Abstract
The inactivation of tumor suppressor genes (TSGs) plays a vital role in the progression of human cancers. Nevertheless, those ubiquitous TSGs have been shown with limited roles in various stages of diverse carcinogenesis. Investigation on identifying unique TSG, especially for early stage of carcinogenesis, is imperative. As such, the search for organ-specific TSGs has emerged as a major strategy in cancer research. Prostate cancer (PCa) has the highest incidence in solid tumors in US males. Cellular prostatic acid phosphatase (cPAcP) is a prostate-specific differentiation antigen. Despite intensive studies over the past several decades on PAcP as a PCa biomarker, the role of cPAcP as a PCa-specific tumor suppressor has only recently been emerged and validated. The mechanism underlying the pivotal role of cPAcP as a prostate-specific TSG is, in part, due to its function as a protein tyrosine phosphatase (PTP) as well as a phosphoinositide phosphatase (PIP), an apparent functional homologue to Phosphatase and tensin homolog (PTEN) in PCa cells. This review is focused on discussing the function of this authentic prostate-specific tumor suppressor and the mechanism behind the loss of cPAcP expression leading to prostate carcinogenesis. We review other phosphatases’ roles as TSGs which regulate oncogenic PI3K signaling in PCa and discuss the functional similarity between cPAcP and PTEN in prostate carcinogenesis.
Keywords: cPAcP, tumor suppressor, prostate cancer, protein tyrosine phosphatase, ErbB-2, phosphoinositide phosphatase
1. Introduction
Protein tyrosine phosphorylation is a key event in cellular signaling that drives cell division, proliferation, differentiation and apoptosis [1]. It is essential to maintain normal physiological phosphorylation signaling as deregulation can lead to dysfunction in cell survival and transformation [2]. The dynamic equilibrium of tyrosine phosphorylation and dephosphorylation is maintained respectively by protein tyrosine kinases (PTKs) and protein tyrosine phosphatases (PTP). The spatial distribution and existence of an almost equal number of coding genes for PTKs and PTPs suggest the substrate specificity and importance in maintaining the optimal level of protein phosphorylation [1–3].
Structural, functional and sequence analyses have classified the PTP superfamily enzyme into (i) Class I cys-based PTPs including ‘classical tyrosine-specific’ and ‘dual specificity phosphatases (DSPs)’, (ii) Class II cys-based low molecular weight acid phosphatases, (iii) Class III cys-based CDC25 and (iv) Asp-based PTPs [2–4] and (v) His-based PTPs [5] (Table 1). Further, the ‘classical tyrosine-specific’ PTPs are divided into two subfamilies: the receptor-type PTP (RPTP), primarily localized at transmembrane region, and the nonreceptor-type PTP (NRPTPs), which localized in the cytosol. The majority of the ‘classical’ tyrosine-specific phosphatases have been hypothesized to possess tumor suppressor function. However, data indicate that some of the RPTPs can function as oncogenes, e.g., RPTPA (RPTPα) in prostate cancer (PCa) [6,7]. DSPs can act both as positive and negative regulators of signal transduction pathways based on its specific dephosphorylation function by either activating or inactivating/suppressive kinases [2,3].
Table 1.
Expression of Protein tyrosine phosphatases (PTPs) including dual specificity phosphatases in prostate tissue.
| PTP type | Approved symbol (based on HUGO gene Nomenclature Committee) | |
|---|---|---|
| 1 | Class I Cys-based PTPs | |
| Classical PTP’s | ||
| Transmembrane receptor-like PTPs | PTPRA; PTPRB; PTPRC; PTPRD; PTPRE; PTPRF; PTPRG; PTPRH; PTPRJ; PTPRK; PTPRM; PTPRN2; PTPRO; PTPRQ; PTPRR; PTPRS; PTPRT; PTPRU; PTPRVP. |
|
| Non-receptor PTPs | PTPN1; PTPN2; PTPN3; PTPN6; PTPN7; PTPN9; PTPN11; PTPN12; PTPN13; PTPN14; PTPN18; PTPN20A; PTPN20B; PTPN21; PTPN22; PTPN23. |
|
| Dual specificity phosphatases | ||
| MAP kinase phosphatases | DUSP1; DUSP2; DUSP4; DUSP5; DUSP6; DUSP7; DUSP8; DUSP9; DUSP10; STYXL1. |
|
| Atypical dual specificity phosphatases | DUPD1; DUSP3; DUSP11; DUSP12; DUSP13; DUSP14; DUSP15; DUSP18; DUSP19; DUSP21; DUSP22; DUSP23; DUSP26; DUSP28; EPM2A; PTPMT1; RNGTT; STYX. |
|
| Slingshots | SSH1; SSH2; SSH3. | |
| PRLs | PTP4A1; PTP4A2; PTP4A3 | |
| CDC14s | CDC14A; CDC14B; PTPDC1. | |
| PTENs | PTEN; TENC1; TNS1. | |
| Myotubularins | MTM1; MTMR1; MTMR2; MTMR3; MTMR4; SBF1; MTMR6; MTMR7; MTMR8; MTMR9; MTMR10; MTMR11; MTMR12; SBF2; MTMR14. |
|
| 2. | Class II Cys-based PTPs | ACP1 (Low molecular weight acid phosphatase) |
| 3. | Class III Cys-based PTPs | CDC25A; CDC25B; CDC25C. |
| 4. | Asp-based PTPs | EYA1; EYA2; EYA3; EYA4. |
| 5. | His-based PTPs | PAcP. |
Individual PTPs were classified and their expression in human prostate tissues were checked respectively in http://www.genenames.org and http://biogps.org and based on the published literature [2–5].
Abbreviations: DSPs, dual specificity phosphatases; MTMs, myotubularins; PAcP, prostatic acid phosphatase; PRLs, phosphatase of regenerating liver; PTEN, phosphatase and tensin homolog; PTP, protein tyrosine phosphatase; PTPR, protein tyrosine phosphatase, transmembrane receptor-type.
PCa has the highest incidence of non-cutaneous malignancy and is the second leading cause of cancer-related death in US males [8]. Androgen deprivation therapy (ADT) is still the gold standard for the metastatic PCa [9]. However, most patients fail to respond after a certain period and eventually develop metastatic castration-resistant prostate cancer (mCR PC) for which there is no curative except taxels and PROVENGE which could prolong one’s life span for a few months [10,11]. One of the mechanisms behind the development of castration resistance is the activation of kinases by tyrosine phosphorylation [12,13]. In past, major efforts have been made on investigating Tyr-phosphorylation regulation of kinases in PCa. Recent advances have moved to search for prostate-specific PTPs and revealed approximately one hundred PTPs expressed in prostate tissue (Table 1) [3–5] in addition to eight ubiquitously known tumor suppressor genes (TSGs) (Rb, p53, PTEN, PP2A, PHLPP, APC, BRCA2 and WT1; Table 2) [14–45]. Further analyses of PTP expression and comparison of closely related PTPs have demonstrated their function to maintain prostate cell homeostasis and revealed cellular prostatic acid phosphatase (cPAcP) as a unique prostate-specific TSG. Although secretory PAcP (sPAcP), an isoform of cPAcP protein, has been recognized as a marker to detect PCa, only recently studies have underscored the importance of cPAcP in PCa growth suppression.
Table 2.
Commonly known tumor suppressor gene expression and its major function in prostate epithelia.
| Gene | Loss of function in prostate cancer |
Prostate cancer associated major function |
Reference(s) |
|---|---|---|---|
| RB1 | Functional mutation | Transcriptional regulator E2F binding; coactivator of androgen receptor. | [14–16] |
| p53 | Functional mutation | Transcription factor; regulates cell cycle and apoptosis. | [17–19] |
| PTEN | Homozygous deletions, loss of heterozygosity (LOH), and inactivating mutations. | Regulation of PI3K/Akt signaling, MAPK activation by EGFR, cell proliferation and apoptosis, and VEGF mediated angiogenesis. | [20–25] |
| PAcP | Decreased expression | Regulates HER-2 and PI3K/Akt signaling. | [12,26–29] |
| PP2A | Decreased expression | Regualtes PI3K/Akt, β-catenin, and FAK signalling. | [30–34] |
| PHLPP | Loss of heterozygosity (LOH) | Regulates PI3K/Akt signaling. | [35–37] |
| APC | Methylation | Regulating β-catenin protein in cytosol. | [38–40] |
| BRCA2 | Mutation | DNA repair; complexes with Rad 51 and other BRCA gene. | [41,42] |
| WT1 | Function mutation; Tumor suppressor activity | Transcription factor. | [43–45] |
Abbreviations: APC, adenomatous polyposis coli; BRCA2, breast cancer 2; EGFR, epidermal growth factor receptor; FAK, focal adhesion kinase; HER-2, human epidermal growth factor receptor 2; MAPK, mitogen-activated protein kinase; p53, phosphoprotein p53; PAcP, prostatic acid phosphatase; PHLPP, PH domain and leucine rich repeat protein phosphatases; PI3K, phosphoinositide 3-kinase; PP2A, protein phosphatase 2A; PTEN, phosphatase and tensin homolog; RB1, retinoblastoma 1; WT1, Wilms tumor 1; VEGF, vascular endothelial growth factor.
The loss of cPAcP has been shown to be an early event in PCa. Understanding of consequences of the early loss of cPAcP in PCa cells will reveal a mechanism underlying the development of PCa and its progression. In this review, we focus on cPAcP, a classically known prostate-specific differentiation antigen, as a prostate-specific tumor suppressor. We emphasize the recent developments in understanding the mechanistic role of cPAcP as a PTP as well as phosphoinositide phosphatase (PIP). We further overview the central function of several other phosphatases as TSGs which regulate phosphoinositide 3-kinase (PI3K)/Akt signaling in PCa. We highlight the significance of cPAcP as a prostate-specific tumor suppressor.
2. Prostatic acid phosphatase
PAcP (E.C.3.1.3.2) is a member of the acid phosphatase superfamily, which hydrolyzes a variety of small organic phosphomonoesters in acidic conditions within the range of pH 4–6 [46–48]. PAcP has been shown to have high levels of expression in normal adult prostate epithelia [49,50]. Recent studies validate very low levels of PAcP expression (less than 2%) in several other non-prostatic tissues by quantitative real-time polymerase chain reaction (qRT-PCR) [51,52].
Prior to puberty, PAcP is expressed at low levels. In normal, well differentiated adult prostate epithelia, PAcP protein is present at a very high level, up to 0.5 mg/gm wet tissue, correlating with decreased cell growth. Hence it has been proposed that cellular PAcP (cPAcP) can be involved in normal prostate cell growth regulation. Since Gutman and colleagues observed elevated PAcP activity in the circulation of PCa patients with bone metastasis, vast clinical studies had provided valuable insight on PAcP as a PCa biomarker until the discovery of prostate-specific antigen (PSA) [53–57]. It should be noted that despite the elevation of circulating PAcP, cellular levels of PAcP (cPAcP) decrease and inversely correlate with PCa progression. Recent advances in functional and mechanistic studies reveal cPAcP’s specific role in maintaining prostate cell homeostasis. Novel substrates and their interactions with cPAcP have been identified by depleting or mutating cPAcP protein. Together these outcomes support the notion that cPAcP functions as a prostate-specific tumor suppressor and its loss of expression can lead to prostate carcinogenesis [12,26,27,58,59].
3. PAcP structure and isoforms
The human PAcP gene is located on chromosome 3q21–23, which spans approximately 51 kb [60,61]. The PAcP gene is comprised of 10 exons, which encode a 386 amino acid precursor. This precursor is posttranslationally modified into a mature 354 amino acid protein with a molecular weight of ~49 kDa by removing a 32 amino acid signal peptide at N-terminal [60,61]. The mature form of human PAcP is a 100 kDa glycoprotein containing two subunits of 50 kDa each with post-translational modifications, which is synthesized in the differentiated columnar epithelia of prostate gland [62–64]. The differential modifications result in two forms of the protein, the cellular and secretory form. Interestingly, a new species of PAcP protein was identified as a result of alternate splicing of the PAcP gene [28] which will be discussed later in section 3.2.
3.1. PAcP protein structure
Structural analyses show human PAcP (hPAcP) protein is a dimer, consisting of two subunits [65] with a larger α/β domain and a smaller α-helical domain in each subunit [65,66]. While it was proposed the PAcP subunits are inactive; serial dilution experiments showed that each subunit exhibits phosphatase activity and though dimerization greatly enhances their specific activity by allosteric, cooperative activation [67].
Initial structural analyses revealed PAcP contains six cysteine residues at positions 129, 183, 281, 314, 319 and 340 [68]. Subsequent three-dimensional structure analyses reveal PAcP can form only two disulfide bonds because cysteine residues 183 and 281 are too far apart to form a disulfide bridge [69]. Additionally, titration experiments confirm PAcP has two reactive free sulfhydryl groups [70]. Hence, it was hypothesized that Cys183 is essential for the PTP activity of PAcP. Nevertheless, biochemical studies revealed histidine plays a critical role in PAcP activity. Site-directed mutagenesis analyses showed His12 and Asp258, but not Cys183 or Cys281, are essential for the PTP activity of PAcP [71]. For the hydrolysis reaction, His12 and Asp258 of PAcP act as a phosphate acceptor and the proton donor, respectively [5,70,72,73]. It should also be noted Asp258 is conserved in the PTP family [5,70]. These results demonstrate PAcP represents a novel class of histidine-dependent PTPs (Table 1) [5]. The detailed description of cPAcP structure and its regulation can be found in other reports [5,11,27].
3.2. PAcP protein isoforms
Several lines of evidence show the existence of different forms of PAcP proteins. For example, biochemical characterizations show a species of PAcP protein purified from prostate tissue exhibits different isoelectric point (pI) values compared to sPAcP [46,74]. Though they exhibit unique antigenic epitope(s), they also share partial cross-reactivities [46]. Immunohistochemical (IHC) staining of normal human prostate archival specimens with anti-PAcP antibody (Ab) show very high levels of PAcP protein in the cytosolic area of differentiated epithelial cells [49,50,75]. While some PAcP proteins are retained intracellularly; others are secreted into the prostatic fluid [76]. Further, immunocytochemical staining with anti-PAcP Ab shows strong, positive staining in the cytosolic area of permeabilized prostate carcinoma cells, but not the intact cells [77]. In addition, prostate cell homogenate contains high PAcP activity in the cytosolic fraction, including the supernatant fraction after 150,000×g ultracentrifugation [12]. It should also be noted that in prostate carcinomas, intracellular PAcP protein levels decrease and inversely correlate with PCa progression [26,27,64] despite the fact that its circulation level can be elevated. The decreased protein level, at least in part, is due to the decrease of mRNA level [27,78,79]. The elevation of circulating PAcP is at least in part due to increased glycosylation including high levels of sialylation, which prolongs its half-life in circulation [48]. It has thus been proposed that the same mRNA encodes for both cellular (cPAcP) and secretory PAcP (sPAcP) proteins. Our recent studies reveal the hPAcP signal peptide can direct differential post-translational modifications in biosynthetic pathways, resulting in different subcellular localizations of PAcP protein (Vishwanatha Lingappa and Ming-Fong Lin, Unpublished observations).
In addition to the known classical PAcP isoforms, cPAcP and sPAcP proteins, a recent study reported the PAcP gene can encode for a third isoform: transmembrane PAcP (TM-PAcP) from alternate splicing of approximately 11 kb between exon 10 and intron 10 [28]. The deduced 417 amino acids of TM-PAcP is predicted to have an endosomal/lysosomal targeting sequence (Y[G\R]NI) separated from a 22 amino acid TM domain in its C-terminus. Although TM-PAcP has been demonstrated to be widely expressed in many mouse tissues including thymus, lung, kidney, spleen, thyroid and fibroblast, with higher levels of expression than in prostate; its expression profile in human tissues other than prostate remains completely unknown [28]. Further analyses revealed that TM-PAcP expression level is not significantly changed in PCa cells, the authors thus concluded that this TM-PAcP does not play a role in prostate carcinogenesis [28].
4. cPAcP protein: as a prostate-specific tumor suppressor
Based on several biochemical features, cPAcP has been proposed as a tumor suppressor. First, PAcP expression is associated with normal prostate differentiation, i.e., slow cell growth. Conversely, cPAcP levels are decreased in PCa cells, lower than in adjacent non-cancerous cells [26,27,80]. Second, the genetic manipulations of PAcP by cDNA and shRNA transfection in PCa cells result in opposite effects on PCa cell proliferation and tumorigenicity [12,26,27,58,81,82]. In parallel, incorporation of purified PAcP protein into PAcP-null PCa cells decreases tyrosine phosphorylation in those cells. Third, ectopic introduction of wt PAcP cDNA expression vector suppresses LNCaP C-81 tumor growth in xenograft animal models [58]. Finally, PAcP-knockout mice develop prostatic intraepithelial neoplasia (PIN) followed by carcinoma in situ in a 12-month period [29,59]. All the evidence together support the notion that decreased cPAcP drives PCa initiation and progression, and cPAcP functions as an authentic tumor suppressor in PCa.
4.1. cPAcP protein in normal and cancerous prostate
Biochemical analyses such as IHC, in situ hybridization and electron microscopy reveal hPAcP is primarily localized in the glandular and ductal regions of differentiated human prostate epithelial cells [64,83–87]. In normal prostate tissue, cPAcP expression is low until puberty, after which PAcP protein level can reach high levels in well-differentiated prostate tissue [49,50]. In prostate adenocarcinomas, cPAcP expression is decreased, lower than adjacent non-cancerous cells, correlating with an increased tumorigenicity and cancer progression [26,50,64,88].
4.2. cPAcP protein as a negative growth regulator
Decreased cPAcP has been characterized to coordinate with activated receptor tyrosine kinases (RTK) in upregulating PCa cell proliferation, and thus is hypothesized to function as a negative growth regulator [27,81]. Hence, it is hypothesized that loss of cPAcP function favors the increased tyrosine phosphorylation of ErbB-2, an epidermal growth factor receptor (EGFR) family member, which in turn activates downstream signaling and promotes PCa cell growth [12,27]. Supportively, in LNCaP and MDA PCa2b androgen-sensitive (AS) PCa cell lines, upon passage, cPAcP expression decreases which correlates with increased growth rates of LNCaP C-81 and MDA PCa2b androgen-independent (AI) cells [12,27,81,89–91]. Further, growth stimuli decrease cPAcP protein level with a concurrent increase in cell proliferation. Conversely, incorporation of purified PAcP protein in PAcP-null PCa cells decreases ErbB-2 tyrosine phosphorylation [92,93]. In histone deacetylase (HDAC) inhibitor-treated PCa cells, growth suppression correlates with cPAcP expression and ErbB-2 dephosphorylation [94]. Consistent with our hypothesis, silencing endogenous PAcP in LNCaP C-33 cells with antisense cDNA or shRNA resulted in enhanced ErbB-2 activation and AI cell proliferation. The introduction of cPAcP cDNA in PAcP-deficient AI LNCaP C-81 cells or PAcP-null PC-3 cells also increases PAcP expression and decreases PCa cell proliferation [12,27,58,77,81]. These results are further supported by the observation of decreased PAcP expression in PCa cells on both mRNA and protein levels when compared to normal epithelia in archival specimens [26,27,64,80,95,96].
4.3. cPAcP protein exhibits the tumor suppression activity on xenograft tumors
Experimental animal studies indicate cPAcP has a potential therapeutic effect against PCa. Supportively, PCa cells expressing cPAcP have low tumorigenicity in soft agar anchorage-independent assay as well as in xenograft animal models [26]. LNCaP C-81 xenograft tumor recapitulates human castration-resistant (CR) PCa phenotype and serves as a useful model for studying the tumor suppressor role of cPAcP [12,26,81,97]. The introduction of single intratumoral injection of cDNA encoding the wild-type PAcP protein, but not PTP-inactive mutant, in pre-established C-81 xenograft tumors results in the suppression of growth and progression of xenograft tumors [58]. Conversely, subcutaneous inoculation of PAcP-knocked down PCa cells in female mice with low circulating testosterone resulted in increased tumorigenicity when compared to the animals injected with control PCa cells expressing endogenous cPAcP [12]. Thus, cPAcP functions as a TSG in culture and xenograft animal models.
4.4. PAcP-knockout mice develop prostatic intraepithelial neoplasia (PIN) and adenocarcinoma in situ
In knockout experiments, mice (C5BL/6J) lacking the exon 3 of the PAcP gene develop prostate hyperplasia at 3 months, prostatic intraepithelial neoplasia (PIN) lesions at 6 months and adenocarcinomas at 12 months of age [29,59]. The close observation of phenotypic changes reveals the similarity of PAcP-knockout mice with PTEN-knockout mice in the stage of PCa progression. In both the conditions with similar genetic (C5BL/6J) background, the knockout mice develop PIN at 3–6 months and adenocarcinoma at 12 months of their age, which mimics human PCa development and progression [29,98,99]. In addition, the histological changes demonstrate the microinvasive properties of PAcP-knockout mice, including bulging of epithelial cells into the stroma with broken fibromuscular sheath [29]. Thus, PAcP functions as a prostate-specific TSG.
4.5. cPAcP vs. TM-PAcP protein as tumor suppressor in prostate
The investigators of the report for studying PAcP-knockout mice, however, proposed that the tumor suppressor effect of PAcP is solely due to the transmembrane variant of PAcP (TM-PAcP) but not cPAcP, the classical PAcP. It should be noted this notion on which form of PAcP isoenzymes as PCa tumor suppressor is totally contradict to their own data [28,29,59]. First, the PAcP-knockout mice were developed by the deletion of exon 3, which is present in both PAcP isoforms, the classical PAcP and the TM-PAcP, and those mouse prostate cells lost the expression of both proteins [29,59,100]. Second, qRT-PCR analyses of PCa specimens revealed that the classical PAcP mRNA, not TM-PAcP mRNA, was significantly decreased in those PCa specimens [28]. It was thus proposed by the same group of investigators that classical PAcP, but not TM-PAcP, is involved in prostate carcinogenesis [28]. Third, TM-PAcP expression level is comparatively low in mouse prostate lobes when compared to the expression in other mouse tissues; while classical PAcP has a predominant expression in prostate than other tissues [28,51,52]. These results suggest the deletion of classical PAcP in knockout mice would have more profound effect on tumor development than TM-PAcP. Finally, the TM-PAcP was observed in membranous prostate structures using a polyclonal PAcP antibody which could potentially cross-react with lysosomal acid phosphatase [101]. This cross-reactivity raises a concern regarding the identity of the detected phosphatase. It is further proposed by the same team of investigators that the phosphatase domain of TM-PAcP localizes extracellularly and hydrolyses AMP in circulation for pain suppression [28,29,100]. This topology of TM-PAcP active domain clearly contradict to the report on observed biochemical properties of the activation of tyrosine phosphorylation as well as phospholipid homeostasis in those PAcP-knockout prostate cells and the colocalization of PAcP with PIP3 in dorsolateral lobe (DL) of prostate [59]. All the above observations together clearly suggest the observed phenotype of PAcP as TSG can be explained only by the loss of both forms of PAcP, if not the classical PAcP alone. Hence, it is even reasonable to suggest the tumor suppressor effect is essentially due to cellular PAcP protein.
5. cPAcP: Mechanism of action in tumor suppression
Biochemically, PAcP is a member of dual specificity phosphatase (DSP) and can dephosphorylate phosphotyrosine, phosphoserine and phosphothreonine with a lower km value of p-Tyr than that of p-Ser or p-Thr [102–105]. While PAcP does not contain the signature motif as the classical DSPs; biochemical characterizations, including site-directed mutagenesis, demonstrate PAcP has histidine and aspartate in its active site [70–72,106–108]. Supportively, initial studies demonstrated that cPAcP is co-purified with the major PTP activity in non-cancerous human prostate tissue [109]. Biochemical studies using purified human PAcP protein [103] and recombinant rat PAcP protein expressed in a baculoviral expression system [110] further support the notion that cPAcP exhibits neutral PTP activity. Incorporation of purified PAcP protein into PAcP-null DU145 cells is associated with phosphotyrosine dephosphorylation [93]. Thus, understanding the reprogramming of PCa cell proliferation by PAcP can aid in the development of novel approaches for PCa therapy.
5.1. cPAcP and ErbB-2 signaling in prostate cancer
Several studies support the notion that elevated ErbB-2 specific activity, but not its amplification plays a critical role in CR PCa progression [82,93,111,112]. In human prostate archival specimens, PAcP enzyme activity, protein level and its mRNA level were decreased in cancer cells, when compared to normal or benign prostate tissue [26,27,80,95,96]. The inverse correlation of cPAcP activity and the level of tyrosyl phosphorylation activity provide the first indication of cPAcP as a PTP in cells [89,103]. Significantly, we have shown PAcP prefers neutral pH for the dephosphorylation of tyrosyl phosphorylated EGFR [113]. EGFR family member activation can directly activate ERK1/2 and/or phosphatidylinositol 3-kinase (PI3K)/Akt pathways and contributes to the survival of CR PCa cells [12,27,111,114–118].
The relationship between ErbB-2 tyrosine phosphorylation level and cPAcP PTP activity has been demonstrated by many intensive studies. In AI LNCaP C-81 and MDA PCa2b-AI PCa cells which have lower endogenous cPAcP level with respect to their AS counterparts and have elevated ErbB-2 tyrosine phosphorylation correlating with increased cell proliferation [12,26,27,119,120]. In parallel, in AS cells, in the presence of phosphatase inhibitors, PAcP activity decreases and inversely correlates with increased protein tyrosine phosphorylation level as well as cell proliferation [119,120]. The in vitro tumorigenic analyses, including anchorage-independent growth assay and xenograft animal experiments on AI LNCaP C-81 cells, further support the notion that PCa cells with lower PAcP and higher ErbB-2 activity will have increased tumorigenic activity [12,58,121]. In addition, the AI LNCaP C-81 PCa cells show enhanced tumor migration and metastasis [122, Ta-Chun Yuan, Fen-Fen Lin and Ming-Fong Lin, unpublished observations]. Conversely, the incorporation of PAcP protein and cDNA into PAcP-null DU145 and PC-3 PCa cells results in decreased Tyr-P of a 185 kDa (ErbB-2) protein [26,93,114]. Intratumor expression of cPAcP by injecting wt PAcP cDNA expression vector, but not the phosphatase inactive mutant, results in tumor suppression and decreased ErbB-2 tyrosine phosphorylation [58]. Importantly, reciprocal co-immunoprecipitation analyses demonstrate interaction between cPAcP and ErbB-2 under non-permissive growth conditions [12]. This interaction by co-immunoprecipitation is decreased upon growth stimulation [119]. Thus, the effect of cPAcP on down-regulation of PCa cell growth is at least in part due to its dephosphorylation of the p-Tyr of ErbB-2 protein in those cells [12,26,71,114,119].
cPAcP may dephosphorylate human ErbB-2 at different sites in PCa cells. In AI human LNCaP C-81 and MDA PCa2b-AI PCa cells in which PAcP expression is decreased, the phosphorylation levels of Tyr1221/2 and Tyr1248 are elevated [12]. Our results clearly show both the autophosphorylation sites of ErbB-2 are activated in PCa cells with low cPAcP activity. Our kinetic analyses upon shRNA transfection shows knockdown of cPAcP is preferentially associated with pY1221/2 phosphorylation followed by pY1248 phosphorylation of ErbB-2. Sharma and his colleagues also demonstrate the peptide, C-DNLpYYWD-N which has a sequence from rat ErbB-2 auto-phosphorylation sites (1197–1203), exhibits the most favorable free energy of binding and interaction [123]. Thus, the cPAcP dephosphorylation model indicates dimeric cPAcP dephosphorylates two autophosphorylated residues on an activated receptor simultaneously because the presence of a second phosphorylated tyrosyl residue at the C-terminus of ErbB-2 can considerably enhance the binding affinity [123]. Alternatively, due to the proximity of Tyr1221/2 and Tyr1248, dephosphorylation of Tyr1248 by PAcP can be secondary to the removal of Tyr1221/2. Thus, understanding the role of PAcP in altering ErbB-2 phosphorylation level may give more insight into cPAcP’s mechanistic role in regulating PCa.
5.2. PAcP and PI3K/Akt survival signaling in prostate cancer
The activation of PI3K/Akt signal that drives cancer cell survival and growth reveals a number of deregulated oncogenes and tumor suppressors. Several studies have established a close relationship between activation of the PI3K/Akt pathway and deregulation of lipid phosphatases such as Phosphatase and tensin homolog (PTEN) in advanced human PCa. The lipid phosphatase PTEN has been demonstrated to regulate PI3K signaling by blocking the activation of downstream Akt survival protein. Additionally, recent advances have revealed PTP’s biological activity goes beyond the dephosphorylation of phosphoproteins. It is hypothesized several tyrosine phosphatases could function as phospholipases in addition to its canonical PTP function [124].
cPAcP biochemically functions as a PTP, is decreased in PCa and its activity is associated with dephosphorylation of ErbB-2 (as discussed in the section 5.1). Thus far, ErbB-2 has been demonstrated as a phosphoprotein substrate of cPAcP and dephosphorylation of ErbB-2 has been described as PAcP’s main function in controlling PCa cell growth [12]. Biochemical studies further reveal PAcP possesses PIP activity and can dephosphorylate phosphatidylinositol 3-phosphate (PI(3)P) from Phosphotidylinositol (3,4,5) phosphate (PIP3) [12,59] (Fig. 1). Colocalization studies further indicate the interaction of cPAcP with PIP3 in dorsolateral lobe of mice [59]. It is proposed that the positively charged side chains in PAcP’s active site may favor the binding of phosphate ion in PIP3. PIP3 is an activator of Akt and is required for its full activation by phosphorylating S473. Our analyses on cPAcP in LNCaP C-33 cells demonstrated the knockdown of cPAcP enhances Akt hyper-activation at S473 and correlates with tumorigenicity [12]. In addition, there is a strong inverse correlation between Akt activation and cPAcP level in AS. vs. AI LNCaP and MDA PCa2b PCa cells. For example, in LNCaP cells where PTEN is mutant, Akt (Ser473) is hyper-activated in AI LNCaP C-81 cells as well as cPAcP-knockdown LNCaP C-33 cells with low cPAcP but not in LNCaP C-33 cells with high cPAcP [12,125]. Similarly, ErbB-2 and Akt activation are observed in MDA PCa2b-AI cells, compared to AS MDA PCa2b cells [12]. These AI cells in which ErbB-2 and Akt are activated have increased tumorigenicity in culture and in xenograft animal models [12,58,121]. Nevertheless, further studies are required to determine the mechanism of Akt hyper-activation in cPAcP-deficient PCa cells and validate cPAcP hydrolyses PIP3 to PIP2.
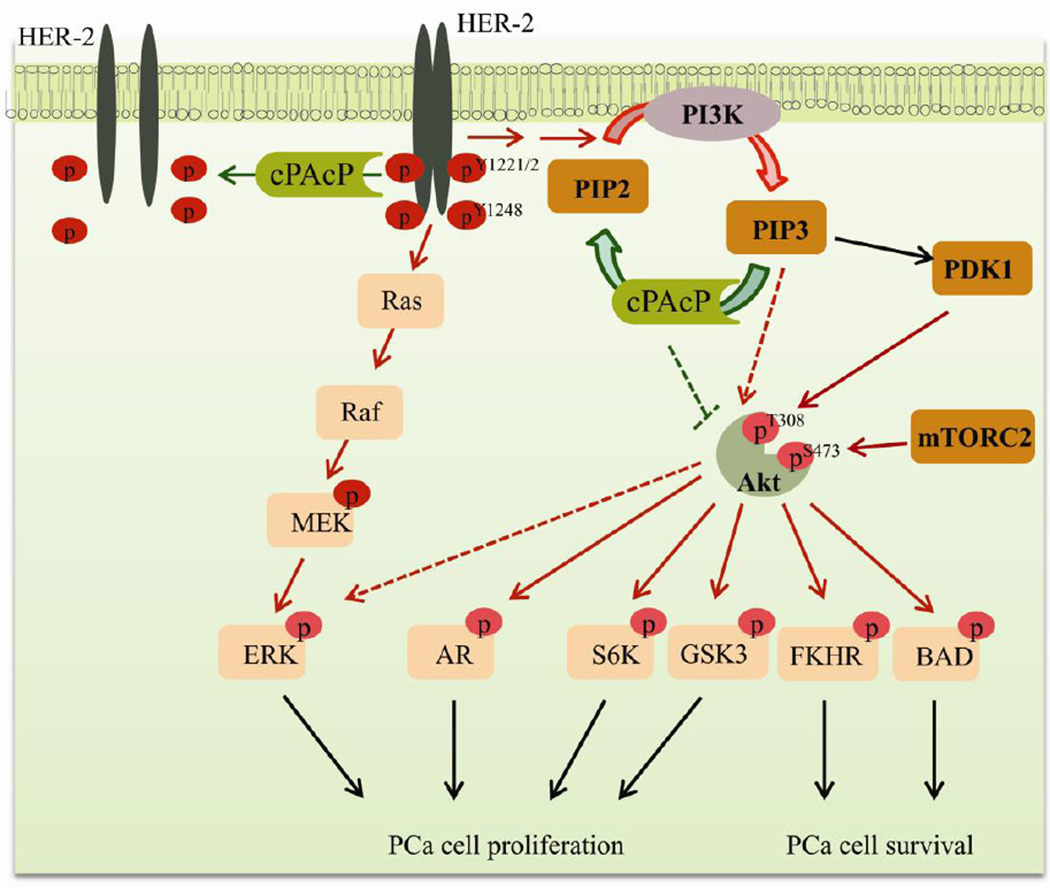
Fig. 1.
A schematic representation of cPAcP interaction with HER-2 and PI3K/Akt pathway in regulating prostate cell homeostasis. cPAcP loss of expression induces carcinogenesis and subsequently prostate cancer (PCa) cell proliferation and progression. Initiation of PCa is accompanied by an early decrease of cPAcP expression in prostate cells resulting in hyperphosphorylation of HER-2 on tyrosine residues including Y1221/2 and Y1248. In addition to the activation of HER-2/MAPK pathway, loss of cPAcP activity can lead to the accumulation of PIP3 and subsequent Akt activation, which results in PCa progression and survival. Akt activation can phosphorylate S6K and GSK3 and leads to prostate cell proliferation. Activated Akt may phosphorylate and sequester FKHR and BAD in cytoplasm which results in survival of PCa cells. In addition, Akt may also phosphorylate AR and ERK which results in inducing PCa cell proliferation. Abbreviations: Akt, v-akt murine thymoma viral oncogene homolog; AR, androgen receptor; BAD, Bcl-2-associated death promoter protein; cPAcP, cellular prostatic acid phosphatase; ERK, extracellular signal-regulated kinases; FKHR, forkhead in rhabdomyosarcoma (also designated FOXO1); GSK3, glycogen synthase kinase 3; HER-2, human epidermal growth factor receptor-2 (also designated ErbB-2/neu); PDK-1, phosphoinositide-dependent kinase 1; PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol 3,4,5-trisphosphate; S6K, ribosomal S6 kinase (a family member of serine/threonine kinases).
6. Other phosphatases as tumor suppressors in prostate cancer
In human prostate cells, PI3K signaling plays an indispensable role in maintaining cell homeostasis, and dysregulation of this signaling results in the pathogenic state. Results of a recent genomic analyses predicted the PI3K pathway is aberrantly regulated in about 40% of primary tumors and almost 100% of metastatic tumors [35]. cPAcP has been proposed to regulate the PI3K signaling in PCa cells. Many other phosphatases are expressed in prostate cells, and their biological functions are in relevant to PI3K signaling in PCa. In the following section, we briefly discuss those protein phosphatases that are involved in regulating Akt activation and may have functional overlapping with cPAcP (Table 3) [12,27,36,37].
Table 3.
Commonly known phosphatase as tumor suppressor in prostate epithelia.
| Phosphatases and its isoforms |
Previous symbols/synonyms | Gene family | Chromosome location |
|---|---|---|---|
| PAcP | 3q21 | ||
| i. PAcP | ACP-3, ACP3, Also classically as ‘ACP2’. | Histidine-dependent DSP. | |
| ii. TM-PAcP | |||
| PTEN | MMAC1, PTEN1, TEP1, BZS, MHAM. | Class I Cys-dependent DSP. | 10q23 |
| PP2A | Serine/threonine protein phosphatase | ||
| i. PPP2CA | Protein phosphatase 2, catalytic subunit, alpha isoform; PP2Calpha. | 5q31.1 | |
| ii. PPP2CB | Protein phosphatase 2, catalytic subunit, beta isoform; PP2Abeta. | 8p12 | |
| iii. PPP2R2A | PP2A regulatory subunit B isoform alpha; B55A; PR52A; PR55A. | 8p21.2 | |
| iv. PPP2R5B | PP2A, regulatory subunit B, beta isoform; PP2A, B subunit; B56B; FLJ35411; PP2A; R5 beta isoform; PR61B. | 11q12 | |
| v. PPP2R2C | PP2A, regulatory subunit B, gamma isoform; IMYPNO; MGC33570; PR52; PR55G. | 4p16.1 | |
| vi. PPP2R1A | PP2A, regulatory subunit A (PR 65), alpha isoform. | 19q13 | |
| PHLPP | |||
| i. PHLPP1 | PLEKHE1, PHLPP. | Mg2+/Mn2+-dependent protein phosphatase. | 18q21.32 |
| ii. PHLPP2 | PHLPPL | 16q22.2 | |
Abbreviations: DSP, dual specificity phosphatase; MMAC1, mutated in multiple advanced cancers 1; PAcP, prostatic acid phosphatase; cPAcP, cellular PAcP; sPAcP, secretory PAcP; PP2A, protein phosphatase 2A; PHLPP, PH domain and leucine rich repeat protein phosphatase; PP2CA, protein phosphatase 2C, alpha isoform; PP2CB, protein phosphatase 2C, betaisoform; PTEN, phosphatase and tensin homolog; TM-PAcP, transmembrane PAcP.
6.1. Phosphatase and tensin homolog (PTEN)
PTEN is a mammalian lipid as well as protein phosphatase and consists of 403 amino acids with the consensus catalytic signature motif required for dephosphorylation. The detailed structure, regulation and mechanism of action of PTEN in maintaining cellular homeostasis can be found elsewhere [126–130]. PTEN Loss of heterozygosity (LOH) and/or mutation occurs most frequently in advanced cancers including PCa [20,21]. The principal function of PTEN is dephosphorylating Phosphotidylinositol (3,4,5) phosphate (PIP3) to Phosphotidylinositol (4,5) phosphate (PIP2) and thus inhibits the activation of Phosphoinositide-dependent kinase 1 (PDK1) and then Akt (Fig. 2). There is evidence to show PTEN deletion and increased level of PIP3 and Akt activation in advanced PCa. The distinctive PIP3 lipid phosphatase activity makes PTEN as an essential molecule in maintaining prostate cellular homeostasis which validates PTEN as a potent tumor suppressor. Further studies show that germline mutations as observed in other cancer types are rare in PCa and hence support the notion that loss of PTEN is a late event in prostate carcinogenesis [22,23].
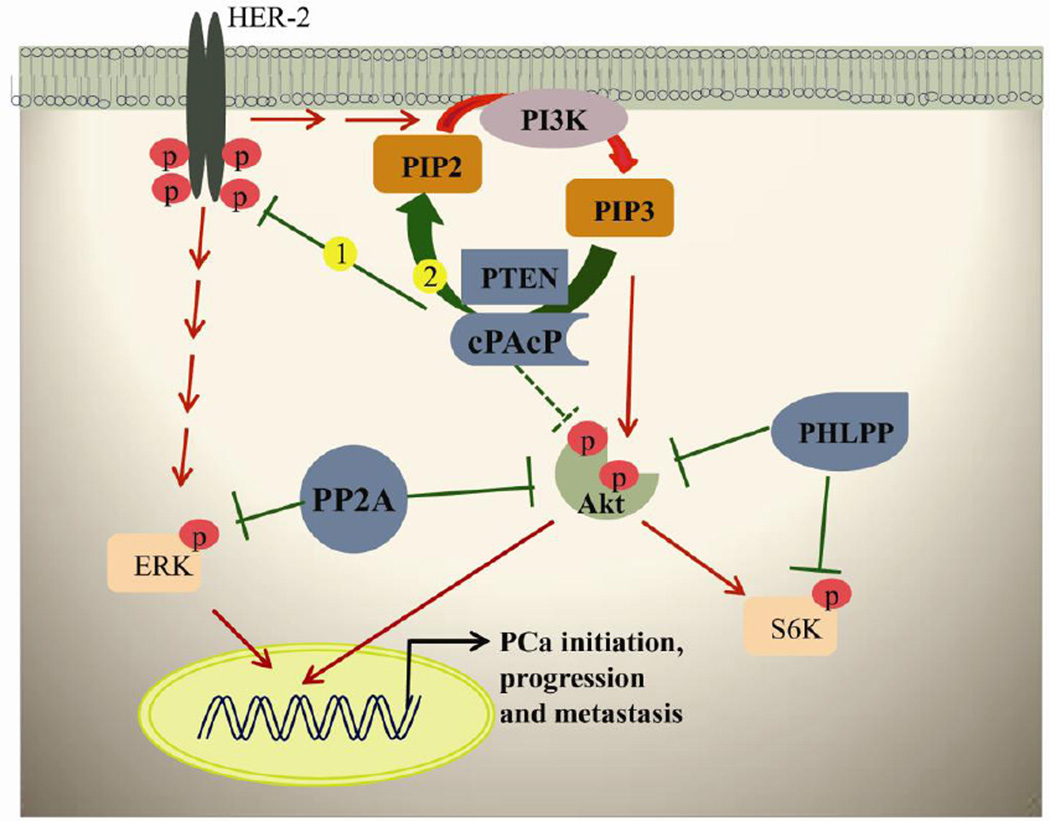
Fig. 2.
A schematic representation of phosphatases in maintaining prostate cell homeostasis. The data indicate two negative regulations of cPAcP in PCa cell proliferation and survival. Early decrease of cPAcP expression results in hyperphosphorylation of HER-2 on tyrosine residues including Y1221/2 and Y1248 and will initiate PCa and progression. Further, ErbB-2 activation can lead to PI3K activation and results in PCa cell survival. Second, cPAcP function loss may result in PIP3 accumulation and subsequent Akt activation which result in mediating downstream signal essential for prostate cell survival. The phosphorylation of PIP3 can be maintained by cPAcP and PTEN by dephosphorylating back into PIP2. PP2A may directly inactivate Akt and ERK in regulating prostate cell homeostasis. PHLPP may also directly inactivate Akt and S6K which results in inhibiting the downstream cell proliferation signals. Note: Phosphatases are indicated by blue. Phosphatases of inhibitory activity are indicated in green solid line. The open dotted line indicates cPAcP may directly dephosphorylate and inactivate Akt. The kinase-induced activity is indicated in red solid arrows. Abbreviations: Akt, v-akt murine thymoma viral oncogene homolog; AR, androgen receptor; cPAcP, cellular prostatic acid phosphatase; ERK, Extracellular signal-regulated kinases; HER-2, human epidermal growth factor receptor-2 (also designated ErbB-2/neu); PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol 3,4,5-trisphosphate; PHLPP, PH domain leucine-rich repeat protein phosphatase; PP2A, protein phosphatase 2A; PTEN, Phosphatase and tensin homolog; S6K, ribosomal S6 kinase (a family member of serine/threonine kinases).
6.2. Protein phosphatase 2A (PP2A)
PP2A is a highly conserved serine/threonine protein phosphatase. Basically, PP2A is a heterotrimeric complex with broad spectrum of substrates [131]. Structurally, PP2A is made of ‘core dimer’ of scaffold (structural) and catalytic subunit, to which variable regulatory subunits associate and form different active holoenzyme complex [132–135]. Currently, based on the association and variable number of regulatory subunits, it is proposed there are approximately 75–100 possible heterotrimeric complexes. It is estimated that PP2A accounts for about 1% of total proteins and is responsible for 90% of total serine/threonine phosphatase activity in any given cell [136]. Studies had also shown that there is an inverse correlation between PP2A and Akt activity in prostate and other cancers [31,32,136,137]. The primary function of PP2A is to dephosphorylate and inactivate Akt and thus inhibits its downstream signaling in PCa (Fig. 2). Recently, PP2A has received much attention in PCa and is proposed to be a potent tumor suppressor [33]. PP2A loses its tumor suppressor functions through diminished expression, mutations and/or somatic alterations either in their scaffold or regulatory subunit [36,138]. In addition, PP2A has been proposed to play a critical role in regulating MAPK signaling in AI PCa cells [31]. Biochemical studies further suggest that the observed Akt elevation in PP2A-knocked down and metastatic PCa cells is due to the B-subunit PR55α, a PP2A regulatory subunit [136–138]. Despite large numbers of functional studies describing PP2A as a tumor suppressor in various cellular models, further preclinical studies are needed to clarify the mechanistic function of PP2A regulatory subunits in PCa. It is also suggested that due to the complexity of the holoenzyme and varied nature of activities in each cell type, more studies on PCa are needed to determine the specific subunit responsible for Akt and other substrates, such as β-catenin and c-MYC dephosphorylation, to delineate the mechanism underlying PP2A as a tumor suppressor.
6.3. Pleckstrin homology domain leucine-rich repeat protein phosphatase (PHLPP)
The tumor suppressor PHLPP is classified as DSP and has three isozymes PHLPP1α, PHLPP1β and PHLPP2 [139] (Table 3). Structural analyses reveled all three PHLPP isozyme has an amino terminus, PH domain, leucine-rich repeat region, phosphatase domain and C-terminal PDZ-binding motif. Genomic analyses show the isozymes PHLPP1 and PHLPP2 are respectively deleted in 7 and 15 % of primary and 43 and 62 % in metastatic PCa patients [35]. Our preliminary data indicated the potential regulation of Akt activation by PHLPP (Yaping Tu and Ming-Fong Lin, unpublished observations). Mechanistic study demonstrated PHLPP has the ability to dephosphorylate S473 and inactivate Akt [37] (Fig. 2). Further, PHLPP has been hypothesized to dephosphorylate PKC and S6K at their hydrophobic phosphorylation motifs [37]. Knockdown and coimmunoprecipitation studies have revealed that both PHLPP isoforms have differential specificities towards Akt isoforms [140] and suggest the interaction between specific Akt and PHLPP isoform may be responsible for its dephosphorylation activity. Further, the PHLPP1-knockout mice develop high grade prostatic intraepithelial neoplasia (PIN) in 9 months of age, but not PCa, thus established the potential of tumor suppressor activity of PHLPP1 protein [36]. Overall, the results support PHLPP has the ability to suppress Akt signaling and can function as a therapeutic target. Given the central role of this phosphatase family in terminating cell survival pathways, more mechanistic studies are needed on the substrates signaling pathways involving PHLPP in PCa.
7. Summary and conclusion
Since the identification of elevated PAcP activity in the circulation of metastatic PCa patients seven decades ago, PAcP has been considered as a surrogate PCa marker until the availability of PSA. Although the sPAcP level is upregulated by androgens, a main driving force behind prostate carcinogenesis; cPAcP is decreased upon androgen stimulation and has been demonstrated to decline in PCa cells in comparison with normal prostate epithelia. Most importantly, analyses by transcriptome-based tissue microarray reveal 90% of PCa tissue specimens of Gleason scores 6–9 have decreased cPAcP expression compared with the adjacent non-cancerous tissue specimens [27]. Clinical data also supports cPAcP is the only known tissue-specific protein which progressively decreases from the premalignant prostatic intraepithelial neoplasia (PIN) stage (Shiv Srivastava and Ming-Fong Lin, Unpublished observations). Furthermore, PCa cells containing higher levels of cPAcP are less tumorigenic when compared to PCa cells with lower cPAcP levels, and PAcP knockout mice have been demonstrated to develop prostate adenocarcinoma.
In PCa, cPAcP and PTEN are apparent functional homologues in regulating prostate cell homeostasis. In addition to both phosphatases exhibiting PTP activity, biochemically, PAcP dephosphorylates PI(3)P, the same site of dephosphorylation by PTEN. The observed phenotype of loss of cPAcP expression with Akt hyper-activation in PTEN-inactive LNCaP C-33 vs. C-81 cells supportively suggests cPAcP may function as a phospholipase in the absence of PTEN. In parallel, cPAcP-knockdown LNCaP C-33 PCa cells show enhanced Akt hyper-activation by S473 phosphorylation [12]. Also, PAcP-knockout mice show PI3K-Akt activation and develop prostate adenocarcinoma with a similar phenotype observed in PTEN-knockout mice [29,59]. Thus, cPAcP behaves as PTEN-functional homologue in PCa cells. Nevertheless, each phosphatase exhibits unique features. Although loss of PTEN in mouse promotes prostate adenocarcinoma, studies on clinical archival specimens show that the majority of PTEN loss is associated with the metastatic state with only about 10–20% of primary PCa, differing from the early loss of cPAcP. Also, cPAcP may directly regulate Akt phosphorylation in addition to ErbB-2; while the PTEN in vivo phosphoprotein substrate requires further identification. Taken together, the data clearly demonstrates cPAcP, a functional homologue to PTEN, is the prostate tissue-specific phosphatase which functions as an authentic tumor suppressor in PCa. Its loss of expression is involved in the early stage of prostate carcinogenesis.
8. Perspectives
In PCa, enormous progress has been made on identifying the genetic cause for the loss-of-function of particular TSGs. Nevertheless, it is not yet known whether the decrease or absence of PAcP in PCa is due to biallelic silencing, point mutation or loss of heterozygocity (LOH). Intriguingly, our recent report demonstrates cPAcP can be regulated epigenetically [94] and hence we suggest the future screening for cPAcP functional loss in a large population would be an active and stimulating area of investigation.
In the present review, we have given mechanistic evidences that cPAcP can dephosphorylate the HER-2 and PI(3)P in PCa cells. Interestingly, recent reports suggest that the blocking of a single EGFR function can be compensated by the overexpression of alternative HERs, establishing an autocrine growth factor loop that maintains downstream signaling and PCa cellular proliferation [141–143]. Although substantial progress has been made toward understanding HER-2 dephosphorylation mechanism by PAcP; it is not yet known whether cPAcP is able to dephosphorylate other HER-2 family members such as EGFR and HER-3. Interestingly, on defining the role of cPAcP in regulating oncogenic PI3K/Akt signaling, PP2A and PHLPP have also been demonstrated as tumor suppressors in PCa by dephosphorylating Akt at T308 or S473 or both. However, when comparing PTEN to other TSGs, further mechanistic studies of PP2A and PHLPP are needed to define their dephosphorylating function in PCa.
Due to the proven importance of the PAcP gene as a TSG in prostate carcinogenesis, investigation of the basic biochemistry and molecular biology of cPAcP, including its interaction with oncogenic proteins, will further unearth the PAcP regulatory mechanisms as a TSG. The information will provide valuable insight into its potential therapeutic applications. Though further investigation is apparently needed, the data may implicate the loss of both cPAcP and PTEN proteins are required to obtain the advanced PCa phenotype, the CR PCa. In parallel, it should also be noted for the remarkable similarity of functional interplay between PTPs and HER-2 family members and between phosphatase and Akt in other cancers for the survival of those cancer cells [126,144–154]. Based on the common theme of dephosphorylation of HER-2 and PIP3s by cPAcP in PCa, similar tissue-specific PTPs and also the ubiquitous protein phosphatases such as PTEN, PP2A and PHLPP can potentially be identified and developed as therapeutic targets for their respective carcinoma.
Acknowledgments
The studies of cPAcP as a prostate-specific tumor suppressor has been supported in part by the National Cancer Institute, National Institutes of Health [R01 CA88184; R01 CA138791]; Department of Defense [W81XWH-08-1-0459]; Nebraska DHHS [LB 506 #2000-19]; and the University of Nebraska Medical Center Bridge Fund. We also thank previous lab members for their efforts on understanding the function and regulation of human prostatic acid phosphatase.
Abbreviations
- AcP
acid phosphatase
- AI
Androgen-independent
- AS
Androgen-sensitive
- cPAcP
cellular prostatic acid phosphatase
- CR PCa
castration-resistant prostate cancer
- DSP
dual specificity phosphatase
- EGFR
epidermal growth factor receptor
- HER-2/ErbB-2/neu
human epidermal growth factor receptor-2
- HDAC
Histone deacetylase
- IHC
Immunohistochemistry
- hPAcP
human PAcP
- PAcP
prostatic acid phosphatase
- PCa
prostate cancer
- PDK1
Phosphoinositide-dependent kinase 1
- pI
isoelectric point
- PIN
prostate intraepithelial neoplasia
- PI3K
phosphoinositide 3-kinase
- PIP
phosphoinositide phosphtase
- PSA
prostate-specific antigen
- PTEN
Phosphatase and tensin homolog
- PTK
protein tyrosine kinase
- PTP
protein tyrosine phosphatase
- p-Tyr
phosphotyrosine
- qRT-PCR
quantitative reverse transcription-polymerase chain reaction
- sPAcP
secretory PAcP
- TM-PAcP
transmembrane PAcP
- TSG
tumor suppressor gene
- Tyr-P
tyrosine phosphorylation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bononi A, Agnoletto C, De Marchi E, Marchi S, Patergnani S, Bonora M, Giorgi C, Missiroli S, Poletti F, Rimessi A, Pinton P. Protein kinases and phosphatases in the control of cell fate. Enzyme Res. 2011;2011:329098. doi: 10.4061/2011/329098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Motiwala T, Jacob ST. Role of protein tyrosine phosphatases in cancer. Prog. Nucleic Acid Res. Mol. Biol. 2006;81:297–329. doi: 10.1016/S0079-6603(06)81008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nat. Rev. Mol. Cell Biol. 2006;7:833–846. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- 4.Alonso A, Sasin J, Bottini N, Friedberg I, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, Mustelin T. Protein tyrosine phosphatases in the human genome. Cell. 2004;117:699–711. doi: 10.1016/j.cell.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 5.Veeramani S, Lee MS, Lin MF. Revisiting histidine-dependent acid phosphatases: A distinct group of tyrosine phosphatases. Trends Biochem. Sci. 2009;34:273–278. doi: 10.1016/j.tibs.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang XQ, Kondrikov D, Yuan TC, Lin FF, Hansen J, Lin MF. Receptor protein tyrosine phosphatase alpha signaling is involved in androgen depletion-induced neuroendocrine differentiation of androgen-sensitive LNCaP human prostate cancer cells. Oncogene. 2003;22:6704–6716. doi: 10.1038/sj.onc.1206764. [DOI] [PubMed] [Google Scholar]
- 7.Yuan TC, Veeramani S, Lin MF. Neuroendocrine-like prostate cancer cells: neuroendocrine transdifferentiation of prostate adenocarcinoma cells. Endocr. Relat. Cancer. 2007;14:531–547. doi: 10.1677/ERC-07-0061. [DOI] [PubMed] [Google Scholar]
- 8.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J. Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 9.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–244. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 10.Madan RA, Pal SK, Sartor O, Dahut WL. Overcoming chemotherapy resistance in prostate cancer. Clin. Cancer Res. 2011;17:3892–3902. doi: 10.1158/1078-0432.CCR-10-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muniyan S, Chaturvedi NK, Dwyer JG, Lagrange CA, Chaney WG, Lin MF. Human prostatic Acid phosphatase: structure, function and regulation. Int. J. Mol. Sci. 2013;14:10438–10464. doi: 10.3390/ijms140510438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chuang TD, Chen SJ, Lin FF, Veeramani S, Kumar S, Batra SK, Tu Y, Lin MF. Human prostatic acid phosphatase, an authentic tyrosine phosphatase, dephosphorylates ErbB-2 and regulates prostate cancer cell growth. J. Biol. Chem. 2010;285:23598–23606. doi: 10.1074/jbc.M109.098301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drake JM, Graham NA, Stoyanova T, Sedghi A, Goldstein AS, Cai H, Smith DA, Zhang H, Komisopoulou E, Huang J, Graeber TG, Witte ON. Oncogene-specific activation of tyrosine kinase networks during prostate cancer progression. Proc. Natl. Acad. Sci. USA. 2012;109:1643–1648. doi: 10.1073/pnas.1120985109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tricoli JV, Gumerlock PH, Yao JL, Chi SG, D'Souza SA, Nestok BR, deVere White RW. Alterations of the retinoblastoma gene in human prostate adenocarcinoma. Genes Chromosomes Cancer. 1996;15:108–114. doi: 10.1002/(SICI)1098-2264(199602)15:2<108::AID-GCC5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Sharma A, Yeow WS, Ertel A, Coleman I, Clegg N, Thangavel C, Morrissey C, Zhang X, Comstock CE, Witkiewicz AK, Gomella L, Knudsen ES, Nelson PS, Knudsen KE. The retinoblastoma tumor suppressor controls androgen signaling and human prostate cancer progression. J. Clin. Invest. 2010;120:4478–4492. doi: 10.1172/JCI44239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun H, Wang Y, Chinnam M, Zhang X, Hayward SW, Foster BA, Nikitin AY, Wills M, Goodrich DW. E2f binding-deficient Rb1 protein suppresses prostate tumor progression in vivo. Proc. Natl. Acad. Sci. USA. 2011;108:704–709. doi: 10.1073/pnas.1015027108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isaacs WB, Carter BS, Ewing CM. Wild-type p53 suppresses growth of human prostate cancer cells containing mutant p53 alleles. Cancer Res. 1991;51:4716–4420. [PubMed] [Google Scholar]
- 18.Massenkeil G, Oberhuber H, Hailemariam S, Sulser T, Diener PA, Bannwart F, Schäfer R, Schwarte-Waldhoff I. P53 mutations and loss of heterozygosity on chromosomes 8p, 16q, 17p, and 18q are confined to advanced prostate cancer. Anticancer Res. 1994;14:2785–2790. [PubMed] [Google Scholar]
- 19.Heidenberg HB, Sesterhenn IA, Gaddipati JP, Weghorst CM, Buzard GS, Moul JW, Srivastava S. Alteration of the tumor suppressor gene p53 in a high fraction of hormone refractory prostate cancer. J. Urol. 1995;154:414–421. doi: 10.1097/00005392-199508000-00024. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 21.Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T, Frye C, Hu R, Swedlund B, Teng DH, Tavtigian SV. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat. Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 22.Cairns P, Okami K, Halachmi S, Halachmi N, Esteller M, Herman JG, Jen J, Isaacs WB, Bova GS, Sidransky D. Frequent inactivation of PTEN/MMAC1 in primary prostate cancer. Cancer Res. 1997;57:4997–5000. [PubMed] [Google Scholar]
- 23.Feilotter HE, Nagai MA, Boag AH, Eng C, Mulligan LM. Analysis of PTEN and the 10q23 region in primary prostate carcinomas. Oncogene. 1998;16:1743–1748. doi: 10.1038/sj.onc.1200205. [DOI] [PubMed] [Google Scholar]
- 24.Deocampo ND, Huang H, Tindall DJ. The role of PTEN in the progression and survival of prostate cancer. Minerva Endocrinol. 2003;28:145–153. [PubMed] [Google Scholar]
- 25.Mulholland DJ, Dedhar S, Wu H, Nelson CC. PTEN and GSK3beta: key regulators of progression to androgen-independent prostate cancer. Oncogene. 2006;25:329–337. doi: 10.1038/sj.onc.1209020. [DOI] [PubMed] [Google Scholar]
- 26.Lin MF, Lee MS, Zhou XW, Andressen JC, Meng TC, Johansson SL, West WW, Taylor RJ, Anderson JR, Lin FF. Decreased expression of cellular prostatic acid phosphatase increases tumorigenicity of human prostate cancer cells. J. Urol. 2001;166:1943–1950. [PubMed] [Google Scholar]
- 27.Veeramani S, Yuan TC, Chen SJ, Lin FF, Petersen JE, Shaheduzzaman S, Srivastava S, MacDonald RG, Lin MF. Cellular prostatic acid phosphatase: A protein tyrosine phosphatase involved in androgen-independent proliferation of prostate cancer. Endocr. Relat. Cancer. 2005;12:805–822. doi: 10.1677/erc.1.00950. [DOI] [PubMed] [Google Scholar]
- 28.Quintero IB, Araujo CL, Pulkka AE, Wirkkala RS, Herrala AM, Eskelinen EL, Jokitalo E, Hellstrom PA, Tuominen HJ, Hirvikoski PP, Vihko PT. Prostatic acid phosphatase is not a prostate specifi c target. Cancer Res. 2007;67:6549–6554. doi: 10.1158/0008-5472.CAN-07-1651. [DOI] [PubMed] [Google Scholar]
- 29.Quintero IB, Herrala AM, Araujo CL, Pulkka AE, Hautaniemi S, Ovaska K, Pryazhnikov E, Kulesskiy E, Ruuth MK, Soini Y, Sormunen RT, Khirug L, Vihko PT. Transmembrane prostatic acid phosphatase (TMPAP) interacts with snapin and deficient mice develop prostate adenocarcinoma. PLoS One. 2013;8:e73072. doi: 10.1371/journal.pone.0073072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh AP, Bafna S, Chaudhary K, Venkatraman G, Smith L, Eudy JD, Johansson SL, Lin MF, Batra SK. Genome-wide expression profiling reveals transcriptomic variation and perturbed gene networks in androgen-dependent and androgen-independent prostate cancer cells. Cancer Lett. 2008;259:28–38. doi: 10.1016/j.canlet.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhardwaj A, Singh S, Srivastava SK, Honkanen RE, Reed E, Singh AP. Modulation of protein phosphatase 2A activity alters androgen-independent growth of prostate cancer cells: therapeutic implications. Mol. Cancer Ther. 2011;10:720–731. doi: 10.1158/1535-7163.MCT-10-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pandey P, Seshacharyulu P, Das S, Rachagani S, Ponnusamy MP, Yan Y, Johansson SL, Datta K, Lin MF, Batra SK. Impaired expression of protein phosphatase 2A subunits enhances metastatic potential of human prostate cancer cells through activation of AKT pathway. Br. J. Cancer. 2013;108:2590–2600. doi: 10.1038/bjc.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seshacharyulu P, Pandey P, Datta K, Batra SK. Phosphatase: PP2A structural importance, regulation and its aberrant expression in cancer. Cancer Lett. 2013;335:9–18. doi: 10.1016/j.canlet.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel R, Gao M, Ahmad I, Fleming J, Singh LB, Rai TS, McKie AB, Seywright M, Barnetson RJ, Edwards J, Sansom OJ, Leung HY. Sprouty2, PTEN, and PP2A interact to regulate prostate cancer progression. J. Clin. Invest. 2013;123:1157–1175. doi: 10.1172/JCI63672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, Antipin Y, Mitsiades N, Landers T, Dolgalev I, Major JE, Wilson M, Socci ND, Lash AE, Heguy A, Eastham JA, Scher HI, Reuter VE, Scardino PT, Sander C, Sawyers CL, Gerald WL. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen M, Pratt CP, Zeeman ME, Schultz N, Taylor BS, O'Neill A, Castillo-Martin M, Nowak DG, Naguib A, Grace DM, Murn J, Navin N, Atwal GS, Sander C, Gerald WL, Cordon-Cardo C, Newton AC, Carver BS, Trotman LC. Identification of PHLPP1 as a tumor suppressor reveals the role of feedback activation in PTEN-mutant prostate cancer progression. Cancer Cell. 2011;20:173–186. doi: 10.1016/j.ccr.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newton AC, Trotman LC. Turning Off AKT: PHLPP as a Drug Target. Annu. Rev. Pharmacol. Toxicol. 2014;54:537–558. doi: 10.1146/annurev-pharmtox-011112-140338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davies G, Jiang WG, Mason MD. The interaction between beta-catenin, GSK3beta and APC after motogen induced cell-cell dissociation, and their involvement in signal transduction pathways in prostate cancer. Int. J. Oncol. 2001;18:843–847. doi: 10.3892/ijo.18.4.843. [DOI] [PubMed] [Google Scholar]
- 39.Enokida H, Shiina H, Urakami S, Igawa M, Ogishima T, Li LC, Kawahara M, Nakagawa M, Kane CJ, Carroll PR, Dahiya R. Multigene methylation analysis for detection and staging of prostate cancer. Clin. Cancer Res. 2005;11:6582–6588. doi: 10.1158/1078-0432.CCR-05-0658. [DOI] [PubMed] [Google Scholar]
- 40.Richiardi L, Fiano V, Grasso C, Zugna D, Delsedime L, Gillio-Tos A, Merletti F. Methylation of APC and GSTP1 in non-neoplastic tissue adjacent to prostate tumour and mortality from prostate cancer. PLoS One. 2013;8:e68162. doi: 10.1371/journal.pone.0068162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edwards SM, Evans DG, Hope Q, Norman AR, Barbachano Y, Bullock S, Kote-Jarai Z, Meitz J, Falconer A, Osin P, Fisher C, Guy M, Jhavar SG, Hall AL, O'Brien LT, Gehr-Swain BN, Wilkinson RA, Forrest MS, Dearnaley DP, Ardern-Jones AT, Page EC, Easton DF, Eeles RA. UK Genetic Prostate Cancer Study Collaborators and BAUS Section of Oncology, Prostate cancer in BRCA2 germline mutation carriers is associated with poorer prognosis. Br. J. Cancer. 2010;103:918–924. doi: 10.1038/sj.bjc.6605822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pasche B, Yi N. Candidate gene association studies: successes and failures. Curr. Opin. Genet. Dev. 2010;20:257–261. doi: 10.1016/j.gde.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dechsukhum C, Ware JL, Ferreira-Gonzalez A, Wilkinson DS, Garrett CT. Detection of a novel truncated WT1 transcript in human neoplasia. Mol. Diagn. 2000;5:117–128. doi: 10.1007/BF03262030. [DOI] [PubMed] [Google Scholar]
- 44.Devilard E, Bladou F, Ramuz O, Karsenty G, Dalès JP, Gravis G, Nguyen C, Bertucci F, Xerri L, Birnbaum D. FGFR1 and WT1 are markers of human prostate cancer progression. BMC Cancer. 2006;6:272. doi: 10.1186/1471-2407-6-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tycko B, Li CM, Buttyan R. The Wnt/beta-catenin pathway in Wilms tumors and prostate cancers. Curr. Mol. Med. 2007;7:479–489. doi: 10.2174/156652407781387118. [DOI] [PubMed] [Google Scholar]
- 46.Vihko P. Human prostatic acid phosphatases: Purification of a minor enzyme and comparisons of the enzymes. Invest. Urol. 1979;16:349–352. [PubMed] [Google Scholar]
- 47.Van Etten RL. Human prostatic acid phosphatase: A histidine phosphatase. Ann. N.Y. Acad. Sci. 1982;390:27–51. doi: 10.1111/j.1749-6632.1982.tb40302.x. [DOI] [PubMed] [Google Scholar]
- 48.Lin MF, Lee CL, Li SS, Chu TM. Purification and characterization of a new human prostatic acid phosphatase isoenzyme. Biochemistry. 1983;22:1055–1062. doi: 10.1021/bi00274a009. [DOI] [PubMed] [Google Scholar]
- 49.Goldfarb DA, Stein BS, Shamszadeh M, Petersen RO. Age-related changes in tissue levels of prostatic acid phosphatase and prostate specific antigen. J. Urol. 1986;136:1266–1269. doi: 10.1016/s0022-5347(17)45310-9. [DOI] [PubMed] [Google Scholar]
- 50.Yam LT. Clinical significance of the human acid phosphatases: A review. Am. J. Med. 1974;56:604–616. doi: 10.1016/0002-9343(74)90630-5. [DOI] [PubMed] [Google Scholar]
- 51.Cunha AC, Weigle B, Kiessling A, Bachmann M, Rieber EP. Tissue-specificity of prostate specific antigens: Comparative analysis of transcript levels in prostate and non-prostatic tissues. Cancer Lett. 2006;236:229–238. doi: 10.1016/j.canlet.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 52.Graddis TJ, McMahan CJ, Tamman J, Page KJ, Trager JB. Prostatic acid phosphatase expression in human tissues. Int. J. Clin. Exp. Pathol. 2011;4:295–306. [PMC free article] [PubMed] [Google Scholar]
- 53.Gutman EB, Sproul EE, Gutman AB. Significance of increased phosphatase activity at the site of osteoplastic metastases secondary to carcinoma of the prostate gland. Am. J. Cancer. 1936;28:485–495. [Google Scholar]
- 54.Huggins C, Hodges CV. Studies on prostatic cancer: the effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–297. [Google Scholar]
- 55.Papsidero LD, Wojcieszyn JW, Horoszewicz JS, Leong SS, Murphy GP, Chu TM. Isolation of prostatic acid phosphatase-binding immunoglobulin G from human sera and its potential for use as a tumor-localizing reagent. Cancer Res. 1980;40:3032–3035. [PubMed] [Google Scholar]
- 56.Wang MC, Valenzuela LA, Murphy GP, Chu TM. Purification of a human prostate specific antigen. Invest. Urol. 1979;17:159–163. [PubMed] [Google Scholar]
- 57.Chu TM, Lin MF. PSA and acid phosphatase in the diagnosis of prostate cancer. J. Clin. Lig. Assay. 1998;21:24–34. [Google Scholar]
- 58.Igawa T, Lin FF, Rao P, Lin MF. Suppression of LNCaP prostate cancer xenograft tumors by a prostate-specific protein tyrosine phosphatase, prostatic acid phosphatase. Prostate. 2003;55:247–258. doi: 10.1002/pros.10240. [DOI] [PubMed] [Google Scholar]
- 59.Vihko PT, Quintero I, Ronka AE, Herrala A, Jantti P, Porvari K, Lindqvist Y, Kaija H, Pulkka A, Vuoristo J, Sormunen R, Soini Y, Halmekytö M, Jänne J, Luokkala T, Kurkela R. Prostatic acid phosphatase (PAcP) is PI(3)P-phosphatase and its inactivation leads to change of cell polarity and invasive prostate cancer. Proc. Amer. Assoc. Cancer Res. 2005;46:5239. [Google Scholar]
- 60.Sharief FS, Lee H, Leuderman MM, Lundwall A, Deaven LL, Lee CL, Li SS. Human prostatic acid phosphatase: cDNA cloning, gene mapping and protein sequence homology with lysosomal acid phosphatase. Biochem. Biophys. Res. Commun. 1989;160:79–86. doi: 10.1016/0006-291x(89)91623-9. [DOI] [PubMed] [Google Scholar]
- 61.Sharief FS, Li SS. Structure of human prostatic acid phosphatase gene. Biochem. Biophys. Res. Commun. 1992;184:1468–1476. doi: 10.1016/s0006-291x(05)80048-8. [DOI] [PubMed] [Google Scholar]
- 62.Drenckhahn D, Waheed A, van Etten R. Demonstration of prostatic-type acid phosphatase in non-lysosomal granules in the crypt epithelium of the human duodenum. Histochemistry. 1987;88:47–52. doi: 10.1007/BF00490166. [DOI] [PubMed] [Google Scholar]
- 63.Risley MJ, Van Etten RL. Structures of the carbohydrate moieties of human prostatic acid phosphatase elucidated by 1H nuclear magnetic resonance spectroscopy. Arch. Biochem. Biophys. 1987;258:404–412. doi: 10.1016/0003-9861(87)90361-4. [DOI] [PubMed] [Google Scholar]
- 64.Hakalahti L, Vihko P, Henttu P, Autio-Harmainen H, Soini Y, Vihko R. Evaluation of PAP and PSA gene expression in prostatic hyperplasia and prostatic carcinoma using northern- blot analyses, in situ hybridization and immunohistochemical stainings with monoclonal and bispecifi c antibodies. Int. J. Cancer. 1993;55:590–597. doi: 10.1002/ijc.2910550413. [DOI] [PubMed] [Google Scholar]
- 65.Kuciel R, Bakalova A, Mazurkiewicz A, Bilska A, Ostrowski W. Is the subunit of prostatic phosphatase active? Reversible denaturation of prostatic acid phosphatase. Biochem. Int. 1990;22:329–334. [PubMed] [Google Scholar]
- 66.Jakob CG, Lewinski K, Kuciel R, Ostrowski W, Lebioda L. Crystal structure of human prostatic acid phosphatase. Prostate. 2000;42:211–218. doi: 10.1002/(sici)1097-0045(20000215)42:3<211::aid-pros7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 67.Luchter-Wasylewska E. Cooperative kinetics of human prostatic acid phosphatase. Biochim. Biophys. Acta. 2001;1548:257–264. doi: 10.1016/s0167-4838(01)00239-4. [DOI] [PubMed] [Google Scholar]
- 68.Van Etten RL, Davidson R, Stevis PE, MacArthur H, Moore DL. Covalent structure, disulfide bonding, and identifi cation of reactive surface and active site residues of human prostatic acid phosphatase. J. Biol. Chem. 1991;266:2313–2319. [PubMed] [Google Scholar]
- 69.Schneider G, Lindqvist Y, Vihko P. Three-dimensional structure of rat acid phosphatase. EMBO J. 1993;12:2609–2615. doi: 10.1002/j.1460-2075.1993.tb05921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ostanin K, Saeed A, van Etten RL. Heterologous expression of human prostatic acid phosphatase and site-directed mutagenesis of the enzyme active site. J. Biol. Chem. 1994;269:8971–8978. [PubMed] [Google Scholar]
- 71.Zhang XQ, Lee MS, Zelivianski S, Lin MF. Characterization of a prostate-specific tyrosine phosphatase by mutagenesis and expression in human prostate cancer cells. J. Biol. Chem. 2001;276:2544–2550. doi: 10.1074/jbc.M006661200. [DOI] [PubMed] [Google Scholar]
- 72.Porvari KS, Herrala AM, Kurkela RM, Taavitsainen PA, Lindqvist Y, Schneider G, Vihko PT. Site-directed mutagenesis of prostatic acid phosphatase. Catalytically important aspartic acid 258, substrate specificity, and oligomerization. J. Biol. Chem. 1994;269:22642–22646. [PubMed] [Google Scholar]
- 73.Ortlund E, LaCount MW, Lebioda L. Crystal structures of human prostatic acid phosphatase in complex with a phosphate ion and alpha-benzylaminobenzylphosphonic acid update the mechanistic picture and offer new insights into inhibitor design. Biochemistry. 2003;42:383–389. doi: 10.1021/bi0265067. [DOI] [PubMed] [Google Scholar]
- 74.Lad PM, Learn DB, Cooper JF, Reisinger DM. Distribution of prostatic acid phosphatase isoenzymes in normal and cancerous states. Clin. Chim. Acta. 1984;141:51–65. doi: 10.1016/0009-8981(84)90166-9. [DOI] [PubMed] [Google Scholar]
- 75.Foti AG, Cooper JF, Herschman H. Prostatic acid phosphatase and prostatic cancer. Recent Results Cancer Res. 1979;67:45–49. doi: 10.1007/978-3-642-81320-7_7. [DOI] [PubMed] [Google Scholar]
- 76.Ronnberg L, Vihko P, Sajanti E, Vihko R. Clomiphene citrate administration to normogonadotropic subfertile men: blood hormone changes and activation of acid phosphatase in seminal fluid. Int. J. Androl. 1981;4:372–378. doi: 10.1111/j.1365-2605.1981.tb00721.x. [DOI] [PubMed] [Google Scholar]
- 77.Lin MF, Garcia-Arenas R, Xia XZ, Biela B, Lin FF. The cellular level of prostatic acid phosphatase and the growth of human prostate carcinoma cells. Differentiation. 1994;57:143–149. doi: 10.1046/j.1432-0436.1994.5720143.x. [DOI] [PubMed] [Google Scholar]
- 78.Lin MF, Garcia-Arenas R. Effect of cell density on androgen regulation of the mRNA level of human prostatic acid phosphatase. Mol. Cell. Endocrinol. 1994;99:R21–R24. doi: 10.1016/0303-7207(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 79.Solin T, Kontturi M, Pohlmann R, Vihko P. Gene expression and prostate specificity of human prostatic acid phosphatase (PAP): evaluation by RNA blot analyses. Biochim. Biophys. Acta. 1990;1048:72–77. doi: 10.1016/0167-4781(90)90024-v. [DOI] [PubMed] [Google Scholar]
- 80.Reif AE, Schlesinger RM, Fish CA, Robinson CM. Acid phosphatase isozymes in cancer of the prostate. Cancer. 1973;31:689–699. doi: 10.1002/1097-0142(197303)31:3<689::aid-cncr2820310331>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 81.Lin MF, Meng TC, Rao PS, Chang C, Schonthal AH, Lin FF. Expression of human prostatic acid phosphatase correlates with androgen-stimulated cell proliferation in prostate cancer cell lines. J. Biol. Chem. 1998;273:5939–5947. doi: 10.1074/jbc.273.10.5939. [DOI] [PubMed] [Google Scholar]
- 82.Meng TC, Lee MS, Lin MF. Interaction between protein tyrosine phosphatase and protein tyrosine kinase is involved in androgen-promoted growth of human prostate cancer cells. Oncogene. 2000;19:2664–2677. doi: 10.1038/sj.onc.1203576. [DOI] [PubMed] [Google Scholar]
- 83.Mori K, Wakasugi C. Immunocytochemical demonstration of prostatic acid phosphatase: different secretion kinetics between normal, hyperplastic and neoplastic prostates. J. Urol. 1985;133:877–883. doi: 10.1016/s0022-5347(17)49271-8. [DOI] [PubMed] [Google Scholar]
- 84.Lilja H, Abrahamsson PA. Three predominant proteins secreted by the human prostate gland. Prostate. 1988;12:29–38. doi: 10.1002/pros.2990120105. [DOI] [PubMed] [Google Scholar]
- 85.Sinha AA, Gleason DF, Wilson MJ, Wick MR, Reddy PK, Blackard CE. Relationship of prostatic acid phosphatase localization in human prostate by a monoclonal antibody with the Gleason grading system. Prostate. 1988;13:1–15. doi: 10.1002/pros.2990130102. [DOI] [PubMed] [Google Scholar]
- 86.Seitz J, Aumüller G. Cytochemistry and biochemistry of acid phosphatases V: Electrophoretic studies on the heterogeneity of acid phosphatases from human prostate, seminal fluid, and leukocytes. Prostate. 1985;7:73–90. doi: 10.1002/pros.2990070109. [DOI] [PubMed] [Google Scholar]
- 87.Lam KW, Li CY, Yam LT, Sun T, Lee G, Ziesmer S. Improved immunohistochemical detection of prostatic acid phosphatase by a monoclonal antibody. Prostate. 1989;15:13–21. doi: 10.1002/pros.2990150103. [DOI] [PubMed] [Google Scholar]
- 88.Dionne FT, Chevalier S, Bleau G, Roberts KD, Chapdelaine A. Induction of acid phosphatase synthesis in canine prostatic epithelial cells in vitro. Mol. Cell. Endocrinol. 1983;33:113–126. doi: 10.1016/0303-7207(83)90060-6. [DOI] [PubMed] [Google Scholar]
- 89.Lin MF, DaVolio J, Garcia-Arenas R. Expression of human prostatic acid phosphatase activity and the growth of prostate carcinoma cells. Cancer Res. 1992;52:4600–4607. [PubMed] [Google Scholar]
- 90.Igawa T, Lin FF, Lee MS, Karan D, Batra SK, Lin MF. Establishment and characterization of androgen-independent human prostate cancer LNCaP cell model. Prostate. 2002;50:222–235. doi: 10.1002/pros.10054. [DOI] [PubMed] [Google Scholar]
- 91.Yuan TC, Lin FF, Veeramani S, Chen SJ, Earp HS, III, Lin MF. ErbB-2 via PYK2 upregulates the adhesive ability of androgen receptor-positive human prostate cancer cells. Oncogene. 2007;26:7552–7559. doi: 10.1038/sj.onc.1210570. [DOI] [PubMed] [Google Scholar]
- 92.Lin MF, DaVolio J, Garcia R. Cationic liposome-mediated incorporation of prostatic acid phosphatase protein into human prostate carcinoma cells. Biochem. Biophys. Res. Commun. 1993;192:413–419. doi: 10.1006/bbrc.1993.1431. [DOI] [PubMed] [Google Scholar]
- 93.Lin MF, Meng TC. Tyrosine phosphorylation of a 185 kDa Phosphoprotein (pp185) inversely correlates with the cellular activity of human prostatic acid phosphatase. Biochem. Biophys. Res. Comm. 1996;226:206–213. doi: 10.1006/bbrc.1996.1334. [DOI] [PubMed] [Google Scholar]
- 94.Chou YW, Chaturvedi NK, Ouyang S, Lin FF, Kaushik D, Wang J, Kim I, Lin MF. Histone deacetylase inhibitor valproic acid suppresses the growth and increases the androgen responsiveness of prostate cancer cells. Cancer Lett. 2011;311:177–186. doi: 10.1016/j.canlet.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Foti AG, Herschman H, Cooper JF. Isozymes of acid phosphatase in normal and cancerous human prostatic tissue. Cancer Res. 1977;37:4120–4124. [PubMed] [Google Scholar]
- 96.Loor R, Wang MC, Valenzuela L, Chu TM. Expression of prostatic acid phosphatase in human prostate cancer. Cancer Lett. 1981;14:63–69. doi: 10.1016/0304-3835(81)90010-0. [DOI] [PubMed] [Google Scholar]
- 97.Dillard PR, Lin MF, Khan SA. Androgen-independent prostate cancer cells acquire the complete steroidogenic potential of synthesizing testosterone from cholesterol. Mol. Cell Endocrinol. 2008;295:115–120. doi: 10.1016/j.mce.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Svensson RU, Haverkamp JM, Thedens DR, Cohen MB, Ratliff TL, Henry MD. Slow disease progression in a C57BL/6 pten-deficient mouse model of prostate cancer. Am. J. Pathol. 2011;179:502–512. doi: 10.1016/j.ajpath.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Roy-Burman P, Wu H, Powell WC, Hagenkord J, Cohen MB. Genetically defined mouse models that mimic natural aspects of human prostate cancer development. Endocr. Relat. Cancer. 2004;11:225–254. doi: 10.1677/erc.0.0110225. [DOI] [PubMed] [Google Scholar]
- 100.Zylka MJ, Sowa NA, Taylor-Blake B, Twomey MA, Herrala A, Voikar V, Vihko P. Prostatic acid phosphatase is an ectonucleotidase and suppresses pain by generating adenosine. Neuron. 2008;60:111–122. doi: 10.1016/j.neuron.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Choe BK, Lillehoj HS, Dong MK, Gleason S, Barron M, Rose NR. Characterization of antigenic sites of human prostatic acid phosphatase. Ann. N. Y. Acad. Sci. 1982;390:16–26. doi: 10.1111/j.1749-6632.1982.tb40301.x. [DOI] [PubMed] [Google Scholar]
- 102.Wasylewska E, Czubak J, Ostrowski WS. Phosphoprotein phosphatase activity of human prostate acid phosphatase. Acta. Biochim. Pol. 1983;30:175–184. [PubMed] [Google Scholar]
- 103.Lin MF, Clinton GM. Human prostatic acid phosphatase has phosphotyrosyl protein phosphatase activity. Biochem. J. 1986;235:351–357. doi: 10.1042/bj2350351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chevalier S, Landry D, Chapdelaine A. Phosphotyrosine phosphatase activity of human and canine acid phosphatases of prostatic origin. Prostate. 1988;12:209–219. doi: 10.1002/pros.2990120304. [DOI] [PubMed] [Google Scholar]
- 105.Lin MF, Clinton GM. Human prostatic acid phosphatase and its phosphotyrosylprotein phosphatase activity. Adv. Protein Phosphatases. 1987;4:199–228. [Google Scholar]
- 106.Vihko P, Virkkunen P, Henttu P, Roiko K, Solin T, Huhtala ML. Molecular cloning and sequence analysis of cDNA encoding human prostatic acid phosphatase. FEBS Lett. 1988;236:275–281. doi: 10.1016/0014-5793(88)80037-1. [DOI] [PubMed] [Google Scholar]
- 107.Roiko K, Janne OA, Vihko P. Primary structure of rat secretory acid phosphatase and comparison to other acid phosphatases. Gene. 1990;89:223–229. doi: 10.1016/0378-1119(90)90009-g. [DOI] [PubMed] [Google Scholar]
- 108.Jackson MD, Denu JM. Molecular reactions of protein phosphatases-insights from structure and chemistry. Chem. Rev. 2001;101:2313–2340. doi: 10.1021/cr000247e. [DOI] [PubMed] [Google Scholar]
- 109.Li HC, Chernoff J, Chen LB, Kirschonbaum A. A phosphotyrosyl-protein phosphatase activity associated with acid phosphatase from human prostate gland. Eur. J. Biochem. 1984;138:45–51. doi: 10.1111/j.1432-1033.1984.tb07879.x. [DOI] [PubMed] [Google Scholar]
- 110.Vihko P, Kurkela R, Porvari K, Herrala A, Lindfors A, Lindqvist Y, Schneider G. Rat acid phosphatase: overexpression of active, secreted enzyme by recombinant baculovirus-infected insect cells, molecular properties, and crystallization. Proc. Natl. Acad. Sci. USA. 1993;90:799–803. doi: 10.1073/pnas.90.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Craft N, Shostak Y, Carey M, Sawyers CL. Mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat. Med. 1999;5:280–285. doi: 10.1038/6495. [DOI] [PubMed] [Google Scholar]
- 112.Yeh S, Lin HK, Kang HY, Thin TH, Lin MF, Chang C. From HER2/Neu signal cascade to androgen receptor and its coactivators: a novel pathway by induction of androgen target genes through MAP kinase in prostate cancer cells. Proc. Natl. Acad. Sci. USA. 1999;96:5458–5463. doi: 10.1073/pnas.96.10.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lin MF, Clinton GM. The epidermal growth factor receptor from prostate cells is dephosphorylated by a prostate-specific phosphotyrosyl phosphatase. Mol. Cell. Biol. 1988;8:5477–5485. doi: 10.1128/mcb.8.12.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Meng TC, Lin MF. Tyrosine phosphorylation of c-ErbB-2 is regulated by the cellular form of prostatic acid phosphatase in human prostate cancer cells. J. Biol. Chem. 1998;273:22096–22104. doi: 10.1074/jbc.273.34.22096. [DOI] [PubMed] [Google Scholar]
- 115.Di Lorenzo G, Tortora G, D'Armiento FP, De Rosa G, Staibano S, Autorino R, D'Armiento M, De Laurentiis M, De Placido S, Catalano G, Bianco AR, Ciardiello F. Expression of epidermal growth factor receptor correlates with disease relapse and progression to androgen-independence in human prostate cancer. Clin. Cancer Res. 2002;8:3438–3444. [PubMed] [Google Scholar]
- 116.Lee MS, Igawa T, Yuan TC, Zhang XQ, Lin FF, Lin MF. ErbB-2 signaling is involved in regulating PSA secretion in androgen-independent human prostate cancer LNCaP C-81 cells. Oncogene. 2003;22:781–796. doi: 10.1038/sj.onc.1206066. [DOI] [PubMed] [Google Scholar]
- 117.Ricciardelli C, Jackson MW, Choong CS, Stahl J, Marshall VR, Horsfall DJ, Tilley WD. Elevated levels of HER-2/neu and androgen receptor in clinically localized prostate cancer identifies metastatic potential. Prostate. 2008;68:830–838. doi: 10.1002/pros.20747. [DOI] [PubMed] [Google Scholar]
- 118.Soler M, Mancini F, Meca-Cortes O, Sánchez-Cid L, Rubio N, López-Fernández S, Lozano JJ, Blanco J, Fernández PL, Thomson TM. HER3 is required for the maintenance of neuregulin-dependent and -independent attributes of malignant progression in prostate cancer cells. Int. J. Cancer. 2009;125:2565–2575. doi: 10.1002/ijc.24651. [DOI] [PubMed] [Google Scholar]
- 119.Veeramani S, Chou YW, Lin FC, Muniyan S, Lin FF, Kumar S, Xie Y, Lele SM, Tu Y, Lin MF. Reactive oxygen species induced by p66Shc longevity protein mediate nongenomic androgen action via tyrosine phosphorylation signaling to enhance tumorigenicity of prostate cancer cells. Free Radic. Biol. Med. 2012;53:95–108. doi: 10.1016/j.freeradbiomed.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Veeramani S, Igawa T, Yuan TC, Lin FF, Lee MS, Lin JS, Johansson SL, Lin MF. Expression of p66(Shc) protein correlates with proliferation of human prostate cancer cells. Oncogene. 2005;24:7203–7212. doi: 10.1038/sj.onc.1208852. [DOI] [PubMed] [Google Scholar]
- 121.Chen SJ, Karan D, Johansson SL, Lin FF, Zeckser J, Singh AP, Batra SK, Lin MF. Prostate-derived factor as a paracrine and autocrine factor for the proliferation of androgen receptor-positive human prostate cancer cells. Prostate. 2007;67:557–571. doi: 10.1002/pros.20551. [DOI] [PubMed] [Google Scholar]
- 122.Radhakrishnan P, Chachadi V, Lin MF, Singh R, Kannagi R, Cheng PW. TNFα enhances the motility and invasiveness of prostatic cancer cells by stimulating the expression of selective glycosyl- and sulfotransferase genes involved in the synthesis of selectin ligands. Biochem. Biophys. Res. Commun. 2011;409:436–441. doi: 10.1016/j.bbrc.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sharma S, Pirila P, Kaija H, Porvari K, Vihko P, Juffer AH. Theoretical investigations of prostatic acid phosphatase. Proteins. 2005;58:295–308. doi: 10.1002/prot.20335. [DOI] [PubMed] [Google Scholar]
- 124.Pulido R, Stoker AW, Hendriks WJ. PTPs emerge as PIPs: protein tyrosine phosphatases with lipid-phosphatase activities in human disease. Hum. Mol. Genet. 2013;22:R66–R76. doi: 10.1093/hmg/ddt347. [DOI] [PubMed] [Google Scholar]
- 125.Lin HK, Hu YC, Yang L, Altuwaijri S, Chen YT, Kang HY, Chang C. Suppression versus induction of androgen receptor functions by the phosphatidylinositol 3-kinase/Akt pathway in prostate cancer LNCaP cells with different passage numbers. J. Biol. Chem. 2003;278:50902–50907. doi: 10.1074/jbc.M300676200. [DOI] [PubMed] [Google Scholar]
- 126.Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat. Rev. Mol. Cell. Biol. 2012;13:283–296. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- 127.Steelman LS, Bertrand FE, McCubrey JA. The complexity of PTEN: mutation, marker and potential target for therapeutic intervention. Expert Opin. Ther. Targets. 2004;8:537–550. doi: 10.1517/14728222.8.6.537. [DOI] [PubMed] [Google Scholar]
- 128.Li L, Ross AH. Why is PTEN an important tumor suppressor? J. Cell Biochem. 2007;102:1368–1374. doi: 10.1002/jcb.21593. [DOI] [PubMed] [Google Scholar]
- 129.Gericke A, Munson M, Ross AH. Regulation of the PTEN phosphatase. Gene. 2006;374:1–9. doi: 10.1016/j.gene.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 130.Chalhoub N, Baker SJ. PTEN and the PI3-kinase pathway in cancer. Annu. Rev. Pathol. 2009;4:127–150. doi: 10.1146/annurev.pathol.4.110807.092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem. J. 2001;353:417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mayer-Jaekel RE, Hemmings BA. Protein phosphatase 2A – a ‘menage a trois’. Trends Cell Biol. 1994;4:287–291. doi: 10.1016/0962-8924(94)90219-4. [DOI] [PubMed] [Google Scholar]
- 133.Kamibayashi C, Estes R, Lickteig RL, Yang SI, Craft C, Mumby MC. Comparison of heterotrimeric protein phosphatase 2A containing different B subunits. J. Biol. Chem. 1994;269:20139–20148. [PubMed] [Google Scholar]
- 134.Cohen PT. Novel protein serine/threonine phosphatases: variety is the spice of life. Trends Biochem. Sci. 1997;22:245–251. doi: 10.1016/s0968-0004(97)01060-8. [DOI] [PubMed] [Google Scholar]
- 135.Arnold HK, Sears RC. Protein phosphatase 2A regulatory subunit B56alpha associates with c-myc and negatively regulates c-myc accumulation. Mol. Cell. Biol. 2006;26:2832–2844. doi: 10.1128/MCB.26.7.2832-2844.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Eichhorn PJ, Creyghton MP, Bernards R. Protein phosphatase 2A regulatory subunits and cancer. Biochim. Biophys. Acta. 2009;1795:1–15. doi: 10.1016/j.bbcan.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 137.Kuo YC, Huang KY, Yang CH, Yang YS, Lee WY, Chiang CW. Regulation of phosphorylation of Thr-308 of Akt, cell proliferation, and survival by the B55alpha regulatory subunit targeting of the protein phosphatase 2A holoenzyme to Akt. J. Biol. Chem. 2008;283:1882–1892. doi: 10.1074/jbc.M709585200. [DOI] [PubMed] [Google Scholar]
- 138.Cheng Y, Liu W, Kim ST, Sun J, Lu L, Sun J, Zheng SL, Isaacs WB, Xu J. Evaluation of PPP2R2A as a prostate cancer susceptibility gene: a comprehensive germline and somatic study. Cancer Genet. 2011;204:375–381. doi: 10.1016/j.cancergen.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Warfel NA, Newton AC. Pleckstrin homology domain leucine-rich repeat protein phosphatase (PHLPP): a new player in cell signaling. J. Biol. Chem. 2012;287:3610–3616. doi: 10.1074/jbc.R111.318675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Brognard J, Sierecki E, Gao T, Newton AC. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol. Cell. 2007;25:917–931. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 141.Carrión-Salip D, Panosa C, Menendez JA, Puig T, Oliveras G, Pandiella A, DeLlorens R, Massaguer A. Androgen-independent prostate cancer cells circumvent EGFR inhibition by overexpression of alternative HER receptors and ligands. Int. J. Oncol. 2012;41:1128–1138. doi: 10.3892/ijo.2012.1509. [DOI] [PubMed] [Google Scholar]
- 142.Chen L, Mooso BA, Jathal MK, Madhav A, Johnson SD, van Spyk E, Mikhailova M, Zierenberg-Ripoll A, Xue L, Vinall RL, deVere White RW, Ghosh PM. Dual EGFR/HER2 inhibition sensitizes prostate cancer cells to androgen withdrawal by suppressing ErbB3. Clin. Cancer Res. 2011;17:6218–6228. doi: 10.1158/1078-0432.CCR-11-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jain A, Penuel E, Mink S, Schmidt J, Hodge A, Favero K, Tindell C, Agus DB. HER kinase axis receptor dimer partner switching occurs in response to EGFR tyrosine kinase inhibition despite failure to block cellular proliferation. Cancer Res. 2010;70:1989–1999. doi: 10.1158/0008-5472.CAN-09-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.van Niekerk CC, Poels LG. Reduced expression of protein tyrosine phosphatase gamma in lung and ovarian tumors. Cancer Lett. 1999;137:61–73. doi: 10.1016/s0304-3835(98)00344-9. [DOI] [PubMed] [Google Scholar]
- 145.Longo FM, Martignetti JA, Le Beau JM, Zhang JS, Barnes JP, Brosius J. Leukocyte common antigen-related receptor-linked tyrosine phosphatase. Regulation of mRNA expression. J. Biol. Chem. 1993;268:26503–26511. [PubMed] [Google Scholar]
- 146.Broggini M, Buraggi G, Brenna A, Riva L, Codegoni AM, Torri V, Lissoni AA, Mangioni C, D'Incalci M. Cell cycle-related phosphatases CDC25A and B expression correlates with survival in ovarian cancer patients. Anticancer Res. 2000;20:4835–4840. [PubMed] [Google Scholar]
- 147.Lee JK, Edderkaoui M, Truong P, Ohno I, Jang KT, Berti A, Pandol SJ, Gukovskaya AS. NADPH oxidase promotes pancreatic cancer cell survival via inhibiting JAK2 dephosphorylation by tyrosine phosphatases. Gastroenterology. 2007;133:1637–1648. doi: 10.1053/j.gastro.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 148.Paul MK, Mukhopadhyay AK. Tyrosine kinase-role and significance in cancer. Int. J. Med. Sci. 2004;1:101–115. doi: 10.7150/ijms.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang Z, Shen D, Parsons DW, Bardelli A, Sager J, Szabo S, Ptak J, Silliman N, Peters BA, van der Heijden MS, Parmigiani G, Yan H, Wang TL, Riggins G, Powell SM, Willson JK, Markowitz S, Kinzler KW, Vogelstein B, Velculescu VE. Mutational analysis of the tyrosine phosphatome in colorectal cancers. Science. 2004;304:1164–1166. doi: 10.1126/science.1096096. [DOI] [PubMed] [Google Scholar]
- 150.Georgescu MM. PTEN Tumor Suppressor Network in PI3K-Akt Pathway Control. Genes Cancer. 2010;1:1170–1177. doi: 10.1177/1947601911407325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Slomovitz BM, Coleman RL. The PI3K/AKT/mTOR pathway as a therapeutic target in endometrial cancer. Clin. Cancer Res. 2012;18:5856–5864. doi: 10.1158/1078-0432.CCR-12-0662. [DOI] [PubMed] [Google Scholar]
- 152.Hafsi S, Pezzino FM, Candido S, Ligresti G, Spandidos DA, Soua Z, McCubrey JA, Travali S, Libra M. Gene alterations in the PI3K/PTEN/AKT pathway as a mechanism of drug-resistance. Int. J. Oncol. 2012;40:639–644. doi: 10.3892/ijo.2011.1312. [DOI] [PubMed] [Google Scholar]
- 153.Demicco EG, Torres KE, Ghadimi MP, Colombo C, Bolshakov S, Hoffman A, Peng T, Bovée JV, Wang WL, Lev D, Lazar AJ. Involvement of the PI3K/Akt pathway in myxoid/round cell liposarcoma. Mod Pathol. 2012;25:212–221. doi: 10.1038/modpathol.2011.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dillon LM, Miller TW. Therapeutic Targeting of Cancers with Loss of PTEN Function. Curr. Drug Targets. 2014;15:65–79. doi: 10.2174/1389450114666140106100909. [DOI] [PMC free article] [PubMed] [Google Scholar]