Abstract
Background
Pre-clinical evaluation of the vascular response of drug eluting stents (DES) is limited especially in the setting of diabetes mellitus (DM) preventing the evaluation of changes in DES design and eluted drugs until after clinical use.
Methods and Results
Cultured human aortic endothelial cells (HAEC) were used to assess the differences between sirolimus (SRL) and its analog, everolimus (EVL), in the setting of hyperglycemia on various cellular functions necessary for endothelial recovery. A diabetic rabbit model of iliac artery stenting was used to compare histologic and morphometric characteristics of the vascular response to everolimus-eluting (EES), sirolimus-eluting (SES) and bare metal stent (BMS) placement. Under hyperglycemic conditions, SRL impaired HAEC endothelial barrier function, migration and proliferation to a greater degree compared with EVL. In our in vivo model of diabetes, endothelialization at 28 days was significantly lower and endothelial integrity was impaired in SES when compared to both EES and BMS. Neointimal area, uncovered struts and fibrin deposition was significantly higher in SES when compared to EES and BMS.
Conclusions
Use of EES results in improved vascular response in our pre-clinical models of diabetes.
Keywords: diabetes mellitus, drug-eluting stents, endothelialization
Symptomatic coronary artery disease (CAD) is a leading cause of morbidity and mortality in patients with diabetes.1, 2 While drug eluting stents (DES) lower the need for target lesion revascularization (TLR) in non-diabetic patients 3, DES use in diabetics is associated with increased risk for TLR (compared to non-diabetics) and with increased risk for late stent thrombosis (ST), a phenomenon whose underlying etiology is associated with delayed endothelialization.4–10 Such adverse outcomes after coronary revascularization have raised concerns over the efficacy and safety of DES in diabetics. 11, 12 The majority of DES used in clinical practice elute sirolimus (SRL) or its analogues (e.g. everolimus, EVL) which inhibit the mammalian target of rapamycin (mTOR), a serine/threonine kinase via binding of FKBP12, a 12-KD FK506 binding protein. Key differences between EVL compared with SRL include increased lipophilicity and decreased binding affinity to FKBP12/12.6 compared with SRL.13, 14 SRL displacement of FKBP12/12.6 from endothelial intracellular calcium release channels can result in endothelial dysfunction through decreased endothelial dependent relaxation responses via activation of protein kinase C and impaired endothelial barrier function.13, 15 This is likely compounded given the association of diabetes with increased PKC activation which is associated with diabetic complications including accelerated atherosclerosis.16 We hypothesize that the use of EVL may lead to decreased endothelial dysfunction when compared with SRL leading to an improved vascular response after DES implantation in the setting of diabetes. In addition to differences in mTOR inhibition, first generation sirolimus eluting stents (SES) compared with newer generation everolimus eluting stents (EES) utilize improved strut construction and more biocompatible polymers in addition to the use of EVL aimed both at improving arterial healing by facilitating stent endothelialization and suppressing neointimal growth to prevent both restenosis and thrombotic complications.17, 18 Clinical trials of newer generation EES compared to SES in diabetics are however limited in scope and do not clearly differentiate whether these design improvements result in overall clinical benefit.19, 20 Pre-clinical evaluation is clearly needed to define the vascular response of DES in the setting of diabetes and evaluate improvements in DES design and/or changes in locally eluted drugs. In this study, we evaluated SRL and EVL using human aortic endothelial cells (HAEC) to compare differences in key cellular processes involved in arterial healing in the setting of hyperglycemia and utilized a diabetic rabbit model of stenting to define vascular responses to EES, SES, and bare metal stents (BMS).
Methods
Diabetic Rabbit Model
22 New Zealand White Male rabbits (3–3.5 kg) were made diabetic by means of a single dose of alloxan (100mg/kg IV). Plasma glucose levels were obtained at baseline prior to alloxan treatment and every four hours for the first 12 hours and every 8 hours for the following 36 hours and daily thereafter. Consistent with previous studies, animals with random plasma glucose consistently > 250 mg/dl between one and two weeks after induction were included in the study. 21 To prevent ketoacidosis, animals with evidence of elevation of blood ketones (monitored daily with reagent strips) were given intra-muscular low dose (1–2 U/day) long-acting Novolin (N) insulin daily. The protocol was approved by the Institutional Animal Care and Committee of Emory University (Atlanta, GA) and all experiments were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. A diagram illustrating the study design is included in the Supplemental Figure 1.
Rabbit Model of Iliac Artery Stenting, Assessment of Stent Morphometry, Histology and Endothelialization
New Zealand White adult rabbits underwent endothelial denudation of both iliac arteries using an angioplasty balloon catheter (Maverick, 3.0 × 12 mm, Boston Scientific, Boston, MA). Subsequently, 3.0 × 12 mm everolimus-eluting stent (EES) (Xience V, Abbott Vascular, Santa Clara, CA), sirolimus-eluting stent (SES) (Cypher, Cordis Corporation, Bridgewater, NJ) or 3.0 × 12 mm bare metal stent with an identical strut backbone to EES (BMS) (Multi-link Vision, Abbott Vascular) were deployed at a target stent-to-artery diameter ratio of 1.3:1 in each iliac artery, respectively. Each rabbit was randomized for BMS and DES (SES, EES) placement in each iliac artery in a 1:1:1 distribution resulting in an equal number of arterial stenting for each stent type. Stented arteries were harvested at 28 days as previously described.22 Confocal light microscopy (LM) was used to assess stent morphometry and histology. The extent of arterial injury at the site of stent struts was graded by the method of Schwartz et al.23 En face scanning electron microscopy (SEM) was used to assess stent endothelialization. See online supplemental materials for further details.
Cell Culture and In Vitro Endothelial Function Assays
Human aortic endothelial cells (HAECs) (Cell Applications, San Diego, CA) were maintained in endothelial cell growth medium (Cell Applications) and passages 4 through 8 were used for all experiments. For all experiments, endothelial cells were washed once in phosphate buffered saline and before appropriate reagents and media were introduced. Transendothelial electrical resistance (TEER) was measured in real-time using ECIS software (Applied Biophysics, Troy, NJ) and is expressed as specific electrical resistance (Ω cm2). Data are presented as the change in resistive portions of the resistance normalized to its value at baseline. Proliferation, apoptosis and migration assays were performed as previously described.24, 25 Further experimental details are available in the online supplemental materials.
Model of mTOR inhibition and Hyperglycemia
Sirolimus dose of 500 nmol/L was chosen consistent with our previous work of tissue concentration after SES implantation. The rapamycin analog, everolimus, was compared at the same concentration to determine its comparative effect on HAEC. Assays were performed under hyperglycemic (30 mmol/L glucose) conditions. Assays were conducted by first exposing HAEC to high glucose for 48 hours followed by sirolimus (500 nmol/L) or everolimus (500 nmol/L) for an additional 24 hours.
Statistical analysis
Statistical analysis was performed with STATA 9.2 (College Station, TX). All comparisons were performed using linear or Poisson regressions to allow correction for intra-class correlations among groups. In addition, conservative Huber/White/Sandwich variance estimates were used given small sample sizes. All dependent variables were tested for normality with the Wilk-Shapiro test either before or after single-parameter log transformation. All regression computations were bootstrapped with 100 random replications per regression model, justifying the use of parametric estimation methods and correcting for intra-class correlations. Comparisons were made between estimated regression coefficients of each independent variable (i.e. bEES or bSES, see supplemental Tables 1–4) or slope/intercept of the regression model. Protection against spuriously significant differences between these two regression coefficients was provided by the Bonferroni Theorem on a per-table or per-figure basis (with n comparisons each) such that our threshold for statistical significance is p< α/n, where α = 0.05. Further statistical details are available in the online supplemental materials.
Results
Endothelial Barrier Function, Cell Proliferation, Migration and Apoptosis under Hyperglycemic Conditions
Cultured human aortic endothelial cells (HAEC) were used to assess the effect of sirolimus (SRL) and everolimus (EVL) in the setting of hyperglycemia on various cellular functions necessary for endothelial recovery. First to confirm the effect of our in vitro hyperglycemic model, we conducted immunobloting for phophorylated Akt. Attenuation of phosphorylation was seen in the setting of hyperglycemia (30 mmol/L) and insulin stimulation when compared to normoglycemia (5 mmol/L) confirming an in vitro model of insulin resistance (Supplemental Figure 2).24
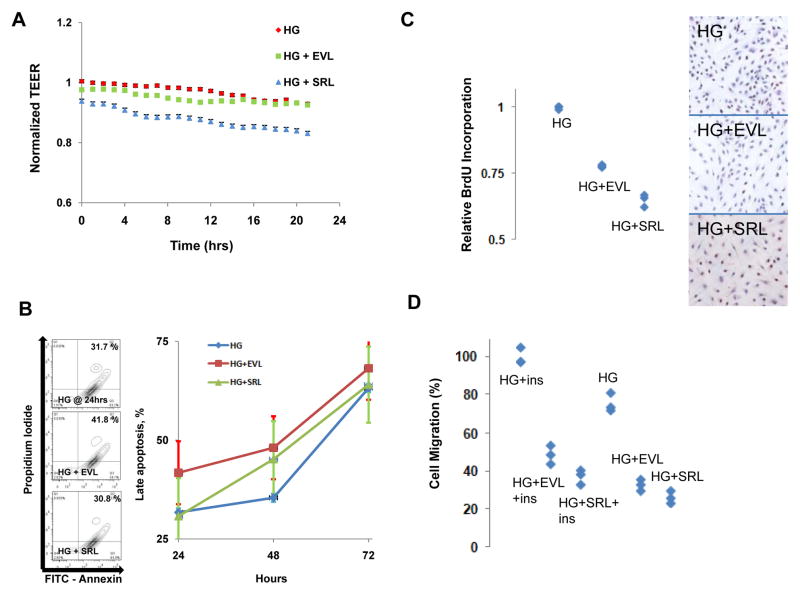
Using regression modeling comparing the regression coefficient between groups, endothelial barrier function (EBF) of HAEC treated with SRL and EVL was compared in the setting of hyperglycemia (HG) using transendothelial electrical resistance (TEER) and showed a significantly impaired TEER in HG+SRL compared with HG+EVL over the measured 24 hour period (Figure 1A). Additionally there was a significant increase in apoptosis with HG+EVL compared with HG+SRL at 24, 48 and 72 hours (Figure 1B). HAEC proliferation was inhibited to a greater extent in HG+SRL compared with HG+EVL at 24 hours (Figure 1C). Migration was significantly decreased by HG+SRL compared with HG+EVL only in the presence of insulin (Figure 1D).
Figure 1. Endothelial Barrier Function, Proliferation, Apoptosis and Migration in Human Aortic Endothelial Cells (HAEC) in Hyperglycemic Environments.
HAEC were incubated in hyperglycemic (30 mM) environment for 72 hours with treatment with everolimus (HG+EVL) or sirolimus (HG+SRL) for 24 hours. (A) HAEC endothelial barrier function was assessed by transendothelial electrical resistance (TEER) showing significantly impaired barrier function with HG+SRL compared with HG+EVL when analyzed with linear regression modeling (n = 3 wells, p = 0.0006, Bonferroni threshold = 0.025). (B) HAEC undergoing late/definite apoptosis were measured by flow cytometry (propridium iodide (PI) and FITC-Annexin positive stained cells) shown quantitatively with examples of bivariate plots at 24 hours shown to the left. Apoptosis in HG+EVL significantly higher at 24, 48 and 72 hours compared with HG+SRL when analyzed with Poisson regression (n = 3, p<0.0005, Bonferroni threshold = 0.017). (C) HAEC proliferation was measured by BrdU incorporation and was significantly decreased by HG+SRL compared with HG+EVL (n = 4, linear regression, p < 0.0005, Bonferroni threshold = 0.017). (D) HAEC migration was measured by modified Boyden chamber assays and expressed as percent cells migrated compared to HG. In the presence of insulin (ins), migration was significantly decreased by HG+SRL compared with HG+EVL (n = 4, linear regression, p < 0.0005, Bonferroni threshold = 0.0083) however not in the absence of insulin (p = 0.0125).
Diabetes induction and insulin signaling
Of the 22 animals that underwent diabetes induction with alloxan, 6 died from overwhelming hypoglycemia consistent with known mortality from alloxan diabetes induction.21 One additional animal did not demonstrate glucose levels >250 mg/dl one week after induction and was excluded. All of the remaining 15 animals underwent stenting 2 weeks after alloxan induction resulting in 30 stented arteries (SES, n = 10; EES, n = 10 and BMS, n = 10). All animals underwent stenting without incidence of dissection, thrombosis, and all stents were patent at follow-up angiography. All animals survived the duration of the study without major complications (i.e. ketoacidosis). Weights also were not different significantly different at the time of diabetic induction and sacrifice within and between different animal groups (Table 1). Animals received similar daily weight-based doses of insulin both within and between groups. Each group maintained similar average daily blood glucose levels well above 250mg/dl throughout the study (Table 1). To confirm the validity of our animal model, mitogenic components of the insulin signaling pathway, Akt and ERK, were examined by immunoblotting in whole iliac arteries of our diabetic model two weeks after alloxan-induction.26 We observed attenuation of Akt (Ser 473) phosphorylation in our diabetic model compared with control animals with ERK (Thr202/Tyr204) phosphorylation remaining preserved (Supplemental Figure 2) recapitulating molecular features of insulin resistance in the vasculature.27, 28
Table 1.
Diabetic Rabbit Average Weight at Alloxan Induction, Sacrifice and Average Daily Blood Glucose Levels after Induction
| BMS (n = 10) | EES (n = 10) | SES (n = 10) | p(bEES=bSES) | |
|---|---|---|---|---|
| Weight (kg) at Induction | 3.3 ± 0.1 | 3.1 ± 0.1 | 3.2 ± 0.2 | 0.11 |
| Weight (kg) at Sacrifice | 3.1 ± 0.2 | 3.2 ± 0.3 | 3.5 ± 0.5 | 0.62 |
| Glucose (mg/dl) ** | 393 ± 34 | 351 ± 78 | 331 ± 51 | 0.91 |
p-value computed after normalizing Log transformation. b is the linear regression coefficient for each group. Bonferroni threshold of significance is 0.0167
Morphometric analysis
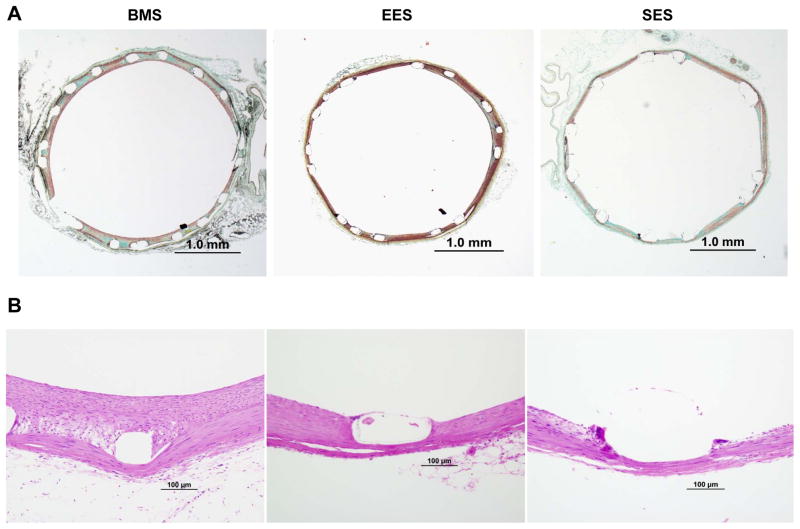
In 28 days stents, neointimal area was significantly lower in EES compared to both SES and BMS however this difference did not persist when normalized to injury score (Table 2, Figure 2A). Internal, external elastic lamina and medial area all were not significantly different between groups (Table 2). Percent stenosis was significantly higher in BMS compared to EES and SES however without significant difference between EES and SES (Table 2).
Table 2.
Morphometric Characteristics of Stents Implanted in a Diabetic Animal Model at 28 days
| BMS (n = 7) | EES (n = 7) | SES (n = 7) | p(bEES=bSES) | |
|---|---|---|---|---|
| IEL area, mm2 | 5.99 ± 0.54 | 5.70 ± 0.45 | 6.22 ± 0.56 | 0.044 |
| EEL area, mm2 | 6.29 ± 0.52 | 5.93 ± 0.51 | 6.51 ± 0.60 | 0.034 |
| Medial area, mm2 | 0.30 ± 0.07 | 0.23 ± 0.08 | 0.23 ± 0.04 | 0.066 |
| Neointimal area, mm2 | 1.08 ± 0.20 | 0.60 ± 0.09 | 0.84 ± 0.18 | 0.004* |
| Stenosis, % | 18.1 ± 3.3 | 10.6 ± 1.8 | 13.4 ± 2.5 | 0.016 |
| Injury score ** | 0.30 ± 0.17 | 0.06 ± 0.06 | 0.13 ± 0.08 | 0.234 |
| Neointimal area/Injury score ** | 8.48 ± 1.57 | 4.72 ± 1.45 | 6.58 ± 1.45 | 0.717 |
p-value < Bonferroni threshold of significance 0.0071.
p-value computed after normalizing log transformation. b is the linear regression coefficient for each group. IEL – internal elastic lamina, EEL – external elastic lamina.
Figure 2. Morphometric and Histologic Examples of 28 day Diabetic Rabbit Iliac Artery Stents.
Low (A) and high power magnification photomicrographs (B) of diabetic rabbit iliac arteries implanted with bare metal stents (BMS), everolimus (EES) and sirolimus eluting stents (SES) at 28 days. Detailed morphometric and histologic examination showed significantly increased neointima area, percent uncovered struts and struts with fibrin with SES compared to EES (see Table 2).
Histology
The percentage of uncovered struts and struts surrounded by fibrin was significantly higher in SES compare with BMS or EES (Table 3). No differences were seen in the number of inflammatory cells and percentage of giant cells between groups (Table 3).
Table 3.
Histologic Characteristics of Stents Implanted in a Diabetic Animal Model at 28 days
| BMS (n = 7) | EES (n = 7) | SES (n = 7) | p(bEES=bSES) | |
|---|---|---|---|---|
| % struts with fibrin ** | 6.8 ± 3.4 | 12.8 ± 11.1 | 27.3 ± 15.9 | 0.008* |
| % uncovered struts ** | 14.6 ± 28.1 | 35.1 ± 30.8 | 67.2 ± 24.6 | 0.005* |
| % struts with giant cells | 45.6 ± 24.0 | 47.8 ± 16.6 | 52.4 ± 22.4 | 0.65 |
| Inflammatory cells ** | 24.3 ± 30.4 | 50.0 ± 39.2 | 65.0 ± 66.6 | 0.68 |
p-value < Bonferroni threshold of significance (0.0125).
p-value computed after normalizing log transformation. b is the linear regression coefficient for each group
Scanning Electron Microscopy
En-Face scanning electron microscopy of 28-day stented segments demonstrated near complete endothelial coverage in arteries implanted with BMS and EES while SES demonstrated significantly decreased coverage over struts (Figure 3A–B). Arteries with SES consistently also showed disrupted endothelial cell junctions compared with BMS and EES (Figure 3A, right panel).
Figure 3. SEM and Quantitative Analysis of 28 day Diabetic Rabbit Iliac Artery Stents.
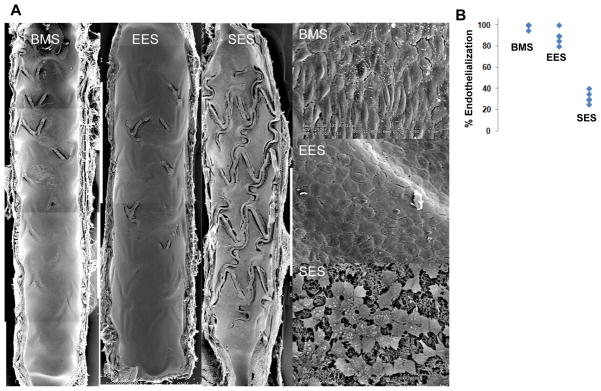
Scanning electron microscopy (SEM) and quantitative analysis of diabetic rabbit iliac arteries implanted with bare metal stents (BMS), everolimus (EES) and sirolimus eluting stents (SES) at 28 days. (A) SEM examination demonstrates near-complete endothelial coverage of BMS and EES however, there is marked absence of intact endothelium over stent struts in SES seen both at low (15x) and high power (200x) with evidence of incomplete cellular junctions. (B) Quantitative analysis of endothelial coverage shows that EES had significantly higher strut coverage compared with SES and similar coverage to BMS when analyzed by linear regression modeling (n = 6, p < 0.0005, Bonferroni threshold = 0.017).
Discussion
This study is the first to compare the vascular response between different -limus based DES in a diabetic animal model of stenting. In this study, 28 day everolimus-eluting stents (EES) were compared to sirolimus-eluting (SES) and bare metal stents (BMS) with an identical strut backbone to EES after placement in the iliac arteries of insulin-dependent diabetic rabbits. Alloxan-induction of diabetes achieved a significant degree of hyperglycemia in our animal model throughout the study period requiring the use long acting insulin (1–2 units of Novolin N) during this period (Table 1) and recapitulated molecular features of insulin resistance (Supplemental Figure 2). In this model, EES displayed an improved vascular response compared to SES with near complete endothelialization/covered strut and less fibrin deposition. In addition robust endothelial barrier integrity comparable to BMS with similar anti-restenosis efficacy to SES was observed. In regards to key cellular functions for endothelialization, EVL demonstrated improved endothelial barrier function, proliferation and migration compared with SRL while conversely having increased apoptosis, all under hyperglycemic conditions. EVL is a 40-O-hydroxyethyl-derivative of SRL which similarly inhibits mTOR through binding to FKBP12/12.6, a ubiquitous 12-KD FK506 binding protein. While EVL displays similar inhibition of mTOR compared with SRL in vivo, in vitro assays previously show decreased affinity of EVL to FKBP12/12.6.14 In this study, we observe poor endothelial barrier function with respect to HG+SRL compared with HG+EVL in the setting of hyperglycemia at 24 hours (Figure 1A) in vitro and correspondingly poor endothelial junctional integrity seen in vivo with SEM analysis of SES compared to EES and BMS (Figure 3A, right panel). While displacement of FKBP12/12.6 by SRL from endothelial intracellular calcium release channels can result in increased intracellular calcium leading to endothelial dysfunction through calcium dependent phosphorylation of protein kinase C13, this suggests that EVL lessen binding affinity to FKBP12/12.6 may lead to improved endothelial function after delivery from DES. We have recently conducted a study showing that SRL disrupts VE Cadherin homeostasis leading to impaired endothelial barrier function likely through displacement of FKBP12/12.6 and increased intracellular calcium rather than mTOR inhibition.15 This mechanism likely works in synergy with protein kinase C phosphorylation seen in diabetes to impair endothelial function which has also been observed in diabetic animal models.29 This study suggests that the less avid FKBP12/12.6 binding with EVL compared with SRL may result in improved endothelial barrier function and integrity explaining the differential vascular response observed between the two drugs.
With respect to proliferation and apoptosis, EVL had higher apoptosis, especially initially at 24 hours, compared with SRL (Figure 1B). However inhibition of HAEC proliferation and migration was greater with SRL compared with EVL treatment. This suggests that SRL may also differentially inhibit Akt compared with EVL as Akt activity is involved in HAEC migration and proliferation.24, 30 Overall our findings suggest that while the efficacy of EES and SES were similar, there were significant differences in endothelialization and endothelial integrity seen in vivo and endothelial barrier function, proliferation and migration in vitro, suggesting that EES/EVL may improve vascular endothelial response in diabetic environment.
Preclinical Findings
Previous studies in a non-diabetic rabbit model showed similar improvement in endothelialization with comparator DES utilizing newer strut constructs (i.e. EES) however these improvements did not persist past 14 days. 22 This differs from our diabetic model where differences were seen at 28 days between EES and SES. Additionally, there was a lesser degree of endothelialization seen with thicker strut SES in our diabetic model (37.7 ± 1.4%) versus historical non-diabetic rabbit model (> 60%) with preservation of endothelialization in thinner strut EES in both models.22 In this study, morphometric and histologic characteristics are more favorable for EES with significant reductions in neointimal area and improvement in the percentage of uncovered struts and struts with fibrin when compared to SES persisting up to 28 days. While this can be partly explained with lower injury scores of thinner constructed EES compared with SES (Figure 4), similar constructed BMS do not maintain these advantages suggesting that this is likely due to components beyond strut construction such as the durable polymer used or eluted drug. There was an observed numeric, but not significant, increase in inflammatory cells with SES compared with EES consistent with an increased inflammatory response which is likely due to polymer biocompatibility as everolimus and sirolimus have equivalent immunosuppressant activity (Figure 4).4, 22, 31 This suggests the improvement of endothelialization/percentage of uncovered struts and endothelial barrier function in newer construction EES are due to the differences in its mTOR inhibition compared with SES (Figure 4).
Figure 4.
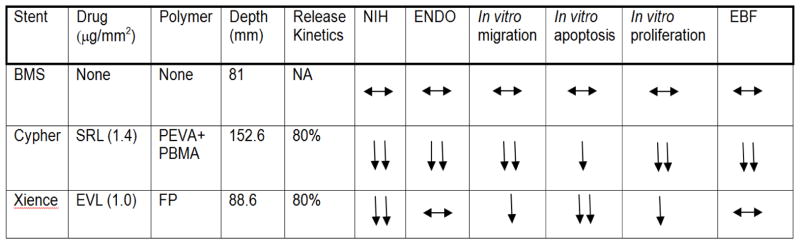
Summary of Vascular Response of Comparator Limus-Based Drug-Eluting and Bare Metal Stents (BMS) In Vivo and In Vitro Diabetic Model.
Abbreviations: SRL – sirolimus, EVL – everolimus, PEVA – polyethylene-co-vinyl acetate, PBMA – poly n-butyl methacrylate, FP – fluropolymer, NIH – neointimal hyperplasia, ENDO – endothelialization, EBF – endothelial barrier function
Clinical Implications
While DES represent a major advance in the treatment of symptomatic coronary artery disease, their use in diabetic patients continue to be associated with poorer outcomes after coronary revascularization.32 Diabetics treated with both SES and EES have higher repeat revascularization rates than non-diabetics and diabetes is an independent risk factor for late stent thrombosis. Our data suggest that both differences in mTOR inhibition and improvements in strut construction in EES improve vascular healing responses as compared to SES while preserving anti-restenotic efficacy. Dedicated clinical trials comparing responses between these two DES in patients with diabetes are generally lacking. The ESSENCE-DIABETES study and subgroup analysis of both the EXCELLENT and ISAR-TEST-4 studies compared SES and EES in patients with diabetes and found improved angiographic findings with EES compared with SES.19, 20, 33 While the ISAR-TEST-4 diabetic subgroup showed a trend towards improved thrombotic complications with EES, these studies overall were not powered to examine late stent thrombosis which might be expected to be improved with EES. Additionally impaired endothelial barrier function may lead to accelerated neoinitimal atherosclerosis (“neoatherosclerosis”) in DES due to various mechanisms, one of which includes –limus based mTOR inhibition.15 While little data exists on neoatherosclerosis in diabetic patients undergoing DES placement, this study suggests neoatherosclerosis is likely augmented in diabetes.16, 34 While durable polymer SES are no longer clinically available, renewed sirolimus use in the setting of bioabsorbable DES may further emphasize these differences in mTOR inhibition.35 Overall our data suggest that changes in both mTOR inhibition and stent design can be evaluated in a pre-clinical diabetic animal model of stenting which interestingly shows improved vascular responses due to the type of mTOR inhibition in addition to improvement to stent design.
Limitations
While our animal model of arterial stenting provides key insights into the vascular response under diabetic conditions, it lacks significant co-morbid conditions such as atherosclerosis. This in turn may underestimate the degree of impaired endothelialization and suggests that the observed improvement in vascular response with everolimus might be more pronounced.36 Another limitation was our inability to measure mTOR activity in the stented arteries. The mTOR complex is upstream to canonical mTOR effectors (i.e. Akt) in addition to those outside the canonical mTOR pathway (i.e. FKBP12.6).15 By measuring mTOR activity, this would allow an indirect measure of –limus efficacy on mTOR related pathways known to effect vascular endothelialization.15
Conclusions
This pre-clinical study, utilizing a diabetic animal model of arterial stenting, suggests that there is an improved vascular response with newer generation DES which is interestingly due to the type of local mTOR inhibition.
Supplementary Material
Acknowledgments
We greatly appreciate the help of John Newell for statistical assistance.
Sources of Funding
This study was supported by the Carlyle Fraser Heart Center, CVPath Inc., American Heart Association and US NIH grant RO1 HL096970-01A.
Abbreviations and Acronyms
- BMS
bare metal stents
- CAD
coronary artery disease
- DES
drug eluting stents
- DM
diabetes mellitus
- EES
everolimus-eluting stents
- EVL
everolimus
- HAEC
human aortic endothelial cells
- mTOR
mammalian target of rapamycin
- LM
light microscopy
- SES
sirolimus-eluting stents
- SEM
scanning electron microscopy
- SRL
sirolimus
- ST
stent thrombosis
- TLR
target lesion revascularization
Footnotes
Disclosures
CVPath Institute has research grants from Medtronic CardioVascular, Abbott Vascular, Terumo Corporation, Atrium Medical, Boston Scientific, and Cordis/Johnson&Johnson, OrbusNeich Medical, and Biosensors International. Dr. Virmani is a consultant for Medtronic CardioVascular, Abbott Vascular, Terumo Corporation, Atrium Medical, W.L. Gore, and Lutonix. AVF has sponsored research agreements with Medtronic CardioVascular and Boston Scientific. He is also an advisory board member to Medtronic CardioVascular. AH is supported with an AHA Postdoctoral Fellowship grant (Greater Southeast Affliate). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the multiple risk factor intervention trial. Diabetes care. 1993;16:434–444. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- 2.Luscher TF, Creager MA, Beckman JA, Cosentino F. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part ii. Circulation. 2003;108:1655–1661. doi: 10.1161/01.CIR.0000089189.70578.E2. [DOI] [PubMed] [Google Scholar]
- 3.Sinning JM, Baumgart D, Werner N, Klauss V, Baer FM, Hartmann F, Drexler H, Motz W, Klues H, Voelker W, Pfannebecker T, Stoll HP, Nickenig G. Five-year results of the multicenter randomized controlled open-label study of the cypher sirolimus-eluting stent in the treatment of diabetic patients with de novo native coronary artery lesions (scorpius) study: A german multicenter investigation on the effectiveness of sirolimus-eluting stents in diabetic patients. American heart journal. 2012;163:446–453. 453 e441. doi: 10.1016/j.ahj.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R. Pathology of drug-eluting stents in humans: Delayed healing and late thrombotic risk. Journal of the American College of Cardiology. 2006;48:193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 5.Wilson GJ, Nakazawa G, Schwartz RS, Huibregtse B, Poff B, Herbst TJ, Baim DS, Virmani R. Comparison of inflammatory response after implantation of sirolimus- and paclitaxel-eluting stents in porcine coronary arteries. Circulation. 2009;120:141–149. 141–142. doi: 10.1161/CIRCULATIONAHA.107.730010. [DOI] [PubMed] [Google Scholar]
- 6.Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Stahle E, Feldman TE, van den Brand M, Bass EJ, Van Dyck N, Leadley K, Dawkins KD, Mohr FW. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. The New England journal of medicine. 2009;360:961–972. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 7.Daemen J, Wenaweser P, Tsuchida K, Abrecht L, Vaina S, Morger C, Kukreja N, Juni P, Sianos G, Hellige G, van Domburg RT, Hess OM, Boersma E, Meier B, Windecker S, Serruys PW. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: Data from a large two-institutional cohort study. Lancet. 2007;369:667–678. doi: 10.1016/S0140-6736(07)60314-6. [DOI] [PubMed] [Google Scholar]
- 8.Finn AV, Palacios IF, Kastrati A, Gold HK. Drug-eluting stents for diabetes mellitus: A rush to judgment? Journal of the American College of Cardiology. 2005;45:479–483. doi: 10.1016/j.jacc.2004.10.060. [DOI] [PubMed] [Google Scholar]
- 9.Stone GW, Kedhi E, Kereiakes DJ, Parise H, Fahy M, Serruys PW, Smits PC. Differential clinical responses to everolimus-eluting and paclitaxel-eluting coronary stents in patients with and without diabetes mellitus. Circulation. 2011;124:893–900. doi: 10.1161/CIRCULATIONAHA.111.031070. [DOI] [PubMed] [Google Scholar]
- 10.Finn AV, Joner M, Nakazawa G, Kolodgie F, Newell J, John MC, Gold HK, Virmani R. Pathological correlates of late drug-eluting stent thrombosis: Strut coverage as a marker of endothelialization. Circulation. 2007;115:2435–2441. doi: 10.1161/CIRCULATIONAHA.107.693739. [DOI] [PubMed] [Google Scholar]
- 11.Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, Airoldi F, Chieffo A, Montorfano M, Carlino M, Michev I, Corvaja N, Briguori C, Gerckens U, Grube E, Colombo A. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA: the journal of the American Medical Association. 2005;293:2126–2130. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- 12.Machecourt J, Danchin N, Lablanche JM, Fauvel JM, Bonnet JL, Marliere S, Foote A, Quesada JL, Eltchaninoff H, Vanzetto G. Risk factors for stent thrombosis after implantation of sirolimus-eluting stents in diabetic and nondiabetic patients: The evastent matched-cohort registry. Journal of the American College of Cardiology. 2007;50:501–508. doi: 10.1016/j.jacc.2007.04.051. [DOI] [PubMed] [Google Scholar]
- 13.Long C, Cook LG, Wu GY, Mitchell BM. Removal of fkbp12/12.6 from endothelial ryanodine receptors leads to an intracellular calcium leak and endothelial dysfunction. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:1580–1586. doi: 10.1161/ATVBAHA.107.144808. [DOI] [PubMed] [Google Scholar]
- 14.Schuler W, Sedrani R, Cottens S, Haberlin B, Schulz M, Schuurman HJ, Zenke G, Zerwes HG, Schreier MH. Sdz rad, a new rapamycin derivative: Pharmacological properties in vitro and in vivo. Transplantation. 1997;64:36–42. doi: 10.1097/00007890-199707150-00008. [DOI] [PubMed] [Google Scholar]
- 15.Habib A, Karmali V, Polavarapu R, Akahori H, Cheng Q, Pachura K, Kolodgie FD, Finn AV. Sirolimus-fkbp12.6 impairs endothelial barrier function through protein kinase c-alpha activation and disruption of the p120-vascular endothelial cadherin interaction. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:2425–2431. doi: 10.1161/ATVBAHA.113.301659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geraldes P, King GL. Activation of protein kinase c isoforms and its impact on diabetic complications. Circulation research. 2010;106:1319–1331. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serruys PW, Silber S, Garg S, van Geuns RJ, Richardt G, Buszman PE, Kelbaek H, van Boven AJ, Hofma SH, Linke A, Klauss V, Wijns W, Macaya C, Garot P, DiMario C, Manoharan G, Kornowski R, Ischinger T, Bartorelli A, Ronden J, Bressers M, Gobbens P, Negoita M, van Leeuwen F, Windecker S. Comparison of zotarolimus-eluting and everolimus-eluting coronary stents. The New England journal of medicine. 2010;363:136–146. doi: 10.1056/NEJMoa1004130. [DOI] [PubMed] [Google Scholar]
- 18.Kolandaivelu K, Swaminathan R, Gibson WJ, Kolachalama VB, Nguyen-Ehrenreich KL, Giddings VL, Coleman L, Wong GK, Edelman ER. Stent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation. 2011;123:1400–1409. doi: 10.1161/CIRCULATIONAHA.110.003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park KW, Chae IH, Lim DS, Han KR, Yang HM, Lee HY, Kang HJ, Koo BK, Ahn T, Yoon JH, Jeong MH, Hong TJ, Chung WY, Jo SH, Choi YJ, Hur SH, Kwon HM, Jeon DW, Kim BO, Park SH, Lee NH, Jeon HK, Gwon HC, Jang YS, Kim HS. Everolimus-eluting versus sirolimus-eluting stents in patients undergoing percutaneous coronary intervention: The excellent (efficacy of xience/promus versus cypher to reduce late loss after stenting) randomized trial. Journal of the American College of Cardiology. 2011;58:1844–1854. doi: 10.1016/j.jacc.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 20.Kim WJ, Lee SW, Park SW, Kim YH, Yun SC, Lee JY, Park DW, Kang SJ, Lee CW, Lee JH, Choi SW, Seong IW, Lee BK, Lee NH, Cho YH, Shin WY, Lee SJ, Hyon MS, Bang DW, Park WJ, Kim HS, Chae JK, Lee K, Park HK, Park CB, Lee SG, Kim MK, Park KH, Choi YJ, Cheong SS, Yang TH, Jang JS, Her SH, Park SJ. Randomized comparison of everolimus-eluting stent versus sirolimus-eluting stent implantation for de novo coronary artery disease in patients with diabetes mellitus (essence-diabetes): Results from the essence-diabetes trial. Circulation. 2011;124:886–892. doi: 10.1161/CIRCULATIONAHA.110.015453. [DOI] [PubMed] [Google Scholar]
- 21.Pomaro DR, Ihara SS, Pinto LE, Ueda I, Casarini DE, Ebihara F, Santos AO, Izar MC, Fonseca FA. High glucose levels abolish antiatherosclerotic benefits of ace inhibition in alloxan-induced diabetes in rabbits. Journal of cardiovascular pharmacology. 2005;45:295–300. doi: 10.1097/01.fjc.0000155384.64350.45. [DOI] [PubMed] [Google Scholar]
- 22.Joner M, Nakazawa G, Finn AV, Quee SC, Coleman L, Acampado E, Wilson PS, Skorija K, Cheng Q, Xu X, Gold HK, Kolodgie FD, Virmani R. Endothelial cell recovery between comparator polymer-based drug-eluting stents. Journal of the American College of Cardiology. 2008;52:333–342. doi: 10.1016/j.jacc.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz RS, Huber KC, Murphy JG, Edwards WD, Camrud AR, Vlietstra RE, Holmes DR. Restenosis and the proportional neointimal response to coronary artery injury: Results in a porcine model. Journal of the American College of Cardiology. 1992;19:267–274. doi: 10.1016/0735-1097(92)90476-4. [DOI] [PubMed] [Google Scholar]
- 24.Patterson C, Mapera S, Li HH, Madamanchi N, Hilliard E, Lineberger R, Herrmann R, Charles P. Comparative effects of paclitaxel and rapamycin on smooth muscle migration and survival: Role of akt-dependent signaling. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:1473–1480. doi: 10.1161/01.ATV.0000223866.42883.3b. [DOI] [PubMed] [Google Scholar]
- 25.Habib A, Karmali V, Polavarapu R, Akahori H, Pachura K, Finn AV. Metformin impairs endothelialization after placement of newer generation drug eluting stents. Atherosclerosis. 2013;229:385–387. doi: 10.1016/j.atherosclerosis.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 27.Jonas M, Edelman ER, Groothuis A, Baker AB, Seifert P, Rogers C. Vascular neointimal formation and signaling pathway activation in response to stent injury in insulin-resistant and diabetic animals. Circulation research. 2005;97:725–733. doi: 10.1161/01.RES.0000183730.52908.C6. [DOI] [PubMed] [Google Scholar]
- 28.Montagnani M, Golovchenko I, Kim I, Koh GY, Goalstone ML, Mundhekar AN, Johansen M, Kucik DF, Quon MJ, Draznin B. Inhibition of phosphatidylinositol 3-kinase enhances mitogenic actions of insulin in endothelial cells. The Journal of biological chemistry. 2002;277:1794–1799. doi: 10.1074/jbc.M103728200. [DOI] [PubMed] [Google Scholar]
- 29.Hamamdzic D, Fenning RS, Patel D, Mohler ER, 3rd, Orlova KA, Wright AC, Llano R, Keane MG, Shannon RP, Birnbaum MJ, Wilensky RL. Akt pathway is hypoactivated by synergistic actions of diabetes mellitus and hypercholesterolemia resulting in advanced coronary artery disease. Am J Physiol Heart Circ Physiol. 2010;299:H699–706. doi: 10.1152/ajpheart.00071.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mtorc2 assembly and akt/pkb. Molecular cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 31.John MC, Wessely R, Kastrati A, Schomig A, Joner M, Uchihashi M, Crimins J, Lajoie S, Kolodgie FD, Gold HK, Virmani R, Finn AV. Differential healing responses in polymer- and nonpolymer-based sirolimus-eluting stents. JACC Cardiovascular interventions. 2008;1:535–544. doi: 10.1016/j.jcin.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Barsness GW, Peterson ED, Ohman EM, Nelson CL, DeLong ER, Reves JG, Smith PK, Anderson RD, Jones RH, Mark DB, Califf RM. Relationship between diabetes mellitus and long-term survival after coronary bypass and angioplasty. Circulation. 1997;96:2551–2556. doi: 10.1161/01.cir.96.8.2551. [DOI] [PubMed] [Google Scholar]
- 33.Kufner S, Byrne RA, Mehilli J, Massberg S, Birkmeier KA, Schulz S, Pache J, Schomig A, Kastrati A. Second-versus first-generation “limus”-eluting stents in diabetic patients with coronary artery disease: A randomized comparison in setting of isar-test-4 trial. Catheter Cardiovasc Interv. 2013;82:E769–76. doi: 10.1002/ccd.24741. [DOI] [PubMed] [Google Scholar]
- 34.Nakazawa G, Otsuka F, Nakano M, Vorpahl M, Yazdani SK, Ladich E, Kolodgie FD, Finn AV, Virmani R. The pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents. Journal of the American College of Cardiology. 2011;57:1314–1322. doi: 10.1016/j.jacc.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ormiston J, Webster M, Stewart J, Vrolix M, Whitbourn R, Donohoe D, Knape C, Lansky A, Attizzani GF, Fitzgerald P, Kandzari DE, Wijns W. First-in-human evaluation of a bioabsorbable polymer-coated sirolimus-eluting stent: Imaging and clinical results of the dessolve i trial (des with sirolimus and a bioabsorbable polymer for the treatment of patients with de novo lesion in the native coronary arteries) JACC Cardiovascular interventions. 2013;6:1026–1034. doi: 10.1016/j.jcin.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 36.Finn AV, John M, Nakazawa G, Polavarapu R, Karmali V, Xu X, Cheng Q, Davis T, Raghunathan C, Acampado E, Ezell T, Lajoie S, Eppihimer M, Kolodgie FD, Virmani R, Gold HK. Differential healing after sirolimus, paclitaxel, and bare metal stent placement in combination with peroxisome proliferator-activator receptor gamma agonists: Requirement for mtor/akt2 in ppargamma activation. Circulation research. 2009;105:1003–1012. doi: 10.1161/CIRCRESAHA.109.200519. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.