Abstract
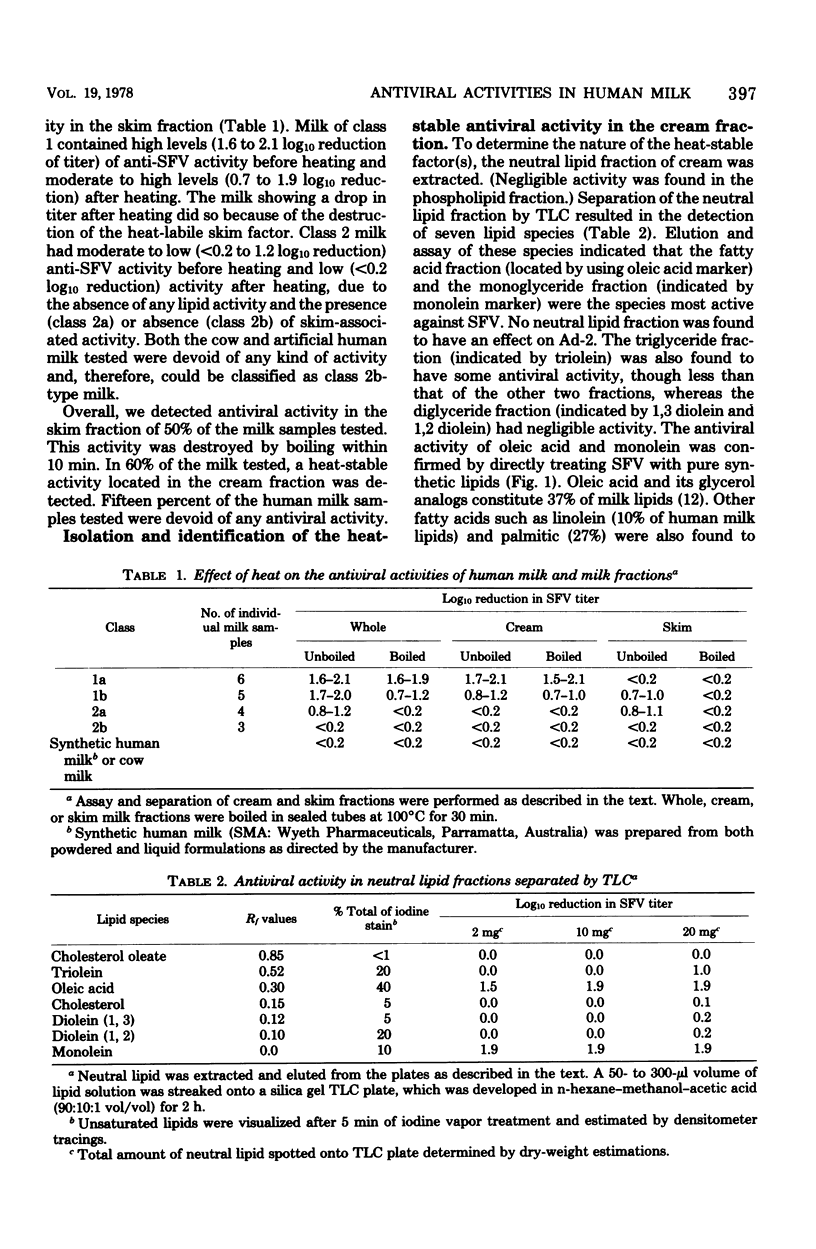
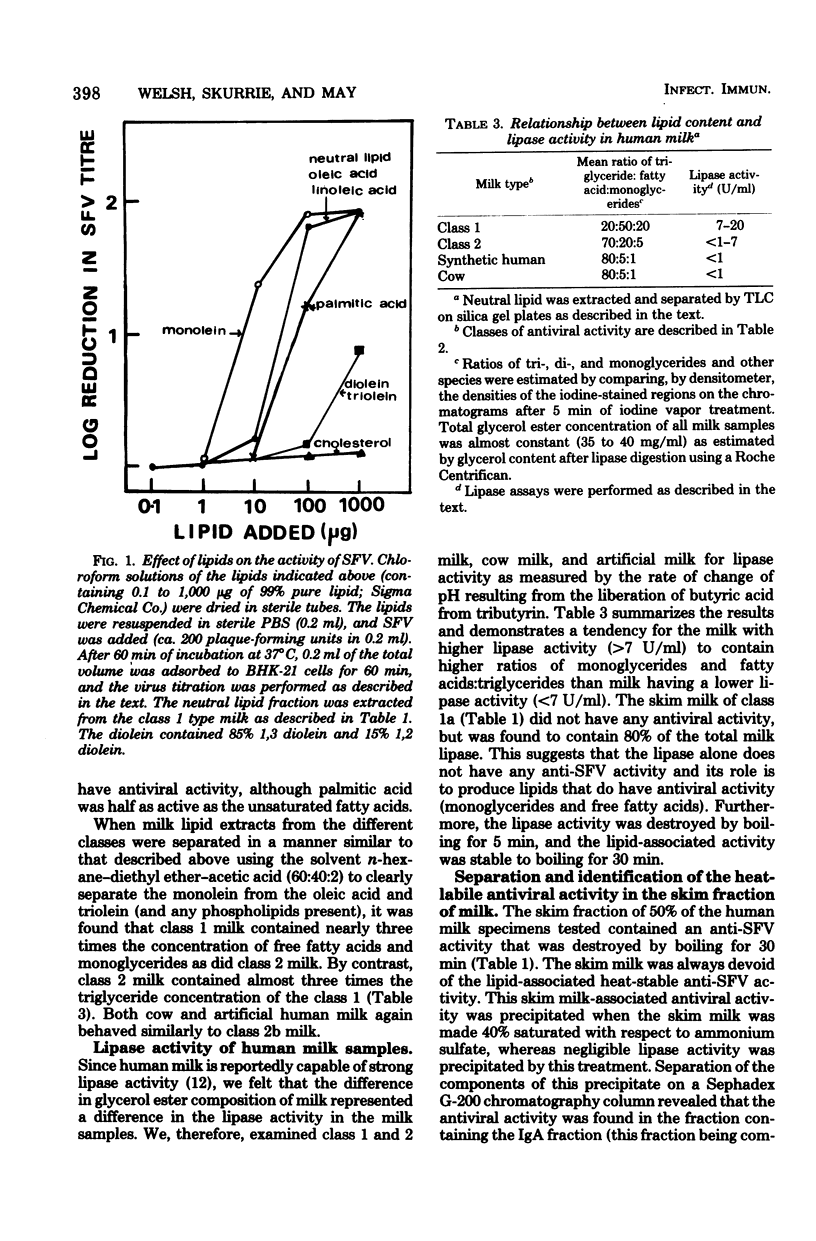
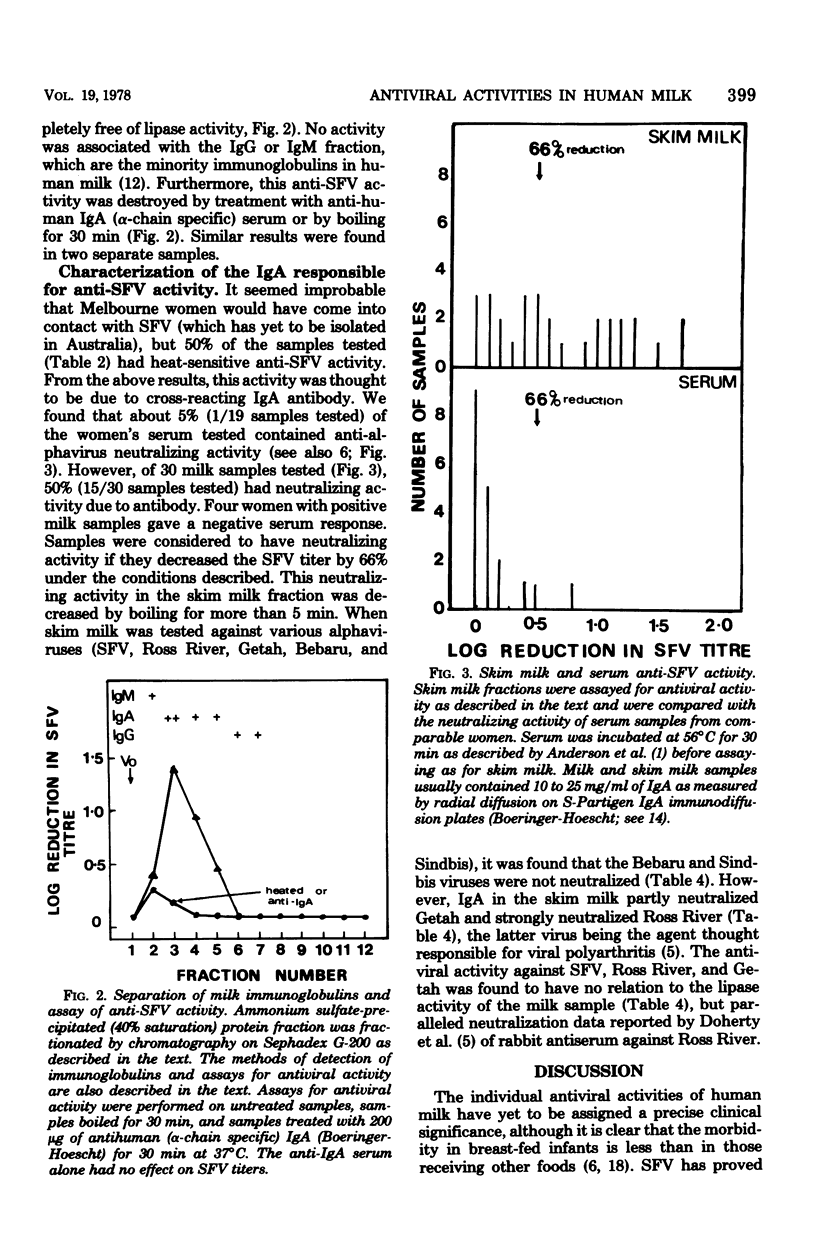
In a simple and reliable assay system, Semliki forest virus (SFV) was used to detect the activity of antiviral factors in human milk. Fractionation of the milk showed that a heat-stable, lipid-associated activity and an immunoglobulin-associated activity were present, either singly or together, in 85% of the human milk samples tested. Cow and synthetic milk showed neither activity. Extraction of the neutral milk lipids allowed the antiviral to be located with the monoglyceride and free fatty acid fractions. The milk low in antiviral lipids and high in triglycerides also lacked a strong lipase activity. The immunoglobulin anti-SFV activity was shown to be due to immunoglobulin A, the major milk immunoglobulin, and appears to be directed against an alphavirus closely related to SFV, possibly Ross River virus.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axel R., Gulati S. C., Spiegelman S. Particles containing RNA-instructed DNA polymerase and virus-related RNA in human breast cancers. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3133–3137. doi: 10.1073/pnas.69.11.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M. R., Padhy L. C., Koshy R., Sirsat S. M., Rich M. A. Human milk samples from different ethnic groups contain RNase that inhibits, and plasma membrane that stimulates, reverse transcription. Nature. 1976 Aug 26;262(5571):802–805. doi: 10.1038/262802a0. [DOI] [PubMed] [Google Scholar]
- Downham M. A., Scott R., Sims D. G., Webb J. K., Gardner P. S. Breast-feeding protects against respiratory syncytial virus infections. Br Med J. 1976 Jul 31;2(6030):274–276. doi: 10.1136/bmj.2.6030.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emödi G., Just M. Interferon production by lymphocytes in human milk. Scand J Immunol. 1974;3(2):157–160. doi: 10.1111/j.1365-3083.1974.tb01243.x. [DOI] [PubMed] [Google Scholar]
- Falkler W. A., Jr, Diwan A. R., Halstead S. B. A lipid inhibitor of dengue virus in human colostrum and milk; with a note on the absence of anti-dengue secretory antibody. Arch Virol. 1975;47(1):3–10. doi: 10.1007/BF01315587. [DOI] [PubMed] [Google Scholar]
- Fieldsteel A. H. Nonspecific antiviral substances in human milk active against arbovirus and murine leukemia virus. Cancer Res. 1974 Apr;34(4):712–715. [PubMed] [Google Scholar]
- Goldman A. S., Smith C. W. Host resistance factors in human milk. J Pediatr. 1973 Jun;82(6):1082–1090. doi: 10.1016/s0022-3476(73)80453-6. [DOI] [PubMed] [Google Scholar]
- György P. The uniqueness of human milk. Biochemical aspects. Am J Clin Nutr. 1971 Aug;24(8):970–975. doi: 10.1093/ajcn/24.8.970. [DOI] [PubMed] [Google Scholar]
- HUMMELER K., GYORGY P., HOOVER J. R., KUHN R. Fractions of human milk and virus multiplication. Science. 1953 Dec 25;118(3078):781–782. doi: 10.1126/science.118.3078.781. [DOI] [PubMed] [Google Scholar]
- MICHAELS R. H. STUDIES OF ANTIVIRAL FACTORS IN HUMAN MILK AND SERUM. J Immunol. 1965 Feb;94:262–271. [PubMed] [Google Scholar]
- Mata L. J., Wyatt R. G. The uniqueness of human milk. Host resistance to infection. Am J Clin Nutr. 1971 Aug;24(8):976–986. doi: 10.1093/ajcn/24.8.976. [DOI] [PubMed] [Google Scholar]
- Matthews T. H., Nair C. D., Lawrence M. K., Tyrrell D. A. Antiviral activity in milk of possible clinical importance. Lancet. 1976 Dec 25;2(8000):1387–1389. doi: 10.1016/s0140-6736(76)91922-x. [DOI] [PubMed] [Google Scholar]
- McCormick J. J., Larson L. J., Rich M. A. RNase inhibition of reverse transcriptase activity in human milk. Nature. 1974 Oct 25;251(5477):737–740. doi: 10.1038/251737a0. [DOI] [PubMed] [Google Scholar]
- ROBINSON M. Infant morbidity and mortality. A study of 3266 infants. Lancet. 1951 Apr 7;1(6658):788–793. doi: 10.1016/s0140-6736(51)92212-x. [DOI] [PubMed] [Google Scholar]
- SABIN A. B., FIELDSTEEL A. H. Antipoliomyelitic activity of human and bovine colostrum and milk. Pediatrics. 1962 Jan;29:105–115. [PubMed] [Google Scholar]
- SILVER R. K., BRAUN G., ZILLIKEN F., WERNER G. H., GYORGY P. Factors in human milk interfering with influenza-virus activities. Science. 1956 May 25;123(3204):932–933. doi: 10.1126/science.123.3204.932. [DOI] [PubMed] [Google Scholar]
- Sarkar N. H., Charney J., Dion A. S., Moore D. H. Effect of human milk on the mouse mammary tumor virus. Cancer Res. 1973 Mar;33(3):626–629. [PubMed] [Google Scholar]
- Sarkar N. H., Moore D. H. On the possibility of a human breast cancer virus. Nature. 1972 Mar 17;236(5342):103–106. doi: 10.1038/236103a0. [DOI] [PubMed] [Google Scholar]



