Background: Existing anthrax postexposure antibiotic treatments are inadequate because they do not clear the high levels of secreted anthrax toxins.
Results: Susceptible cells treated with anthrax toxin receptor-targeted siRNAs became resistant to anthrax toxin-mediated cytotoxicity.
Conclusion: RNAi-targeted silencing of anthrax toxin receptors prevents toxins from entering target cells and inducing pathogenesis.
Significance: Toxin receptor-targeted RNAi can be developed as a postexposure treatment against anthrax.
Keywords: Anthrax Toxin, Bacterial Toxin, Macrophage, Receptor, RNA Interference (RNAi), RNA Silencing
Abstract
Anthrax spores can be aerosolized and dispersed as a bioweapon. Current postexposure treatments are inadequate at later stages of infection, when high levels of anthrax toxins are present. Anthrax toxins enter cells via two identified anthrax toxin receptors: tumor endothelial marker 8 (TEM8) and capillary morphogenesis protein 2 (CMG2). We hypothesized that host cells would be protected from anthrax toxins if anthrax toxin receptor expression was effectively silenced using RNA interference (RNAi) technology. Thus, anthrax toxin receptors in mouse and human macrophages were silenced using targeted siRNAs or blocked with specific antibody prior to challenge with anthrax lethal toxin. Viability assays were used to assess protection in macrophages treated with specific siRNA or antibody as compared with untreated cells. Silencing CMG2 using targeted siRNAs provided almost complete protection against anthrax lethal toxin-induced cytotoxicity and death in murine and human macrophages. The same results were obtained by prebinding cells with specific antibody prior to treatment with anthrax lethal toxin. In addition, TEM8-targeted siRNAs also offered significant protection against lethal toxin in human macrophage-like cells. Furthermore, silencing CMG2, TEM8, or both receptors in combination was also protective against MEK2 cleavage by lethal toxin or adenylyl cyclase activity by edema toxin in human kidney cells. Thus, anthrax toxin receptor-targeted RNAi has the potential to be developed as a life-saving, postexposure therapy against anthrax.
Introduction
Bacillus anthracis is the etiological agent responsible for anthrax. B. anthracis is a Gram-positive, rod-shaped bacterium capable of forming stable and easily dispersible spores that can be developed and used as a bioweapon (1–3). Alveolar macrophages will ingest the B. anthracis spores following exposure via inhalation and transport these spores to draining lymph nodes, where they germinate (3, 4) and produce virulence factors: a poly-d-glutamic acid capsule surrounding the vegetative form of the bacterium and anthrax toxins (4, 5).
B. anthracis secretes two binary toxins: 1) lethal toxin (LeTx),2 which cleaves mitogen-activated protein kinase kinases (MAPKKs) and leads to cell lysis, and 2) edema toxin (EdTx), which elevates intracellular cyclic adenosine monophosphate (cAMP) levels, leading to swelling or edema (5, 6). Both toxins have protective antigen (PA) in common bound to either lethal factor (LF) or edema factor (EF). PA is responsible for host cell receptor binding and internalization of toxin complexes, binding to either of two identified anthrax toxin receptors (ANTXRs): tumor endothelial marker 8 (TEM8/ANTXR1) (7) and capillary morphogenesis protein 2 (CMG2/ANTXR2) (8). ANTXRs are implicated in angiogenesis, binding of extracellular matrix (ECM) proteins, maintenance of ECM homeostasis, and regulation of matrix metalloproteinase activity (9).
Inhalational anthrax is a leading bioterrorist threat (1, 3) and is fatal when left untreated (1). An anthrax vaccine has been licensed for human use in the United States (AVA or Biothrax, from Emergent Biosolutions, Rockville, MD). BioThrax is a protein subunit vaccine produced from culture filtrates of a non-virulent, noncapsulated strain (V770-NP1-R) with a very complicated administration schedule; it requires five intramuscular injections (at 0, 1, 6, 12, and 18 months) followed by annual boosters (10). Postexposure treatment for inhalational anthrax includes 60-day antibiotic therapy and a one-dose vaccination of AVA shortly after exposure. However, this treatment is unreliable at later stages of infection, when large amounts of anthrax toxins have been produced (3). Although antibiotics help clear the bacterial infection, they do not directly eliminate anthrax toxins, although it is possible that certain antibiotics inhibit anthrax toxins because they inhibit protein or RNA synthesis (11).
In this study, we tested if we could use ANTXR-targeted siRNAs to protect susceptible cells against 1) LeTx-induced cell death, 2) LeTx-mediated MAPKK cleavage, and 3) EdTx-provoked elevation of intracellular cAMP. Effective siRNA-targeted silencing of ANTXRs could provide for postexposure prophylaxis that is viable for both early and late stage anthrax infections.
EXPERIMENTAL PROCEDURES
Cell Culture
Raw 264.7 cells (TIB-71, ATCC, Manassas, VA) and AD293 cells were maintained in DMEM (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Sigma-Aldrich), 100 units/ml penicillin, and 100 μg/ml streptomycin (complete DMEM). THP-1 cells (TIB-202, ATCC) were maintained in RPMI (Invitrogen) supplemented with 10% heat-inactivated FBS, 2 mm l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. Phorbol 12-myristate 13-acetate (PMA) was purchased from Sigma and used to differentiate THP-1 cells at 10 nm unless otherwise indicated.
siRNA Transfections
Initial experiments were performed with Raw 264.7 cells seeded in 24-well culture plates at 2 × 105 cells/well in a 0.5-ml total volume of antibiotic-free DMEM, 10% FBS 1 day prior to transfection with siRNAs. siRNAs targeted to murine Tem8 (si-mTEM8) and Cmg2 (si-mCMG2) were purchased from Santa Cruz Biotechnology, Inc. Detailed sequence information is shown in Table 1. GFP siRNA (siGFP; sense strand, 5′-GGCAUCAAGUAUCGGAAGAdTdT-3′) was custom-ordered from Invitrogen. The siGFP was used as an irrelevant, control siRNA for our studies because the Raw 264.7 cells used did not contain the GFP gene. All siRNAs were delivered to Raw 264.7 cells using Lipofectamine RNAiMax reagent (Invitrogen) as per the manufacturer's protocol. Later experiments were downscaled to a 96-well format, with 1.25 × 104 cells seeded per well in a 0.1-ml total volume of antibiotic-free medium. In these experiments, Raw 264.7 cells were transfected with siRNAs 1 day after seeding, incubated for 48 h, transfected again with a 48-h incubation, and challenged with LeTx. For initial experiments using the THP-1 cell line, cells were cultured in 6-well plates with 10 nm PMA for 3 days before using 36, 72, or 150 pmol of siRNA to optimize silencing efficiency. siRNAs targeted to human TEM8 and human CMG2 were also purchased from Santa Cruz Biotechnology, and detailed sequences are shown in Table 1. For experiments in 96-well format, 1 × 105 cells were seeded per well in 0.1-ml total volume of antibiotic-free medium with 10 nm PMA for 3 days. These cells were then treated with 3 pmol of siRNA for 24 h. For experiments using AD293 cells, the cells were seeded at 5 × 105 cells/well in 6-well plates and treated with 150 pmol of each siRNA for 24 h.
TABLE 1.
Anthrax receptor-targeted siRNA duplexes
| siRNA (catalog no.) | Duplex | Duplex sequences |
|---|---|---|
| si-mCMG2 (sc-60232) | A | Sense: GUGUGACAGUGUAUCUUCAtt |
| Antisense: UGAAGAUACACUGUCACACtt | ||
| B | Sense: CGACAUGAGAGGUGAUGAAtt | |
| Antisense: UUCAUCACCUCUCAUGUCGtt | ||
| C | Sense: GAAGGAAAUAGCUCAGAUAtt | |
| Antisense: UAUCUGAGCUAUUUCCUUCtt | ||
| si-hCMG2 (sc-60231) | A | Sense: GAAGAACCUUUGCCUACUAtt |
| Antisense: UAGUAGGCAAAGGUUCUUCtt | ||
| B | Sense: CUACCUUGGUUAUGAUUCAtt | |
| Antisense: UGAAUCAUAACCAAGGUAGtt | ||
| C | Sense: GAACUGGUAUAGACAAUGAtt | |
| Antisense: UCAUUGUCUAUACCAGUUCtt | ||
| si-mTEM8 (sc-40201) | A | Sense: CCACAGUAGAUGCCUCUUAtt |
| Antisense: UAAGAGGCAUCUACUGUGGtt | ||
| B | Sense: GCAACCUACUAAUGAUUCAtt | |
| Antisense: UGAAUCAUUAGUAGGUUGCtt | ||
| C | Sense: GAACCAAGAUGCUGGUGUUtt | |
| Antisense: AACACCAGCAUCUUGGUUCtt | ||
| si-hTEM8 (sc-44144) | A | Sense: GGAUUUGACCUGUACUUCAtt |
| Antisense: UGAAGUACAGGUCAAAUCCtt | ||
| B | Sense: CCACUGGAAUGAAAUCUAUtt | |
| Antisense: AUAGAUUUCAUUCCAGUGGtt | ||
| C | Sense: GGAACAGUUGGCUCACAAAtt | |
| Antisense: UUUGUGAGCCAACUGUUCCtt |
Detection of Surface Proteins by Flow Cytometry
Cells were collected and washed with PBS containing 2% FBS. They were stained with anti-mouse CD14-APC (BD Biosciences), primary goat polyclonal antibody to CMG2 (Abcam, Cambridge, MA) with secondary chicken anti-goat Alexa Fluor 488 (Invitrogen), and/or primary rabbit polyclonal to TEM8 with secondary donkey anti-rabbit PE (both from Abcam). Data were acquired using the Gallios flow cytometer (Beckman Coulter, Miami, FL) and analyzed using FlowJo version 7.6.5 or version X.0.6 software (Tree Star, Ashland, OR).
LeTx Challenge and Viability Assay
To challenge cells, LeTx solutions were prepared by incubating 320 ng/ml recombinant LF (BEI Resources, Manassas, VA) in PBS for 10 min. Recombinant PA (BEI Resources) was then added at 480 ng/ml and incubated for another 10 min. LF + PA (LeTx) solutions were then added to Raw 264.7 cells in 96-well plates at 100 μl/well and were incubated for 7 h at 37 °C and 5% CO2. Some cells were incubated with 100 μl/well PBS instead of LeTx and were used as viable cell control samples. Following toxin treatments, a thiazolyl blue tetrazolium bromide (MTT) colorimetric assay was used to assess cell viability as described previously (12–15), with some modification. Briefly, 20 μl of MTT was added to each well and incubated for 2 h at 37 °C and 5% CO2. Then, the medium was carefully removed, and 200 μl/well of dimethyl sulfoxide was added to solubilize the formazan crystals formed. The absorbance was read at 570 nm using the PowerWave XS2 spectrophotometer (BioTek, Winooski, VT). Readings were normalized to viable cell control samples for each experiment. Although initial experiments were performed with 320 ng/ml recombinant LF and 480 ng/ml PA, these toxins were frequently titrated for cytoxicity in both Raw 264.7 and differentiated THP-1 cells and used at their most effective concentrations. AD293 cells were treated with LeTx (1 μg/ml LF + 1.5 μg/ml PA) for 2 days.
Antibody Blocking of CMG2 Receptors to Inhibit LeTx Toxicity
Raw 264.7 cells were seeded at 1.25 × 105 cells/well in 100 μl/well complete DMEM and incubated overnight. The cells were then treated with 1 μg/ml goat anti-CMG2 polyclonal antibody (ab101711, Abcam, Inc., Cambridge, MA) or goat anti-mouse IgG2a (Bethyl Laboratories, Inc., Montgomery, TX) as a negative control for 30 min on ice to allow for binding. LeTx challenge, followed by assessment of cell viability, proceeded as described above.
Reverse Transcription-Polymerase Chain Reaction
RNA was isolated from cells using the QiaShredder and RNeasy Mini kits from Qiagen (Valencia, CA). Superscript III (Invitrogen) or ProtoScript Moloney murine leukemia virus (New England BioLabs, Inc., Ipswich, MA) reverse transcriptases were used for first strand cDNA synthesis from 0.5 μg of total RNA/sample. Murine Tem8 (258 bp), Cmg2 (364 bp), and glyceraldehyde-3-phosphate dehydrogenase (Gapdh; 239 bp) fragments were then amplified for 36 cycles using specific primers as described in Table 2 and described by Young et al. (16). Human TEM8 (256 bp), CMG2 (344 bp), and GAPDH (930 bp) fragments were similarly amplified and are also described in Table 2.
TABLE 2.
Primers for RT-PCR of anthrax toxin receptors and GAPDH transcripts
| Primer name | Sequence | Reference |
|---|---|---|
| mANTXR1 F | 5′-GAACAGATCCGACAAGGCCTAGAAG-3′ | Ref. 16 |
| mANTXR1 R | 5′-GAACAGATCCGACAAGGCCTAGAAG-3′ | Ref. 16 |
| mANTXR2 F | 5′-CTGACAGAGAGATTTGTGAGC-3′ | Ref. 16 |
| mANTXR2 R | 5′-GCAATTCTTTCCAGCTGA-3′ | Ref. 16 |
| hANTXR1 F | 5′-GAACAAATCCGTCAAGGCCTAGAAG-3′ | Ref. 16 |
| hANTXR1 R | 5′-TCATTGAAATCTTTCACACCAAC-3′ | Ref. 16 |
| mGAPDH F | 5′-TGAAGGTCGGTGTGAACGGATTTGGC-3′ | Ref. 16 |
| mGAPDH R | 5′-TAGTGGGGTCTCGCTCCTGGAAGATG-3′ | Ref. 16 |
| hANTXR2 F | 5′-CTTGCGGAGAGATTTGTGAGC-3′ | Ref. 16 |
| hANTXR2 R | 5′-GCAATTCTTTCAAGCTG-3′ | Ref. 16 |
| hGAPDH F | 5′-CAGCCGAGCCACATCGCTGA-3′ | Accession no. AF261085.1 |
| hGAPDH R | 5′-CAATGCCAGCCCCAGCGTCA-3′ | Accession no. AF261085.1 |
MEK2 Cleavage Assays
Because LeTx cleaves the N-terminal portion of MEKS, we monitored cleavage by Western blot analysis using an antibody raised against the N terminus of MEK2 as described before by others (17, 18). AD293 cells cultured in 6-well plates were treated with 2 ml of LeTx (1 μg/ml LF + 1.5 μg/ml PA) for up to 2 h. The cells were then lysed using Cell Lysis Buffer (Cell Signal Technology, Boston, MA) with 1 μm PMSF protease inhibitor. 15 μg of protein lysates were loaded and separated on 10% SDS-PAGE and then transferred onto nitrocellulose membrane using a semidry transblot apparatus (Bio-Rad). The membrane was blocked in PBS with 1% Tween (PBST) containing 5% nonfat milk for 1 h and incubated with rabbit polyclonal anti-MEK2 antibody (Abgent, San Diego, CA) or mouse monoclonal anti-β-actin (Cell Signal Technology) at 4 °C overnight. After washing with PBST, the membrane was incubated with alkaline phosphatase-conjugated anti-rabbit or anti-mouse IgG at room temperature for 1 h. After washing, the membrane was developed with 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium substrate (Sigma).
Measuring EdTx Activity by cAMP-specific ELISA
AD293 cells were cultured in 6-well plates and treated with siRNAs for 24 h as before. The culture medium was then removed and replaced with EdTx (0.25 μg/ml EF + 1 μg/ml PA) for 1 h as we have described previously (14). The recombinant EF from B. anthracis was acquired from BEI Resources. Next, the toxin solutions were aspirated, and the cells were lysed by the addition of 1 ml of 0.1 m HCl for 10 min. Finally, cAMP concentrations were measured using a commercial cAMP ELISA kit as per the manufacturer's protocol (Enzo Life Sciences, Farmingdale, NY). Each sample was measured in duplicate wells. ELISA plates were read at 405 nm using the FlexStation 3 microplate reader with SoftMax Pro version 5.4.1 software (Molecular Devices, Sunnyvale, CA).
Statistical Analyses
GraphPad Prism version 5.04 was used to perform statistical analyses. Two-tailed, one-way, analysis of variance (ANOVA) was performed between groups and was followed by Dunnett's multiple comparison tests. A p value of less than 0.05 was considered significant.
RESULTS
Anthrax Toxin Receptor Transcript and Protein Expressions in the Raw 264.7 Murine Macrophage Cell Line
Transcript expression of the two anthrax receptors, Tem8 and Cmg2, was evaluated in the murine Raw 264.7 cell line via RT-PCR methods using species-specific primers. Tem8 and Cmg2 gene amplifications were based on previously described methods (16) that were optimized here for maximum amplification using annealing temperature gradient PCR. Optimal amplifications of murine Cmg2 and Gapdh genes were achieved at an annealing temperature of 55 °C (Fig. 1A). However, the Tem8 transcript was not detectable in Raw 264.7 cells. The next step was to verify surface CMG2 protein expression in these cells. Unlike other studies, we employed flow cytometry for surface expression rather than Western blot because truly, only surface expression levels of receptors are relevant for this study. Using flow cytometry, we confirmed that 1) Raw 264.7 cells were of monocytic lineage because the majority (84%) of cells expressed CD14 by direct staining methods, and 2) over 40% of Raw 264.7 cells expressed surface CMG2 protein by indirect staining methods (Fig. 1B). Thus, we determined that in these cells, CMG2 would be the most suitable target for gene silencing to prevent LeTx entry and cell death.
FIGURE 1.
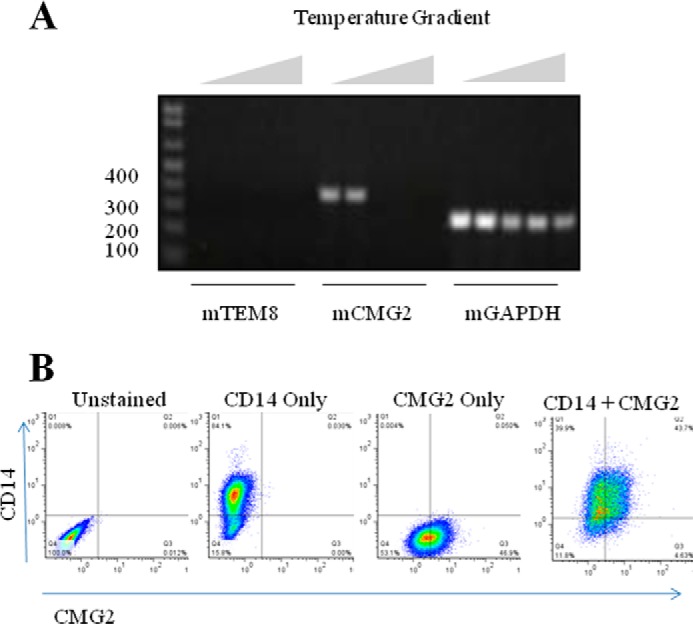
Anthrax toxin receptor expression in Raw 264.7 macrophage. Total RNA was isolated from Raw 264.7 cells, and Superscript III reverse transcriptase was used to synthesize cDNA. A, gradient PCR (55–68.5 °C) was used to amplify cDNA fragments corresponding to murine Tem8 (258 bp), Cmg2 (364 bp), and Gapdh (239 bp). B, flow cytometry methods were employed to measure expression of surface proteins. Representative plots are shown for cell samples that were unstained, stained for CD14 expression alone, stained for CMG2 expression alone, or dual stained for CD14 and CMG2 expression.
Effects of Anthrax LeTx-mediated Toxicity in Anthrax Receptor-silenced Raw 264.7 Cells
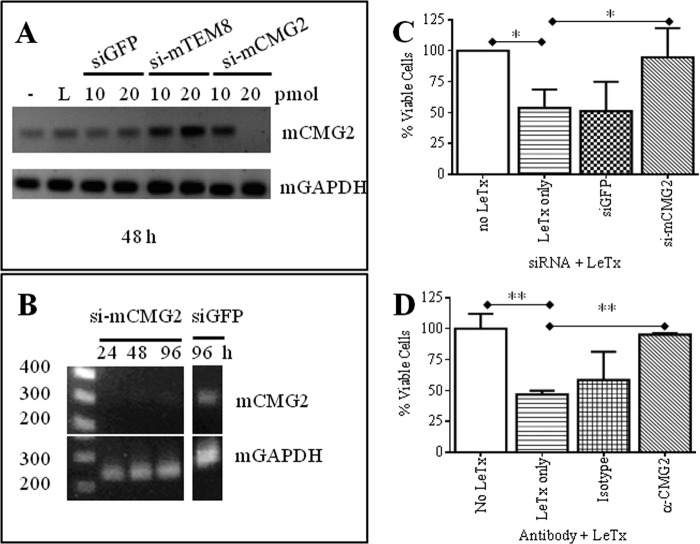
Experiments to optimize specific and effective silencing of Cmg2 transcript expression were performed in 24-well culture plates to provide sufficient sample amounts for subsequent total RNA extraction. Cells were mock-transfected or transfected with Lipofectamine RNAiMAX reagent, siGFP or si-mTEM8 as nonspecific siRNA controls, and si-mCMG2. RT-PCR analyses revealed that 20 pmol of si-mCMG2 specifically and effectively silenced Cmg2 mRNA expression at 48 h post-transfection (Fig. 2A). RT-PCR analyses using Gapdh as a housekeeping gene were performed in parallel, and results are also shown. We next monitored Cmg2 transcript expression in Raw 264.7 cells treated with 20 pmol of si-mCMG2 from 24–96 h post-transfection. Cells were treated with siRNAs at time 0 and at 48 h post-transfection. RT-PCR analyses verified that Cmg2 transcript expression was silenced during this time period, whereas levels of Gapdh transcript remained stable (Fig. 2B). As expected, siGFP did not silence Cmg2 transcript expression.
FIGURE 2.
siRNA-targeted silencing of CMG2 and evaluation of anthrax LeTx toxicity in Raw 264.7 cells. A, Raw 264.7 cells were cultured in 24-well plates and treated as follows: 1) untransfected (−); 2) RNAiMAX alone (L), 3) siGFP, 10 and 20 pmol; 4) si-mTEM8, 10 and 20 pmol; and 5) si-mCMG2, 10 and 20 pmol. Total RNAs from these cells were isolated after 48 h, and RT-PCR was performed to amplify mCMG2 and mGAPDH fragments. B, 24–96 h post-transfection. Cells were treated with siRNAs at times 0 and at 48 h post-transfection. C, cells were cultured in 96-well plates and were transfected twice with 5 pmol of siGFP or si-mCMG2 or mock-transfected and then challenged with anthrax LeTx 48 h after that last transfection. Data were normalized to cell viability controls (no LeTx) in each experiment. The mean ± S.D. (error bars) of four experiments, performed in triplicates, is shown for all groups. One-way ANOVA revealed the groups to be significantly different (p = 0.0033), and Dunnett post hoc comparisons with mock-transfected, LeTx-treated cells (LeTx only) were performed. D, anti-CMG2 or isotype control antibodies were allowed to bind to Raw 264.7 cells prior to the addition of LeTx. One-way ANOVA revealed the groups to be significantly different (p = 0.0022), and Dunnett post hoc comparisons with cells treated with LeTx only were performed. *, p < 0.05; **, p < 0.01.
Next, we determined whether Cmg2-silenced Raw 264.7 cells were protected against LeTx under the conditions described above. Cell viability was assessed following 7-h LeTx treatments (Fig. 2C). Initially, LeTx was added for 24 h after siRNA treatments, but these treatments were not protective against LeTx (data not shown). However, after two siRNA treatments, protection was shown against LeTx in cells that were treated with Cmg2-specific siRNAs over untreated cells. Cells that were treated with LeTx alone (LeTx only) were 54% viable when compared with cells not treated with toxin. To control for nonspecific siRNA effects, GFP-targeted siRNAs were used to treat cells, and as expected, cell viability after toxin treatment was similar to that of cells that were treated with LeTx only (51%). In contrast, cells treated with two doses of Cmg2-targeted siRNAs within a 96-h time span were 95% viable after LeTx treatment. Thus, specific silencing of Cmg2 via targeted siRNAs offered almost complete protection against LeTx. Furthermore, the groups tested were significantly different, as determined by two-tailed, one-way ANOVA (p = 0.0033). Moreover, Dunnett post hoc comparisons of each group with the LeTx only group revealed that the differences in cell viability between the Cmg2-silenced group and the LeTx only group were statistically significant (p < 0.05).
To confirm these exciting results, a CMG2-specific antibody-blocking assay was used to show that the hindrance of CMG2 in Raw 264.7 led to the inability of LeTx to induce cytoxicity. The anti-CMG2 goat polyclonal antibody from Abcam (ab101711) was chosen for this assay because it was raised against a peptide sequence, HEGLKLANEQIQK, found within the anthrax toxin-binding region (NP_477520.2; NP_001139266.1) and is reactive in humans, mice, and rats. As a negative control, a goat anti-mouse IgG2a antibody was used in this assay. One-way ANOVA revealed the treatment groups to be significantly different (p = 0.0022). As before, the addition of LeTx to Raw 264.7 cells led to an approximately 50% reduction in cell viability as compared with cells not treated with toxin (Fig. 3D). Cells treated with goat anti-mouse IgG2a were also susceptible to LeTx and were only 58% viable. In contrast, cells treated with anti-CMG2 antibody were 95% viable and thus were protected from toxicity.
FIGURE 3.
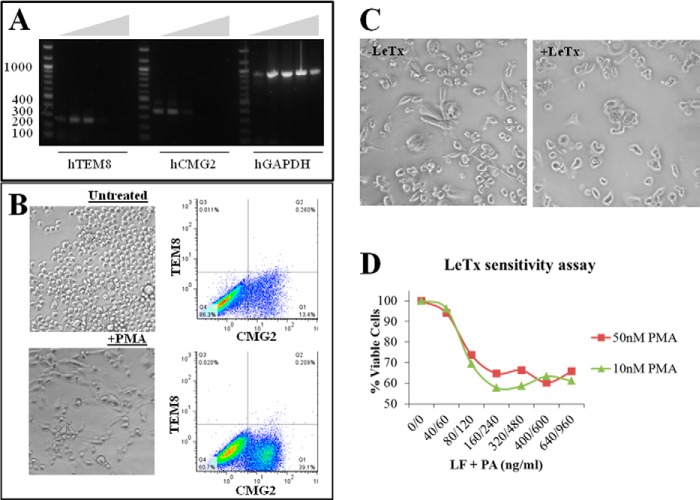
Differentiation of THP-1 cells and LeTx sensitivity. A, total RNA was isolated from THP-1 cells and Superscript III reverse transcriptase was used to synthesize cDNA. Gradient PCR (55–69.1 °C) was used to amplify cDNA fragments corresponding to human TEM8 (256 bp), CMG2 (344 bp), and GAPDH (930 bp). B, comparison of THP-1 (top) and PMA (10 nm)-differentiated THP-1 (bottom) morphology and surface expression of ANTXRs. Brightfield images were taken at ×20,000 magnification using a Nikon Eclipse Ti microscope. C, effects of LeTx in PMA (10 nm)-differentiated (right) compared with untreated cells (−LeTx, left). D, titration of LeTx in THP-1 cells treated with 10 and 50 nm PMA.
Testing Anthrax LeTx-mediated Toxicity in Anthrax Receptor-silenced Human Macrophage-like Cells
After showing that it was possible to protect murine macrophages against LeTx by silencing CMG2, we wanted to test if this approach could be applied successfully to human cells. First, we assessed ANTXR transcript expression in human THP-1 cells. Optimal amplifications of human TEM8, CMG2, and GAPDH genes were reached at an annealing temperature of 56.7 °C (Fig. 3A). However, attempts to induce cytoxicity in THP-1, as mediated by LeTx, were unsuccessful (data not shown). These results were consistent with published work that shows that human monocyte cell lines, including THP-1, are not susceptible to LeTx-mediated death (20). In contrast, when differentiated with 10 nm PMA into adherent macrophage-like cells, these cells up-regulate surface CMG2 protein expression (Fig. 3B) and become sensitive to LeTx (Fig. 3, C and D) (20). Of note, TEM8 levels remained unchanged after PMA treatment. In addition, increasing PMA concentration to 50 nm did not induce greater sensitivity of the THP-1 cells to LeTx. In light of these findings, our main focus was once again to silence CMG2 expression. Therefore, differentiated THP-1 cells cultured in 6-well plates were treated with varying amounts of CMG2-targeted siRNAs to determine the optimal amount of siRNA needed to effectively silence CMG2 transcript expression. By RT-PCR analysis, it was confirmed that silencing was dose-dependent and that 150 pmol was most effective after 24 h (Fig. 4A). When scaling down the experiment for the 96-well format, 3 pmol of siRNAs were sufficient to treat the differentiated THP-1 cells. The treated cells were challenged with LeTx, and viability by treatment groups was statistically different (p < 0.0012). Treatment of differentiated THP-1 cells with CMG2-targeted siRNAs was highly protective against LeTx cytotoxicity, helping to maintain 92% (p < 0.01) viability in these cells as compared with 64% viability in cells treated with LeTx only (Fig. 4B). The addition of TEM8-targeted siRNAs was also protective against LeTx in these cells, preserving 83% viability (p < 0.05) as compared with the LeTx only samples. Although cells treated with GFP-targeted siRNAs were 76% viable, this result was not significantly different from that for cells treated with LeTx only.
FIGURE 4.
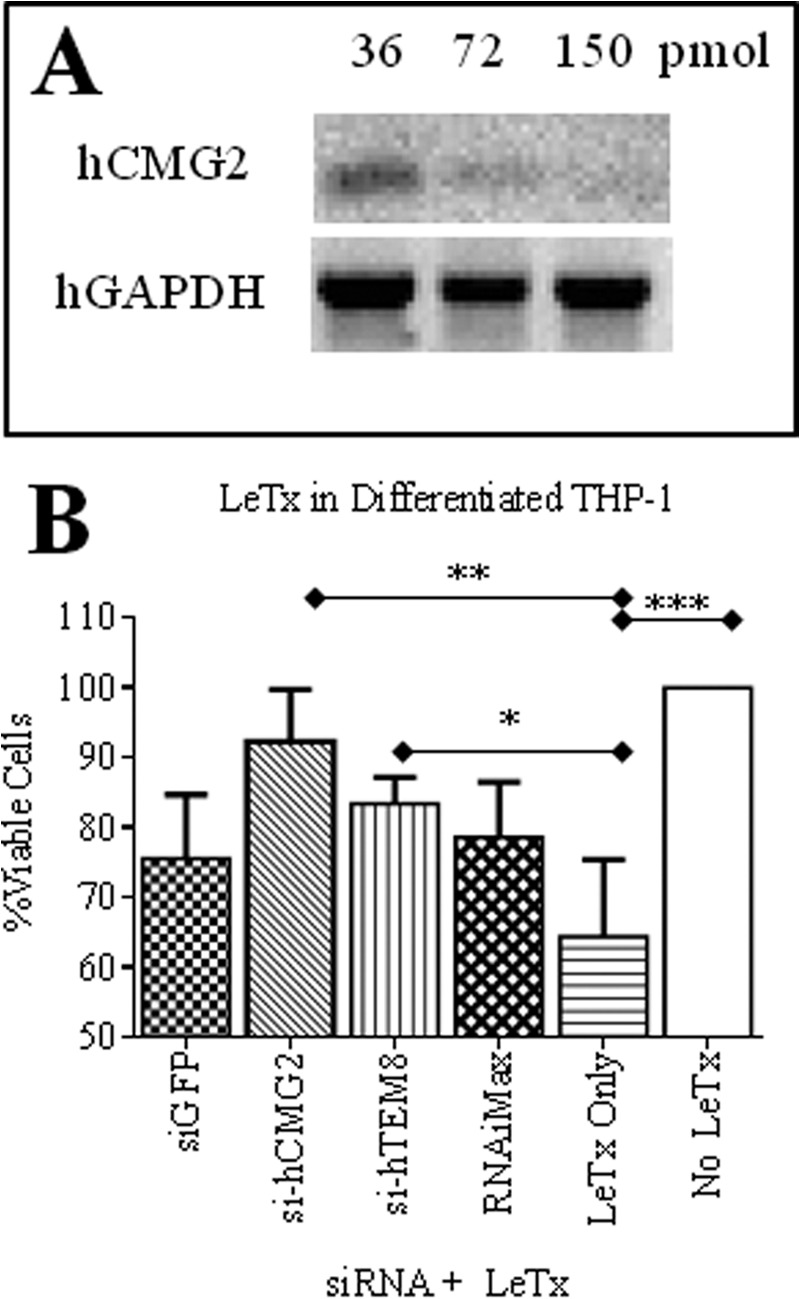
siRNA-targeted silencing of ANTXRs and evaluation of anthrax LeTx toxicity in differentiated THP-1 cells. A, differentiated THP-1 cells were treated for 24 h with different amounts of si-hCMG2. RT-PCR for hCMG2 and hGAPDH transcript expression was then performed from RNA extractions. B, THP-1 cells were cultured in 96-well plates with 10 nm PMA for 2 days. Then they were transfected with 3 pmol of siRNAs or mock-transfected. After 24 h, they were challenged with anthrax LeTx for 48 h. MTT was added, and cells were incubated for an additional day to evaluate cell viability. Data were normalized to cell viability controls (no LeTx) in each experiment. The mean ± S.D. (error bars) of three experiments, performed in replicates of six per group, is shown for each group. One-way ANOVA revealed the groups to be significantly different (p = 0.0012), and Dunnett post hoc comparisons with mock-transfected, LeTx-treated cells (LeTx only) were performed (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
Protection against Anthrax Toxins in Human Kidney Cells
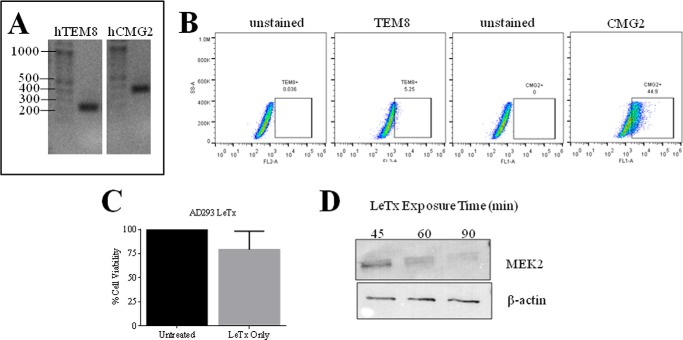
It was also important for us to evaluate protection by siRNA silencing of ANTXRs in other cell types targeted by anthrax disease. The kidneys are among the organs that are harmed in human cases of inhalational anthrax, with evidence of intratubular bleeding and tubular necrosis found by histopathology (21). Based on a previous study, we knew that human embryonic kidney 293 (HEK293) cells were susceptible to cleavage of MEK1 by LeTx and, thus, deduced that these cells had to express at least one of two ANTXRs. For our studies, we used AD293 cells, which are derived from the HEK293 cell line (22). We found that AD293 cells expressed both TEM8 and CMG2 transcripts, as determined by RT-PCR methods (Fig. 5A). By FACS analysis, it was confirmed that the AD293 cells expressed both TEM8 (5% of cells) and CMG2 (45% of cells) surface protein (Fig. 5B). LeTx did not induce cell death in AD293 (Fig. 5C), but we report that it cleaves MEK2 (Fig. 5D). Of importance, LeTx cleavage of MEK2 was reproducibly inhibited when cells were pretreated for 24 h with siRNAs against human TEM8, CMG2, or both receptors (Fig. 6A). Moreover, silencing both receptors led to more optimal protection against MEK2 cleavage than silencing a single receptor in this human kidney cell line. Targeted silencing of ANTXRs also protected AD293 cells against the effects of EdTx. Intracellular cAMP levels rose to 25.9 pmol/ml in cells treated with EdTx for 1 h, whereas only 1.89 pmol/ml cAMP was detected in control cells (Fig. 6B). However, this elevation in cAMP was significantly inhibited in cells pretreated with si-hTEM8 (8.37 pmol/ml) or si-hCMG2 (9.43 pmol/ml). Protection against EdTx was optimal when both receptors were silenced (3.54 pmol/ml).
FIGURE 5.
ANTXR expression in AD293 human embryonic kidney cells and susceptibility to LeTx. A, total RNA was extracted from AD293 cells, and ProtoScript Moloney murine leukemia virus reverse transcriptase was used to synthesize cDNA. PCR was then used to amplify TEM8 (256 bp) and CMG2 (344 bp). B, flow cytometry methods were employed to measure expression of surface TEM8 and CMG2. C, AD293 cells were treated with LeTx (1 μg/ml LF + 1.5 μg/ml PA) for 2 days, and cell viability was assessed by an MTT assay. The mean ± S.D. (error bars) of three separate experiments performed in triplicates is shown. D, MEK2 cleavage and β-actin expression after LeTx exposure for 45, 60, and 90 min are shown by Western blot.
FIGURE 6.
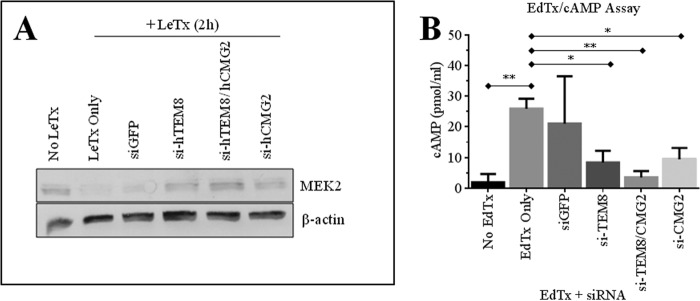
Evaluation of anthrax toxin-mediated MEK2 cleavage and intracellular cAMP in ANTXR-silenced cells. AD293 cells were cultured in 6-well plates and treated with 150 pmol siRNAs for 24 h prior to treatment with anthrax toxins. A, cells were treated with LeTx for 2 h. MEK2 cleavage and β-actin expression were assayed by Western blot. The figure is representative of three separate experiments. B, cells were treated with EdTx (0.25 μg/ml EF + 1 μg/ml PA) for 1 h. Cells were then lysed with 0.1 m HCl, and intracellular cAMP was measured using a commercial ELISA kit. Results (mean ± S.D. (error bars)) from three experiments are shown. One-way ANOVA revealed the groups to be significantly different (p = 0.0057), and Dunnett post hoc comparisons with mock-transfected, EdTx-treated cells (EdTx only) were performed (*, p < 0.05; **, p < 0.01).
DISCUSSION
The goal of our study was to evaluate ANTXR-targeted siRNAs as a potential therapy against anthrax disease. We began our study in Raw 264.7 murine macrophages because they undergo rapid apoptosis in response to LeTx and, thus, provide an easy model to test any molecular therapy designed to block entry of anthrax toxins and the downstream effects of these toxins. Of note, the Raw 264.7 cell line is commonly used in anthrax toxin neutralization assays; these assays are typically used to measure neutralizing antibody levels elicited by anthrax vaccines or to test immunotherapies against anthrax (23). We found that these murine macrophages were significantly protected from LeTx-induced cell death by silencing Cmg2 transcript expression via targeted siRNA therapy. These observations were further substantiated using anti-CMG2 antibodies to block the PA-binding domain of CMG2, which also resulted in protection of murine macrophages against LeTx. It was not possible for us to evaluate silencing of Tem8 in Raw 264.7 cells because Tem8 transcript levels were undetectable in these cells. Poor or undetectable levels of Tem8 transcripts in Raw 264.7 cells have been previously reported (16, 24). Furthermore, primary mouse and human alveolar macrophages also express marginal levels of TEM8 protein (24). However, although TEM8 may not be important as an ANTXR in mouse macrophages, it may be more noteworthy in the anthrax toxin entry of other cell types, as is the case in differentiated THP-1 cells.
Use of human TEM8- and human CMG2-targeted siRNAs was found to be protective against LeTx challenge in human macrophage-like cells, albeit silencing CMG2 was the most effective treatment, and offered almost complete protection against cytotoxicity. This was not surprising because these cells expressed much more CMG2 on their cell surface than TEM8. Furthermore, there was an increase in CMG2 in PMA-differentiated THP-1 macrophage-like cells, which were sensitive to LeTx intoxication over THP-1 monocytic cells that were resistant to toxin. Although it has been shown that differentiated THP-1 cells are sensitive to LeTx (20), it has not been shown that increased expression of CMG2, but not TEM8, may be responsible for this increased sensitivity in these cells. Hence, it appears that in these human macrophages, CMG2 functions as the major anthrax toxin receptor too.
Protective results using ANTXR-targeted siRNA therapies against anthrax toxins were not limited to macrophages. We evaluated protection following ANTXR silencing in the AD293 human embryonic kidney cell line. The kidneys are targets of anthrax pathogenesis during human inhalational anthrax; capillaritis, intratubular bleeding, and tubular necrosis are observed by histopathology (21). Hemorrhaging in the kidney is also observed in animal models of gastrointestinal anthrax (25). The AD293 cells expressed both receptors abundantly, and silencing either receptor, or both, protected against the enzymatic activities of LeTx and EdTx. The finding that AD293 cells were protected by the siRNA against EdTx challenge was exciting because EdTx challenge in mice results in altered kidney function (26) and kidney lesions (27). We now had proof that targeting ANTXRs for silencing resulted in protection against the main activities attributed to the anthrax toxins: cytotoxicity, MAPKK cleavage, and adenylyl cyclase activity to increase intracellular cAMP.
The roles of CMG2 and TEM8 as anthrax toxin receptors have been firmly established by others in vitro (7, 8) and using in vivo mouse models (28, 29). In the absence of these receptors, host cells are resistant to anthrax toxins. More recently, Cmg2-null (Cmg2−/−) and cell type-specific Cmg2−/− mice have been generated to elucidate the tissue targets of anthrax toxin-induced lethality and to clarify the role of myeloid cells in disease caused by anthrax. Some of the results were surprising. For instance, endothelial cells were thought to be target cells of lethality based on loss of barrier function (30) and apoptosis (31, 32) in vitro and measurable vascular leakage following administration of LeTx in mice in vivo (1). Furthermore, humans commonly manifest pleural effusions following exposure to inhalational anthrax (1). However, mice with Cmg2−/− endothelial cells were still as sensitive to LeTx as normal mice, indicating that LeTx targeting of EC is not lethal in mice (33). In contrast, LeTx targeting of cardiomyocytes and vascular smooth muscle cells was essential for lethality; cardiomyocyte Cmg2−/− or cardiomyocyte and smooth muscle cell Cmg2−/− mice were resistant to LeTx-induced death. EdTx, on the other hand, targets the intestinal epithelial cells and hepatocytes (33).
Another surprising result was that mice generated with Cmg2−/− myeloid cells were still sensitive to LeTx, whereas complete Cmg2−/− mice were resistant to LeTx (28). However, mice with Cmg2−/− myeloid cells were completely resistant to infection following anthrax spore challenge. A possible explanation is that anthrax toxins disable myeloid cells, including neutrophils and macrophages, thus evading the immune system and allowing for the establishment of infection (28). Anthrax toxins can suppress immune function in monocytes, macrophages, dendritic cells, B lymphocytes, and T lymphocytes (34) because their activities interfere with various signaling pathways, including those of the innate immunity (35). Following establishment, the bacteria continue to multiply and produce more anthrax toxins to damage susceptible cells (28). Thus, a CMG2- or TEM8-targeted molecular therapy could serve to 1) hinder establishment of host infection in B. anthracis in the early stages of infection and 2) protect susceptible cells from anthrax toxin-induced lethality in the later stages of infection.
Indeed, ANTXRs are an obvious target for therapy, and approaches that have been presented previously by others include the use of decoy receptors (7, 8) and RNAi (36, 37). We show for the first time that efficient and effective silencing of ANTXRs using specific siRNAs can significantly protect mouse and human macrophages from anthrax toxins in vitro. Thus, we have provided proof-of-concept evidence for an effective strategy that can be further developed in vivo, first in animal models. In addition, an advantage to using siRNAs against host determinants of pathogenesis, such as the ANTXRs, is that unlike in the case of antibiotic treatments, we do not need to worry about bacterial strains acquiring mutations through natural selection or through bioengineering that result in antibiotic resistance. siRNAs targeted against ANTXRs also have a similar advantage over antitoxins that are selected against specific epitopes that can mutate and render these antitoxins futile.
In this study, we also showed that using anti-CMG2 antibody to block LeTx binding to CMG2 was effective in protecting against LeTx cytotoxicity. However, using siRNA as a therapy against disease offers several advantages over the use of antibody-based therapies. Beyond cost effectiveness and the high level of specificity of using siRNA, we can also circumvent unwanted immune responses associated with use of antibody therapies (38, 39). Furthermore, several siRNAs can easily be designed, synthesized, and characterized to maximize specificity and reduce undesirable nonspecific effects for each target.
These studies on preventing LeTx cytotoxicity are especially relevant because LeTx challenge in mice leads to mortality associated with hypoxia, vascular collapse, and shock, all of which are symptoms also observed in cases of human inhalational anthrax (40). The next challenge in using these siRNA therapies is efficient delivery for effective therapy in vivo. It would be beneficial to conduct future studies using Cmg2- and Tem8-targeted siRNAs in a murine model of pulmonary anthrax and eventually in New Zealand White rabbits, a more appropriate nonhuman primate model to study inhalational anthrax (41, 42). Once we determine the most appropriate delivery methods for our siRNAs, we can further evaluate whether these treatments can provide protection against lethality when used prior to anthrax spore infection, early on during infection, or in later stages of infection. We would also need to evaluate safety in vivo because ANTXRs are involved in angiogenesis and ECM homeostasis (9). However, it is known that Tem8- or Cmg2-null or knock-out mice are viable, develop normally, and survive into adulthood (19, 43–45). Phenotypically, the main defect in these mice is excessive ECM protein deposition (19, 43). Aberrant ECM deposition in the cervix and uterus of females has been associated with fertility defects observed in the females of null or knock-out mice (19). Thus, a possible side effect of silencing ANTXRs would be increased ECM deposition for the transient period in which these receptors are silenced. This would be a minor and transient inconvenience, not comparable with the pathogenesis and lethality of anthrax disease caused by anthrax toxins.
This work was supported by a grant (to M. Z.) from the Paul L. Foster School of Medicine at Texas Tech University Health Sciences Center, El Paso.
- LeTx
- lethal toxin
- EdTx
- edema toxin
- MAPKK
- MAPK kinase
- PA
- protective antigen
- LF
- lethal factor
- EF
- edema factor
- ECM
- extracellular matrix
- PMA
- phorbol 12-myristate 13-acetate
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- ANOVA
- analysis of variance.
REFERENCES
- 1. Grabenstein J. D. (2008) Vaccines: countering anthrax: vaccines and immunoglobulins. Clin. Infect. Dis. 46, 129–136 [DOI] [PubMed] [Google Scholar]
- 2. Franz D. R. (2009) Preparedness for an anthrax attack. Mol. Aspects Med. 30, 503–510 [DOI] [PubMed] [Google Scholar]
- 3. Bouzianas D. G. (2009) Medical countermeasures to protect humans from anthrax bioterrorism. Trends Microbiol. 17, 522–528 [DOI] [PubMed] [Google Scholar]
- 4. Hudson M. J., Beyer W., Böhm R., Fasanella A., Garofolo G., Golinski R., Goossens P. L., Hahn U., Hallis B., King A., Mock M., Montecucco C., Ozin A., Tonello F., Kaufmann S. H. (2008) Bacillus anthracis: balancing innocent research with dual-use potential. Int. J. Med. Microbiol. 298, 345–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bradley K. A., Young J. A. (2003) Anthrax toxin receptor proteins. Biochem. Pharmacol. 65, 309–314 [DOI] [PubMed] [Google Scholar]
- 6. Sweeney D. A., Hicks C. W., Cui X., Li Y., Eichacker P. Q. (2011) Anthrax infection. Am. J. Respir. Crit. Care Med. 184, 1333–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bradley K. A., Mogridge J., Mourez M., Collier R. J., Young J. A. (2001) Identification of the cellular receptor for anthrax toxin. Nature 414, 225–229 [DOI] [PubMed] [Google Scholar]
- 8. Scobie H. M., Rainey G. J., Bradley K. A., Young J. A. (2003) Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc. Natl. Acad. Sci. U.S.A. 100, 5170–5174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reeves C., Charles-Horvath P., Kitajewski J. (2013) Studies in mice reveal a role for anthrax toxin receptors in matrix metalloproteinase function and extracellular matrix homeostasis. Toxins 5, 315–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Emergent BioSolutions (2013) Vaccine product sheet: BioThrax® anthrax vaccine: adsorbed dosage and administration, Emergent BioSolutions, Rockville, MD [Google Scholar]
- 11. Sherer K., Li Y., Cui X., Eichacker P. Q. (2007) Lethal and edema toxins in the pathogenesis of Bacillus anthracis septic shock: implications for therapy. Am. J. Respir. Crit. Care Med. 175, 211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Twentyman P. R., Luscombe M. (1987) A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br. J. Cancer 56, 279–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xu Q., Zeng M. (2008) Detoxified lethal toxin as a potential mucosal vaccine against anthrax. Clin. Vaccine Immunol. 15, 612–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zeng M., Xu Q., Hesek E. D., Pichichero M. E. (2006) N-fragment of edema factor as a candidate antigen for immunization against anthrax. Vaccine 24, 662–670 [DOI] [PubMed] [Google Scholar]
- 15. Zeng M., Xu Q., Pichichero M. E. (2007) Protection against anthrax by needle-free mucosal immunization with human anthrax vaccine. Vaccine 25, 3588–3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Young J. J., Bromberg-White J. L., Zylstra C., Church J. T., Boguslawski E., Resau J. H., Williams B. O., Duesbery N. S. (2007) LRP5 and LRP6 are not required for protective antigen-mediated internalization or lethality of anthrax lethal toxin. PLoS Pathog. 3, e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pellizzari R., Guidi-Rontani C., Vitale G., Mock M., Montecucco C. (1999) Anthrax lethal factor cleaves MKK3 in macrophages and inhibits the LPS/IFNγ-induced release of NO and TNFα. FEBS Lett. 462, 199–204 [DOI] [PubMed] [Google Scholar]
- 18. Pellizzari R., Guidi-Rontani C., Vitale G., Mock M., Montecucco C. (2000) Lethal factor of Bacillus anthracis cleaves the N terminus of MAPKKs: analysis of the intracellular consequences in macrophages. Int. J. Med. Microbiol. 290, 421–427 [DOI] [PubMed] [Google Scholar]
- 19. Reeves C. V., Wang X., Charles-Horvath P. C., Vink J. Y., Borisenko V. Y., Young J. A., Kitajewski J. K. (2012) Anthrax toxin receptor 2 functions in ECM homeostasis of the murine reproductive tract and promotes MMP activity. PLoS One 7, e34862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kassam A., Der S. D., Mogridge J. (2005) Differentiation of human monocytic cell lines confers susceptibility to Bacillus anthracis lethal toxin. Cell. Microbiol. 7, 281–292 [DOI] [PubMed] [Google Scholar]
- 21. Grinberg L. M., Abramova F. A., Yampolskaya O. V., Walker D. H., Smith J. H. (2001) Quantitative pathology of inhalational anthrax I: quantitative microscopic findings. Mod. Pathol. 14, 482–495 [DOI] [PubMed] [Google Scholar]
- 22. Zhang J., Chen J., Liu L., Ji C., Gu S., Ying K., Mao Y. (2006) Different gene expression profiles of AD293 and HEK293 cell lines that show contrasting susceptibility to apoptosis induced by overexpression of Bim L. Acta Biochim. Pol. 53, 525–530 [PubMed] [Google Scholar]
- 23. Ngundi M. M., Meade B. D., Lin T. L., Tang W. J., Burns D. L. (2010) Comparison of three anthrax toxin neutralization assays. Clin. Vaccine Immunol. 17, 895–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu W., Mehta H., Chakrabarty K., Booth J. L., Duggan E. S., Patel K. B., Ballard J. D., Coggeshall K. M., Metcalf J. P. (2009) Resistance of human alveolar macrophages to Bacillus anthracis lethal toxin. J. Immunol. 183, 5799–5806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xie T., Sun C., Uslu K., Auth R. D., Fang H., Ouyang W., Frucht D. M. (2013) A new murine model for gastrointestinal anthrax infection. PLoS One 8, e66943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sastalla I., Tang S., Crown D., Liu S., Eckhaus M. A., Hewlett I. K., Leppla S. H., Moayeri M. (2012) Anthrax edema toxin impairs clearance in mice. Infect. Immun. 80, 529–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Firoved A. M., Miller G. F., Moayeri M., Kakkar R., Shen Y., Wiggins J. F., McNally E. M., Tang W. J., Leppla S. H. (2005) Bacillus anthracis edema toxin causes extensive tissue lesions and rapid lethality in mice. Am. J. Pathol. 167, 1309–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu S., Miller-Randolph S., Crown D., Moayeri M., Sastalla I., Okugawa S., Leppla S. H. (2010) Anthrax toxin targeting of myeloid cells through the CMG2 receptor is essential for establishment of Bacillus anthracis infections in mice. Cell Host Microbe 8, 455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu S., Zhang Y., Hoover B., Leppla S. H. (2013) The receptors that mediate the direct lethality of anthrax toxin. Toxins 5, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Warfel J. M., Steele A. D., D'Agnillo F. (2005) Anthrax lethal toxin induces endothelial barrier dysfunction. Am. J. Pathol. 166, 1871–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kirby J. E. (2004) Anthrax lethal toxin induces human endothelial cell apoptosis. Infect. Immun. 72, 430–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rolando M., Stefani C., Flatau G., Auberger P., Mettouchi A., Mhlanga M., Rapp U., Galmiche A., Lemichez E. (2010) Transcriptome dysregulation by anthrax lethal toxin plays a key role in induction of human endothelial cell cytotoxicity. Cell. Microbiol. 12, 891–905 [DOI] [PubMed] [Google Scholar]
- 33. Liu S., Zhang Y., Moayeri M., Liu J., Crown D., Fattah R. J., Wein A. N., Yu Z. X., Finkel T., Leppla S. H. (2013) Key tissue targets responsible for anthrax-toxin-induced lethality. Nature 501, 63–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tournier J. N., Rossi Paccani S., Quesnel-Hellmann A., Baldari C. T. (2009) Anthrax toxins: a weapon to systematically dismantle the host immune defenses. Mol. Aspects Med. 30, 456–466 [DOI] [PubMed] [Google Scholar]
- 35. Moayeri M., Leppla S. H. (2009) Cellular and systemic effects of anthrax lethal toxin and edema toxin. Mol. Aspects Med. 30, 439–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Abrami L., Bischofberger M., Kunz B., Groux R., van der Goot F. G. (2010) Endocytosis of the anthrax toxin is mediated by clathrin, actin and unconventional adaptors. PLoS Pathog. 6, e1000792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Abrami L., Kunz B., Deuquet J., Bafico A., Davidson G., van der Goot F. G. (2008) Functional interactions between anthrax toxin receptors and the WNT signalling protein LRP6. Cell. Microbiol. 10, 2509–2519 [DOI] [PubMed] [Google Scholar]
- 38. Choi B., Hwang Y., Kwon H. J., Lee E. S., Park K. S., Bang D., Lee S., Sohn S. (2008) Tumor necrosis factor α small interfering RNA decreases herpes simplex virus-induced inflammation in a mouse model. J. Dermatol. Sci. 52, 87–97 [DOI] [PubMed] [Google Scholar]
- 39. Pluvinet R., Pétriz J., Torras J., Herrero-Fresneda I., Cruzado J. M., Grinyó J. M., Aran J. M. (2004) RNAi-mediated silencing of CD40 prevents leukocyte adhesion on CD154-activated endothelial cells. Blood 104, 3642–3646 [DOI] [PubMed] [Google Scholar]
- 40. Turk B. E. (2007) Manipulation of host signalling pathways by anthrax toxins. Biochem. J. 402, 405–417 [DOI] [PubMed] [Google Scholar]
- 41. Comer J. E., Ray B. D., Henning L. N., Stark G. V., Barnewall R. E., Mott J. M., Meister G. T. (2012) Characterization of a therapeutic model of inhalational anthrax using an increase in body temperature in New Zealand white rabbits as a trigger for treatment. Clin. Vaccine Immunol. 19, 1517–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lawrence W. S., Hardcastle J. M., Brining D. L., Weaver L. E., Ponce C., Whorton E. B., Peterson J. W. (2009) The physiologic responses of Dutch belted rabbits infected with inhalational anthrax. Comp. Med. 59, 257–265 [PMC free article] [PubMed] [Google Scholar]
- 43. Cullen M., Seaman S., Chaudhary A., Yang M. Y., Hilton M. B., Logsdon D., Haines D. C., Tessarollo L., St Croix B. (2009) Host-derived tumor endothelial marker 8 promotes the growth of melanoma. Cancer Res. 69, 6021–6026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chaudhary A., Hilton M. B., Seaman S., Haines D. C., Stevenson S., Lemotte P. K., Tschantz W. R., Zhang X. M., Saha S., Fleming T., St Croix B. (2012) TEM8/ANTXR1 blockade inhibits pathological angiogenesis and potentiates tumoricidal responses against multiple cancer types. Cancer Cell 21, 212–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu S., Crown D., Miller-Randolph S., Moayeri M., Wang H., Hu H., Morley T., Leppla S. H. (2009) Capillary morphogenesis protein-2 is the major receptor mediating lethality of anthrax toxin in vivo. Proc. Natl. Acad. Sci. U.S.A. 106, 12424–12429 [DOI] [PMC free article] [PubMed] [Google Scholar]