Background: GPR120 (FFAR4) is a GPCR activated by long chain fatty acids.
Results: Palmitate- and DHA-stimulated glucagon secretion is markedly reduced from GPR120 KO islets.
Conclusion: GPR120 is a nutrient sensor that is activated endogenously by both saturated and unsaturated long chain fatty acids.
Significance: Targeting GPR120 for diabetes may impact glucose homeostasis in part through altering glucagon secretion and islet function.
Keywords: Fatty Acid, G Protein-coupled Receptor (GPCR), Lipid, Pancreatic Islet, Polyunsaturated Fatty Acid (PUFA), GPR120, GPR40, Long Chain Fatty Acids, Alpha Cell, Glucagon
Abstract
GPR40 (FFAR1) and GPR120 (FFAR4) are G-protein-coupled receptors (GPCRs) that are activated by long chain fatty acids (LCFAs). GPR40 is expressed at high levels in islets and mediates the ability of LCFAs to potentiate glucose-stimulated insulin secretion (GSIS). GPR120 is expressed at high levels in colon, adipose, and pituitary, and at more modest levels in pancreatic islets. The role of GPR120 in islets has not been explored extensively. Here, we confirm that saturated (e.g. palmitic acid) and unsaturated (e.g. docosahexaenoic acid (DHA)) LCFAs engage GPR120 and demonstrate that palmitate- and DHA-potentiated glucagon secretion are greatly reduced in isolated GPR120 KO islets. Remarkably, LCFA potentiated glucagon secretion is similarly reduced in GPR40 KO islets. Compensatory changes in mRNA expression of GPR120 in GPR40 KO islets, and vice versa, do not explain that LCFA potentiated glucagon secretion seemingly involves both receptors. LCFA-potentiated GSIS remains intact in GPR120 KO islets. Consistent with previous reports, GPR120 KO mice are hyperglycemic and glucose intolerant; however, our KO mice display evidence of a hyperactive counter-regulatory response rather than insulin resistance during insulin tolerance tests. An arginine stimulation test and a glucagon challenge confirmed both increases in glucagon secretion and liver glucagon sensitivity in GPR120 KO mice relative to WT mice. Our findings demonstrate that GPR120 is a nutrient sensor that is activated endogenously by both saturated and unsaturated long chain fatty acids and that an altered glucagon axis likely contributes to the impaired glucose homeostasis observed in GPR120 KO mice.
Introduction
Type 2 diabetes is characterized by insulin resistance and loss of β cell function. A family of five G-protein-coupled receptors (GPCR)2 that are activated by fatty acids of varying chain lengths has been identified and characterized as drug targets for diabetes (1). Of particular interest is GPR120 (FFAR4), which is engaged by long chain fatty acids (LCFAs) regardless of the degree of saturation, is coupled to Gαq/11 and is expressed predominantly in tissues that contribute to glucose homeostasis including colon, adipose, pituitary, and islets (2). Recent data suggest that engagement of GPR120 might result in both enhanced β cell function and improved insulin sensitivity (3, 4).
GPR120 was originally identified as the receptor that mediates GLP-1 secretion from the colon in response to the unsaturated LCFA α-linolenic acid (5). GLP-1 is an incretin hormone that promotes insulin secretion in humans and is suggested to increase β cell survival and proliferation in preclinical models (6). More recently, GPR120 was proposed to mediate the ability of ω-3 fatty acids (omega-3, n-3) to reduce inflammation and prevent insulin resistance in a diet-induced model of diabetes (3). Deletion of GPR120 from mice results in glucose intolerance and obesity, and consistent with this observation, a mutation that inhibits GPR120 signaling is associated with an increased risk of obesity in European populations (7). A study comparing normal and diabetic human islets revealed that reductions in GPR120 expression are associated with increased HbA1c levels; a clinical end point used as a measure of glucose levels over longer periods of time (4). The combination of these data suggest that GPR120 agonists might improve β cell function and insulin sensitivity, with the additional potential to reduce body weight, making GPR120 an attractive target for the treatment of metabolic disease.
The goal of this series of studies was to gain a better understanding of the role of GPR120 in pancreatic islets and the extent to which this role may contribute to the metabolic phenotype of GPR120 KO mice. Although several groups have demonstrated that GPR120 is expressed in islets (1, 4, 8), the acute role of GPR120 in islets has yet to be examined. LCFAs, which are thought to be endogenous ligands for GPR120, have well-characterized acute effects on hormone secretion from islet α- and β-cells. They amplify glucagon secretion from α-cells, with saturated fatty acids potentiating glucagon secretion more potently than unsaturated variants of identical chain length (9). LCFAs also potentiate insulin secretion from β cells and like the incretins, their effect is glucose-dependent, only enhancing secretion when stimulating levels of glucose are present (10–12). GPR40 is a major contributor to the increase in insulin secretion caused by LCFAs. The ability of linoleic or palmitic acid to acutely amplify GSIS is markedly reduced in islets isolated from GPR40 KO mice (11, 13) or in islets targeted with GPR40 antisense (14). Moreover, in vivo increases in insulin secretion typically observed after intralipid infusion are greatly reduced in GPR40 KO mice (15). The synthetic GPR40 agonist, TAK-875, increases GSIS in rodent and human islets and results in significant reductions in HbA1c in patients with type 2 diabetes (16).
Here, we demonstrate that the acute effects of LCFAs on hormone secretion from GPR120 KO islets are intact in β-cells and greatly reduced from α-cells. Although GPR120 is frequently referred to as an ω-3 or unsaturated fatty acid receptor, our data suggest that both saturated and unsaturated LCFAs engage GPR120 endogenously. Surprisingly, LCFA potentiated glucagon secretion is similarly reduced in GPR40 KO islets, suggesting that both receptors contribute to LCFA potentiated glucagon secretion. Consistent with published data, we find that GPR120 KO mice have impaired glucose tolerance; however, our data suggest that aberrant glucose control may be due to increased glucagon-mediated hepatic glucose production rather than from impairment in insulin sensitivity as has been previously proposed (3, 7).
EXPERIMENTAL PROCEDURES
Materials
All chemicals, peptides, and kits were purchased from Sigma-Aldrich unless otherwise noted.
Knock-out Mice
GPR120 and GPR40 knock-out mice were purchased from Taconic. All GPR120 mice were backcrossed onto C57Bl/6 for 6 generations or greater prior to experimentation. All GPR40 mice were backcrossed onto C57Bl/6 for 12 generations. Mice were maintained on a standard chow diet (LabDiet; 5008) unless otherwise noted. 60% high fat diet was purchased from Research Diets (RD12492). All experiments were approved by the Johnson & Johnson institutional animal care and use committee.
Metabolic Analyses
Glucose (1.5 g/kg), insulin (Humulin®, Eli Lilly) (0.75 units/kg), pyruvate (2 g/kg), and arginine (1.25 g/kg) tolerance tests were performed by intraperitoneal injection (IP). The glucagon challenge was performed by subcutaneous injection of glucagon (10 μg/kg) 15 min following intraperitoneal injection of somatostatin (10 mg/kg). Glucose was measured from tail blood at the specified time points using the Bayer Ascensia Breeze 2 blood glucose monitoring system. For plasma insulin measurements during a glucose tolerance test, fasting and 15-min plasma insulin levels were measured from 10 μl of plasma collected from the tail using an electochemiluminescent assay (Mesoscale Diagnostics). For measurement of glucagon prior to and during arginine tolerance tests, blood was collected from the tail and measured using Luminex Bead technology (Millipore).
Quantitative RT-PCR/Splicing Analysis
RNA was isolated using the GenElute mammalian total RNA isolation miniprep kit and reverse transcribed using the high capacity cDNA reverse transcription kit (Applied Biosystems) according to the manufacturer's instructions. cDNA was then combined with stock taqman primer probesets to the gene of interest and amplified with TaqMan advanced universal PCR master mix (Applied Biosystems) using an ABI 7500 fast real-time PCR system. Expression was normalized to 18s. Humans islet mRNA and α-cell mRNA derived from human embryonic stem cells were a kind gift of Betalogics (17). The human islet mRNA was a single pooled sample from several subjects. For analysis of GPR120 splice site expression, primers flanking the putative splice site were designed (Applied Biosystems) (5′-GTGCCAGGACTGGTCATTGT-3′ and 5′-GTGAGCCTCTTCCTTGATGC-3′), and cDNA was amplified as previously described (18).
Fatty Acid Preparation
DHA and palmitic acid were purchased from Sigma-Aldrich. These fatty acids were prepared as salts through the combination of equimolar amounts of fatty acid and sodium hydroxide in 100% ethanol. The ethanol was evaporated overnight, and fatty acid salts were dissolved in water on a hot plate at 20 mm. The 20 mm fatty acid solution was then complexed with bovine serum albumin (BSA) by adding an equal volume of 4 mm (26.4%) fatty acid-free BSA (Sigma Aldrich) and incubating at 37 °C for a minimum of 1 h. 10 mm fatty acid:2 mm BSA solutions were prepared fresh for each experiment.
Islet Isolation
Islets were isolated from mice by injecting Liberase TL (Roche Applied Science) into the pancreatic duct, surgically removing the infused pancreas and placing it into 50-ml conical tubes containing 4 ml of Liberase TL in Hank's balanced salt solution (HBSS). Mouse pancreata were incubated for 15 min in a 37 °C water bath. The digested pancreata were than washed three times in a 10% FBS/HBSS solution, and islets were isolated from acinar tissue on a Histopaque gradient. After three additional washes, islets were handpicked under a dissecting microscope and cultured in RPMI 1640 containing 11.1 mm glucose, 10% fetal bovine serum, 2 mm l-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, and 250 ng/ml amphotericin b (Invitrogen) for 18 h at 37 °C and 5% CO2 to allow for recovery from the isolation process.
Glucagon/Insulin Secretion Studies
For all islet studies, Krebs-ringer buffer (2.6 mm calcium chloride, 98.5 mm sodium chloride, 4 mm potassium chloride, 1.2 mm potassium phosphate, 1.2 mm magnesium sulfate, 25.9 mm sodium bicarbonate, 20 mm HEPES) was oxygenated with 95% O2/5% CO2 for 20 min immediately prior to each experiment, and subsequently supplemented with 0.2% BSA and varying concentrations of glucose (stated in figures).
For analysis of glucagon secretion, islets were washed three times in 12 mm glucose KRB and then allowed to equilibrate in this buffer for 1 h at 37 °C. 10 islets were then handpicked, placed into a well on a 96-well plate containing 200 μl of 1 mm glucose KRB supplemented with 2–5 mm l-arginine, 2 mm l-glutamine, and the indicated stimulus, and incubated for 90 min at 37 °C. Supernatant was collected and assayed for glucagon using an electochemiluminescent assay. Each condition was replicated 4–6 times per experiment.
For analysis of insulin secretion, islets were washed three times in 2.75 mm glucose KRB and then allowed to equilibrate for 1 h at 37 °C. 10 islets were then handpicked, placed into a well on a 96-well plate containing 200 μl of either 2.67 or 16.67 mm glucose KRB supplemented with the indicated stimulus, and incubated for 1 h 37 °C. Supernatant was collected and assayed for insulin using an electochemiluminescent assay. Each condition was replicated 4–6 times per experiment. For analysis of total insulin and glucagon content, islets were lysed in RIPA buffer and assayed using electrochemiluminescent assays.
Immunohistochemistry
Pancreata were formalin fixed, paraffin embedded, and sectioned at 5-μm thickness. Sections were double labeled with rabbit insulin (Cell Signaling) and mouse glucagon (Chemicon Interanational, Inc.) antibodies at 1:400 and 1:1000 dilutions, respectively. Goat anti-rabbit biotinylated IgG and donkey anti-mouse biotinylated IgG were used as secondary antibodies at 1:2000 dilutions (Chemicon). The immune-reactivity was visualized by ABC reagent (Vector) and diaminobenzidine (DAKO) for insulin or ImmPACT SG (Vector) for glucagon followed by counterstaining with nuclear fast red. A light microscope equipped with a Q Image camera was used to capture images.
Calcium Mobilization
Human GPR120S and GPR40 expressing cells were plated at 10,000 cells per well with 20 μl of media in black/clear 384-well plates. After overnight culture, calcium mobilization assays were run using a BD calcium assay kit according to the manufacturer's protocol with the exception that 2.5 mm probenecid was included. Varying concentrations of fatty acids dissolved in DMSO were added, and fluorescence changes were monitored via a FLIPR Tetra instrument (Molecular Devices).
Statistical Analysis
Data are presented as means ± S.E. Differences between groups were analyzed by the t test. When 3 or more groups were included in the study, one-way ANOVA was used. Bonferroni post-hoc tests were performed to compare between groups of interest. For in vivo studies (e.g. GTT, ITT, PTT glucagon challenge), two-way ANOVA analysis was used. p < 0.05 was considered significant. Individual experiments, that are representative of a series of experiments, are presented.
RESULTS
GPR120S Predominates in Islets and Other Tissues
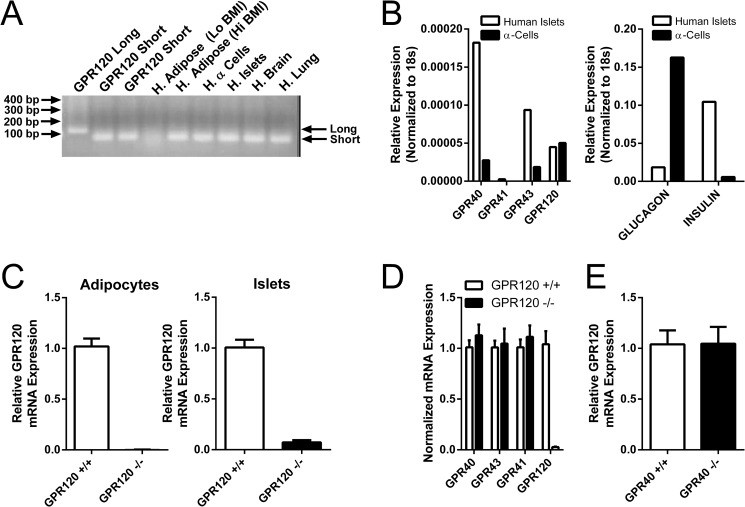
Several groups have reported the expression of GPR120 in human islets and rodent islet cell lines (1, 4, 8). Analysis of GPR120 sequences in public databases suggests the presence of an alternative exon within the 3rd intracellular loop of GPR120 such that a short (GPR120S) and a long (GPR120L) isoform with a 16-amino acid insertion may be produced in humans (5, 19). Studies exploring the expression profile of the distinct isoforms in different tissues or disease states have not been performed. To confirm expression of GPR120 in human islets and to determine which of the GPR120 splice isoforms predominates, primers that flank the putative splice insert were used to amplify GPR120 from human islet cDNA, cDNA from α-cell progenitor cells differentiated from human embryonic stem cells (17) and from cDNA where high levels of GPR120 expression has been reported. For reference, cDNA from cell lines stably transfected with GPR120L or GPR120S (2 distinct lines) were amplified in parallel. The amplified product from every tissue analyzed co-migrated with the product amplified from cells expressing GPR120S (Fig. 1A). No amplified product co-migrating with GPR120L was detected (Fig. 1A). A similarly designed quantitative RT-PCR analysis evaluating 72 human tissues or tissue regions with GPR120S or GPR120L-specific TaqMan primer/probe sets did not identify a single tissue that expresses the GPR120L isoform (data not shown). These data suggest that GPR120S is the predominant isoform in the human tissues previously reported to have abundant GPR120 expression.
FIGURE 1.
Expression of GPR120 in human and mouse islets. A, expression of GPR120S and GPR120L was examined by RT-PCR using primers that flank the putative splice insert and cDNA from human (H.) islets, adipose, brain, lung, and α cell progenitor cells differentiated from human embryonic stem cells. For reference, cDNA from cell lines stably transfected with either GPR120L (GPR120 Long) or GPR120S (GPR120 Short) was amplified in parallel to generate products that migrated as two distinct bands. The amplified product from every cDNA sample analyzed migrated exactly with the product amplified from cells expressing GPR120S. B, expression of GPR40, GPR41, GPR43, and GPR120 (left panel), as well as glucagon and insulin (right panel), was analyzed by quantitative PCR using cDNA from human islets and α cell progenitor cells differentiated from human embryonic stem cells. The α cells were enriched in glucagon mRNA relative to the human islets. Both the human islets and the α cells expressed GPR40, GPR43, and GPR120. C, expression of GPR120 in adipocytes and islets isolated from GPR120 WT and GPR120 KO mice was evaluated by quantitative RT-PCR. GPR120 mRNA was detected in islets and adipocytes isolated from GPR120 WT mice but not from GPR120 KO mice. D, effect of GPR120 deletion on the expression of GPR40 (FFAR1), GPR41 (FFAR3), and GPR43 (FFAR2) in islets isolated from GPR120 WT and GPR120 KO mice was assessed. No differences were observed in the expression of GPR40, GPR41, and GPR43 between GPR120 WT and GPR120 KO islets. E, expression of GPR120 in islets isolated from GPR40 WT and GPR40 KO mice was evaluated by quantitative PCR. Identical levels of GPR120 mRNA were detected in islets isolated from GPR40 WT and GPR40 KO mice.
GPR120 Expression in Human and Mouse Islets
Expression of GPR120 and the other FFAR family members GPR40, GPR41, and GPR43 were examined by quantitative RT-PCR in a pooled sample of human islets and in α cells differentiated from human embryonic stem cells (Fig. 1B, left panel). Determination of the relative expression of insulin and glucagon mRNA in either population demonstrated a marked enrichment in glucagon expression in the α cells (Fig. 1B, right panel). GPR40, GPR43, and GPR120 were expressed in both human islets and the human α cells (Fig. 1B, left panel).
Mice produce only one GPR120 isoform, orthologous to GPR120S. Quantitative RT-PCR confirmed expression of GPR120 mRNA in wild-type (WT) mouse islets and adipocytes (Fig. 1C). GPR120 mRNA transcripts were not detected in adipocytes and islets isolated from GPR120 KO mice (Fig. 1C). To determine whether there are compensatory changes in the expression of other fatty acid receptors in GPR120 KO islets, mouse islet RNA from GPR120 WT and GPR120 KO islets was analyzed for changes in the expression of the other free fatty acid receptors. GPR40 (FFAR1), GPR43 (FFAR2), and GPR41 (FFAR3) expression was similar in GPR120 WT and GPR120 KO islets (Fig. 1D).
Islet Function and Architecture in GPR120 KO Mice
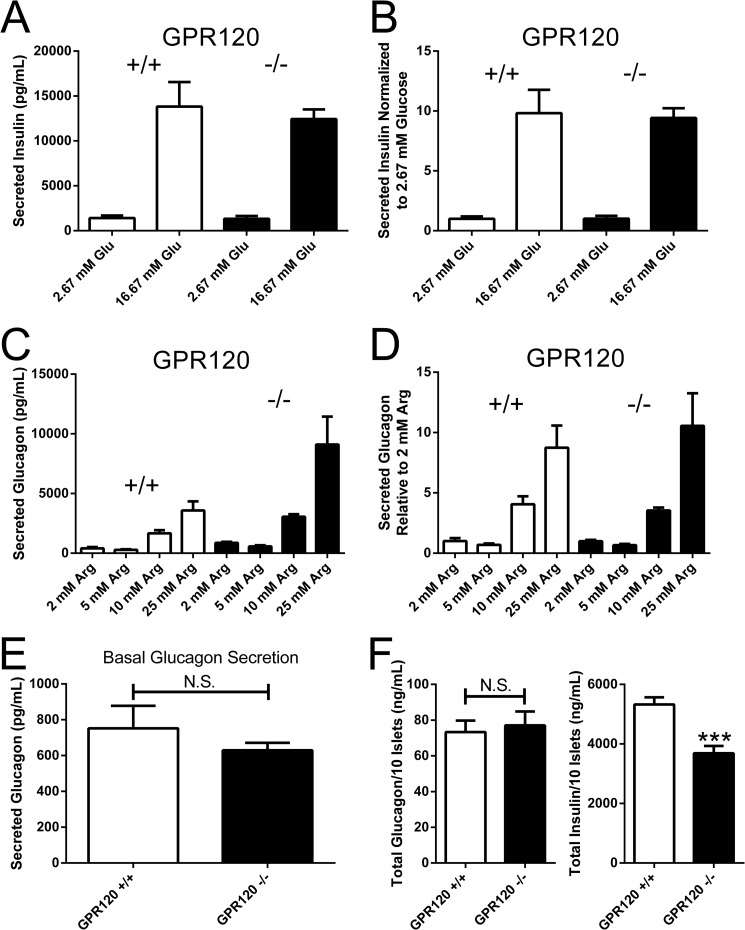
The expression of GPR120 in islets suggests a role for this receptor in the regulation of fatty acid potentiated hormone secretion from islet cell sub-types. Before exploring whether there were changes in the ability of fatty acids to potentiate hormone secretion from islet α and β cells in GPR120 KO mice, we sought to provide evidence that islets isolated from GPR120 KO mice have the capacity to respond to other nutrient stimuli. High glucose (16.67 mm) stimulated insulin secretion similarly in GPR120 WT and KO islets (Fig. 2A). Expressed as a stimulation index, high glucose increased insulin secretion relative to low glucose (2.75 mm) 9.8-fold in islets isolated from GPR120 WT mice and 9.4-fold in islets isolated from GPR120 KO mice (Fig. 2B). Similarly, arginine potentiated glucagon secretion in both GPR120 WT and GPR120 KO islets (Fig. 2C). Expressed as a stimulation index relative to 2 mm arginine (basal secretion), increasing doses of arginine potentiated glucagon secretion to the same degree in islets isolated from both GPR120 WT and GPR120 KO mice (Fig. 2D). Taken together, these data suggest that the stimulus-secretion coupling machinery in both the α and β cells of GPR120 KO islets is functional and not different from WT islets.
FIGURE 2.
Glucose-stimulated insulin secretion (GSIS) and arginine-potentiated glucagon secretion from GPR120 WT and KO islets. A, ability of islets to secrete insulin in response to glucose was compared in islets isolated from GPR120 WT and KO mice. GPR120 WT islets secreted 1408 ± 278.9 pg/ml insulin at basal glucose levels (2.67 mm) and secreted 13824 ± 2738 pg/ml insulin in response to 16.67 mm glucose. GPR120 KO islets secreted 1321 ± 315.9 pg/ml insulin at basal glucose levels (2.67 mm) and secreted 12441 ± 1073 pg/ml insulin in response to 16.67 mm glucose. B, expressed as a stimulation index, 16.67 mm glucose increased insulin secretion relative to 2.67 mm glucose 9.8 ± 1.9-fold in GPR120 WT islets and 9.4 ± .8-fold in GPR120 KO islets. C, ability of islets to secrete glucagon in response to arginine was compared in islets isolated from GPR120 WT and KO mice. GPR120 WT islets secreted 410 ± 106 pg/ml glucagon at basal arginine levels (2 mm) and secreted 286 ± 43 pg/ml, 1664 ± 270 pg/ml and 3586 ± 756 pg/ml glucagon in response to 5, 10, and 25 mm arginine. GPR120 KO islets secreted 863 ± 92 pg/ml glucagon at basal arginine levels (2 mm) and secreted 575 ± 91 pg/ml, 3057 ± 209 pg/ml, and 9106 ± 2340 pg/ml glucagon in response to 5, 10, and 25 mm arginine. D, expressed as a stimulation index, 5, 10, and 25 mm arginine increased glucagon secretion relative to 2 mm arginine 0.7 ± 0.1-fold, 4.1 ± 0.7-fold, and 8.7 ± 1.8- fold, respectively, from GPR120 WT islets and 0.7 ± 0.1-fold, 3.5 ± .2-fold, and 10.5 ± 2.7-fold, respectively, from GPR120 KO islets. E, to determine if basal glucagon secretion (2 mm arginine) is different between GPR120 WT and GPR120 KO islets, basal glucagon secretion was combined across all experiments performed. GPR120 WT islets secreted 752 ± 125.6 pg/ml and GPR120 KO islets secreted 629.2 ± 42.1 pg/ml glucagon at basal arginine levels (p = 0.4, n = 24–25). F, total glucagon and insulin content was compared in islets isolated from GPR120 WT and GPR120 KO mice on normal chow diet. GPR120 WT islets contained 73.3 ± 6.4 ng/ml and GPR120 KO islets contained 77.1 ± 7.8 ng/ml glucagon per 10 islets (p = 0.71, n = 17–22). GPR120 WT islets contained 5327 ± 241 ng/ml and GPR120 KO islets contained 3687 ± 243 ng/ml insulin per 10 islets (p < 0.0001, n = 17–22).
The apparent increase in glucagon secretion under basal conditions and in response to arginine in GPR120 KO islets relative to GPR120 WT islets was not consistently observed from experiment to experiment. Analysis of basal glucagon secretion (2 mm arginine) across all experiments did not reveal differences between islets isolated from GPR120 WT and GPR120 KO mice (Fig. 2E). The variable nature of islet isolations and the challenges associated with matching the size of islets between distinct genotypes, even though isolated in parallel, likely contributed to the observed differences. To control for these differences, we normalized fatty acid potentiated insulin and glucagon secretion to basal secretion in the experiments described below.
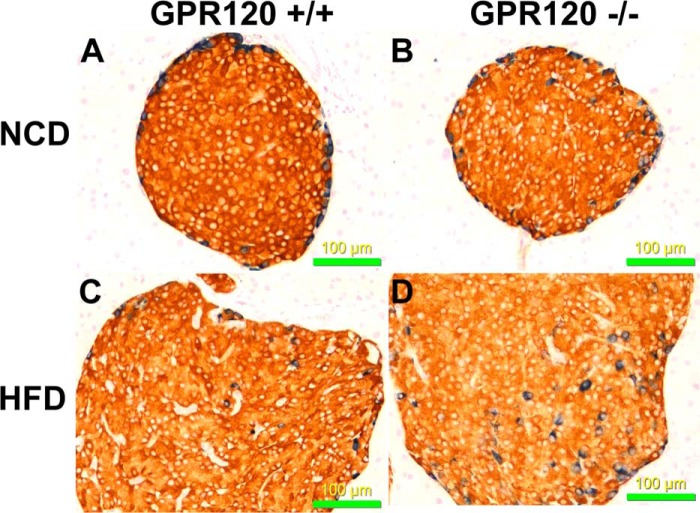
To determine if the overall organization of cells within islets in GPR120 KO mice is normal, immunohistochemistry was performed on pancreas sections from GPR120 WT and GPR120 KO mice either on normal chow or on a 60% high fat diet using insulin and glucagon antibodies (Fig. 3; insulin, red; glucagon, blue). Islets from both genotypes were intact and generally similar in terms of overall architecture (Fig. 3). The only qualitative difference we observed was an increase in the presence of α cells within the core of GPR120 KO islets relative to GPR120 WT islets. This was apparent in GPR120 KO mice on both normal chow and 60% high fat diet. It should be noted, however, that these studies were not designed or powered appropriately to quantitate islet number, hormone expression, or the presence of α cells in the islet core. As a result, the observation of increased core α cells was not assessed as has been previously described (20). We did investigate total islet glucagon and insulin content in isolated islets from GPR120 WT and GPR120 KO islets. While there was no difference in glucagon content between islets isolated from GPR120 WT and KO mice (Fig. 2F, left panel), there was a 31% decrease in insulin content in GPR120 KO islets relative to GPR120 WT islets (Fig. 2F, right panel).
FIGURE 3.
Immunohistochemical analysis of pancreas sections from GPR120 WT and KO mice. To compare the morphology of islets from GPR120 WT and GPR120 KO mice on NCD and HFD, immunohistochemical localization studies were performed on pancreas sections using antibodies to insulin (red) and glucagon (blue). A, islets from GPR120 WT mice on NCD; B, islets from GPR120 KO mice on NCD; C, islets from GPR120 WT mice on HFD; D, islets from GPR120 KO mice on HFD. Photographs shown are all at ×200 magnification.
Saturated and Unsaturated LCFAs Mobilize Calcium in GPR120 Overexpressing Cells
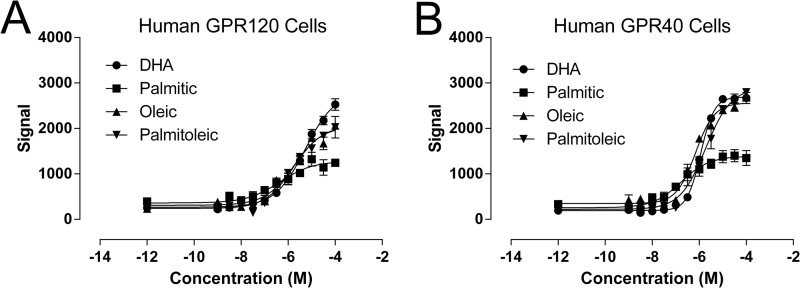
Initial reports suggested that both saturated (e.g. palmitic acid) and unsaturated LCFAs (e.g. DHA, palmitoleic, oleic acid) lead to increases in calcium mobilization in cells expressing GPR120 (5). To confirm that both saturated and unsaturated fatty acids activate GPR120, calcium mobilization was monitored in cells overexpressing GPR120 following treatment with fatty acids containing varying degrees of saturation. As a positive control, the identical experiment was performed in cells overexpressing GPR40, a receptor that is known to be engaged by both saturated and unsaturated LCFAs. Palmitic acid, oleic acid, palmitoleic acid, and DHA solubilized in DMSO caused dose-dependent increases in intracellular calcium levels in both GPR120- (Fig. 4A) and GPR40-overexpressing cells (Fig. 4B). The relative insolubility of palmitic acid at high concentrations hampered our ability to determine its maximal efficacy in comparison to the other fatty acids tested, which remained in solution for the duration of the assay. To increase solubility and to mimic physiological conditions in subsequent islet experiments, fatty acids were prepared as salts in a 5:1 molar mixture with fatty-acid free bovine serum albumin. Since a significant fraction of the fatty acids bind to BSA and only the unbound fatty acids can interact with the receptor, higher concentrations of fatty acid:BSA mixtures are required for GPR120 activation than might be predicted from experiments where fatty acids are solubilized only with DMSO.
FIGURE 4.
Saturated and LCFAs increase calcium mobilization in cells overexpressing GPR120 and GPR40. The effect of the saturated fatty acid, palmitic acid and the effects of the unsaturated fatty acids DHA, oleic, and palmitoleic acid on intracellular Ca2+ levels were examined in cells overexpressing either the human GPR120S receptor (A) or the human GPR40 receptor (B). Average pEC50 values obtained in GPR120S-expressing cells were: palmitic acid, −6.4 ± 0.3; DHA, −5.3 ± 0.1; oleic acid, −5.7 ± 0.2; and palmitoleic acid, −5.8 ± 0.1). Average pEC50 values obtained in GPR40-expressing cells were: palmitic acid, −6.8 ± 0.2; DHA, −5.9 ± 0.03; oleic acid, −6.2 ± 0.1, and palmitoleic acid, −5.7 ± 0.1.
LCFA-Potentiated Glucagon Secretion Is Markedly Reduced in GPR120 KO Islets
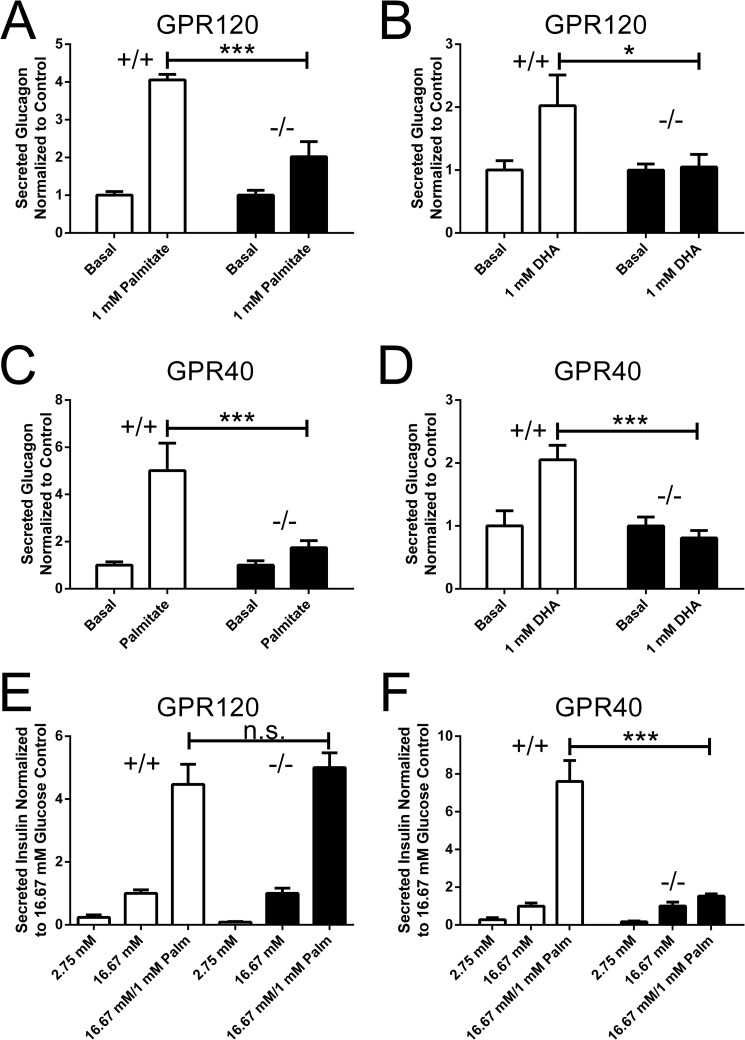
LCFAs have previously been demonstrated to potentiate glucagon secretion from α cells with saturated fatty acids increasing glucagon secretion to a greater extent than unsaturated fatty acids of identical chain length (9). Our observation that GPR120 is expressed in human and mouse islets and in α cells differentiated from human embryonic stem cells, prompted us to ask whether GPR120 contributes to the ability of LCFAs to potentiate glucagon secretion in an acute paradigm. To address this question, islets were isolated from GPR120 WT and GPR120 KO mice, and glucagon secretion was measured after treatment with palmitate or DHA. The ability of palmitate and DHA to potentiate glucagon secretion from GPR120 KO islets was attenuated compared with that from WT islets. Palmitate increased glucagon secretion 4.1-fold in GPR120 WT islets relative to BSA compared with only 2-fold in GPR120 KO islets (Fig. 5A). DHA increased glucagon secretion 2-fold relative to BSA from GPR120 WT islets but did not produce any increase in glucagon secretion in GPR120 KO islets (Fig. 5B). These data suggest that GPR120 regulates LCFA potentiated glucagon secretion and that both saturated and unsaturated LCFA can engage GPR120 endogenously in mouse α cells.
FIGURE 5.
Long chain fatty acid potentiated glucagon and insulin secretion in islets isolated from GPR120 and GPR40 KO mice. The effect of palmitate (A) and DHA (B) on glucagon secretion was assessed in GPR120 WT and KO islets. A, glucagon secretion from GPR120 WT islets was increased 4.1 ± 0.1-fold relative to BSA in response to palmitate whereas glucagon secretion from GPR120 KO islets was increased only 2.0 ± 0.4-fold (p < 0.001, n = 6 compared with WT islets). B, glucagon secretion from GPR120 WT islets was increased 2.0 ± 0.5-fold relative to BSA in response to DHA but DHA failed to increase glucagon secretion from GPR120 KO islets (p < 0.05, n = 5 compared with WT islets). The effect of palmitate (C) and DHA (D) on glucagon secretion was similarly assessed in GPR40 WT and GPR40 KO islets. C, glucagon secretion from GPR40 WT islets was increased 5.0 ± 1.1-fold relative to BSA in response to palmitate whereas glucagon secretion from GPR40 KO islets increased only 1.7 ± 0.2-fold in response to palmitate (p < 0.001, n = 6 compared with WT islets). D, glucagon secretion from GPR40 WT islets increased 2.1 ± 0.2-fold relative to BSA in response to DHA. DHA failed to increase glucagon secretion from GPR40 KO islets (p < 0.001, n = 6 compared with WT islets). The ability of palmitate to potentiate GSIS secretion was analyzed in GPR120 KO islets (E) and GPR40 KO islets (F). E, palmitate treatment increased GSIS from GPR120 WT islets 4.5 ± 0.6-fold relative to BSA and increased GSIS from GPR120 KO islets 5 ± 0.5-fold (p = n.s., n = 5 compared with WT islets). F, palmitate treatment increased GSIS from GPR40 WT islets 7.6 ± 1.1-fold relative to BSA and only increased GSIS from GPR40 KO islets by 1.5 ± 0.1-fold (p < 0.001, n = 6 compared with WT islets).
Since GPR40 is engaged by the same fatty acids that activate GPR120 and is important for LCFA-potentiated GSIS, we hypothesized that GPR40 and GPR120 would have distinct cell type specific roles in mediating hormone secretion from islet cells. To understand if GPR40 plays a role in LCFA-potentiated glucagon secretion, islets were isolated from GPR40 WT and KO mice and glucagon secretion was measured after treatment with palmitate or DHA. Surprisingly, both palmitate- and DHA-potentiated glucagon secretion were reduced in GPR40 KO islets. In GPR40 WT islets, palmitate-potentiated glucagon secretion 5-fold relative to BSA versus only 1.7-fold from GPR40 KO islets (Fig. 5C). DHA increased glucagon secretion 2.1-fold from GPR40 WT islets relative to BSA but was unable to potentiate glucagon secretion from GPR40 KO islets (Fig. 5D). Altered GPR120 mRNA expression was not observed in islets isolated from GPR40 KO mice (Fig. 1D), nor were changes in GPR40 expression observed in islets isolated from GPR120 KO mice (Fig. 1E). Taken together, these data suggest that both GPR120 and GPR40 regulate LCFA potentiated glucagon secretion and that both saturated and unsaturated LCFA can engage GPR120 endogenously in mouse α cells.
GPR120 Is Not Required for LCFA Potentiated GSIS
To determine if GPR40 and GPR120 are both involved in LCFA-potentiated GSIS from β cells, islets from GPR120 WT, GPR120 KO, GPR40 WT, and GPR40 KO mice were isolated, and the ability of palmitate to potentiate GSIS was evaluated. Palmitate-potentiated GSIS was fully preserved in GPR120 KO islets. Palmitate increased GSIS by 4.5-fold relative to BSA from GPR120 WT islets and by 5-fold from GPR120 KO islets (Fig. 5E). Consistent with previous reports (11, 13), palmitate-potentiated GSIS was markedly reduced from GPR40 KO islets (Fig. 5F). Palmitate increased GSIS by 6.6-fold relative to BSA from GPR40 WT islets versus only 1.8-fold from GPR40 KO islets (Fig. 5F). These data demonstrate that GPR40, and not GPR120, is the key receptor mediating the acute potentiation of GSIS by palmitate in the β-cell.
Obesity, Hyperglycemia, and Glucose Intolerance in GPR120 KO Mice
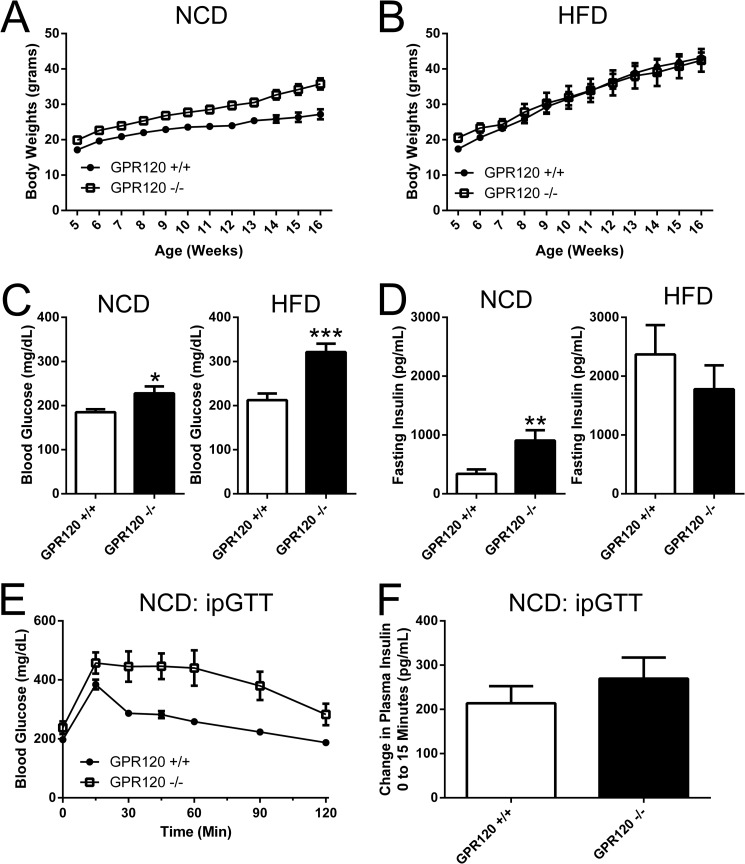
The metabolic phenotype of our GPR120 KO mice is largely in line with previous reports suggesting that GPR120 KO mice are obese and glucose intolerant (3, 7). A cohort of GPR120 WT and GPR120 KO were placed either on a normal chow diet (NCD) or a 60% high fat diet (HFD) and body weights were then collected weekly from 5 weeks through 16 weeks of age. At 5 weeks, the GPR120 KO mice on NCD were 16% heavier than GPR120 WT mice on NCD (Fig. 6A) and the group of GPR120 KO mice initiated on HFD at this time were 18% heavier than GPR120 WT mice (Fig. 6B). On NCD, this difference in weight persisted and further increased as the mice aged. By four months of age, GPR120 KO mice on NCD were 31.4% heavier than GPR120 WT mice (Fig. 6A). However by four months of age on HFD, the initial difference in body weights was no longer apparent, and GPR120 WT mice actually weighed 2% more than GPR120 on HFD (Fig. 6B).
FIGURE 6.
Metabolic characteristics of GPR120 KO mice. A and B, body weights were monitored weekly from 5 weeks through 16 weeks of age in a cohort of GPR120 WT and GPR120 KO mice on NCD and HFD. A, in GPR120 WT mice on NCD, average body weight increased from 17.1 to 27.2 grams from 5 through 16 weeks, whereas in GPR120 KO mice on NCD, average body weight increased from 19.9 to 35.8 grams. B, in GPR120 WT mice on HFD, average body weight increased from 17.4 to 43.4 grams and in GPR120 KO mice on HFD, average body weight increased from 20.6 to 42.4 grams. 6-hour fasting glucose (C) and insulin (D) levels were also evaluated. Average 6-h fasting glucose (FG) was 185 ± 7 mg/dL in GPR120 WT on NCD versus 228 ± 16 mg/dL in GPR120 KO mice on NCD (p < 0.05, n = 6 compared with WT). In mice on HFD, average 6 h FG was 212 ± 15 mg/dL in GPR120 WT mice versus 321 ± 19 mg/dL in GPR120 KO mice (p < 0.001, n = 9 compared with WT). Average 6 h fasting insulin (FI) was 339 ± 76 pg/ml in GPR120 WT mice on NCD versus 906 ± 175 pg/ml in GPR120 KO mice on NCD (p < 0.01, n = 6 compared with WT). In mice on HFD, average 6 h FI was 2370 ± 498 pg/ml in GPR120 WT mice, and 1777 ± 407.9 pg/ml in GPR120 KO mice (p = n.s., n = 9 compared with WT). E, an intraperitoneal glucose tolerance test (GTT) was performed on GPR120 WT and GPR120 KO mice on NCD. A significant increase in the glucose excursion was observed in GPR120 KO mice on NCD relative to GPR120 WT mice (p < 0.001, n = 6 compared with WT). F, insulin was measured prior to (0 min) and during (15 min) the ipGTT. Insulin increased 213.8 ± 38.7 pg/ml from 0 to 15 min in GPR120 WT mice and increased 269.6 ± 47.5 pg/ml from 0 to 15 min in GPR120 KO mice (p = .4, n = 6).
Parameters associated with glucose homeostasis were also analyzed in GPR120 WT and GPR120 KO mice on NCD and HFD. On NCD, GPR120 KO mice are significantly hyperglycemic and hyperinsulinemic relative to GPR120 WT mice. Average 6-h fasting glucose was increased by 48 mg/dL (Fig. 6C) and average 6-h fasting insulin increased by 569 pg/ml (Fig. 6D) in GPR120 KO mice on NCD relative to GPR120 WT mice. GPR120 KO mice on HFD are significantly hyperglycemic but not hyperinsulinemic relative to GPR120 WT mice. Average 6 h fasting glucose increased by 109 mg/dL relative to that in GPR120 WT mice (Fig. 6C), and although the difference was not statistically significant 6 h fasting insulin was surprisingly decreased by 593 pg/ml relative to GPR120 WT mice (Fig. 6D). An intraperitoneal glucose tolerance test (IPGTT) was performed to further understand the ability of GPR120 KO mice on NCD to dispose of glucose. A marked increase in the glucose excursion was observed during the IPGTT in GPR120 KO mice relative to GPR120 WT mice on NCD (Fig. 6E). Analysis of plasma insulin levels prior to (0 min) and during (15 min) the ipGTT revealed that insulin increases to a similar extent in both GPR120 WT and KO mice (Fig. 6F).
Insulin Sensitivity in GPR120 KO Mice
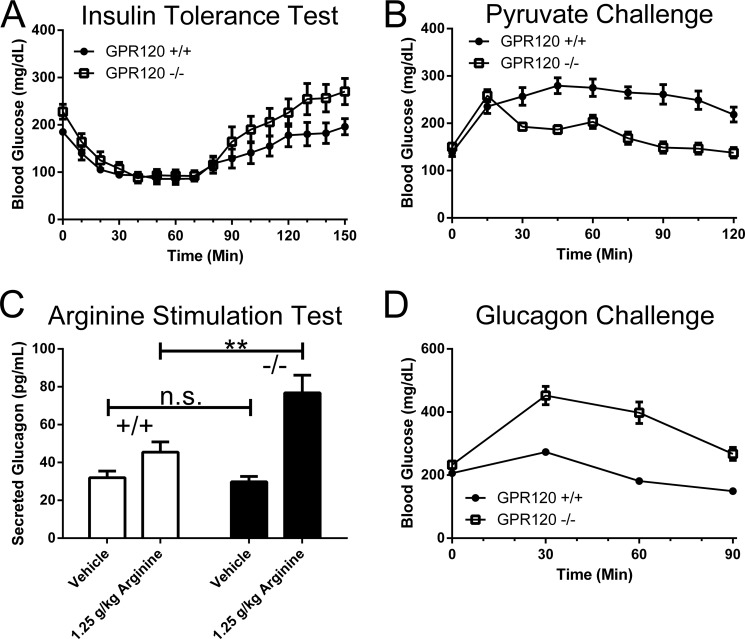
The combination of hyperglycemia and glucose intolerance with hyperinsulinemia observed in GPR120 KO mice on NCD is usually indicative of tissue insulin resistance. Intraperitoneal insulin tolerance tests (ITTs) were therefore performed to assess whole body insulin sensitivity. After injection with insulin, glucose levels in GPR120 KO mice decreased and plateaued at the same glucose levels as in WT mice despite starting 40 mg/dL higher (Fig. 7A). The results of the ITT do not suggest that GPR120 KO mice are insulin resistant relative to GPR120 WT mice. We noticed during the portion of the ITT (70–120 min) where counter-regulatory hormones are increased and act on the liver to increase glucose levels back toward baseline levels, that blood glucose levels appeared to increase much faster in GPR120 KO mice than in GPR120 WT mice (Fig. 7A, 70–120 min). This is consistent with previously published hyperinsulinemic-euglycemic clamp data indicating increased endogenous glucose production in GPR120 KO mice (3). The deficits in glucose homeostasis observed in GPR120 KO mice might therefore be caused by increased hepatic glucose production rather than by insulin resistance per se.
FIGURE 7.
Evaluation of insulin sensitivity and hepatic glucose tolerance in GPR120 KO mice on a NCD. A, an ITT was performed in GPR120 WT and GPR120 KO mice on NCD to assess insulin sensitivity. A significant difference in the glucose excursion was observed in GPR120 KO mice relative to GPR120 WT mice (p < 0.05, n = 6). B, a pyruvate tolerance test was performed to understand gluconeogenesis in GPR120 KO mice on NCD. A significant decrease in gluconeogenesis, reflected as a decrease in glucose excursion following pyruvate injection, was observed in GPR120 KO mice relative to GPR120 WT mice on NCD (p < 0.001, n = 6). C, glucagon secretion was measured in GPR120 WT and KO mice on NCD after an intraperitoneal injection of arginine. Basal glucagon secretion did not differ significantly between GPR120 WT and GPR120 KO mice on NCD (31.9 ± 3.5 pg/ml in WT versus 29.8 ± 2.8 pg/ml in KO) whereas arginine stimulated glucagon secretion was markedly increased in GPR120 KO mice relative to GPR120 WT mice on NCD (45.5 ± 5.4 pg/ml in WT versus 76.77 ± 9.4 in KO mice; p < 0.01, n = 10). D, to assess liver glucagon sensitivity, a glucagon challenge was performed in GPR120 WT and KO mice on NCD. Glucagon increased glucose levels over time to a much greater degree in GPR120 KO mice relative to GPR120 WT mice (p < 0.001, n = 13–15).
Reduced Gluconeogensis in GPR120 KO Mice
Hepatic glucose production is mediated either by gluconeogenesis or glycogenolysis. To explore whether increased gluconeogenesis contributes to glucose intolerance in GPR120 KO mice on NCD, a pyruvate tolerance test was performed. Surprisingly, a significant decrease in the glucose excursion was observed after pyruvate injection in GPR120 KO mice relative to GPR120 WT mice (Fig. 7B). The observation of decreased gluconeogenesis following a pyruvate challenge in GPR120 KO mice is surprising given the degree of glucose intolerance observed in GPR120 KO mice.
Increased Glucagon Secretion and Glucagon Sensitivity in GPR120 KO Mice
Glucagon released from α cells acts primarily to increase hepatic glucose production via increased glycogenolysis with little to no acute effect on gluconeogenesis (21). To explore the possible contributions of the glucagon axis to altered glucose homeostasis in GPR120 KO mice, the capacity of α cells to secrete glucagon in vivo was assessed following an arginine challenge. There was no observed difference in baseline glucagon levels between WT and GPR120KO mice (Fig. 7C). Arginine potentiated glucagon release to a much greater degree in GPR120 KO mice than in GPR120 WT mice, resulting in a 167% increase in glucagon release in GPR120 KO mice relative to basal levels versus only a 42% increase in glucagon release in GPR120 WT mice (Fig. 7C). To further understand the glucagon axis in GPR120 KO mice, a glucagon challenge was performed to determine hepatic glucagon sensitivity. Prior to performing the glucagon challenge, mice were injected intraperitoneally with somatostatin to inhibit endogenous glucagon and insulin production. Consistent with increased glucagon sensitivity, a significant increase in the glucose excursion was observed during the glucagon challenge in GPR120 KO mice relative to GPR120 WT mice (Fig. 7D). The combination of increased glucagon secretion and increased hepatic glucagon sensitivity likely contributes to the impaired glucose homeostasis observed in GPR120 KO mice.
DISCUSSION
Unsaturated LCFAs have anti-diabetic and GPR120-dependent effects in adipocytes, macrophages, and enteroendocrine cells (3, 5). Although GPR120 is expressed in islets (1, 4, 8) and LCFAs regulate hormone secretion from islet α and β cells (9, 12), it was not clear whether GPR120 mediates these effects. Here, we explored the role of GPR120 in mouse islets using LCFAs and demonstrate that the potentiating effects of LCFAs on glucagon secretion are markedly reduced from GPR120 KO islets regardless of whether the LCFA is saturated or unsaturated. Similar GPR120-dependent effects on insulin secretion from β cells were not identified. We further explored the contribution of the glucagon axis to the glucose intolerance observed in GPR120 KO mice based on observations of an enhanced rate at which glucose returned to baseline in GPR120 KO mice during insulin tolerance tests. Both glucagon secretion and hepatic glucagon sensitivity are increased in vivo in GPR120 KO mice, suggesting that an increase in hepatic glucose production contributes to their metabolic phenotype. Our findings uncover a role for GPR120 in the islets, suggest saturated as well as unsaturated LCFAs are endogenous GPR120 ligands and implicate the liver as a key integrator of GPR120-dependent signals arising from islets and perhaps, other GPR120-expressing tissues that exert effects on glucose metabolism.
Expression of GPR120 in Islets
An initial report using MIN-6 cells as a surrogate for islet β cells suggested limited expression of the GPR120 gene in islets (5). Follow-up studies have since revealed GPR120 expression in a subset of islet cell lines including βTC-6 cells (data not shown) as well as in mouse and human islets ((Fig. 1) and Refs. 1, 4). In humans, increased GPR120 expression in islets is associated with increased insulin secretion and reduced HbA1C, and GPR120 expression is significantly reduced in islets isolated from patients with type 2 diabetes relative to patients with normoglycemia (4). The localization of GPR120 within islets has not been extensively explored. Our observation that GPR120 mRNA is expressed in α cells differentiated from human embryonic stem cells (Fig. 1B) combined with our observation that LCFA potentiated glucagon secretion is markedly reduced from GPR120 KO islets (Fig. 5, A and B) suggests that there is some expression in islet α cells. We sought to identify the localization of the GPR120 protein within islets, as has been previously described in taste cells (22), but could not identify a commercially available antibody that was specific for GPR120 (data not shown).
The human GPR120 gene is different than the rodent gene in that it is subject to alternative splicing. We explored the isoform-specific expression of GPR120 in human islets and other GPR120 expressing tissues. In human islets and in α cells differentiated from human embryonic stem cells, only the GPR120 isoform lacking the splice insert (GPR120S) appears to be expressed (Fig. 1A). The isoform containing the splice insert (GPR120L) was not detected in islets or in any of the other GPR120 expressing tissues (e.g. adipose or lung) we evaluated using conventional PCR methods. This is the first description of the relative expression of GPR120S and GPR120L in human tissues. The reports describing the existence of GPR120L do not mention the tissue from which it was cloned (5, 19). Whether tissues can be identified that express GPR120L is of significant interest because GPR120L signals exclusively through β-arrestin upon stimulation with agonists whereas GPR120S signals both through Gαq/11 mediated increases in calcium mobilization and via β-arrestin pathways (19). A broader expression study on human tissues from both normal and diseased states would likely shed light on whether GPR120L is expressed in tissues not evaluated here and whether the observed differences in signaling are relevant to GPR120 biology.
Islet Architecture and Function in GPR120 KO Mice
Islets from GPR120 KO mice secreted both insulin and glucagon in response to non-fatty acid secretogogues to the same degree as GPR120 WT mice (Fig. 2, B and D). While islet glucagon content was similar between genotypes, insulin content was markedly reduced in GPR120 KO mice relative to GPR120 WT mice (Fig. 2F). Analysis of the architecture of islets using immunohistochemistry did not reveal any gross abnormalities in GPR120 KO mice relative to GPR120 WT mice regardless of whether the mice were fed normal chow or high fat diets. The only difference we observed was an increase in the presence of α cells within the core of islets in GPR120 KO mice on both normal chow and high fat diets relative to GPR120 WT mice (Fig. 3). It should be noted, however, that these immunohistochemistry studies were not designed or powered appropriately to quantitate islet number, hormone expression or the presence of α cells in the islet core. As a result, the observation of increased core α cells was not assessed as has been previously described (20). This observation is common in rodent models where stress increases the demand for insulin and is likely the result of the metabolic dysregulation in GPR120 KO mice (23). The generation of islet-specific GPR120 KO mice could help clarify whether our observations of reduced islet insulin content and an increased presence of core α cells in GPR120 KO mice is caused by metabolic stress or the loss of GPR120 within the islet.
GPR120 Mediates the Effects of Unsaturated and Saturated LCFA-potentiated Glucagon Secretion
Research on GPR120 to date has relied heavily on the use of unsaturated LCFAs to interrogate its role in tissues important for glucose homeostasis. Despite the initial de-orphanization of GPR120 suggesting that it could be activated by saturated fatty acids such as myristic, palmitic, and stearic acids (5), unsaturated ω-3 fatty acids (e.g. DHA and α-linolenic acid) have primarily been used to demonstrate roles for GPR120 in mediating GLP-1 release from enteroendocrine cells, in the regulation of glucose uptake in adipocytes and in its ability to engage anti-inflammatory and insulin-sensitizing mechanisms in macrophages (3, 5). A synthetic agonist (GW9508), which activates both GPR120 and GPR40, albeit with 100-fold more selectivity for GPR40, has been used to support observations made with ω-3 fatty acids in tissues that lack GPR40 expression (3, 24).
Here, we clearly demonstrate that both saturated and unsaturated fatty acids lead to increases in calcium mobilization in cells expressing either GPR120 or GPR40. In the context of exploring an acute role for GPR120 in islets and specifically α cells, we investigated whether GPR120 is important for both palmitate- and DHA-potentiated glucagon secretion. Our results reveal that GPR120 is involved in mediating LCFA-potentiated glucagon secretion and suggest that both saturated and unsaturated LCFAs are endogenous ligands for GPR120 in the islet (Fig. 5, A and B). The finding that GPR120 mediates glucagon secretion from α-cells in response to LCFAs is consistent with observations that increases in intracellular calcium are required for palmitate-potentiated glucagon secretion from mouse islets (25).
Whether both saturated and unsaturated LCFAs similarly activate GPR120 in adipocytes, macrophages, and enteroendocrine cells or whether unsaturated fatty acids engage the receptor differently than saturated fatty acids is unclear. The latter hypothesis is intriguing in light of the recent identification of allosteric agonists to GPR40. Like GPR120, GPR40 is coupled to Gαq/11 and is activated by both saturated and unsaturated LCFAs. GPR40 is highly expressed in islet β cells and is required for the potentiating effects of LCFAs on glucose-stimulated insulin secretion (GSIS) (11, 13), a finding that is confirmed here (Fig. 5F) and supported by data suggesting synthetic GPR40 agonists similarly enhance GSIS both ex vivo and in vivo (26). GPR40 has been suggested to have 3 allosteric sites. Full agonists that bind to one site are very efficacious at increasing both GLP-1 and insulin release in vivo and ex vivo whereas partial agonists that bind to another site are comparably less efficacious at inducing insulin release and fail to have any effect on GLP-1 release (27). The differences in efficacy of saturated versus unsaturated fatty acids on glucagon secretion are suggestive that different LCFA species can engage secretory pathways in α-cells differently (Fig. 5), this may be explained in-part by differences in the metabolism of the different LCFA species. However, the finding that GPR120 is activated endogenously by both species of LCFAs and the finding by Oh et al. that GPR120 is required for the anti-inflammatory and insulin-sensitizing effects of omega-3 fatty acids in DIO mice (3), an effect not observed with saturated fatty acids in rodents (28), supports the design of future experiments to explore whether saturated and unsaturated fatty acids engage GPR120-dependent signaling cascades differently in different tissues.
Both GPR40 and GPR120 In Are Important for LCFA-potentiated Hormone Release from α Cells but Not β Cells
We explored the possibility that GPR120 might also contribute to the potentiating effects of LCFAs on GSIS but did not find evidence that it played a role in the ability of palmitate to potentiate GSIS.
Similarly, we explored the effects of LCFAs on glucagon secretion from GPR40 KO islets. Previous reports indicated that GPR40 is expressed in α cells (29) and although the synthetic GPR40 agonist, TAK-875, does not appear to increase glucagon secretion from rodent or human islets (26), antisense experiments suggest that GPR40 is required for LCFA potentiated glucagon secretion (29, 30). We found that like GPR120, GPR40 can mediate the potentiating effects of both palmitate and DHA on glucagon secretion from α cells (Fig. 5, C and D). At least at the mRNA level, changes in the expression of GPR40 and GPR120 in KO islets do not appear to explain the involvement of both receptors for LCFA-potentiated glucagon secretion. There is some evidence in taste cells that both receptors are required as taste preference for LCFAs is reduced in both GPR120 and GPR40 KO mice (31). The implication that both receptors are involved in the regulation of LCFA-potentiated glucagon secretion to the interpretation of our results is unclear. The development of GPR120-specific antibodies may shed light on whether cell surface expression of GPR40 or GPR120 is altered in KO islets. It is also possible that both receptors independently contribute to a fraction of LCFA potentiated glucagon secretion. The development of GPR120 agonists and a more thorough characterization of the different classes of GPR40 agonists may help to confirm the extent to which each of the receptors contributes to increased glucagon secretion from α cells. The development of tissue-specific GPR120/GPR40 double knock-out mice might provide additional insight as to the role of these receptors in the α cell.
The Glucagon Axis Contributes to the Glucose Intolerance Observed in GPR120 KO Mice
Metabolic dysregulation in the GPR120 KO mice has previously been described. The severity of the phenotype and the diet on which it is most pronounced varies between reports. Oh et al. observe glucose intolerance in GPR120 KO mice on NCD but not on HFD but, do not observe any differences in body weight between genotypes regardless of diet (3). Ichimura et al. observe glucose intolerance on HFD but not on NCD and similarly, observe increased body weight in GPR120 KO mice on HFD but not on NCD (7). In our hands, GPR120 KO mice on both NCD and HFD are hyperglycemic and glucose intolerant (Fig. 6, C and E). The degree of hyperglycemia and glucose intolerance appeared to be more severe in our GPR120 KO mice on HFD. Our GPR120 KO mice are significantly heavier than WT mice on NCD (Fig. 6A). On HFD, the body weights of GPR120 KO mice are similar to WT mice through 4 months of age (Fig. 6B). An increase does however become apparent in GPR120 KO mice relative to GPR120 WT mice by 6 months of age (20 weeks on HFD, data not shown). It is not clear what contributes to the differences reported between the three independent analyses of GPR120 KO mice on NCD and HFD. It is not uncommon for phenotypes to vary between research groups; in fact, marked differences have been reported for GPR40 KO mice (13, 32). The observed differences between our 3 groups may relate to the extent of backcrossing, the method of KO generation or even differences in housing.
Analysis of the source of glucose intolerance observed by Oh et al. and Ichimura et al. in GPR120 KO mice is purported to be related to insulin resistance. Our data are somewhat conflicting with respect to insulin resistance and when taken together do not appear to support insulin resistance as the cause of glucose intolerance in our GPR120 KO mice. Consistent with the concept of increased insulin resistance, our GPR120 KO mice on NCD are hyperinsulinemic and exhibit an increase in the expression of inflammatory markers within adipose tissue (Fig. 6D; data not shown). On the other hand, our GPR120 KO mice on HFD are hyperglycemic but have insulin levels similar to those of WT mice on HFD (Fig. 6, C and D). Further, glucose levels reach a similar nadir in both GPR120 WT and KO mice on NCD during insulin tolerance tests despite a higher starting baseline glucose level in GPR120 KO mice (Fig. 7A). Moreover, insulin-mediated akt phosphorylation is identical in the skeletal muscle, liver, and adipose tissue of GPR120 WT and KO mice on NCD after injection of insulin via the vena cava (data not shown). The increased rate of return of glucose to baseline during an insulin tolerance test in GPR120 KO mice relative to WT mice pointed to an enhanced counter-regulatory response as a contributor to the observed glucose intolerance (Fig. 7A). In agreement with this hypothesis, not only is the glucose profile during the ITT performed by Ichimura et al. strikingly similar to that peformed here but the report by Oh et al. revealed markedly increased endogenous glucose production in GPR120 KO mice during the course of a euglycemic-hyperinsulinemic clamp study (3, 7).
To gain a better understanding of glucose production by the liver, gluconeogenesis and glycogenolysis were evaluated in GPR120 KO mice on NCD. Our results were somewhat contradictory in that the glucose excursion was reduced following a pyruvate challenge, suggesting reduced gluconeogenesis, and the glucose excursion was markedly increased following a glucagon challenge, suggesting increased glycogenolysis (Fig. 7, B and D). Increased arginine-potentiated glucagon release was also observed in GPR120 KO mice (Fig. 7C). Although unlikely since GLP-1 does not appear to be different between GPR120 WT and KO mice (3), we cannot exclude the possibility that altered GLP-1 release did not contribute to the observed increase in glucagon. GLP-1 is known to have inhibitory effects on glucagon secretion and GPR120 activation is reported to regulate GLP-1 secretion (5, 33). The net effect of increased hepatic glucagon sensitivity on a background of increased stimulated glucagon secretion versus the reduction in gluconeogenesis is difficult to assess with the tools used here. These data combined with the increase in endogenous glucose production observed previously are suggestive that an altered glucagon axis contributes significantly to the metabolic phenotype of GPR120 KO mice (3). At a minimum, our observations suggest that hepatocytes, which do not express GPR120 (2, 5, 34) and data not shown), may be a key integrator of hormonal and metabolic alterations arising from GPR120 signaling in other tissues like colon, adipose, pancreas, and pituitary.
Closing Remarks
Here, we show for the first time that GPR120 may play an important role in islet function, specifically in the regulation of glucagon release. Elevation of glucagon is a central feature in type 2 diabetes contributing to hyperglycemia by driving increased glucose production from the liver. Integration of our observations in islets with respect to the GPR120 KO mouse phenotype requires further investigation and may involve compensatory mechanisms. The development of potent and selective GPR120 synthetic agonists and antagonists should help to better understand the extent of glucose lowering that can be achieved via modulation of hepatic glucose production by GPR120-dependent mechanisms.
Acknowledgments
We thank Austin Bell, Veronica Moreno, Joseph Gunnet, Peter Haug, Hong Hua, Jason Clapper, Wilmelenne Columna, Keith Demarest, and members of the Janssen CVM therapeutic area for their support of these studies.
Footnotes
- GPCR
- G-protein-coupled receptor
- LCFA
- long chain fatty acid
- IPGTT
- intraperitoneal glucose tolerance test
- ITT
- insulin tolerance test
- GSIS
- glucose-stimulated insulin secretion
- DHA
- docosahexaenoic acid
- NCD
- normal chow diet
- HFD
- high fat diet
- KO
- knockout.
REFERENCES
- 1. Kebede M. A., Alquier T., Latour M. G., Poitout V. (2009) Lipid receptors and islet function: therapeutic implications? Diabetes Obes. Metab. 11, 10–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Regard J. B., Sato I. T., Coughlin S. R. (2008) Anatomical profiling of G protein-coupled receptor expression. Cell 135, 561–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oh D. Y., Talukdar S., Bae E. J., Imamura T., Morinaga H., Fan W., Li P., Lu W. J., Watkins S. M., Olefsky J. M. (2010) GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 142, 687–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taneera J., Lang S., Sharma A., Fadista J., Zhou Y., Ahlqvist E., Jonsson A., Lyssenko V., Vikman P., Hansson O., Parikh H., Korsgren O., Soni A., Krus U., Zhang E., Jing X. J., Esguerra J. L., Wollheim C. B., Salehi A., Rosengren A., Renström E., Groop L. (2012) A systems genetics approach identifies genes and pathways for type 2 diabetes in human islets. Cell Metab. 16, 122–134 [DOI] [PubMed] [Google Scholar]
- 5. Hirasawa A., Tsumaya K., Awaji T., Katsuma S., Adachi T., Yamada M., Sugimoto Y., Miyazaki S., Tsujimoto G. (2005) Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat. Med. 11, 90–94 [DOI] [PubMed] [Google Scholar]
- 6. Drucker D. J. (2013) Incretin action in the pancreas: Potential promise, possible perils, and pathological pitfalls. Diabetes 62, 3316–3323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ichimura A., Hirasawa A., Poulain-Godefroy O., Bonnefond A., Hara T., Yengo L., Kimura I., Leloire A., Liu N., Iida K., Choquet H., Besnard P., Lecoeur C., Vivequin S., Ayukawa K., Takeuchi M., Ozawa K., Tauber M., Maffeis C., Morandi A., Buzzetti R., Elliott P., Pouta A., Jarvelin M. R., Körner A., Kiess W., Pigeyre M., Caiazzo R., Van Hul W., Van Gaal L., Horber F., Balkau B., Lévy-Marchal C., Rouskas K., Kouvatsi A., Hebebrand J., Hinney A., Scherag A., Pattou F., Meyre D., Koshimizu T. A., Wolowczuk I., Tsujimoto G., Froguel P. (2012) Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature 483, 350–354 [DOI] [PubMed] [Google Scholar]
- 8. Morgan N. G., Dhayal S. (2009) G-protein coupled receptors mediating long chain fatty acid signalling in the pancreatic β-cell. Biochem. Pharmacol. 78, 1419–1427 [DOI] [PubMed] [Google Scholar]
- 9. Hong J., Abudula R., Chen J., Jeppesen P. B., Dyrskog S. E., Xiao J., Colombo M., Hermansen K. (2005) The short-term effect of fatty acids on glucagon secretion is influenced by their chain length, spatial configuration, and degree of unsaturation: studies in vitro. Metabolism 54, 1329–1336 [DOI] [PubMed] [Google Scholar]
- 10. Alcázar O., Qiu-yue Z., Giné E., Tamarit-Rodriguez J. (1997) Stimulation of islet protein kinase C translocation by palmitate requires metabolism of the fatty acid. Diabetes 46, 1153–1158 [DOI] [PubMed] [Google Scholar]
- 11. Latour M. G., Alquier T., Oseid E., Tremblay C., Jetton T. L., Luo J., Lin D. C., Poitout V. (2007) GPR40 is necessary but not sufficient for fatty acid stimulation of insulin secretion in vivo. Diabetes 56, 1087–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Warnotte C., Gilon P., Nenquin M., Henquin J. C. (1994) Mechanisms of the stimulation of insulin release by saturated fatty acids. A study of palmitate effects in mouse β-cells. Diabetes 43, 703–711 [DOI] [PubMed] [Google Scholar]
- 13. Steneberg P., Rubins N., Bartoov-Shifman R., Walker M. D., Edlund H. (2005) The FFA receptor GPR40 links hyperinsulinemia, hepatic steatosis, and impaired glucose homeostasis in mouse. Cell Metab. 1, 245–258 [DOI] [PubMed] [Google Scholar]
- 14. Salehi A., Flodgren E., Nilsson N. E., Jimenez-Feltstrom J., Miyazaki J., Owman C., Olde B. (2005) Free fatty acid receptor 1 (FFA(1)R/GPR40) and its involvement in fatty-acid-stimulated insulin secretion. Cell Tissue Res. 322, 207–215 [DOI] [PubMed] [Google Scholar]
- 15. Kebede M., Alquier T., Latour M. G., Semache M., Tremblay C., Poitout V. (2008) The fatty acid receptor GPR40 plays a role in insulin secretion in vivo after high-fat feeding. Diabetes 57, 2432–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burant C. F., Viswanathan P., Marcinak J., Cao C., Vakilynejad M., Xie B., Leifke E. (2012) TAK-875 versus placebo or glimepiride in type 2 diabetes mellitus: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 379, 1403–1411 [DOI] [PubMed] [Google Scholar]
- 17. Rezania A., Riedel M. J., Wideman R. D., Karanu F., Ao Z., Warnock G. L., Kieffer T. J. (2011) Production of functional glucagon-secreting alpha-cells from human embryonic stem cells. Diabetes 60, 239–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Suckow A. T., Comoletti D., Waldrop M. A., Mosedale M., Egodage S., Taylor P., Chessler S. D. (2008) Expression of neurexin, neuroligin, and their cytoplasmic binding partners in the pancreatic β-cells and the involvement of neuroligin in insulin secretion. Endocrinology 149, 6006–6017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Watson S. J., Brown A. J., Holliday N. D. (2012) Differential signaling by splice variants of the human free fatty acid receptor GPR120. Mol. Pharmacol. 81, 631–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang C., Suckow A. T., Chessler S. D. Altered pancreatic islet function and morphology in mice lacking the β-cell surface protein neuroligin-2. PLoS One 8, e65711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ramnanan C. J., Edgerton D. S., Kraft G., Cherrington A. D. (2011) Physiologic action of glucagon on liver glucose metabolism. Diabetes Obes. Metab. 13, 118–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matsumura S., Eguchi A., Mizushige T., Kitabayashi N., Tsuzuki S., Inoue K., Fushiki T. (2009) Colocalization of GPR120 with phospholipase-Cβ2 and α-gustducin in the taste bud cells in mice. Neurosci. Lett. 450, 186–190 [DOI] [PubMed] [Google Scholar]
- 23. Kharouta M., Miller K., Kim A., Wojcik P., Kilimnik G., Dey A., Steiner D. F., Hara M. (2009) No mantle formation in rodent islets –the prototype of islet revisited. Diabetes Res. Clin. Pract. 85, 252–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Briscoe C. P., Peat A. J., McKeown S. C., Corbett D. F., Goetz A. S., Littleton T. R., McCoy D. C., Kenakin T. P., Andrews J. L., Ammala C., Fornwald J. A., Ignar D. M., Jenkinson S. (2006) Pharmacological regulation of insulin secretion in MIN6 cells through the fatty acid receptor GPR40: identification of agonist and antagonist small molecules. Br. J. Pharmacol. 148, 619–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Olofsson C. S., Salehi A., Göpel S. O., Holm C., Rorsman P. (2004) Palmitate stimulation of glucagon secretion in mouse pancreatic alpha-cells results from activation of L-type calcium channels and elevation of cytoplasmic calcium. Diabetes 53, 2836–2843 [DOI] [PubMed] [Google Scholar]
- 26. Yashiro H., Tsujihata Y., Takeuchi K., Hazama M., Johnson P. R., Rorsman P. (2012) The effects of TAK-875, a selective G protein-coupled receptor 40/free fatty acid 1 agonist, on insulin and glucagon secretion in isolated rat and human islets. J. Pharmacol. Exp. Therap. 340, 483–489 [DOI] [PubMed] [Google Scholar]
- 27. Luo J., Swaminath G., Brown S. P., Zhang J., Guo Q., Chen M., Nguyen K., Tran T., Miao L., Dransfield P. J., Vimolratana M., Houze J. B., Wong S., Toteva M., Shan B., Li F., Zhuang R., Lin D. C. (2012) A potent class of GPR40 full agonists engages the enteroinsular axis to promote glucose control in rodents. PLoS One 7, e46300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Holland W. L., Bikman B. T., Wang L. P., Yuguang G., Sargent K. M., Bulchand S., Knotts T. A., Shui G., Clegg D. J., Wenk M. R., Pagliassotti M. J., Scherer P. E., Summers S. A. (2011) Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J. Clin. Investig. 121, 1858–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Flodgren E., Olde B., Meidute-Abaraviciene S., Winzell M. S., Ahrén B., Salehi A. (2007) GPR40 is expressed in glucagon producing cells and affects glucagon secretion. Biochem. Biophys. Res. Commun. 354, 240–245 [DOI] [PubMed] [Google Scholar]
- 30. Wang L., Zhao Y., Gui B., Fu R., Ma F., Yu J., Qu P., Dong L., Chen C. (2011) Acute stimulation of glucagon secretion by linoleic acid results from GPR40 activation and [Ca2+]i increase in pancreatic islet {alpha}-cells. J. Endocrinol. 210, 173–179 [DOI] [PubMed] [Google Scholar]
- 31. Cartoni C., Yasumatsu K., Ohkuri T., Shigemura N., Yoshida R., Godinot N., le Coutre J., Ninomiya Y., Damak S. (2010) Taste preference for fatty acids is mediated by GPR40 and GPR120. J. Neurosci. 30, 8376–8382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lan H., Hoos L. M., Liu L., Tetzloff G., Hu W., Abbondanzo S. J., Vassileva G., Gustafson E. L., Hedrick J. A., Davis H. R. (2008) Lack of FFAR1/GPR40 does not protect mice from high-fat diet-induced metabolic disease. Diabetes 57, 2999–3006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. De Marinis Y. Z., Salehi A., Ward C. E., Zhang Q., Abdulkader F., Bengtsson M., Braha O., Braun M., Ramracheya R., Amisten S., Habib A. M., Moritoh Y., Zhang E., Reimann F., Rosengren A. H., Shibasaki T., Gribble F., Renström E., Seino S., Eliasson L., Rorsman P. (2010) GLP-1 inhibits and adrenaline stimulates glucagon release by differential modulation of N- and L-type Ca2+ channel-dependent exocytosis. Cell Metab. 11, 543–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Raptis D. A., Limani P., Jang J. H., Ungethüm U., Tschuor C., Graf R., Humar B., Clavien P. A. (2014) GPR120 on Kupffer cells mediates hepatoprotective effects of omega3-fatty acids. J. Hepatol. 60, 625–632 [DOI] [PubMed] [Google Scholar]