRNA under physiological conditions. Because not much is known about post-transcriptional regulation of Nox expression (68), this hypothesis has to be verified in further experiments. According to our results we postulate that during systemic inflammation molecular pathways leading to the development of atherogenesis through induction of oxidative stress are only partially activated by TNF-α.
Altogether we have demonstrated that oxidative stress, in particular increased Nox2 activity, promotes the development of atherosclerosis in models of systemic inflammatory diseases as RA. Moreover we provide evidence that TNF-α alone is not the driving force in atherogenic processes. As the standard therapeutics in RA, TNF-α antagonists, and disease modifying anti-rheumatic drugs have only a limited success in prevention of cardiovascular events, the additional use of antioxidative drugs could be an alternative. In numerous clinical trials the usage of nutritional supplements (such as β-carotene, selenium, vitamin C, and vitamin E) as antioxidative substances in the prevention of coronary heart disease and stroke resulted in conflicting data (positive, neutral, and negative) (69, 70). Therefore more specific drugs acting via down-regulation of Nox could be promising.
This work was supported by Federal Ministry of Education and Research Grant BMBF 01EO1003, Innovation Foundation of the State of Rhineland-Palatinate Grant 961-386261/917K, and Deutsche Forschungsgemeinschaft Grant LI 1759/1-1 (to H. K.).
- AS
- atherosclerosis
- CD68
- cluster of differentiation 68
- CIA
- collagen-induced arthritis
- CTSS
- cathepsin S
- DRB
- 6-dichloro-1-ribofuranosylbenzimidazole
- eNOS
- endothelial nitric-oxide synthase
- ICAM-1
- intercellular adhesion molecule 1
- Ier3
- immediate early response 3
- iNOS
- inducible nitric-oxide synthase
- Mip-1α (CCL3)
- macrophage inflammatory protein-1α
- Nox
- NADPH oxidase
- RA
- rheumatoid arthritis
- RONS
- reactive oxygen and nitrogen species
- S100A8
- S100 calcium-binding protein A8
- SPP1
- osteopontin
- TTP
- tristetraprolin
- VASP
- vasodilator-stimulated phosphoprotein
- VCAM-1
- vascular cell adhesion molecule 1
- qRT
- quantitative RT
- PDBu
- phorbol 12,13-dibutyrate
- ANOVA
- analysis of variance.
REFERENCES
- 1. Hansson G. K., Robertson A. K., Söderberg-Nauclér C. (2006) Inflammation and atherosclerosis. Annu. Rev. Pathol. 1, 297–329 [DOI] [PubMed] [Google Scholar]
- 2. Dai X. Y., Cai Y., Sun W., Ding Y., Wang W., Kong W., Tang C., Zhu Y., Xu M. J., Wang X. (2014) Intermedin inhibits macrophage foam-cell formation via tristetraprolin-mediated decay of CD36 mRNA. Cardiovasc. Res. 101, 297–305 [DOI] [PubMed] [Google Scholar]
- 3. Jamal Uddin M., Joe Y., Zheng M., Blackshear P. J., Ryter S. W., Park J. W., Chung H. T. (2013) A functional link between heme oxygenase-1 and tristetraprolin in the anti-inflammatory effects of nicotine. Free Radic. Biol. Med. 65, 1331–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cao H., Cao F., Roussel A. M., Anderson R. A. (2013) Quantitative PCR for glucose transporter and tristetraprolin family gene expression in cultured mouse adipocytes and macrophages. In Vitro Cell. Dev. Biol. Anim. 49, 759–770 [DOI] [PubMed] [Google Scholar]
- 5. Galkina E., Ley K. (2009) Immune and inflammatory mechanisms of atherosclerosis (*). Annu. Rev. Immunol. 27, 165–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aviña-Zubieta J. A., Choi H. K., Sadatsafavi M., Etminan M., Esdaile J. M., Lacaille D. (2008) Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 59, 1690–1697 [DOI] [PubMed] [Google Scholar]
- 7. Roman M. J., Moeller E., Davis A., Paget S. A., Crow M. K., Lockshin M. D., Sammaritano L., Devereux R. B., Schwartz J. E., Levine D. M., Salmon J. E. (2006) Preclinical carotid atherosclerosis in patients with rheumatoid arthritis. Ann. Intern. Med. 144, 249–256 [DOI] [PubMed] [Google Scholar]
- 8. Maradit-Kremers H., Nicola P. J., Crowson C. S., Ballman K. V., Gabriel S. E. (2005) Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum. 52, 722–732 [DOI] [PubMed] [Google Scholar]
- 9. del Rincón I. D., Williams K., Stern M. P., Freeman G. L., Escalante A. (2001) High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 44, 2737–2745 [DOI] [PubMed] [Google Scholar]
- 10. Gabriel S. E. (2008) Cardiovascular morbidity and mortality in rheumatoid arthritis. Am. J. Med. 121, S9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iademarco M. F., Barks J. L., Dean D. C. (1995) Regulation of vascular cell adhesion molecule-1 expression by IL-4 and TNF-α in cultured endothelial cells. J. Clin. Invest. 95, 264–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jayakody L., Kappagoda T., Senaratne M. P., Thomson A. B. (1988) Impairment of endothelium-dependent relaxation: an early marker for atherosclerosis in the rabbit. Br. J. Pharmacol. 94, 335–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Griendling K. K., FitzGerald G. A. (2003) Oxidative stress and cardiovascular injury: part II: animal and human studies. Circulation 108, 2034–2040 [DOI] [PubMed] [Google Scholar]
- 14. Münzel T., Daiber A., Ullrich V., Mülsch A. (2005) Vascular consequences of endothelial nitric oxide synthase uncoupling for the activity and expression of the soluble guanylyl cyclase and the cGMP-dependent protein kinase. Arterioscler. Thromb. Vasc. Biol. 25, 1551–1557 [DOI] [PubMed] [Google Scholar]
- 15. Förstermann U., Münzel T. (2006) Endothelial nitric-oxide synthase in vascular disease: from marvel to menace. Circulation 113, 1708–1714 [DOI] [PubMed] [Google Scholar]
- 16. Bosello S., Santoliquido A., Zoli A., Di Campli C., Flore R., Tondi P., Ferraccioli G. (2008) TNF-alpha blockade induces a reversible but transient effect on endothelial dysfunction in patients with long-standing severe rheumatoid arthritis. Clin. Rheumatol. 27, 833–839 [DOI] [PubMed] [Google Scholar]
- 17. Chen C. Y., Shyu A. B. (1995) AU-rich elements: characterization and importance in mRNA degradation. Trends Biochem. Sci. 20, 465–470 [DOI] [PubMed] [Google Scholar]
- 18. Brooks S. A., Blackshear P. J. (2013) Tristetraprolin (TTP): interactions with mRNA and proteins, and current thoughts on mechanisms of action. Biochim. Biophys. Acta 1829, 666–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carballo E., Lai W. S., Blackshear P. J. (1998) Feedback inhibition of macrophage tumor necrosis factor-α production by tristetraprolin. Science 281, 1001–1005 [DOI] [PubMed] [Google Scholar]
- 20. Kang J. G., Amar M. J., Remaley A. T., Kwon J., Blackshear P. J., Wang P. Y., Hwang P. M. (2011) Zinc finger protein tristetraprolin interacts with CCL3 mRNA and regulates tissue inflammation. J. Immunol. 187, 2696–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blackshear P. J. (2002) Tristetraprolin and other CCCH tandem zinc-finger proteins in the regulation of mRNA turnover. Biochem. Soc. Trans. 30, 945–952 [DOI] [PubMed] [Google Scholar]
- 22. Ghosh S., Hoenerhoff M. J., Clayton N., Myers P., Stumpo D. J., Maronpot R. R., Blackshear P. J. (2010) Left-sided cardiac valvulitis in tristetraprolin-deficient mice: the role of tumor necrosis factor α. Am. J. Pathol. 176, 1484–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Taylor G. A., Carballo E., Lee D. M., Lai W. S., Thompson M. J., Patel D. D., Schenkman D. I., Gilkeson G. S., Broxmeyer H. E., Haynes B. F., Blackshear P. J. (1996) A pathogenetic role for TNF α in the syndrome of cachexia, arthritis, and autoimmunity resulting from tristetraprolin (TTP) deficiency. Immunity 4, 445–454 [DOI] [PubMed] [Google Scholar]
- 24. Ridker P. M., Rifai N., Pfeffer M., Sacks F., Lepage S., Braunwald E. (2000) Elevation of tumor necrosis factor-α and increased risk of recurrent coronary events after myocardial infarction. Circulation 101, 2149–2153 [DOI] [PubMed] [Google Scholar]
- 25. Mori L., Loetscher H., Kakimoto K., Bluethmann H., Steinmetz M. (1992) Expression of a transgenic T cell receptor β chain enhances collagen-induced arthritis. J. Exp. Med. 176, 381–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schmidt N., Art J., Forsch I., Werner A., Erkel G., Jung M., Horke S., Kleinert H., Pautz A. (2012) The anti-inflammatory fungal compound (S)-curvularin reduces proinflammatory gene expression in an in vivo model of rheumatoid arthritis. J. Pharmacol. Exp. Ther. 343, 106–114 [DOI] [PubMed] [Google Scholar]
- 27. Ray A., Dittel B. N. (2010) Isolation of mouse peritoneal cavity cells. J. Vis. Exp. 35, e1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li H., Hergert S. M., Schäfer S. C., Brausch I., Yao Y., Huang Q., Mang C., Lehr H. A., Förstermann U. (2005) Midostaurin up-regulates eNOS gene expression and preserves eNOS function in the microcirculation of the mouse. Nitric Oxide 12, 231–236 [DOI] [PubMed] [Google Scholar]
- 29. Friedewald W. T., Levy R. I., Fredrickson D. S. (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18, 499–502 [PubMed] [Google Scholar]
- 30. Chomczynski P., Sacchi N. (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162, 156–159 [DOI] [PubMed] [Google Scholar]
- 31. Schmidt N., Pautz A., Art J., Rauschkolb P., Jung M., Erkel G., Goldring M. B., Kleinert H. (2010) Transcriptional and post-transcriptional regulation of iNOS expression in human chondrocytes. Biochem. Pharmacol. 79, 722–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Siuda D., Zechner U., El Hajj N., Prawitt D., Langer D., Xia N., Horke S., Pautz A., Kleinert H., Förstermann U., Li H. (2012) Transcriptional regulation of Nox4 by histone deacetylases in human endothelial cells. Basic Res. Cardiol. 107, 283. [DOI] [PubMed] [Google Scholar]
- 33. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−[/Delta][/Delta] C(T)) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 34. Daiber A., August M., Baldus S., Wendt M., Oelze M., Sydow K., Kleschyov A. L., Munzel T. (2004) Measurement of NAD(P)H oxidase-derived superoxide with the luminol analogue L-012. Free Radic. Biol. Med. 36, 101–111 [DOI] [PubMed] [Google Scholar]
- 35. Oelze M., Daiber A., Brandes R. P., Hortmann M., Wenzel P., Hink U., Schulz E., Mollnau H., von Sandersleben A., Kleschyov A. L., Mülsch A., Li H., Förstermann U., Münzel T. (2006) Nebivolol inhibits superoxide formation by NADPH oxidase and endothelial dysfunction in angiotensin II-treated rats. Hypertension 48, 677–684 [DOI] [PubMed] [Google Scholar]
- 36. Schuhmacher S., Wenzel P., Schulz E., Oelze M., Mang C., Kamuf J., Gori T., Jansen T., Knorr M., Karbach S., Hortmann M., Mäthner F., Bhatnagar A., Förstermann U., Li H., Münzel T., Daiber A. (2010) Pentaerythritol tetranitrate improves angiotensin II-induced vascular dysfunction via induction of heme oxygenase-1. Hypertension 55, 897–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Knorr M., Hausding M., Kröller-Schuhmacher S., Steven S., Oelze M., Heeren T., Scholz A., Gori T., Wenzel P., Schulz E., Daiber A., Münzel T. (2011) Nitroglycerin-induced endothelial dysfunction and tolerance involve adverse phosphorylation and S-glutathionylation of endothelial nitric oxide synthase: beneficial effects of therapy with the AT1 receptor blocker telmisartan. Arterioscler. Thromb. Vasc. Biol. 31, 2223–2231 [DOI] [PubMed] [Google Scholar]
- 38. Sukhova G. K., Zhang Y., Pan J. H., Wada Y., Yamamoto T., Naito M., Kodama T., Tsimikas S., Witztum J. L., Lu M. L., Sakara Y., Chin M. T., Libby P., Shi G. P. (2003) Deficiency of cathepsin S reduces atherosclerosis in LDL receptor-deficient mice. J. Clin. Invest. 111, 897–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Scatena M., Liaw L., Giachelli C. M. (2007) Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler. Thromb. Vasc. Biol. 27, 2302–2309 [DOI] [PubMed] [Google Scholar]
- 40. Bobryshev Y. V., Lord R. S. (2002) Expression of heat shock protein-70 by dendritic cells in the arterial intima and its potential significance in atherogenesis. J. Vasc. Surg. 35, 368–375 [DOI] [PubMed] [Google Scholar]
- 41. Pendyala S., Gorshkova I. A., Usatyuk P. V., He D., Pennathur A., Lambeth J. D., Thannickal V. J., Natarajan V. (2009) Role of Nox4 and Nox2 in hyperoxia-induced reactive oxygen species generation and migration of human lung endothelial cells. Antioxid. Redox Signal. 11, 747–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brånén L., Hovgaard L., Nitulescu M., Bengtsson E., Nilsson J., Jovinge S. (2004) Inhibition of tumor necrosis factor-α reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol. 24, 2137–2142 [DOI] [PubMed] [Google Scholar]
- 43. Yoshida L. S., Tsunawaki S. (2008) Expression of NADPH oxidases and enhanced H2O2-generating activity in human coronary artery endothelial cells upon induction with tumor necrosis factor-α. Int. Immunopharmacol. 8, 1377–1385 [DOI] [PubMed] [Google Scholar]
- 44. Moe K. T., Yin N. O., Naylynn T. M., Khairunnisa K., Wutyi M. A., Gu Y., Atan M. S., Wong M. C., Koh T. H., Wong P. (2011) Nox2 and Nox4 mediate tumour necrosis factor-α-induced ventricular remodelling in mice. J. Cell. Mol. Med. 15, 2601–2613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Goodson N., Marks J., Lunt M., Symmons D. (2005) Cardiovascular admissions and mortality in an inception cohort of patients with rheumatoid arthritis with onset in the 1980s and 1990s. Ann. Rheum. Dis. 64, 1595–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee H. H., Yang S. S., Vo M. T., Cho W. J., Lee B. J., Leem S. H., Lee S. H., Cha H. J., Park J. W. (2013) Tristetraprolin down-regulates IL-23 expression in colon cancer cells. Mol. Cells 36, 571–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gonzalez-Gay M. A., Gonzalez-Juanatey C., Lopez-Diaz M. J., Piñeiro A., Garcia-Porrua C., Miranda-Filloy J. A., Ollier W. E., Martin J., Llorca J. (2007) HLA-DRB1 and persistent chronic inflammation contribute to cardiovascular events and cardiovascular mortality in patients with rheumatoid arthritis. Arthritis Rheum. 57, 125–132 [DOI] [PubMed] [Google Scholar]
- 48. Patino W. D., Kang J. G., Matoba S., Mian O. Y., Gochuico B. R., Hwang P. M. (2006) Atherosclerotic plaque macrophage transcriptional regulators are expressed in blood and modulated by tristetraprolin. Circ. Res. 98, 1282–1289 [DOI] [PubMed] [Google Scholar]
- 49. Zhang H., Taylor W. R., Joseph G., Caracciolo V., Gonzales D. M., Sidell N., Seli E., Blackshear P. J., Kallen C. B. (2013) mRNA-binding protein ZFP36 is expressed in atherosclerotic lesions and reduces inflammation in aortic endothelial cells. Arterioscler. Thromb. Vasc. Biol. 33, 1212–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kerekes G., Szekanecz Z., Dér H., Sándor Z., Lakos G., Muszbek L., Csipö I., Sipka S., Seres I., Paragh G., Kappelmayer J., Szomják E., Veres K., Szegedi G., Shoenfeld Y., Soltész P. (2008) Endothelial dysfunction and atherosclerosis in rheumatoid arthritis: a multiparametric analysis using imaging techniques and laboratory markers of inflammation and autoimmunity. J. Rheumatol. 35, 398–406 [PubMed] [Google Scholar]
- 51. Dharmashankar K., Widlansky M. E. (2010) Vascular endothelial function and hypertension: insights and directions. Curr. Hypertens. Rep. 12, 448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lai W. S., Parker J. S., Grissom S. F., Stumpo D. J., Blackshear P. J. (2006) Novel mRNA targets for tristetraprolin (TTP) identified by global analysis of stabilized transcripts in TTP-deficient fibroblasts. Mol. Cell. Biol. 26, 9196–9208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sommer S. L., Berndt T. J., Frank E., Patel J. B., Redfield M. M., Dong X., Griffin M. D., Grande J. P., van Deursen J. M., Sieck G. C., Romero J. C., Kumar R. (2006) Elevated blood pressure and cardiac hypertrophy after ablation of the gly96/IEX-1 gene. J. Appl. Physiol. 100, 707–716 [DOI] [PubMed] [Google Scholar]
- 54. Mangan S. H., Van Campenhout A., Rush C., Golledge J. (2007) Osteoprotegerin up-regulates endothelial cell adhesion molecule response to tumor necrosis factor-α associated with induction of angiopoietin-2. Cardiovasc. Res. 76, 494–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang J., Guo H., Mi Z., Gao C., Bhattacharya S., Li J., Kuo P. C. (2009) EF1A1-actin interactions alter mRNA stability to determine differential osteopontin expression in HepG2 and Hep3B cells. Exp. Cell Res. 315, 304–312 [DOI] [PubMed] [Google Scholar]
- 56. Ishmael F. T., Fang X., Galdiero M. R., Atasoy U., Rigby W. F., Gorospe M., Cheadle C., Stellato C. (2008) Role of the RNA-binding protein tristetraprolin in glucocorticoid-mediated gene regulation. J. Immunol. 180, 8342–8353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. McMurray R. W. (1996) Adhesion molecules in autoimmune disease. Semin. Arthritis Rheum. 25, 215–233 [DOI] [PubMed] [Google Scholar]
- 58. Crook M. F., Southgate K. M., Newby A. C. (2002) Both ICAM-1- and VCAM-1-integrin interactions are important in mediating monocyte adhesion to human saphenous vein. J. Vasc. Res. 39, 221–229 [DOI] [PubMed] [Google Scholar]
- 59. Carballo E., Blackshear P. J. (2001) Roles of tumor necrosis factor-α receptor subtypes in the pathogenesis of the tristetraprolin-deficiency syndrome. Blood 98, 2389–2395 [DOI] [PubMed] [Google Scholar]
- 60. Greenberg J. D., Kremer J. M., Curtis J. R., Hochberg M. C., Reed G., Tsao P., Farkouh M. E., Nasir A., Setoguchi S., Solomon D. H., and CORRONA Investigators (2011) Tumour necrosis factor antagonist use and associated risk reduction of cardiovascular events among patients with rheumatoid arthritis. Ann. Rheum. Dis. 70, 576–582 [DOI] [PubMed] [Google Scholar]
- 61. Al-Aly Z., Pan H., Zeringue A., Xian H., McDonald J. R., El-Achkar T. M., Eisen S. (2011) Tumor necrosis factor-α blockade, cardiovascular outcomes, and survival in rheumatoid arthritis. Transl. Res. 157, 10–18 [DOI] [PubMed] [Google Scholar]
- 62. Kundu S., Ghosh P., Datta S., Ghosh A., Chattopadhyay S., Chatterjee M. (2012) Oxidative stress as a potential biomarker for determining disease activity in patients with rheumatoid arthritis. Free Radic. Res. 46, 1482–1489 [DOI] [PubMed] [Google Scholar]
- 63. Ikonomidis I., Lekakis J. P., Nikolaou M., Paraskevaidis I., Andreadou I., Kaplanoglou T., Katsimbri P., Skarantavos G., Soucacos P. N., Kremastinos D. T. (2008) Inhibition of interleukin-1 by anakinra improves vascular and left ventricular function in patients with rheumatoid arthritis. Circulation 117, 2662–2669 [DOI] [PubMed] [Google Scholar]
- 64. Carrick D. M., Lai W. S., Blackshear P. J. (2004) The tandem CCCH zinc finger protein tristetraprolin and its relevance to cytokine mRNA turnover and arthritis. Arthritis Res. Ther. 6, 248–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Huo Y., Rangarajan P., Ling E. A., Dheen S. T. (2011) Dexamethasone inhibits the Nox-dependent ROS production via suppression of MKP-1-dependent MAPK pathways in activated microglia. BMC Neurosci. 12, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lim H., Kim D., Lee S. J. (2013) Toll-like receptor 2 mediates peripheral nerve injury-induced NADPH oxidase 2 expression in spinal cord microglia. J. Biol. Chem. 288, 7572–7579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sandler H., Stoecklin G. (2008) Control of mRNA decay by phosphorylation of tristetraprolin. Biochem. Soc. Trans. 36, 491–496 [DOI] [PubMed] [Google Scholar]
- 68. Lambeth J. D., Kawahara T., Diebold B. (2007) Regulation of Nox and Duox enzymatic activity and expression. Free Radic. Biol. Med. 43, 319–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Badimon L., Vilahur G., Padro T. (2010) Nutraceuticals and atherosclerosis: human trials. Cardiovasc. Therap. 28, 202–215 [DOI] [PubMed] [Google Scholar]
- 70. Schramm A., Matusik P., Osmenda G., Guzik T. J. (2012) Targeting NADPH oxidases in vascular pharmacology. Vascul. Pharmacol. 56, 216–231 [DOI] [PMC free article] [PubMed] [Google Scholar]