Abstract
Objectives
Fat is digested in the intestine into free fatty acids (FFAs), which are detergents and therefore toxic to cells at micromolar concentration. The mucosal barrier protects cells in the adult intestine, but this barrier may not be fully developed in premature infants. Lipase-digested infant formula, but not fresh human milk, has elevated FFAs and is cytotoxic to intestinal cells, and therefore could contribute to intestinal injury in necrotizing enterocolitis (NEC). But even infants exclusively fed breast milk may develop NEC. Our objective was to determine if stored milk and milk from donor milk banks (DM) could also become cytotoxic, especially after digestion.
Methods
We exposed cultured rat intestinal epithelial cells or human neutrophils to DM and milk collected fresh and stored at 4 or −20 °C for up to 12 weeks and then treated for 2 hours (37°C) with 0.1 or 1 mg/ml pancreatic lipase and/or trypsin and chymotrypsin.
Results
DM and milk stored 3 days (at 4 or −20 °C) and then digested were cytotoxic. Storage at −20 °C for 8 and 12 weeks resulted in an additional increase in cytotoxicity. Protease digestion decreased, but did not eliminate cell death.
Conclusions
Current storage practices may allow milk to become cytotoxic and contribute to intestinal damage in NEC.
Additional keywords: Lipase, necrotizing enterocolitis, breast milk donor banks, necrosis, intestinal cell damage
Introduction
In many ways, the infant intestine is more vulnerable to damage than the mature intestine. One example of this is necrotizing enterocolitis (NEC), the leading cause of gastrointestinal related death in premature infants. NEC is characterized by intestinal hemorrhagic necrosis and is rarely seen before onset of enteral nutrition (1). This vulnerability to intestinal damage likely results from the immature state at birth of the intestinal mucosal barrier, observable as increased permeability to luminal content compared to mature intestines (2, 3). The intestines of premature infants are even more permeable (3), suggesting, unsurprisingly, they are even less mature.
The intestinal mucosal barrier controls permeability and is comprised of an inner barrier of epithelial cells with tight inter-epithelial junctions and an outer barrier of attached and secreted mucin (i.e. mucus). In the mature intestine, the mucosal barrier serves to prevent pathogens in the intestinal lumen from entering the body. The mucin component also prevents digestive enzymes and certain products of food digestion from directly contacting and damaging the epithelium. One such product is free fatty acid (FFA) released by lipase digestion of ingested fats. As detergents, FFAs may insert themselves into and disrupt (4) all types of cell membranes (5, 6) (i.e. a receptor independent event). As a result, they may be cytotoxic in even micromolar concentration (7). Yet FFA concentrations in the intestinal lumen after a meal may easily surpass 10 mM in humans (8). The mucin barrier prevents FFAs from damaging the epithelium (9). However, it has been shown that at higher concentrations (e.g. 40 mM), FFAs cause villi damage even in healthy, mature intestine (9). Likewise, inhibition of the enzymes that create and release FFAs prevents intestinal hemorrhagic necrosis and improves mortality after mucosal barrier disruption by intestinal ischemia in adult rats (7).
In neonates the mucin barrier is still forming and less mature intestines may be damaged by lower concentrations of FFA (e.g. 5 mM) (10). Mouse models suggest that the ability of the intestine to replenish its mucin barrier after a challenge is deficient in pre-term neonates (11). This possibility is supported by findings of fewer mucin-containing goblet cells in histological sections from NEC infants in humans, suggesting depletion of mucin stores (11). In contrast, addition of dietary epidermal growth factor (EGF), a mucin-stimulating factor, protects against NEC in mouse models (12, 13). If mucin deficit is a major factor predisposing the premature infant to NEC, then it becomes critical to determine what dietary factors increase FFA concentration and thus cytotoxicity in the intestinal lumen.
There is a 10-fold higher risk for development of NEC in premature infants fed infant formula instead of breast milk (14). We have shown that infant formula digested in vitro generates 10 times the concentration of unbound (i.e. not attached to protein and therefore cytotoxic) FFA generated by fresh human milk under the same digestion conditions (5). Furthermore, the digested formulas but not the digested fresh human milk were cytotoxic to multiple cell types, since they cause physical disruption of lipid membranes. This evidence suggests that the increased risk for NEC associated with formula feeding could be attributable to concentrations of FFA after digestion exceeding the protective capacity of the mucin barrier.
However, milk from donor banks has not always performed better than formula in preventing NEC (15, 16) nor does exclusive breast milk feeding provide full protection from NEC or other GI problems (14, 17). One possible reason may be the practice of storing breast milk. Breast milk contains bile salt-sensitive lipase (BSSL) and lipoprotein lipase (LPL), and, if stored, forms FFAs that taste “soapy” (18). This may occur even if the milk is stored frozen at −20 °C (19). Stored breast milk has been shown to become cytotoxic to a number of cell types and this death was attributed to FFAs (6), but the focus was on the effects this could have on immune cells or pathogens present in the milk, rather than on potential damage to the intestine. Nor have previous studies taken into account the additional FFA formation and thus cytotoxicity that may occur during digestion in an infant’s intestine. Current guidelines suggest that parents may store milk up to eight days at 4 °C or 6 to 12 months at −20 °C (20) and allow milk banks 3 to 6 months of milk storage at −20 °C prior to pasteurization (21). This extended storage could lead to cytotoxicity in donor milk that may be detrimental to the premature infant’s intestine. Even otherwise healthy full-term neonates could potentially be affected by cytotoxicity in their mother’s own stored milk if their mucosal barrier is not fully developed.
The objectives of this study were to: 1) determine if intestinal epithelial cells (IECs) can be killed by stored breast milk, 2) investigate general cytotoxicity as a function of storage time, storage temperature, and subsequent digestion, and 3) determine if donor milk (DM) obtained from human milk banks is cytotoxic with or without subsequent digestion.
Materials and Methods
To achieve our objectives we obtained DM and collected fresh milk from volunteers and stored it at 4°, −20°, or −80° C for varying periods then digested the milk with pancreatic lipase and/or proteases. When possible, we tested cytotoxicity on cultured IECs. However, that assay is limited in that cells were not always available at the same time as fresh human milk and because the presence of even small amounts of protease would cause cell detachment from the culture wells. Since the latter interferes with quantification of cell death, the exposure time of IECs to milk was limited to 5 minutes (well below the expected in vivo exposure time of ~50 min, based on gastric emptying rate (22)). Therefore for some studies a general assay of cytotoxicity was performed using 60-minute exposure to rapidly obtainable, freshly isolated human neutrophils as the test cell type.
Ethics Statement
The Institutional Review Board of the University of California, San Diego, approved all protocols involving human subjects.
Human Milk Collection and Storage
Fresh human milk was obtained from healthy volunteer mothers after written consent. Participants were asked to pump a full expression to allow mixing of fore and hind milk. Milk was kept at 4°C and aliquoted within 2 hours. Aliquots for the fresh milk group were immediately digested for measurements of cytotoxicity on human neutrophils, an uncultured human cell source available on short notice. Milk that was not slated for immediate testing was stored at 4 °C, −20 °C, and/or −80 °C. For selected studies, duplicate aliquots of milk received 0.25 mg/ml of the lipase inhibitor, orlistat (Sigma-Aldrich; St. Louis, MO), prior to storage. Because IECs were not always available when fresh milk arrived, the primary determination of cytotoxicity at fresh, 3 day, and 7 day time points was performed using the general cytotoxicity (e.g. neutrophil) assay. The “fresh” and “7 day” time points were then replicated on IECs using milk stored at −80 °C until the day of the assay or thawed and stored at 4 °C 7 days prior to the IEC assay. To study long-term storage at −20 °C, fresh milk was stored for 0, 4, 8, or 12 weeks at −20 °C before transfer to −80 °C prior to testing on IECs (all samples assayed on the same day).
Raw (N=6) and pasteurized (i.e. as provided for consumption; N=6) DM was obtained from the San Jose Human Milk Bank. DM was frozen and shipped in batches to the milk bank by mothers after collection over an 8 to 130 day period. The milk remained frozen at −20 °C until pasteurization by the Milk Bank between 2 and 16 days of receipt by the bank before it was shipped to us (written communication from milk bank). Samples were shipped frozen on dry ice. DM was immediately thawed and aliquoted upon delivery (6 to 8 days after pasteurization). Within minutes, raw and pasteurized DMs were assayed for lipase activity and pasteurized DMs were digested and tested for cytotoxicity on neutrophils. The remaining aliquots were frozen (−80 °C) for later (14 days) cytotoxicity measurements on IECs.
Milk Digestion with Pancreatic Lipase and Protease
For studies involving digestion, human milk was incubated for 2 h (37 °C) (similar to the transit time through an infant jejunum) after mixing 4:1 (v:v) with PBS, porcine pancreatic lipase (Sigma Aldrich), bovine pancreatic trypsin and chymotrypsin (Sigma Aldrich), or lipase, trypsin, and chymotrypsin. Final enzyme concentrations were either 0.1 (this, along with reducing exposure time to 5 minutes, was designed to reduce IEC cell detachment) or 1 mg/ml. These concentrations bracket and are on the order of concentrations in the intestinal lumen of human infants (23). Note we avoided the use of bile salts in the digestion, as bile salt concentration is highly variable in neonates and often well below the critical micelle concentration (i.e. the theoretical concentration at which bile would be able to assist in digestion) (24).
After digestion, selected samples were defatted (centrifugation at 16000g, 20 min, 4 °C, collecting the supernatant under the solid fat layer). Defatting to remove fat globules was required for all cytotoxicity assays involving neutrophils to allow measurement by flow cytometry and for all plate reader assays (i.e. lipase activity and FFA concentration measurements).
Some samples were filtered after digestion and defatting through glass fiber pre-filters (Pall-Gellman; Port Washington, NY) to remove unbound FFAs, as previously described (5).
Cytotoxicity Assays
Freshly isolated human neutrophil and cultured rat IEC cytotoxicity measurements were performed as previously described (5). In brief, after a 60 minute exposure to sample for neutrophils (to approximate the expected bolus transit time of food passing through the intestine as suggested by gastric emptying rate (22)) or a 5 minute exposure for IECs (to avoid cell detachment), death was determined using propidium iodide (PI; Sigma), a fluorescent life/death indicator, and analyzed immediately via flow cytometer (neutrophils) or fluorescent imaging (IECs). % Cell death was calculated as the percentage of gated cells that were PI-positive in the neutrophil assay and by using the ratio of PI-positive cells to the average number of PI-positive cells in wells treated with 0.1% Triton X-100 in PBS for 30 seconds (100% cell death). A sample was defined as cytotoxic if it caused more than 17% (neutrophils) or 5% (IECs) cell death (i.e. at least one standard deviation above the mean death of cells exposed to PBS alone − 10±7% and 1.6±1.7%, respectively).
Lipase Activity Assay
Lipase activity of defatted milk was assayed fresh, after 1 week of refrigeration (4 °C) or freezing (−20 °C), after long-term (16±9 weeks) storage at −80 °C, and in the raw and pasteurized DM upon arrival. Lipase activity was determined via digestion of the fluorescent substrate, O-pivaloyloxymethyl umbelliferone (C-POM; Invitrogen; Carlsbad, CA) as previously described (7).
FFA Measurement
Total FFA concentration was determined in a 96-well plate using the Free Fatty Acids Half-Micro Test kit (Roche Applied Sciences; Indianapolis, IN). All samples were pre-diluted 10-fold to bring concentrations within the linear range of the assay (<1 mM), with reported values corrected to the original concentrations. The kit measures total FFAs regardless of binding to fatty acid binding proteins such as bovine serum albumin (BSA) (5). Filtration through glass fiber pre-filters removes unbound but not bound FFAs (5) and reduces cytotoxicity (7, 25). Therefore, to determine the approximate amounts of bound and unbound FFAs, we filtered samples through 3 glass fiber filters in series, as previously described (5). Filtered samples were then assayed for total FFAs yielding the bound FFA concentration of the original unfiltered sample.
Statistics
Data are presented as means ± standard deviation. Single or multi-factor ANOVA (with repeated measures for paired samples) was completed for experiments with multiple groups (α=0.05) followed by Bonferroni correction for pair-wise t-test comparisons. Statistical analysis was performed in Excel and SPSS (IBM corporation; Armonk, NY).
Results
Cytotoxicity of digested fresh vs. stored breast milk to human neutrophils
Defatted fresh human milk was on average not cytotoxic in the neutrophil assay, regardless of digestion group (only 1 of 12 samples became cytotoxic after lipase digestion; Table 1). However, milk stored for 3 days at 4 °C or −20 °C showed a significant increase in cytotoxicity after lipase digestion, with more cell death in the 4 °C group than in the −20 °C group. After 1 week of storage, 11 of 12 milk samples stored at 4 °C and 10 of 12 samples stored at −20 °C were cytotoxic after lipase digestion. Neutrophils exposed to 4 °C, but not −80 °C, stored milk (1 week) show visible disruptions of the cell membrane (e.g. large blebs) (Figure 1), similar to previously observed bleb formations that precede cell membrane rupture and death by a FFA mechanism (5, 7, 25).
Table 1.
| with PBS | with Protease | with Lipase | with Lipase + Protease |
|
|---|---|---|---|---|
| Fresh Milk c | 3 ± 2 (0 of 12) | 2 ± 1 (0 of 12)§ | 6 ± 7 (1 of 12) | 7 ± 12 (1 of 12) |
| Stored 3 days (4°C) | 5 ± 4 (0 of 12) | 3 ± 2 (0 of 12) | 43 ± 23 (10 of 12)*‡ | 28 ± 10 (10 of 12)*§‡ |
| Stored 7 days (4°C) | 47 ± 38 (7 of 12)‡ | 17 ± 17 (5 of 12)§‡ | 50 ± 19 (12 of 12)‡ | 39 ± 19 (10 of 12)§‡ |
| Stored 3 days (−20°C) | 3 ± 1 (0 of 12) | 2 ± 1 (0 of 12)§^ | 18 ± 12 (7 of 12)*‡^ | 13 ± 11 (3 of 12)*^ |
| Stored 7 days (−20°C) | 4 ± 2 (0 of 12)^ | 2 ± 1 (0 of 12)§^ | 27 ± 15 (10 of 12)*‡^ | 22 ± 19 (6 of 12)*‡^ |
upper number: Mean % ± SD; lower number in parentheses: Fraction of samples that were cytotoxic - i.e. with death greater than 17%
Samples skimmed after digestion (2 h, 37 °C). Enzyme concentrations: 1 mg/ml. N=12 breast milk donors
Fresh milk data from reference (Penn et al., 2012)
lipase digestion increased death (p<0.003)
protease digestion decreased death (p<0.01)
increased death vs. fresh milk (p<0.01)
less death when stored at −20 °C vs. 4 °C (p<0.01)
p<0.0125 considered significant
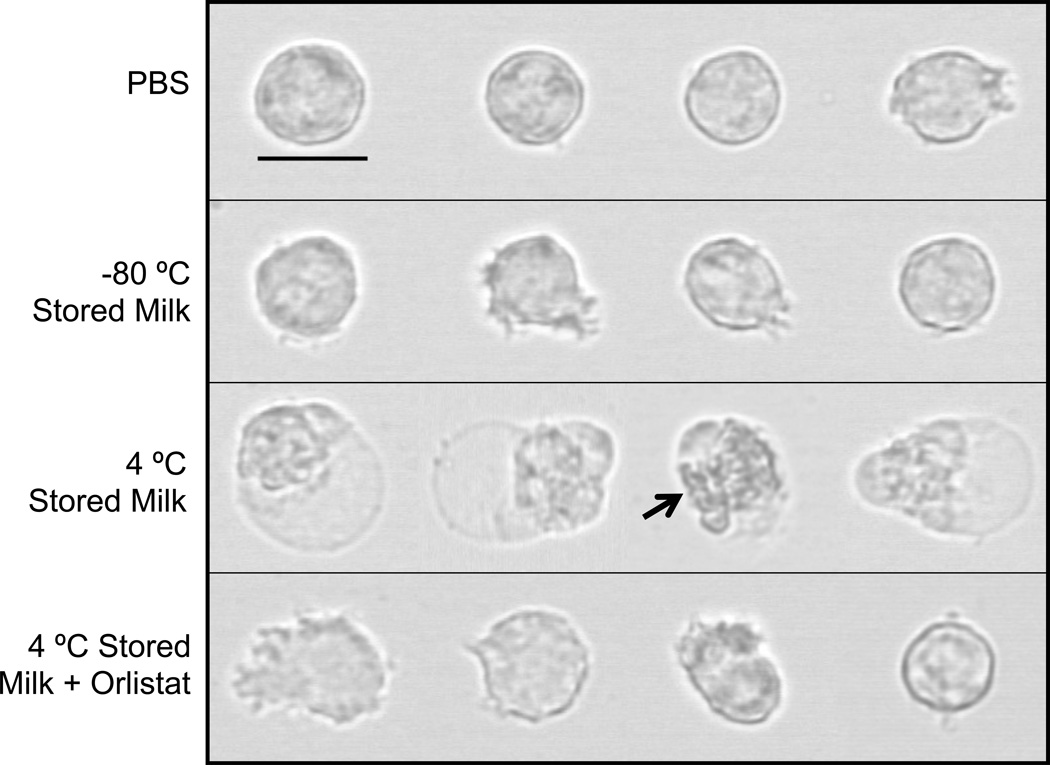
Figure 1. Neutrophils exposed to stored milk.
Representative images of human neutrophils exposed for 30 minutes, prior to glutaraldehyde fixation, to PBS (control) or human breast milk stored for 1 week at −80 °C, 4 °C, or at 4 °C with orlistat pretreatment. Neutrophils in the PBS, −80 °C, and 4 °C + Orlistat groups were either round or had pseudopods. In contrast, all of the observed cells in the 4 °C group, showed either blebbing or had the appearance of cells whose blebs were ruptured (arrow). Bar = 10 µm (800x).
Protease digestion, shown previously to increase the capacity of breast milk to resist death from exogenously added FFA (5), either decreased the cell death and/or the number of samples that were cytotoxic in stored milk (Table 1). This did not appear to be due to digestion of endogenous or exogenous lipase since lipase activity was unchanged with protease addition (1259±585 vs. 1196±487 fluorescent units in the 7 day, 4 °C, milk with lipase vs. lipase + protease groups). Lipase- and protease-only controls were not cytotoxic to neutrophils (not shown).
The post-natal age of the child at the time of milk donation (range: 22–507 days) did not correlate to the cytotoxicity of milk stored 7 days at 4 °C (PBS digestion, correlation coefficient = 0.15, p=0.32) or initial lipase activity in the sample (correlation coefficient = −0.01, p=0.49). Mother’s age at time of expression was unavailable for 2 samples, but we did not find a correlation between mother’s age and cytotoxicity of milk stored 7 days at 4 °C (PBS digestion, correlation coefficient = −0.17, p=0.32) or initial lipase activity in the sample (correlation coefficient = 0.13, p=0.35) in the remaining 10 samples. Mothers’ race was primarily Caucasian (7 Caucasian/non-Hispanic, 1 Black, 1 Asian, 1 half-Asian/half-Caucasian, 2 unavailable), so no effects of race are determinable for this data.
FFA Cytotoxic Mechanism
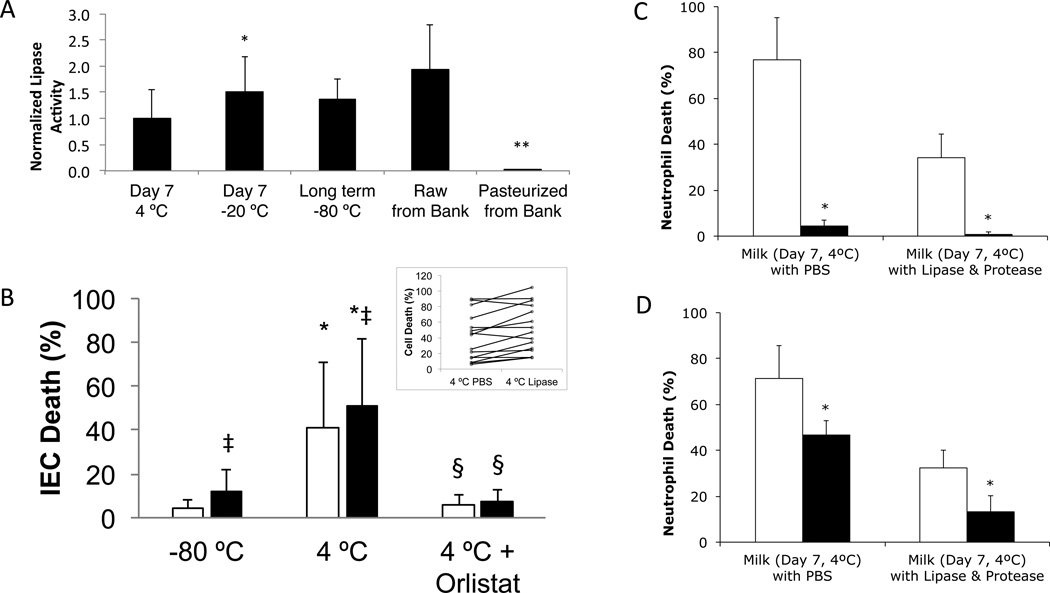
We measured lipase activity in samples stored for 7 days at 4 °C or −20 °C, or long-term at −80 °C, normalized by their activities when fresh, as well as that of raw and pasteurized DM (Figure 2A). Fresh milk alone had 3.1±1.4 times the activity as 1 mg/ml of exogenous pancreatic lipase (not shown). Pasteurized DM had no detectable lipase activity, in agreement with previous studies (26), but all other samples had activity. Activity was significantly elevated with freezing (−20 °C) compared to refrigeration (4 °C). Raw DM and −80 °C stored milk also trended towards increased activity compared to 4 °C (p=0.046 for raw DM; p=0.076 for −80 °C stored milk). This agrees with previous studies showing lipase activation by cooling (2 °C) cow’s milk (27) and by freezing human milk (BSSL normally needs bile salt to become active, but this requirement is lost after freezing) (28). No correlation was seen between cytotoxicity of the Milk with PBS after 7 days storage at 4 °C and the initial (correlation coefficient = −0.04, p=0.45) or final lipase activity (correlation coefficient = −0.35, p=0.16), suggesting that variations in cytotoxicity were due more to differences in substrate (e.g. milk fat globules) between samples than differences in lipase activity.
Figure 2. IEC death after 4 °C storage, lipase activity and effects of lipase inhibition or hydrophobic filtering on cell death.
(A) Milks stored (duration and temperature as shown) or obtained from the milk bank before and after pasteurization. Values normalized by activity prior to storage (or the mean of the fresh milk activity, in the case of DM). N=12 donors of fresh milk, N=6 batches of raw DM and 6 batches of pasteurized DM. * p < 0.0007 vs. Day 7 4°C milk. **p< 0.003 vs. raw milk or Day 7 4°C milk. (B) Milk stored at −80 °C or 7 days at 4 °C (with or without orlistat) before transfer to −80 °C (total storage time 13 ± 5 days – min 7, max 20), then digested (PBS (white) or 0.1 mg/ml lipase (black)) and exposed to IEC for 5 minutes. N=15; ‡ p<0.006 vs. without lipase; * p<0.0007 vs. −80 °C storage only; § p<0.0007 vs. without orlistat. Inset: raw data from Day 7 4°C milk digested with PBS or Lipase. (C&D) Milk stored 7 days at 4 °C (non-cytotoxic samples excluded) (C) with (black) or without (white) orlistat pretreatment or (D) before (white) or after (black) removing unbound FFAs by filtering three times with glass fibers. (C) * p<0.02 (N=3 for milk w/ PBS; N=4 for milk w/ lipase and protease groups), (D) * p<0.05 (N=3 in milk w/ PBS; N=5 in milk w/ lipase and protease).
Despite a relatively short 5 minutes exposure, milk stored at 4 °C for 7 days killed 41% of IECs, as compared to 5% from milk stored at −80 °C (Figure 2B). Exogenous lipase digestion increased both of these values a small but significant (by paired t-test) amount (see inset for raw data of 4 °C groups). Cytotoxicity was prevented when lipase was inhibited with orlistat prior to storage at 4 °C. Thus, as with digested formula (5), FFAs created by lipase digestion in stored milk are cytotoxic to intestinal cells. We confirmed that lipase inhibition would also prevent cytotoxicity to neutrophils (Figure 2C) and that passage of cytotoxic stored milk through glass fiber filters, which bind and remove unbound, but not bound, FFAs (5), significantly reduced cytotoxicity (Figure 2D).
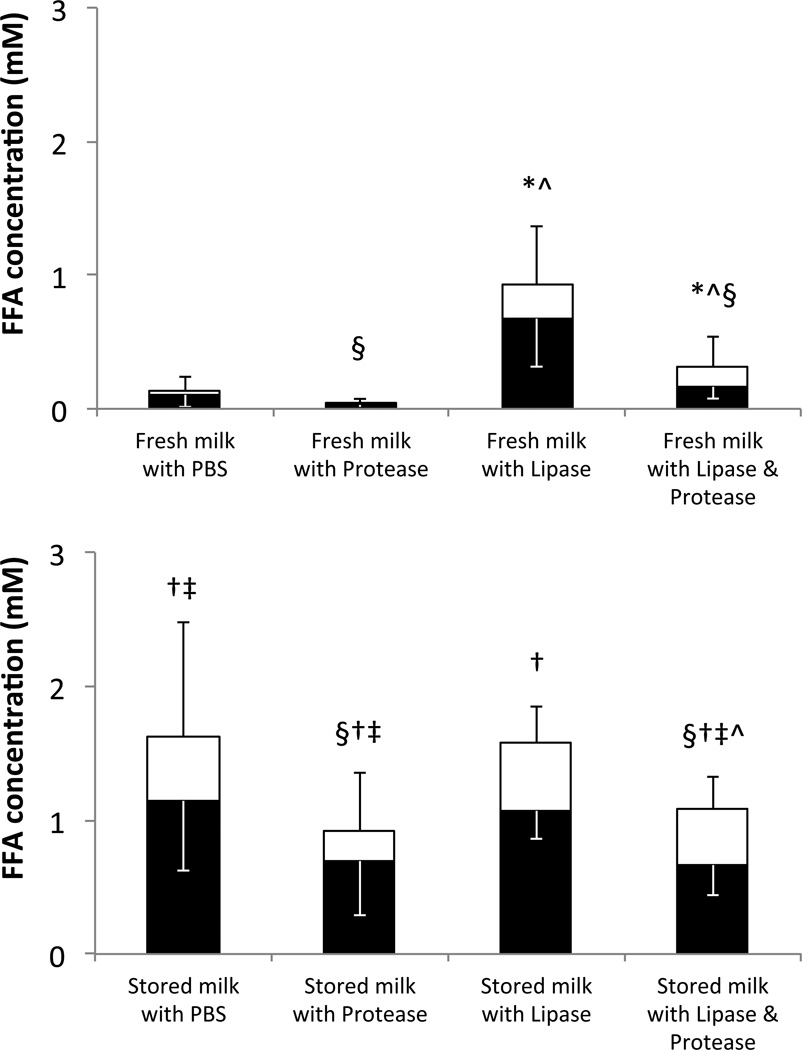
We measured total and unbound FFAs in aliquots of the same defatted solutions that were previously tested for cytotoxicity on human neutrophils (Table 1) and stored at –80 °C until FFA analysis. We detected little to no FFAs in fresh milk incubated with PBS or proteases (Figure 3). Pancreatic lipase digestion significantly increased the total and unbound FFAs in fresh breast milk. Milk stored for 7 days at 4 °C had significantly higher unbound and total FFA levels compared to fresh milk in all cases, except for unbound FFAs in the Milk with Lipase groups which approached, but did not reach significance (P=0.02). Lipase digestion of stored milk increased neither the total nor the unbound concentrations of FFAs over the Stored milk with PBS control unless protease digestion also occurred, in agreement with the cytotoxicity findings (Table 1). It is possible that fat digestion or FFA aqueous solubility in breast milk are near their maximal levels in the stored milk already, minimizing the effect of exogenous lipase unless proteases are present to increase the binding of FFA to proteins (see below) removing them from solution and making room for additional FFA (in vivo, solubility is increased via FFA incorporation into bile acid micelles (29)). Protease digestion decreased total FFA levels in all cases. Overall, unbound FFA concentration correlated significantly with cytotoxicity (correlation coefficient = 0.706, p<0.001).
Figure 3. Effect of storage on FFA concentrations.
Total (full columns) and bound (black portion) FFA concentrations in (top) fresh milk or (bottom milk stored for 7 days at 4 °C after digestion and subsequent defatting. The differences between the total and bound FFAs (white portion) represent the concentrations of unbound FFAs. * p<0.0006 for total FFAs (or ^ p<0.016 for unbound FFAs) vs. without lipase digestion. † p<8×10−4 for total FFAs (‡ p<0.008 for unbound FFAs) vs. fresh milk. § p<0.009, significant decrease in total FFA with protease digestion. Some values of unbound FFAs approached, but did not reach significance (i.e. p<0.0167): stored milk with lipase vs. fresh milk with lipase (p=0.02) and stored milk with PBS vs. stored milk with protease (p=0.05). N=12. Fresh milk data from reference (5).
Long-term milk storage at −20 °C
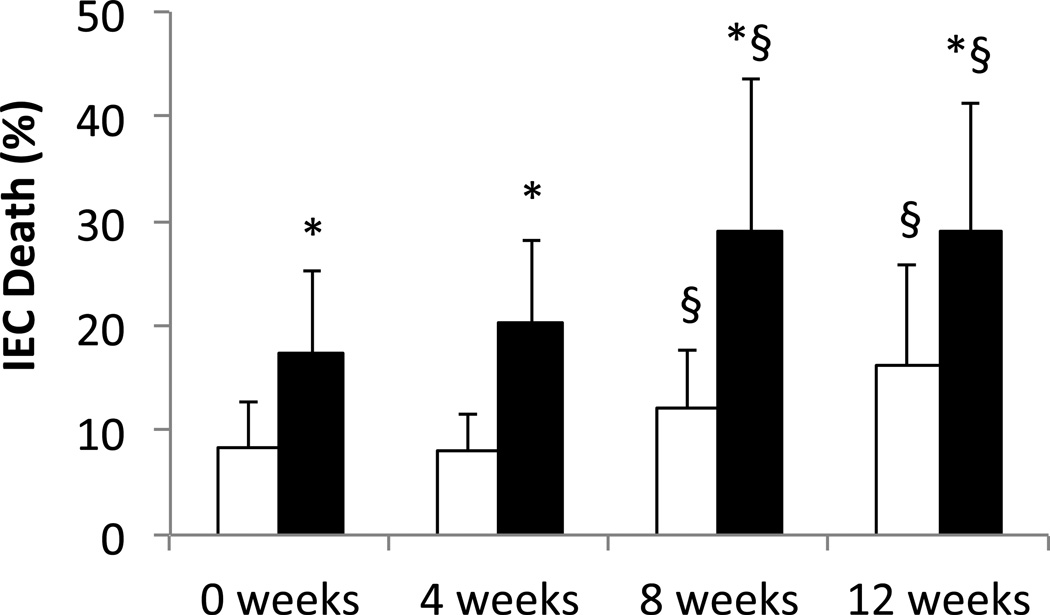
To explore the effects of long-term milk storage at −20 °C on IEC cytotoxicity, we stored fresh milk at −20 °C for 0, 4, 8, or 12 weeks, transferring the aliquots to −80 °C until simultaneous digestion and testing on IECs (total storage = 20.7 ± 8.3 weeks, max 37.6 weeks). Every group, including the 0 week group that was kept at −80 °C for the duration, increased their cytotoxicity with lipase digestion (Figure 4 also apparent in Figure 2B). This suggests that frozen milk, even at −80 °C, loses some of the resistance to lipolysis present in fresh milk, subject to variations between donors and/or assay conditions. However, after 8 weeks we saw an additional cytotoxic effect of storage at −20 °C. This increase was seen even in the absence of exogenous pancreatic lipase digestion.
Figure 4. Effect of −20°C milk storage on IEC death.
Fresh milk was aliquoted and stored at either −80°C (0 weeks) or −20°C for 4, 8, or 12 weeks before being moved to −80°C until testing (total storage time = 20.7 ± 8.3 weeks, max 37.6). After thawing, milk was digested with PBS (white) or lipase (black) (0.1 mg/ml final). N=17. * p<0.0007 vs. Control; § p<0.02 vs. 0 weeks.
To determine whether the secondary increase in cytotoxicity during storage at −20 °C is due to retention of residual lipase activity even while frozen, we tested the effect of lipase inhibition on milk prior to storage. Milk from 7 individual donors was stored 28 days or approximately 2 months (60.1 ± 3.0 days) at −20 °C with or without 0.25 mg/ml orlistat, then moved to −80 °C before thawing and immediate defatting and testing for cytotoxicity to neutrophils without additional digestion or incubation at 37 °C (total storage time 33.1 ± 3.0 and 61.1 ± 3.0 days, respectively). Aliquots of the defatted milk were immediately returned to −80 °C until testing for FFA concentration (total storage time 38.1 ± 3.0 and 68.1 ± 3.0 days, respectively). We found no appreciable cell death at either time point (3 ± 1% with or without orlistat at 28 day time point, 2 ± 1% with or without orlistat at 2 month time point). At 28 days we found approximately the same low concentration of FFAs as we observed in the fresh milk group (Figure 3) in both groups (0.13 ± 0.12 mM FFA without orlistat and 0.10 ± 0.04 mM FFA with orlistat). However, at the 2-month time point, FFAs were doubled (0.22 ± 0.11 mM FFA), and this increase was prevented by pretreatment with orlistat (0.10 ± 0.04 mM FFA; p<0.011).
Milk from Donor Bank
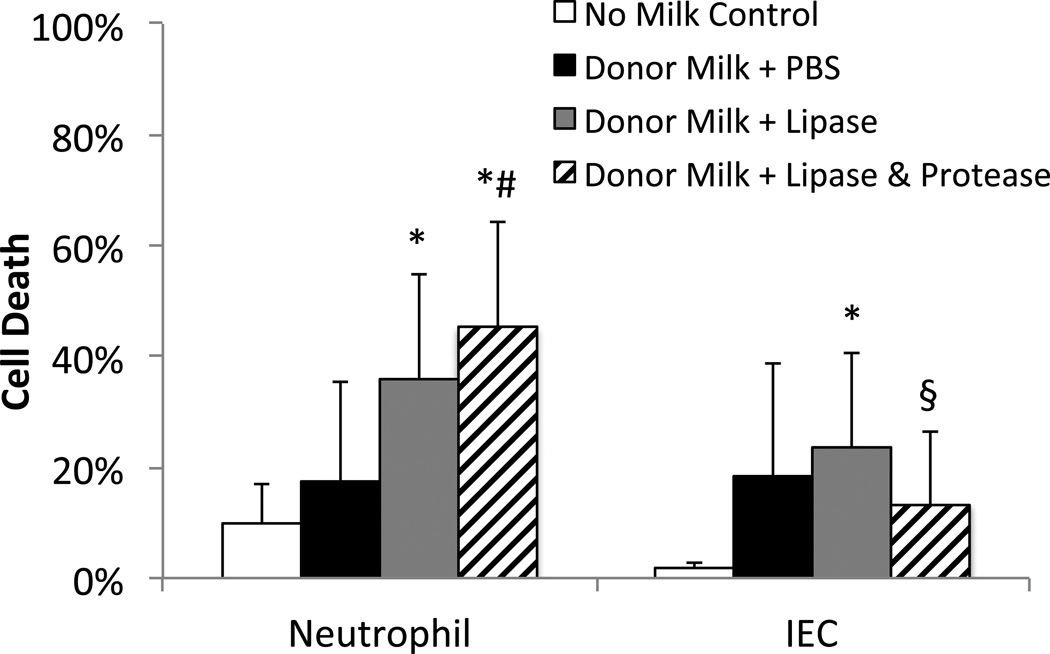
Given that 8 weeks of storage at −20 °C can increase FFAs and cytotoxicity and that milk banks allow donors to store milk for longer periods before shipping to their facilities, we determined the cytotoxicity of DM, with and without exogenous lipase digestion. We found that 2 of 6 DM samples were initially cytotoxic to neutrophils, but after lipase digestion all 6 samples were cytotoxic (Figure 5 left). The IEC assay gave similar results for the control and lipase digested DM although by that method, 4 of the samples were considered cytotoxic to start with, increasing to 5 of 6 with lipase digestion (Figure 5 right). The addition of proteases to the digestion of DM resulted in a decrease in cytotoxicity by IEC assay, but increased cytotoxicity as measured by the neutrophil assay, possibly due to the higher protease concentration and extra ~1.5 hours of digestion (55 minutes longer incubation with neutrophils plus ~30 minutes for defatting step) in that assay (see below).
Figure 5. Cytotoxicity of milk from a donor milk bank.
Milk was assayed with (A) neutrophils the day it arrived from the milk bank and with (B) IECs 2 weeks later (−80°C in interim). After thawing, milk was digested with PBS (black), lipase (grey), or lipase, trypsin, and chymotrypsin (striped) (1 mg/ml for neutrophil assay, 0.1 mg/ml for IEC assay) and defatted (neutrophil assay only), before exposure to cells (60 minutes for neutrophils, 5 minutes for IECs). As a no milk control (white), neutrophils (30 replicates) were incubated for 60 minutes with PBS and IECs (7 replicates) were kept in normal media until their propidium iodide steps. N=6 donor milks, p<0.025 considered significant. (A) * p<0.02 vs. PBS control, # p<0.02 vs. Donor Milk + Lipase. (B) * p<0.025 vs. no milk control, § p<0.025 vs. Donor Milk + Lipase.
FFA binding capacity as measured by cytotoxicity response to exogenous FFA
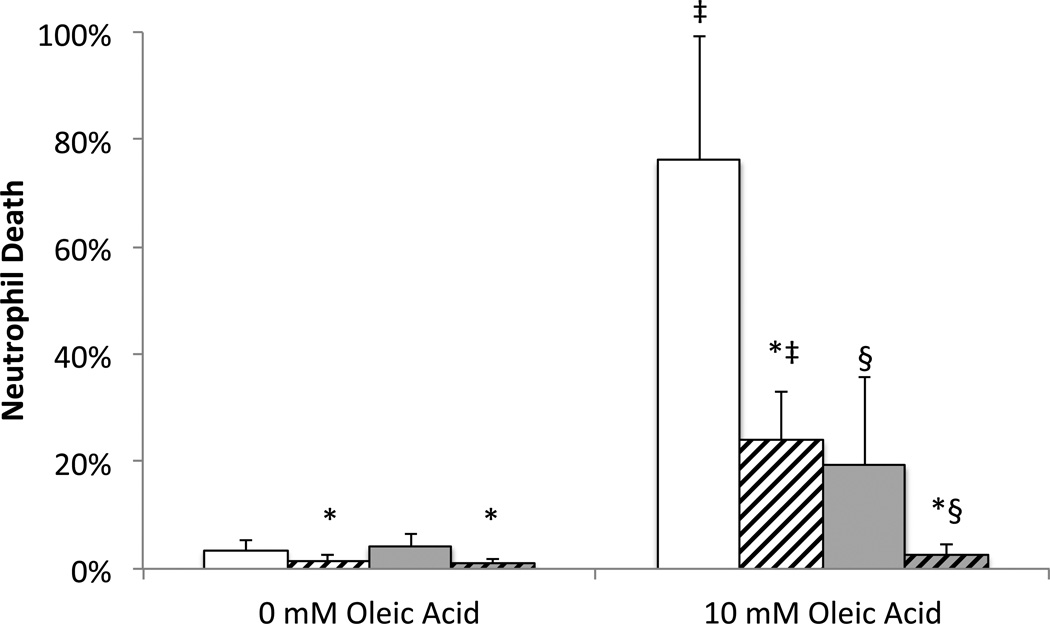
Because FFAs are likely to insert themselves into milk fat globule membranes, the fat layer itself may provide some protection from cytotoxicity. We therefore compared the ability of defatted and whole milk to lower the cytotoxicity of exogenous oleic acid (e.g. via binding the FFA) and determined how those capacities are affected by protease digestion. Similar to previous results with milk (5), digestion by proteases significantly improved the ability of milk to lower the cytotoxicity of exogenous FFA, whether or not the milk was defatted prior to oleic acid addition (Figure 6). This suggests that protease digestion increases FFA binding capacity not just in whole milk but also in the components that remain in defatted milk. There was also a significant protection against cytotoxicity if oleic acid was added to milk before, as opposed to after, defatting. This suggests that the fat layer and/or pellet also have a significant FFA binding capacity. There was a significant interaction by ANOVA between defatting and digestion, implying that the binding capacity of the fat layer/pellet is also increased by protease digestion. This agrees with the FFA measurement (Figure 3) showing a decrease in total FFA concentration in milk digested by proteases before defatting, despite no decrease in lipase activity.
Figure 6. Effect of protease digestion and defatting on FFA binding capacity.
Milk from −80 °C storage (20.9 ± 11.3 weeks; min 10.1, max 41.4) was placed in PBS (solid) or digested with 1 mg/ml proteases (slashed). 0 mM or 10 mM oleic acid (in ethanol, 1.5% final) was then added after (white or before (gray) defatting, prior to 60 minute incubation with neutrophils. N=9 milk donors; p<0.017 considered significant. * p<0.01 versus without protease; ‡ p<0.007 versus no oleic acid; § p<0.0002 versus oleic acid added after spinning.
To determine if the differential response to protease digestion shown in Figure 5 could be the result of differences in the degree of protein digestion, we kinetically tested the effect of protease digestion on FFA binding capacity in DM. 5mM exogenous oleic acid was added to defatted DM, which was then digested for 0, 0.5, 1, 2, or 4 hours with 1 mg/ml trypsin and chymotrypsin before inhibiting protease activity with 1 mM phenylmethanesulfonylfluoride and incubating with neutrophils. Unlike in Figure 5 left we saw a significant decrease in neutrophil cytotoxicity compared to 0 hours digestion (59±11%) at 0.5 (47±11%, p<0.0001) and 1 hours (46±11%, p<0.015) digestion (N=6). However, this was followed by a near significant increase in cytotoxicity at the 2-hour time point (55±21%, p=0.052 versus 1-hour time point, with one sample having a 10% increase in cytotoxicity compared to its 0-hour time point) and a second significant decrease at 4 hours (33±11%, p<0.01 versus 2-hour time point). The complexity of the kinetics suggests that more than one protein is likely to be involved in this process.
Discussion
The current results indicate that 1) stored human milk is capable of killing IECs with as little as 5 minutes exposure, 2) 3 days storage at either 4 °C or −20 °C will cause milk to become cytotoxic after lipase digestion similar to that in the infant intestine, and 3) some milk from donor banks is already cytotoxic upon distribution and, unlike fresh milk, gains significant cytotoxicity after lipase digestion.
Cytotoxicity could be prevented by pre-treatment with the lipase inhibitor, orlistat, prior to milk storage, confirming that the products of lipolysis are the cause of cell death. Cytotoxicity could be decreased by protease digestion, and by filtering through glass fiber pre-filters, a procedure that removes unbound FFAs but not FFAs bound to proteins such as albumin (5, 7). Both total and unbound FFAs are increased in stored milk and unbound FFA concentration correlates with levels of cell death. These findings support the hypothesis that unbound FFAs are the main cytotoxic mediator in stored milk, though monoglycerides may also contribute.
We observed membrane bleb formation and rupture and eventually total cell destruction, similar to that observed with other FFA sources (7), and consistent with the idea of physical disruption of the bi-lipid membrane. Cell destruction by FFAs is cell type independent (e.g. epithelial cells (5), endothelial cells (5), leukocytes (6), red cells (6)). Though neutrophils were used here primarily as a convenient measure of general cytotoxicity, neutrophils are present in intestinal tissue and, like other cell types in the intestine that remain to be investigated, may be affected by FFA cytotoxicity when the mucosal barrier is breached. In our previous work, the cell sources were human, rat, and bovine, all of which gave qualitatively similar results (5), making it unlikely that there are major species differences with regards to response of cells exposed to FFAs.
Freezer stored milk (−20 °C) caused cell death that appears to increase in a biphasic fashion. In the first phase, we saw that freezing for 3 or more days resulted in increased cytotoxicity after subsequent exogenous lipase digestion. We saw no increase in cytotoxicity, and levels of FFA that approximated that of fresh milk, in milk stored for 4 weeks at −20 °C without subsequent digestion, suggesting that no appreciable fat digestion occurs while milk is frozen for less than 4 weeks. Therefore, the increased cytotoxicity versus fresh milk we observed at 3 and 7 days (Table 1) after exogenous lipase digestion is likely due to an increase in the susceptibility of the milk fat to lipolysis (e.g. disruption of the milk fat globule membrane). Alternatively, BSSL activated by freezing (28) (Figure 2A) may interact synergistically with the exogenous lipase to increase lipolysis.
The secondary increase in cytotoxicity of milk stored for 8 and 12 weeks at −20 °C but not milk kept at −80 °C is likely the result of lipase activity while the milk is frozen, since FFA concentration was doubled in milk stored at −20 °C for 8 weeks without orlistat. Partial digestion of milk fat or the milk fat globule membrane may also prime the fat for increased lipolysis after thawing. The non-negligible death in milk stored long-term −80 °C (Figure 4 0 week Control group) and the small but significant increase in cytotoxicity with exogenous lipase digestion after short-term storage at −80 °C (Figure 2B) suggest that the same changes to milk that occur at −20 °C may also occur at −80 °C, but to a lesser extent that varies between individuals.
Two-thirds of the milk samples obtained from the donor bank were cytotoxic to IECs without additional lipid digestion, increasing to five-sixths after exogenous lipase digestion. This is in line with our findings of increased cytotoxicity compared to fresh milk after long-term storage at −20 °C.
In vivo intestinal environment
Though we tried to match factors such as digestion time and temperature, enzyme concentrations, and cell exposure time, our in vitro protocols are not, of course, a perfect representation of digestion as it occurs in an infant in vivo. For example, lipase profiles change during early development and in response to weaning (30). For this study, we chose to use pancreatic lipase so our findings would be comparable with our previous study comparing formula and fresh milk (5). This is likely to match the conditions in infants that have begun to wean, but are still consuming some human milk, but may not match conditions in infants on a milk-only diet, as prior to weaning most of the lipase activity in a neonatal intestine is from pancreatic lipase-related protein 2 (PLRP2) or BSSL (30). PLRP2 may be less efficient at lipid digestion in milk than pancreatic lipase (31), which could lead to lower FFA levels and cytotoxicity in the intestine than demonstrated in the current experiments. In contrast, lingual and gastric lipases, also not included in this study, may prime milk fats for easier digestion in the intestine, leading to higher FFA levels and greater cytotoxicity. Interestingly, since BSSL and PLRP2 have a lower affinity for large saturated fatty acids like palmitic acid (32, 33), which is almost exclusively located at the sn-2 position in human milk triglycerides and is found there over 3 times as often as any other fatty acid (34), and since pancreatic lipase also avoids cleaving the sn-2 triglyceride position, it is unlikely that the composition of FFAs will differ substantially regardless of which lipase dominates. Though we clearly demonstrate here that subsequent lipase digestion may cause non-cytotoxic stored milk to become cytotoxic (Table 1), testing cytotoxicity in infant intestinal aspirates will be necessary to resolve the question of the degree to which digestion increases stored milk cytotoxicity in-vivo.
Likewise, we did not include bile acids in our digestions, as their concentration is low and variable in neonates (24). However, since bile acids are small, amphiphilic molecules like FFAs, and are themselves cytotoxic (35), with the in vivo purposes of activating BSSL (28), emulsifying fats, and increasing FFA solubility and transport of FFAs across the mucin barrier by incorporating them into bile acid micelles (36), it is likely that bile acids would only increase overall cytotoxicity from stored milk.
Lastly, while milk supplied to donor banks is expected to come from later in lactation and while older infants could also be affected by milk stored later in lactation, it is possible that colostrum obtained in the first week or two may develop cytotoxicity either faster or slower with storage than the milk used for this study.
Protease Digestion of Stored Milk
Similar to our findings with fresh milk and a few infant formulas (5), cytotoxicity of stored milk also decreased significantly with protease digestion, which increases the FFA binding capacity of the milk. This is opposite to the effects of protease digestion on FFA binding proteins we have previously studied (e.g. albumin and the binding proteins in intestinal wall homogenates) (7, 25), suggesting the involvement of a specialized protein. One possible candidate is the casein micelle, which is disrupted upon protease digestion (37) to expose hydrophobic portions of the individual casein molecules and which is known to bind to hydrophobic lipid after disruption (31, 38). Alternatively, partial digestion could induce the conformational change in alpha lactalbumin that allows it to bind FFAs after the removal of its calcium ion (39).
This putative specialized protein is likely not the only protein involved in this process, however, as Figure 4 indicates that milk has the capacity to bind mM quantities of FFA even without protease digestion. For example, serum albumin is also present in human milk, and as mentioned above is capable of binding FFAs unless first digested with protease (7). A simultaneous decrease in binding capacity of some proteins and increase in binding capacity of other proteins with protease digestion could easily explain our findings in Figure 5 and the kinetic experiment on DM. We have preliminary data suggesting that the protection provided by protease digestion of milk eventually disappears altogether as protease digestion continues. It is possible that the pasteurization step that occurs during preparation of DM may accelerate the rate at which the protective protein is degraded compared to the unpasteurized milk in Table 1. The effect of protease on the fat layer may also have an effect.
Cytotoxicity reduction strategies
Our findings suggest a number of possible strategies to limit cytotoxicity in stored breast milk. Storage of breast milk at −80 °C rather than −20 °C or above, or earlier pasteurization for donor milk to denature the lipase, may reduce the potential production of cytotoxic mediators prior to, and during, digestion of the milk. Since freezing activates milk lipases, more limited storage of thawed milk before consumption or pasteurization may also serve as a preventive measure. If pancreatic insufficiency is present, pretreatment of breast milk with trypsin and/or chymotrypsin may decrease cytotoxicity. Guidelines for milk storage times at 4 °C or −20 °C could be more limited. If long-term storage is required and colder storage is unavailable, the efficacy and safety of glass fiber filtration or addition of a lipase inhibitor could be considered.
In summary, we determined that milk stored according to current practices, whether obtained from a fresh source or from a donor bank can become cytotoxic, though to a lesser degree than nearly every infant formula we previously tested (5). It remains to be examined to what degree the cytotoxicity is increased during digestion in-vivo, however, the incidence of cytotoxicity in stored milk may explain why NEC can occur in milk fed infants and why DM has not always performed better than formula in preventing NEC.
Acknowledgements
We wish to thank Tiffany Lai for help in recruiting milk donors, Lynn Han and Leena Kurre for help in analysis of IEC death, Parth Chokshi for assistance with IEC culturing, and Dr. Emily Blumenthal for her statistical assistance.
Funding Disclosure: Supported by NIH grants NS071580 and GM85072.
Footnotes
Conflicts of Interest
The authors have no financial interests or conflicts of interest to declare related to the contents of this manuscript
References
- 1.Anand RJ, Leaphart CL, Mollen KP, et al. The role of the intestinal barrier in the pathogenesis of necrotizing enterocolitis. Shock. 2007;27(2):124–133. doi: 10.1097/01.shk.0000239774.02904.65. [DOI] [PubMed] [Google Scholar]
- 2.Catassi C, Bonucci A, Coppa GV, et al. Intestinal permeability changes during the first month: effect of natural versus artificial feeding. J Pediatr Gastroenterol Nutr. 1995;21(4):383–386. doi: 10.1097/00005176-199511000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Weaver LT, Laker MF, Nelson R. Intestinal permeability in the newborn. Arch Dis Child. 1984;59(3):236–241. doi: 10.1136/adc.59.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badwey JA, Curnutte JT, Robinson JM, et al. Effects of free fatty acids on release of superoxide and on change of shape by human neutrophils. Reversibility by albumin. J Biol Chem. 1984;259(12):7870–7877. [PubMed] [Google Scholar]
- 5.Penn AH, Altshuler AE, Small JW, et al. Digested formula but not digested fresh human milk causes death of intestinal cells in vitro: implications for necrotizing enterocolitis. Pediatric Research. 2012 doi: 10.1038/pr.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakaguchi M, Tomomasa T, Kuroume T. Cytolytic action of stored human milk on blood cells in vitro. J Perinat Med. 1995;23(4):293–300. doi: 10.1515/jpme.1995.23.4.293. [DOI] [PubMed] [Google Scholar]
- 7.Penn AH, Schmid-Schönbein GW. The intestine as source of cytotoxic mediators in shock: free fatty acids and degradation of lipid-binding proteins. Am J Physiol Heart Circ Physiol. 2008;294(4):H1779–H1792. doi: 10.1152/ajpheart.00902.2007. [DOI] [PubMed] [Google Scholar]
- 8.Hofmann AF, Borgstroem B. The Intraluminal Phase of Fat Digestion in Man: The Lipid Content of the Micellar and Oil Phases of Intestinal Content Obtained during Fat Digestion and Absorption. J Clin Invest. 1964;43:247–257. doi: 10.1172/JCI104909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishikawa S, Cepinskas G, Specian RD, et al. Epidermal growth factor attenuates jejunal mucosal injury induced by oleic acid: role of mucus. Am J Physiol. 1994;267(6 Pt 1):G1067–G1077. doi: 10.1152/ajpgi.1994.267.6.G1067. [DOI] [PubMed] [Google Scholar]
- 10.Velasquez OR, Tso P, Crissinger KD. Fatty acid-induced injury in developing piglet intestine: effect of degree of saturation and carbon chain length. Pediatr Res. 1993;33(6):543–547. doi: 10.1203/00006450-199306000-00001. [DOI] [PubMed] [Google Scholar]
- 11.McElroy SJ, Prince LS, Weitkamp JH, et al. Tumor necrosis factor receptor 1-dependent depletion of mucus in immature small intestine: a potential role in neonatal necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2011;301(4):G656–G666. doi: 10.1152/ajpgi.00550.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark JA, Doelle SM, Halpern MD, et al. Intestinal barrier failure during experimental necrotizing enterocolitis: protective effect of EGF treatment. Am J Physiol Gastrointest Liver Physiol. 2006;291(5):G938–G949. doi: 10.1152/ajpgi.00090.2006. [DOI] [PubMed] [Google Scholar]
- 13.Halpern MD, Holubec H, Saunders TA, et al. Bile acids induce ileal damage during experimental necrotizing enterocolitis. Gastroenterology. 2006;130(2):359–372. doi: 10.1053/j.gastro.2005.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet. 1990;336(8730):1519–1523. doi: 10.1016/0140-6736(90)93304-8. [DOI] [PubMed] [Google Scholar]
- 15.Boyd CA, Quigley MA, Brocklehurst P. Donor breast milk versus infant formula for preterm infants: systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2007;92(3):F169–F175. doi: 10.1136/adc.2005.089490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schanler RJ, Lau C, Hurst NM, et al. Randomized trial of donor human milk versus preterm formula as substitutes for mothers' own milk in the feeding of extremely premature infants. Pediatrics. 2005;116(2):400–406. doi: 10.1542/peds.2004-1974. [DOI] [PubMed] [Google Scholar]
- 17.Kolacek S, Puntis JW, Lloyd DR, et al. Ontogeny of pancreatic exocrine function. Arch Dis Child. 1990;65(2):178–181. doi: 10.1136/adc.65.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deeth HCaF-G, CH . Lipolytic Enzymes and Hydrolytic Rancidity. In: Fox PFaM, PLH, editors. Advanced Dairy Chemistry, Volume 2: Lipids. New York: Springer; 2006. [Google Scholar]
- 19.Bitman J, Wood DL, Mehta NR, et al. Lipolysis of triglycerides of human milk during storage at low temperatures: a note of caution. J Pediatr Gastroenterol Nutr. 1983;2(3):521–524. doi: 10.1097/00005176-198302030-00021. [DOI] [PubMed] [Google Scholar]
- 20.Eglash A. ABM clinical protocol #8: human milk storage information for home use for full-term infants (original protocol March 2004;revision #1 March 2010) Breastfeed Med. 2010;5(3):127–130. doi: 10.1089/bfm.2010.9988. [DOI] [PubMed] [Google Scholar]
- 21.Arslanoglu S, Bertino E, Tonetto P, et al. Guidelines for the establishment and operation of a donor human milk bank. J Matern Fetal Neonatal Med. 2010;23(Suppl 2):1–20. doi: 10.3109/14767058.2010.512414. [DOI] [PubMed] [Google Scholar]
- 22.Van Den Driessche M, Peeters K, Marien P, et al. Gastric emptying in formula-fed and breast-fed infants measured with the 13C-octanoic acid breath test. J Pediatr Gastroenterol Nutr. 1999;29(1):46–51. doi: 10.1097/00005176-199907000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Norman A, Strandvik B, Ojamae O. Bile acids and pancreatic enzymes during absorption in the newborn. Acta Paediatr Scand. 1972;61(5):571–576. doi: 10.1111/j.1651-2227.1972.tb15947.x. [DOI] [PubMed] [Google Scholar]
- 24.Andersson EL, Hernell O, Blackberg L, et al. BSSL and PLRP2: key enzymes for lipid digestion in the newborn examined using the Caco-2 cell line. J Lipid Res. 2011;52(11):1949–1956. doi: 10.1194/jlr.M015685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Penn AH, Hugli TE, Schmid-Schönbein GW. Pancreatic enzymes generate cytotoxic mediators in the intestine. Shock. 2007;27(3):296–304. doi: 10.1097/01.shk.0000235139.20775.7f. [DOI] [PubMed] [Google Scholar]
- 26.Henderson TR, Fay TN, Hamosh M. Effect of pasteurization on long chain polyunsaturated fatty acid levels and enzyme activities of human milk. J Pediatr. 1998;132(5):876–878. doi: 10.1016/s0022-3476(98)70323-3. [DOI] [PubMed] [Google Scholar]
- 27.Krukovsky VN, Sharp PF. Effect of the properties of the fat and of the fat globule surface on lipolytic activity in milk. Journal of Dairy Science. 1940;23:1109–1118. [Google Scholar]
- 28.Mehta NR, Jones JB, Hamosh M. Lipases in preterm human milk: ontogeny and physiologic significance. J Pediatr Gastroenterol Nutr. 1982;1(3):317–326. doi: 10.1097/00005176-198201030-00007. [DOI] [PubMed] [Google Scholar]
- 29.Tso P, Nauli A, Lo CM. Enterocyte fatty acid uptake and intestinal fatty acid-binding protein. Biochem Soc Trans. 2004;32(Pt 1):75–78. doi: 10.1042/bst0320075. [DOI] [PubMed] [Google Scholar]
- 30.Li X, Lindquist S, Lowe M, et al. Bile salt-stimulated lipase and pancreatic lipase-related protein 2 are the dominating lipases in neonatal fat digestion in mice and rats. Pediatr Res. 2007;62(5):537–541. doi: 10.1203/PDR.0b013e3181559e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berton A, Sebban-Kreuzer C, Rouvellac S, et al. Individual and combined action of pancreatic lipase and pancreatic lipase-related proteins 1 and 2 on native versus homogenized milk fat globules. Mol Nutr Food Res. 2009;53(12):1592–1602. doi: 10.1002/mnfr.200800563. [DOI] [PubMed] [Google Scholar]
- 32.Wang CS, Kuksis A, Manganaro F, et al. Studies on the substrate specificity of purified human milk bile salt-activated lipase. J Biol Chem. 1983;258(15):9197–9202. [PubMed] [Google Scholar]
- 33.Xiao X, Ross LE, Miller RA, et al. Kinetic properties of mouse pancreatic lipase-related protein-2 suggest the mouse may not model human fat digestion. J Lipid Res. 2011;52(5):982–990. doi: 10.1194/jlr.M014290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Innis SM, Dyer R, Nelson CM. Evidence that palmitic acid is absorbed as sn-2 monoacylglycerol from human milk by breast-fed infants. Lipids. 1994;29(8):541–545. doi: 10.1007/BF02536625. [DOI] [PubMed] [Google Scholar]
- 35.Campbell NB, Ruaux CG, Shifflett DE, et al. Physiological concentrations of bile salts inhibit recovery of ischemic-injured porcine ileum. Am J Physiol Gastrointest Liver Physiol. 2004;287(2):G399–G407. doi: 10.1152/ajpgi.00310.2003. [DOI] [PubMed] [Google Scholar]
- 36.Thomson AB, Keelan M, Garg ML, et al. Intestinal aspects of lipid absorption: in review. Can J Physiol Pharmacol. 1989;67(3):179–191. doi: 10.1139/y89-031. [DOI] [PubMed] [Google Scholar]
- 37.Ono T, Takagi Y, Kunishi I. Casein phosphopeptides from casein micelles by successive digestion with pepsin and trypsin. Biosci Biotechnol Biochem. 1998;62(1):16–21. doi: 10.1271/bbb.62.16. [DOI] [PubMed] [Google Scholar]
- 38.Michalski MC, Januel C. Does homogenization affect the health properties of cow's milk? Trends in Food Science & Technology. 2006;17:423–437. [Google Scholar]
- 39.Barbana C, Perez MD, Pocovi C, et al. Interaction of human alpha-lactalbumin with fatty acids: determination of binding parameters. Biochemistry (Mosc) 2008;73(6):711–716. doi: 10.1134/s0006297908060126. [DOI] [PubMed] [Google Scholar]