Abstract
Introduction and hypothesis
This study describes a technique to quantify muscle fascicle directions in the levator ani (LA) and tests the null hypothesis that the in vivo fascicle directions for each LA subdivision subtend the same parasagittal angle relative to a horizontal reference axis.
Methods
Visible muscle fascicle direction in the each of the three LA muscle subdivisions, the pubovisceral (PVM; synonymous with pubococcygeal), puborectal (PRM), and iliococcygeal (ICM) muscles, as well as the external anal sphincter (EAS), were measured on 3-T sagittal MRI images in a convenience sample of 14 healthy women in whom muscle fascicles were visible. Mean ± standard deviation (SD) angle values relative to the horizontal were calculated for each muscle subdivision. Repeated measures ANOVA and post-hoc paired t tests were used to compare muscle groups.
Results
Pubovisceral muscle fiber inclination was 41±8.0°, PRM was −19±10.1°, ICM was 33±8.8°, and EAS was −43±6.4°. These fascicle directions were statistically different (p<0.001). Pairwise comparisons among levator subdivisions showed angle differences of 60° between PVM and PRM, and 52° between ICM and PRM. An 84° difference existed between PVM and EAS. The smallest angle difference between levator divisions was between PVM and ICM 8°. The difference between PRM and EAS was 24°. All pairwise comparisons were significant (p<0.001).
Conclusions
The null hypothesis that muscle fascicle inclinations are similar in the three subdivisions of the levator ani and the external anal sphincter was rejected. The largest difference in levator subdivision inclination, 60°, was found between the PVM and PRM.
Keywords: Muscle fascicle, MRI, Levator ani, External anal sphincter, Female, Anatomy
Introduction
The importance of the levator ani (LA) for pelvic organ support is well established [1, 2]: for example, injury to the LA muscle is seen in 55 % of women with prolapse, yet only in 16 % of women with normal support [1]. The LA has three subdivisions: pubovisceral (also known as pubococcygeal), puborectal, and iliococcygeal [3, 4]. Injury to the pubovisceral subdivision is associated with prolapse, but the puborectal subdivision is not [5, 6]. Our understanding of the mechanical effects that result from loss of the pubovisceral muscle, and why that is linked to the development of prolapse, is presently poor. In part this because there is little information on the differences in morphology between the levator subdivisions.
The mechanical effect of muscle contraction for a muscle such as the LA is determined by the line-of-action of its fibers1 and their pennation angle (if any), their degree of activation, and physiological cross-sectional area [7]. Therefore, the functional consequence of LA muscle injury depends on the region of muscle affected. Data concerning the muscle line-of-action are lacking for the LA muscle and specifically for each of its three subdivisions.
High-resolution magnetic resonance imaging (MRI) allows a detailed view of the LA muscle in living women [8–10]. Recently, diffusion tensor imaging (DTI) has allowed visualization of fiber direction in certain portions of the levator [11–13]. However, at present only some regions of the muscle have yielded analyzable tracts and specific angles have not been determined.
Two functional characteristics of the LA muscle have been described: its ability to lift (vertically in the standing posture) the pelvic organs, and its action in squeezing the levator hiatus closed (horizontally in the standing position) [14, 15]. It is unclear, at present, how much each component of the levator contributes to each of these two actions. The goal of this study was to measure the fiber direction in the three subdivisions of the LA muscle, as well as that of the adjacent external anal sphincter, in living women. We tested the null hypothesis that there is no significant difference in mean muscle fiber angle among LA subdivisions.
Materials and methods
Image acquisition
A convenience sample of pelvic floor MRIs was selected for this research project. To be included, women had to have normal pelvic organ support on examination and there could not be significant LA muscle damage, as determined by review of the scans. Scans were drawn from two IRB-approved studies (Evaluating Maternal Recovery from Labor and Delivery; EMRLD, IRB 2005-0011 and Organ Prolapse And Levator; OPAL2 study, IRB 1999-0395). The EMRLD study evaluated women at approximately 7 weeks and 7 months after vaginal delivery to study LA muscle injury and the 7-month images of a low-risk for injury comparison group were used. The OPAL2 project was a case–control study evaluating structural changes involved in anterior vaginal wall support. The scans selected for this project were from the women recruited as controls.
The scans of a total of 34 women were reviewed for inclusion. All of these women had been noted to have clearly visible muscle anatomy. Of these 34 women, 14 had sufficient fiber visibility on the sagittal images to allow fiber direction to be measured in all three subdivisions of the LA muscle and the external anal sphincter (Fig. 1). Images from the sagittal plane were selected because they most closely parallel the line-of-action of the levator fibers that run predominantly in a ventral–caudal direction.
Fig. 1.
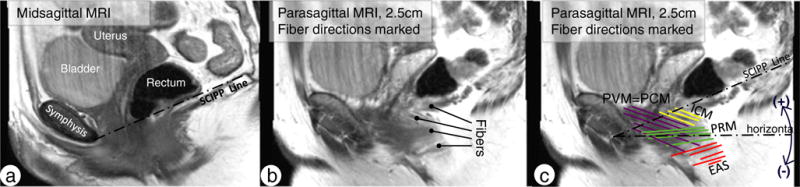
a Midsagittal MRI view of the muscles from the left side of the pelvis. The sacrococcygeal inferior pubic point (SCIPP) line is drawn in the midsagittal plane and transposed to all parasagittal slides. b Fibers are demonstrated (lines with round tips) on a parasagittal slide. c Fiber directions were marked and evaluated in respect of the individual SCIPP line and expressed as the angle to the average horizontal line, which is 34° below the SCIPP line with the Pelvic Inclination Correction System (PICS) system. Fiber orientations subtending an angle clockwise to the horizontal line have a negative sign; those with an angle counter-clockwise to the horizontal line have a positive sign. PVM pubovisceral muscle, PCM pubococcygeal muscle, ICM iliococcygeus muscle, PRM puborectal muscle, EAS external anal sphincter muscle
Full details of the MRI acquisitions have been previously published [16, 17].
Image analysis and fiber angle measurement
Sequential sagittal images between the ischial spines, which mark the lateral-most extent of the levator, were evaluated by the first author (C.B.) and reviewed by the senior author (J.O.L.D.) to confirm the absence of significant muscle injury and adequate fiber visibility. There was an average of 12 sagittal images (range 9–17) containing visible fibers for each woman. The mid-sagittal image was used to establish the sacrococcygeal inferior pubic point line (SCIPP), but not for fiber mapping because it is not parallel to the muscle fibers in that location. The images with visible muscle fibers were imported into PowerPoint and a straight line was placed along representative fibers following their visible direction (Fig. 1c). Muscle fibers were color-coded according to the three Terminologia Anatomica-listed major subdivisions of the LA muscle, namely in the pubovisceral (or pubococcygeal) muscle (PVM), the puborectal muscle (PRM), and the iliococcygeal muscle (ICM), as well as the external anal sphincter (EAS), based on our prior work identifying these subdivisions on MRI images [10, 18]. The EAS, a pelvic floor muscle that is not part of the LA, was fiber coded, as its fibers were clearly visible on MRI.
The Terminologia Anatomica-listed subdivisions are named according to their origin–insertion pair combination, which facilitated the logic of the fiber tracing.
Information regarding whether they came from the left or right side of the pelvis was also noted.
The Pelvic Inclination Correction System (PICS) was used as an axis system to record LA fiber angles [19] relative to the horizontal in the sagittal plane when standing. This system accounts for differences in how the pelvis might be orientated in the scanner and allows angles relative to the longitudinal axis of the body to be established. The horizontal axis was designated as representing zero degrees, with the origin at the inferior pubic point. The angles of the lines that had been placed on the individual sagittal images were determined using Matlab (Version R2012a; MathWorks, Natick, MA, USA) relative to the sacrococcygeal line and then transformed to the PICS coordinate system. The line-of-action of each individual fiber will be referred to as “fiber angle”. Angles above the horizontal line were assigned a positive sign (counterclockwise) and those below were assigned a negative sign (clockwise).
Data management and statistics
For the demographics, descriptive statistics were calculated, including the mean and standard deviation (SD) for the normally distributed data, with the median and range (minimum to maximum) being used instead for non-normally distributed data.
To determine the direction in which the muscle fibers run in each muscle subdivision for each woman, we took the mean of the directions of individual fibers sampled within the muscle subdivisions for each individual. This will be referred to as the “subdivision angle” to distinguish it from “fiber angle,” as previously defined. Prior to data reduction, variation in the fiber angle within each muscle subdivision was evaluated per person by descriptive statistics. Fiber directions were normally distributed. The subdivision angles for the 14 subjects were expressed as means and SD.
To see if the left and right sides had muscle directions and could be combined, we examined the difference in fiber angles between the two sides for each subdivision. We made the arbitrary assumption that a difference below 10° would not be mechanically significant. For each subdivision, the difference was below this value and so the values for the fiber angles for the two sides of each subdivision were combined for analysis. We chose this level because the difference in force developed when muscle fibers contracting with unit force is compared with a second set of identical and similarly contracting fibers oriented at an angle of 10° to the first set differs by 1.5 %, a value we judged to be physiologically trivial in this context.
Comparisons of the subdivision angles were made using repeated measures ANOVA (Greenhouse–Geisser test). Statistical analysis was performed using IBM SPSS Statistics 20 (SPSS, Chicago, IL, USA). In other comparisons a p value of less than 0.05 was considered statistically significant. For multiple pairwise comparisons between individual subdivisions a post-hoc paired t test with Bonferroni correction indicated that a p value of ≤0.008 would be appropriate.
Results
The women in the study had a mean age ± SD of 35.9±11.4 years, BMI 25.4±3.9 kg/m2, a median parity of 1 (range 1–4), and median of 1 vaginal delivery (0–3). Thirteen women were Caucasian and 1 woman was Asian. None of the women had undergone hysterectomy.
The mean numbers of subdivision fibers that were sampled in each woman were as follows: for the PVM, 52±3.2 (SD); PRM, 20±2.2; ICM, 54±3; and the EAS, 16±1.5. The range of subdivision angles for the ICM and PRM was 29° and for the PVM was 28°, while the EAS was 24° (Table 1). Mean angle differences between the right and left sides for the PVM were 4.1°; for the PRM, 6.2°; the ICM, 1.9°; and the EAS 0.01°.
Table 1.
Mean (SD) subdivision angles (in degrees) for the pubovisceral muscle (PVM); puborectal muscle (PRM); iliococcygeus muscle (ICM); external anal sphincter muscle (EAS)
| PVM | PRM | ICM | EAS | |
|---|---|---|---|---|
| Mean | 40.7° | −19.0° | 33.1° | −42.9° |
| SD | 8.0° | 10.1° | 8.8° | 6.4° |
| Minimum | 27° | −4° | 18° | −32° |
| Maximum | 55° | −33° | 47° | −56° |
| Range minimum to maximum | 28° | 29° | 29° | 24° |
The mean (SD) subdivision angles for the four muscle groups are reported in Table 1 and shown graphically in Fig. 2. The subdivision angles in the PVM, PRM, ICM, and EAS muscle were significantly different (p<0.001, ANOVA); thus, the null hypothesis was rejected. The muscle subdivision angles relative to the horizontal ranged from 41° for the PVM to −43° for the EAS. Pairwise comparisons of the mean difference in subdivision angles between the PVM and the PRM was 60° (p<0.001), between the ICM and the PRM it was 52° (p<0.001), and between the PVM and the EAS it was 84° (p<0.001). The smallest mean angle differences were found between the PVM and ICM 8° (p<0.001) and PRM and EAS 24° (p<0.001).
Fig. 2.
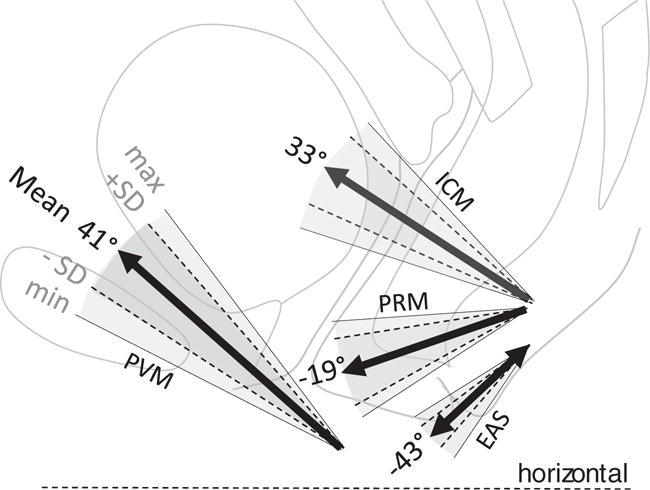
The thick arrow displays the mean direction to the horizontal line in a two-dimensional graphic. The dashed line is the horizontal line from which the angles are measured. Angles above the horizontal line have a “+” sign and those below the horizontal line a “−” sign. On MRI, the PVM was found medial to the PRM; for graphical reasons their lines of actions are depicted in the same plane
Discussion
The angle at which an individual muscle fiber shortens establishes the line of action for the muscle [6]. The large difference in mean fiber angle, 60°, between the PVM and the PRM, suggests that they might have two different mechanical actions. These can be understood within the context of the standing position (Fig. 3). The first action is to develop a “closing” force, acting in a horizontal direction so as to close the levator hiatus, thereby creating a vaginal high pressure zone [15, 20]. The second action involves the generation of a vertically oriented “lifting” force, acting on the perineal tissues and pelvic organs against the action of gravity [14, 21]. For both muscles, note that the horizontal closing vector is larger than the vector acting in the vertical plane. In the latter, the PVM has a substantial lifting component while the small PRM component actually acts in a downward (caudal) direction so that it has no “lifting” action. This would indicate that both the PVM and PRM contribute to hiatal closure, but only the PVM contributes to perineal elevation in the normal woman. The third part of the LA, the ICM, has a direction similar to that of the PVM. The justification for considering it separate from the PVM is because it is inserted differently. The EAS, although not part of the LA, is attached to the anococcygeal ligament; thus, there may be some movement in that direction in addition to its constrictive effects.
Fig. 3.
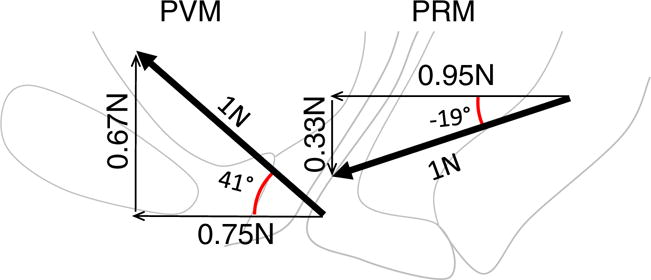
Horizontal and vertical components of the PVM and PRM in the standing position. The thick arrows show the average direction of the lines of action of the PVM and PRM muscles relative to the horizontal with a theoretical 1 N force. Thin lines indicate the portion of each force related to a closing and lifting function. (Note: vectors are shown larger than the background anatomy to avoid an overlap in the display)
The attachment points of these muscle fibers often overlap [4] and so the resulting movement of structures will depend on the interaction of the different muscles. Additionally, the fibers within the muscle subdivisions themselves are not entirely parallel. For example, the PRM showed a range of fiber directions of 29° and the action is a summation of the action of the component fibers that should occur along the mean. Of course, the topography of the pelvic floor changes with pelvic organ prolapse and LA injury [14], and so further research will be needed to determine the action vectors in these specific situations.
These data help inform a discussion of the mechanical consequences of LA muscle injury after vaginal delivery. Since this injury involves the pubovisceral, but not the puborectal muscle component of the muscle [6], our results suggest that the injury would reduce both the vertical “lifting” component of the LA muscle force, as well as the hiatal constriction effect. The action of the puborectal muscle is mainly a constricting effect with no elevating effect. Loss of pubovisceral muscle, therefore, would directly affect lifting. This may be mitigated by the fact that the ICM may remain intact with PVM injury, thereby allowing some elevation, although not as much as with an intact PVM. The loss of elevating forces is consistent with the finding that perineal structures are lower in women with PVM injury even in the absence of prolapse [14]. Loss of its constricting component might be lessened by the action of the remaining PRM. In fact, hypertrophy is seen in the PRM portion of the levator that decussates behind the rectum in women with PVM injury [22]. This suggests compensatory hypertrophy that could somewhat mitigate the loss of levator closure, but not the loss of elevatory function.
This study adds quantitative information to the qualitative information already established about LA muscle anatomy. The three muscle components recognized by Terminologia Anatomica [3], pubococcygeal (or pubovisceral), puborectal, and iliococcygeal, are widely accepted, despite the wide variation in terminology used by individual investigators to describe this anatomy [23]. Dissections and histology in fetal and newborn tissues have provided excellent detail on the overall architecture of the muscle [4, 24]. In addition, Janda et al. have completed in vitro mapping of the LA muscle for the purposes of computer modeling [25]. This detailed work, however, was performed on cadaver material, which is known to have an altered shape owing to both the loss of muscle tone and, importantly, the large abdominal pressures generated during embalming [26]. By quantifying the direction of the fibers in living women with a physiological muscle tone, the present study provides quantitative data on the lines of action of these muscle subdivisions that have not been available hitherto.
The directions of muscle fibers have also been demonstrated using the fiber tracking capabilities of diffusion tensor imaging [11, 13]. This technique holds great potential for mapping levator fibers. At present, however, it has not been possible to adequately establish fiber angles throughout the three muscle divisions. It is also possible that as the resolution of 3D pelvic floor ultrasound improves the mapping of fiber directions may be enabled with use of this technique. To date, endoanal MR imaging showed sex- and age-related variations in the length and thickness of the anal sphincter and PRM [27].
Several limitations of our study should be kept in mind while interpreting the results. First, this study was based on a small convenience sample of healthy women with easily visible muscle fibers. It is a weakness of MRI that less than half of the screened patients (14 out of 34) showed sufficient image quality to trace fibers.
Second, in the future it would be of interest to classify muscle fiber orientations for a specific age group, parity status, race/ethnic groups because there are known racial differences in LA muscle [28]. Additionally, muscle fiber angle comparison between multiparous women with no evidence of LA injury and nulliparous women would be of value as there might be some differences induced by pregnancy or labor. Third, changes in muscle fiber directions that occur with injury and/or defects in specific portions of the LA need to be studied in the presence and absence of prolapse. Because data concerning the mean and standard deviation of muscle fiber directions in the levator subdivisions were not available when this project started, no power calculation was possible at the beginning of the study. However, this does not affect the statistically significant differences we found.
Magnetic resonance imaging examination of muscle has its inherent limitations. Conventional MRI is not the appropriate method for measuring fiber curvature because the data are discontinuous and censored by the sequential nature of the images being taken at 5-mm intervals. This raises the issue of the quality of fiber tracking. This issue was found in an MRI study of the soleus muscle in which the multipennate fiber pattern, often oriented oblique to the slice, as well as the volume averaging of capillary structures, decreased the accuracy of fiber orientation measurements [29]. The sheet-like nature of the LA muscles indicates that this should not be a major factor affecting our results. Similar issues have been found with DTI, where the multipennate fiber pattern decreases the accuracy of fiber orientation measurements [13]. However, these discrepancies are relatively small and would not be responsible for the large differences found in our study. Finally, for the present study, we were interested in the morphology of normal muscle whereby the effect of the two sides contracting simultaneously are resolved to a single force in the midsagittal plane. In the future, however, if muscles with a unilateral defect are studied [30], resolving to a single force may not be valid.
These results provide quantitative data to help understand different lifting and constricting effects of individual subdivisions of the LA muscle. The distinct difference in orientation between the PVM and the PRM as well as that between the ICM and the PRM supports consideration of the LA as three distinct regions, with different names and functions. The PVM and ICM have similar fiber directions and their different anatomy is justified by their different insertions. Knowing the angles at which these muscle divisions act can now be used to evaluate the mechanical effects of losing one or more portions of the levator ani.
Acknowledgments
We gratefully acknowledge the intellectual contribution of Catherine Brandon, MD, Musculoskeletal Division, Department of Radiology, University of Michigan, and her assistance in obtaining the high-quality MR images for this study, and Prof. Burkhardt Seifert, Division of Biostatistics, University of Zurich, for his contribution to the data analysis.
Funding EMRLD (Evaluating Maternal Recovery from Labor & Delivery) funded by NIH ORWH SCOR P50 HD044406, OPAL II funded by RO1 HD 38665; grant to CB from the Swiss National Science Foundation.
Footnotes
In the rest of this paper we use the term muscle “fiber” instead of muscle fascicle because the term muscle “fiber” is more commonly used in the clinical literature. Given the assumption that the direction of the fiber is collinear with its fascicle as visualized on MRI this seems acceptable.
Institutional review board approval IRB University of Michigan 2005-0011 (EMRLD) and IRB 1999-0395.
Conflicts of interest None.
Contributor Information
Cornelia Betschart, Email: cornelia.betschart@gmx.ch, Department of Obstetrics and Gynecology, University of Michigan, Ann Arbor, MI, USA, Department of Obstetrics and Gynecology, University of Zurich, Frauenklinikstrasse 10, 8091 Zurich, Switzerland.
Jinyong Kim, Department of Mechanical Engineering, University of Michigan, Ann Arbor, MI, USA.
Janis M. Miller, Department of Obstetrics and Gynecology, University of Michigan, Ann Arbor, MI, USA, School of Nursing, University of Michigan, Ann Arbor, MI, USA
James A. Ashton-Miller, Department of Mechanical Engineering, University of Michigan, Ann Arbor, MI, USA
John O. L. DeLancey, Department of Obstetrics and Gynecology, University of Michigan, Ann Arbor, MI, USA
References
- 1.DeLancey JO, Morgan DM, Fenner DE, Kearney R, Guire K, Miller JM, Hussain H, Umek W, Hsu Y, Ashton-Miller JA. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet Gynecol. 2007;109(2 Pt 1):295–302. doi: 10.1097/01.AOG.0000250901.57095.ba. [DOI] [PubMed] [Google Scholar]
- 2.Dietz HP, Simpson JM. Levator trauma is associated with pelvic organ prolapse. BJOG. 2008;115(8):979–984. doi: 10.1111/j.1471-0528.2008.01751.x. [DOI] [PubMed] [Google Scholar]
- 3.Terminologia Anatomica: International Anatomical Terminology. Stuttgart: Thieme; 1998. http://www.unifr.ch/ifaa/ [Google Scholar]
- 4.Lawson J. Pelvic anatomy. I. Pelvic floor muscles. Ann R Coll Surg Engl. 1974;54:244–252. [PMC free article] [PubMed] [Google Scholar]
- 5.Margulies RU, Huebner M, DeLancey JO. Origin and insertion points involved in levator ani muscle defects. Am J Obstet Gynecol. 2007;196:251–255. doi: 10.1016/j.ajog.2006.10.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeLancey JO, Sørensen HC, Lewicky-Gaupp C, Smith TM. Comparison of the puborectal muscle on MRI in women with POP and levator ani defects with those with normal support and no defect. Int Urogynecol J. 2012;23(1):73–77. doi: 10.1007/s00192-011-1527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacIntosh BR, Gardiner PF, McComas AJ. Skeletal muscle: form and function. Human Kinetics; Champaign, IL: 2006. Muscle architecture and muscle fiber anatomy; Chapter 11: Muscle contraction; pp. 151–174.pp. 3–21. [Google Scholar]
- 8.Law YM, Fielding JR. MRI of pelvic floor dysfunction: review. AJR Am J Roentgenol. 2008;191(6 Suppl):S45–S53. doi: 10.2214/AJR.07.7096. [DOI] [PubMed] [Google Scholar]
- 9.Morris VC, Murray MP, DeLancey JO, Ashton-Miller JA. A comparison of the effect of age on levator ani and obturator internus muscle cross-sectional areas and volumes in nulliparous women. Neurourol Urodyn. 2012;31(4):481–486. doi: 10.1002/nau.21208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Margulies RU, Hsu Y, Kearney R, Stein T, Umek WH, DeLancey JO. Appearance of the levator ani muscle subdivisions in magnetic resonance images. Obstet Gynecol. 2006;107(5):1064–1069. doi: 10.1097/01.AOG.0000214952.28605.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zijat FM, Froeling M, van der Paardt MP, Lakeman MME, Bipat S, Montauban van Swijndregt AD, Strijkers GJ, Nederveen AJ, Stoker J. Feasibility of diffusion tensor imaging (DTI) with fibre tractography of the normal female pelvic floor. Eur Radiol. 2011;21:1243–1249. doi: 10.1007/s00330-010-2044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brandão S, Da Roza T, Parente M, Ferreira HA, Mascarenhas T, Ramos I, Jorge RN. Magnetic resonance tractography as a means to evaluate pubovisceral muscle fibers. IUGA; Brisbane: 2012. Abstract 293. [Google Scholar]
- 13.Rousset P, Delmas V, Buy JN, Rahmouni A, Vadrot D, Deux JF. In vivo visualization of the levator ani muscle subdivisions using MR fiber tractography with diffusion tensor imaging. J Anat. 2012;221(3):221–228. doi: 10.1111/j.1469-7580.2012.01538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark NA, Brincat CA, Yousuf AA, DeLancey JO. Levator defects affect perineal position independently of prolapse status. Am J Obstet Gynecol. 2010;203(6):595.e17–595.e22. doi: 10.1016/j.ajog.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guaderrama NM, Nager CW, Liu J, Pretorius DH, Mittal RK. The vaginal pressure profile. Neurourol Urodyn. 2005;24(3):243–247. doi: 10.1002/nau.20112. [DOI] [PubMed] [Google Scholar]
- 16.Miller JM, Brandon C, Jacobson JA, Low LK, Zielinski R, Ashton-Miller J, Delancey JO. MRI findings in patients considered high risk for pelvic floor injury studied serially after vaginal childbirth. AJR Am J Roentgenol. 2010;195(3):786–791. doi: 10.2214/AJR.09.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brandon C, Jacobson J, Low L, Park L, DeLancey JO, Miller J. Pubic bone injuries in primiparous women: magnetic resonance imaging in detection and differential diagnosis of structural injury. Ultrasound Obstet Gynecol. 2012;39(4):444–451. doi: 10.1002/uog.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu Y, Fenner DE, Weadock WJ, DeLancey JO. Magnetic resonance imaging and 3-dimensional analysis of external anal sphincter anatomy. Obstet Gynecol. 2005;106(6):1259–1265. doi: 10.1097/01.AOG.0000189084.82449.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Betschart C, Chen L, Ashton-Miller JA, DeLancey JO. On pelvic reference lines and the MR evaluation of genital prolapse: a proposal for standardization using the Pelvic Inclination Correction System. Int Urogynecol J. 2013;24(9):1421–1428. doi: 10.1007/s00192-013-2100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raizada V, Bhargava V, Jung SA, Karstens A, Pretorius D, Krysl P, Mittal RK. Dynamic assessment of the vaginal high-pressure zone using high-definition manometery, 3-dimensional ultrasound, and magnetic resonance imaging of the pelvic floor muscles. Am J Obstet Gynecol. 2010;203(2):172.e1–8. doi: 10.1016/j.ajog.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewicky-Gaupp C, Yousuf A, Larson KA, Fenner DE, DeLancey JO. Structural position of the posterior vagina and pelvic floor in women with and without posterior vaginal prolapse. Am J Obstet Gynecol. 2010;202(5):497.e1–497.e6. doi: 10.1016/j.ajog.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu Y, Chen L, Huebner M, Ashton-Miller JA, DeLancey JO. Quantification of levator ani cross-sectional area differences between women with and those without prolapse. Obstet Gynecol. 2006;108(4):879–883. doi: 10.1097/01.AOG.0000233153.75175.34. [DOI] [PubMed] [Google Scholar]
- 23.Kearney R, Sawhney R, DeLancey JO. Levator ani muscle anatomy evaluated by origin-insertion pairs. Obstet Gynecol. 2004;104(1):168–173. doi: 10.1097/01.AOG.0000128906.61529.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts WH, Harrison CW, Mitchell DA, Fischer HF. The levator ani muscle and the nerve supply of its puborectalis component. Clin Anat. 1988;1:256–283. [Google Scholar]
- 25.Janda S, van der Helm FC, de Blok SB. Measuring morphological parameters of the pelvic floor for finite element modeling purposes. J Biomech. 2003;36(6):749–757. doi: 10.1016/s0021-9290(03)00008-3. [DOI] [PubMed] [Google Scholar]
- 26.Richter K. Lebendige Anatomie der Vagina. Geburtshilfe Frauenheilkd. 1966;26:1213–1223. [PubMed] [Google Scholar]
- 27.Rociu E, Stoker J, Eijkemans MJ, Laméris JS. Normal anal sphincter anatomy and age- and sex-related variations at high-spatial-resolution endoanal MR imaging. Radiology. 2000;217(2):395–401. doi: 10.1148/radiology.217.2.r00nv13395. [DOI] [PubMed] [Google Scholar]
- 28.Zacharin RF. “A Chinese anatomy”—the supporting tissues of the Chinese and Occidental female compared and contrasted. Aust N Z J Obstet Gynaecol. 1977;17:1–11. [Google Scholar]
- 29.Hodgson JA, Finni T, Lai AM, Edgerton VR, Sinha S. Influence of structure on the tissue dynamics of the human soleus muscle observed in MRI studies during isometric contractions. J Morphol. 2006;267(5):584–601. doi: 10.1002/jmor.10421. [DOI] [PubMed] [Google Scholar]
- 30.Chen L, Hsu Y, Ashton-Miller JA, DeLancey JO. Measurement of the pubic portion of the levator ani muscle in women with unilateral defects in 3-D models from MR images. Int J Gynecol Obstet. 2006;92(3):234–241. doi: 10.1016/j.ijgo.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


