Abstract
Background
HIV-associated neurocognitive disorder (HAND) is an independent predictor of early mortality and is associated with many difficulties in activities of daily living. We sought to determine the prevalence of and risk factors for HAND in HIV-infected Koreans. In addition, we investigated the performance of screening tools and components of neuropsychological (NP) tests for diagnosing HAND.
Methods
HIV-infected patients were enrolled consecutively from two different urban teaching hospitals in Seoul, South Korea between March 2012 and September 2012. Participants completed a detailed NP assessment of six cognitive domains commonly affected by HIV. The Frascati criteria were used for diagnosing HAND. Four key questions, international HIV dementia scale (IHDS) and MoCA-K were also assessed as potential tools for screening for HAND.
Results
Among the 194 participants, the prevalence of HAND was 26.3%. Asymptomatic neurocognitive impairment, minor neurocognitive disorder accounted for 52.9% and 47.1% of the patients with HAND, respectively. In multivariate analysis, hemoglobin levels ≤13g/dL (p=0.046) and the current use of protease inhibitor-based regimen (p=0.031) were independent risk factors for HAND. The sensitivity and specificity of IHDS were 72.6% and 60.8%, and MoCA-K were 52.9% and 73.4%, respectively. IHDS (p<0.001) and MoCA-K (p<0.001) were both useful for screening for HAND. Among NP tests, the sensitivity and specificity of the Grooved Pegboard Test were 90.2% and 72.0%, and the Wisconsin Card Sorting Test were 61.2% and 84.4%, respectively.
Conclusions
HAND is a prevalent comorbidity in HIV-infected Koreans. Active screening and diagnosis with useful tools, like IHDS, MoCA-K and Grooved Pegboard Test, could be used to identify this important complication.
Keywords: HAND, HIV, risk factors, screening tool, NP tests
Introduction
Human immunodeficiency virus (HIV) may enter the central nervous system (CNS) early after infection [1], and although it does not directly infect neurons, it is frequently associated with structural and functional brain abnormalities [2-5]. This CNS infection can lead to neurocognitive impairment. This HIV-associated neurocognitive disorder (HAND) strongly predicts a wide variety of difficulties in activities of daily living, like employment, automobile driving, medication adherence, financial management, shopping, cooking, and use of public transportation [6]. Moreover, viral levels in the cerebrospinal fluid (CSF) have been associated with HAND in HIV-unsuppressed patients with AIDS [7], while a suppression of CSF HIV can correspond with improvement in cognition [8]. However, in the era of combination antiretroviral therapy (cART), HAND in Western countries is still common (16∼52%), even when cART is successful with suppression of viral load [9-14]. The extent to which cART leads to a reduction in the incidence and prevalence of HIV-related cognitive impairment remains unclear. Additionally, most neurocognitive research in HIV-infected individuals has been conducted from western populations, and the burden of HAND in Korean HIV-infected individuals is so far unknown. Furthermore, there are few studies concerning how to screen HIV-infected individuals for HAND and the diagnostic validation of neuropsychological (NP) tests for diagnosing HAND among HIV-infected Asian persons.
Methods
Study design
Two hundred HIV-infected patients who were 18 years old or older were recruited consecutively in Severance Hospital and Korea University Guro Hospital between March 2012 and September 2012 in Seoul, Korea. All subjects received and acknowledged informed consent and received standardized neurological, NP, and functional assessments at two study sites. This study was approved by the Institutional Review Board of the hospital (IRB #4-2011-0630).
The exclusion criteria were (1) recent and/or significant traumatic brain injury; (2) neurologic disorder not related to HIV infection; (3) infections that can affect the CNS; (4) significant CNS opportunistic infection based upon history and/or neuromedical examination; (5) current or past psychotic disorder; (6) significant substance use, including greater than three alcoholic drinks per day daily over the last month, or recreational drug use greater than one time per week during the last month; (7) symptoms of a current, active infection, body temperature of > 38.5°C at the time of recruitment or current treatment for a serious, systemic infection within 3 months; (8) color blindness; (9) hearing deficit that appears to affect auditory comprehension [15].
Six of two hundred subjects were excluded due to five confounding comorbidity and one withdrawing early from the study. Enrolled participants had demographical, clinical, and neuropsychological assessments. At baseline visits, neuromedical history and standardized exam, neurobehavioral testing, structured evaluation of selected psychiatric variables were evaluated. The following variables were also assessed: age at first visit, gender, body mass index (BMI), hemoglobin (Hb) level, degree of education, duration of HIV diagnosis, Centers for Disease Control and Prevention (CDC) classification, reported mode of transmission, prior acquired immunodeficiency syndrome (AIDS) diagnosis, hepatitis B or C co-infection, antiretroviral therapy regimen (2 nucleoside analog reverse transcriptase inhibitors (NRTIs) + non-nucleoside reverse transcriptase inhibitor (NNRTI), 2 NRTIs + protease inhibitor (PI), or other combination), CNS penetration effectiveness (CPE) score[16], initial CD4+ T cell count, pre-cART CD4+ T cell count, current CD4+ T cell count, lowest CD4+ T cell count, initial viral load (VL), pre-cART VL, current VL, and highest VL. Additionally, a clinician administered the depression questionnaire[17] and interview for assessing depression and substance use history. The Karnofsky performance status scale[18] was used to assess functional impairments. Also, eight questions designed by authors in Korean based on the suggestions of Antinori et al.[15] were used to assess functional activities; these were translated as: “Is it hard to take medication in correct dosages at correct time?”; “Is it hard to manage financial matters independently (budgets, writes checks, pays rent and bills, goes to bank)?”; “Is it hard to maintain house alone or with occasional assistance?”; “Is it hard to maintain personal schedules?”; “Do you have more mistakes in business?”; “Do you need more times than ever for finishing the same amount of work?; “Do you need more efforts than ever for doing well?; “Are you poorer at best working?”.
Neuromedical evaluation
Neuromedical assessments were standardized between study sites and included multiple, linked evaluations. These evaluations included the following elements:
Medical history: Standardized forms to record medical and neurological symptoms. Medical conditions were classified using an International Classification of Diseases 9th Revision, Clinical Modification (ICD-9-CM) codes, and HIV disease stage was classified according to 1993 CDC guidelines[19]. Staff also administered a brief questionnaire to collect information about HIV risk behavior.
Current Medications, Medication History, and Adherence: A clinician recorded prescribed medications taken for longer than one month. Detailed information about past and present ART use was completed. Each site used the Adherence Scale to Anti-HIV Medications form to collect ART adherence data [20].
Neurological and General Physical Examinations: The research clinician performed a standardized neurological, medical examination that included assessment of vital signs height and weight, mental status, cranial nerves, motor, sensory and cerebellar function, reflexes and gait. The medical staff also performs a general physical examination and note health-related functioning.
Assessment of screening tool
Four key questions proposed by the Asia, Australia, Africa and Middle East (AAAME) HAND Advisory Board as a screening tool (unpublished data) to evaluate an early potential of neurocognitive impairment and depression were applied to participants: “Are you slower in your thinking processes?”; “Are you more forgetful?”; “Is it harder to organize things?” and “Are you less able to find pleasure in things you used to enjoy?” Also, international HIV dementia scale (IHDS) [21] and Montreal Cognitive Assessment (MoCA)–K [22] were evaluated as screening tools for diagnosing HAND. The most useful cut-off values of each screening tests was also investigated.
Neuropsychological tests
In order to establish rates of HAND, we used NP test norms based upon the HIV-negative participants[23-27]. The neurobehavioral evaluation assessed six ability domains (Supplement table 1). These are well-established measures that have been used in numerous studies of HIV/AIDS. In addition to individual NP test results, the battery facilitates a clinical determination of level of impairment within each domain, as well as on global level of impairment based upon the population-specific normative standards. This approach has shown good inter-rater reliability, even across raters at different institutions [28].
Diagnosis of HAND
Subjects completed a detailed NP assessment measuring their functioning in six cognitive domains known to be commonly affected by HIV. The Frascati criteria were used for diagnosing HAND, classified into asymptomatic neurocognitive impairment (ANI), minor neurocognitive disorder (MND) and HIV-associated dementia (HAD) [15].
Statistical analyses
Independent t-test or Chi-square test was used to measure differences of each variable between neurocognitive impaired and non-impaired subjects. To identify independent factors associated with HAND, multivariate logistic regression analysis was performed with the variables that had a significant association with HAND on univariate analysis (p<0.05). Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were then calculated in comparison with the reference diagnosis, which was based on cases of HAND. In addition, we conducted a receiver operating characteristic (ROC) curve analysis to compare the predictive accuracy of screening tools and the area under the curve (AUC) was calculated. The 95% confidence interval (CI) was calculated using the Wilson score method. All p-values will be 2-tailed and p<0.05 will be considered statistically significant. All analyses will be performed using SPSS for Windows 12.0 (SPSS, Chicago, Illinois, USA).
Results
General characteristics of study subjects
Of the 194 enrolled subjects, 93.8% were male, and the mean age (range) was 45.12 (21-72) years. The most common exposure category was men having sex with men (MSM) (52.6%), followed by heterosexual contact (27.3%). The duration of education (mean±standard deviation (SD)) was 13.4±3.3 years, and 31.9%, and 27.9% of the subjects were at the clinical CDC stages B and C of HIV disease, respectively. Average current CD4+ T cell counts (cells/mm3, mean±SD) were 481.4±236.0 and current viral load (mean±SD, log10 copies/mL) were 2.0±1.4 (Table 1).
Table 1. Baseline characteristics and factors associated HAND in HIV-infected individuals.
| Variables | Total (N=194) | HAND (N= 51) | No HAND (N= 143) | p-value | Multivariate analysis | |
|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | p-value | |||||
| Age (years, range) | 45.12 (21-72) | 44.39 (21-70) | 45.38 (25-72) | 0.581 | ||
| Male gender (%) | 182 (93.8) | 49 (96.1) | 133 (93.0) | 0.735 | ||
| BMI (Kg/mm2) | 23.0±4.5 | 22.1±2.9 | 23.3±4.9 | 0.105 | ||
| Hemoglobin ≤ 13.0g/dL (%) | 15 (29.4) | 22 (15.4) | 0.029 | 2.287 (1.014-5.162) | 0.046 | |
| Education | ||||||
| Mean (years) | 13.4±3.3 | 12.8±3.4 | 13.6±3.3 | 0.188 | ||
| Mode of transmission (%) | ||||||
| Homosexual contacts | 102 (52.6) | 24 (47.1) | 78 (54.5) | 0.358 | ||
| Heterosexual contacts | 53 (27.3) | 17 (33.3) | 36 (25.2) | 0.262 | ||
| Unknown and others | 39 (20.1) | 10 (19.6) | 29 (20.3) | |||
| Duration of HIV infection (years) | 77.7±70.1 | 73.4±52.5 | 79.3±75.5 | 0.606 | ||
| Previous AIDS diagnosis | 55 (28.4) | 18 (35.3) | 37 (25.9) | 0.200 | ||
| AIDS at diagnosis | ||||||
| No | 76 | 17 (33.3) | 59 (41.3) | 0.320 | ||
| Yes | 118 | 34 (66.7) | 84 (58.7) | 0.320 | ||
| Initial CD4+ T cell count, mean±SD, cells/mm3 | 243.8±188.3 | 233.8±220.7 | 247.3±176.0 | 0.668 | ||
| Initial VL, mean±SD, log10 copies/mL | 4.8±1.2 | 4.7±1.3 | 4.8±176.0 | 0.797 | ||
| Pre-cART CD4+ T cell count, mean±SD, cells/mm3 | 186.4±136.0 | 176.5±167.5 | 189.8±123.8 | 0.601 | ||
| Pre-cART VL, mean±SD, log10 copies/mL | 5.0±0.9 | 5.0±0.9 | 5.0±0.9 | 0.965 | ||
| Current CD4+ T cell count, mean±SD, cells/mm3 | 481.4±236.0 | 444.5±259.2 | 494.6±226.6 | 0.194 | 0.999 (0.997-1.001) | 0.225 |
| Current VL, mean±SD, log10 copies/mL | 2.0±1.4 | 2.4±1.7 | 1.9±1.2 | 0.069 | ||
| Nadir CD4+ T cell count, mean±SD, cells/mm3 | 187.0±138.0 | 171.8±167.2 | 192.4±126.3 | 0.365 | ||
| Highest VL, mean±SD, log10 copies/mL | 5.0±0.9 | 5.0±0.9 | 4.9±1.0 | 0.566 | ||
| Current cART (%) | ||||||
| No treatment | 20 (10.3) | 7 (13.7) | 13 (9.1) | 0.350 | ||
| 2NRTI+PI | 104 (59.8) | 32 (72.7) | 72 (55.4) | 0.043 | 2.323 (1.078-5.005) | 0.031 |
| 2NRTI+NNRTI | 49 (28.2) | 7 (15.9) | 42 (32.3) | 0.037 | ||
| 2NRTI+II | 12 (6.9) | 3 (6.8) | 9 (6.9) | 1.000 | ||
| 3NRTI | 3 (1.7) | 0 (0.0) | 3 (2.3) | 0.572 | ||
| Other | 6 (3.4) | 2 (4.5) | 4 (3.1) | |||
| CPE rank | 6.7±1.3 | 6.7±1.1 | 0.961 | |||
| HCV co-infection (%) | 6 (3.7) | 3 (6.7) | 3 (2.6) | 0.350 | ||
| HBV co-infection (%) | 9 (5.3) | 2 (4.3) | 7 (5.6) | 1.000 | ||
HAND, HIV associated neurocognitive disorder; BMI, body mass index; AIDS, acquired immune deficiency syndrome; HIV, human immunodeficiency virus; cART, combination antiretroviral therapy; IQR, interquartile range; VL, viral load; PI, protease inhibitor; NNRTI, non-nucleoside reverse-transcriptase inhibitor; II, integrase inhibitor; CPE, central nervous system penetration effectiveness; HCV, hepatitis C virus; HBV, hepatitis B virus
Prevalence and clinical characteristics of HAND
The overall prevalence of HAND in the study cohort was 26.3%. Of the 51 participants with HAND, ANI and MND comprised 52.9% and 47.1%, respectively. No individuals were diagnosed with HAD. Of those with HAND, the mean age (range) was 44.39 (21-70) years, and 96.1% of them were male. The most common exposure category was MSM (47.1%), followed by heterosexual contact (33.3%). The duration of education (mean±SD) was 12.8±3.4 years. The mean hemoglobin (Hb) level was 13.6±1.8 g/dL with 15% having Hb below 13.0g/dL. Initial and current CD4+ T cell counts (cells/mm3, mean±SD) were 233.8±220.7 and 444.5±259.2, respectively. Initial and current viral load (mean±SD, log10 copies/mL) were 4.7±1.3 and 2.4±1.7, respectively, and 71.7% had the cART regimen of 2NRTI+PI (Table 1). Also, 82% of study participants with HAND receiving cART in our cohort had suppressed viral loads (<50 copies/ml). Among six domains in cognitive functioning in the individuals with HAND, impairment of sensory perceptual/motor skills were the most common (90.2%), followed by abstraction/executive (78.0%) and memory (learning and recall) (48.0%) (Table 2).
Table 2. Impairment of 6 domains in cognitive functioning in 51 Korean HIV-infected individuals with HAND.
| Domain | No. of impairment (%) in HAND |
|---|---|
| Verbal/language | 2 (3.9) |
| Attention/Working Memory | 3 (5.9) |
| Abstraction/Executive | 39 (78.0) |
| Memory (learning and recall) | 24 (48.0) |
| Speed of information processing | 1 (2.0) |
| Sensory perceptual/motor skills | 46 (90.2) |
HAND, HIV-associated neurocognitive disorder
In univariate analysis, Hb levels ≤13g/dL (p=0.029) and current use of a PI-based regimen (p=0.043) were the only factors significantly associated with HAND. The observed significance remained for both Hb levels (p=0.046) and current use of PI-based regimen in multivariate analysis, (p=0.031) (Table 1).
The performance of screening tests for HAND
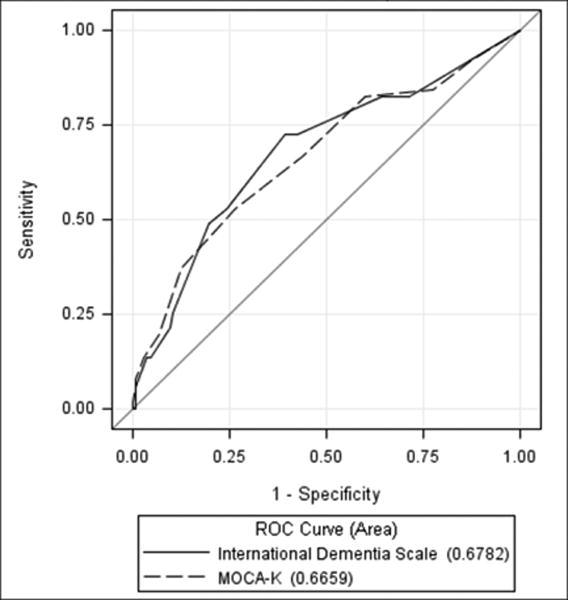
The sensitivity and specificity of IHDS were 72.6% and 60.8%, and MoCA-K were 52.9% and 73.4%, respectively (Table 3). The MoCA-K and IHDS screening tests significantly correlated with HAND, and a cut-off value of ≤25 and ≤10 was the most useful in diagnosing HAND, respectively (Table 3 and Supplement Tables 2 and 3). To compare the predictive accuracy of MoCA-K and IHDS as screening tools, a ROC analysis was performed (Figure 1), which demonstrated that the area under the curves of IHDS and MoCA-K for diagnosing HAND were 0.678 (p<0.001) and 0.666 (p<0.001), respectively. However, the developed ‘four key questions’ used as a screening tool did not significantly correlate with diagnosis of HAND by standard methods (Supplement Table 4).
Table 3. Diagnostic performance of IHDS and MoCA-K.
| NP test | Sensitivity | Specificity | Accuracy |
|---|---|---|---|
| IHDS ≤10 | 72.55(60.30-84.80) | 60.84(52.84-68.84) | 63.92(57.16-70.68) |
| MoCA-K ≤25 | 52.94(39.24-66.64) | 73.43(66.19-80.67) | 68.04(61.48-74.60) |
| p-value for comparison | 0.0244 | 0.0071 | 0.3134 |
IHDS, International HIV dementia scale; MoCA-K, Montreal Cognitive Assessment -K
Figure 1. The AUCs of IHDS and MoCA-K for diagnosing HAND.
NP tests for diagnosing HAND
Diagnostic characteristics for each NP test for diagnosing HAND in HIV-infected Koreans are provided in Table 4. Among NP tests, the Grooved Pegboard Test had the highest sensitivity and modest specificity (90.2% and 72.0%). The next highest scoring NP test was the Wisconsin Card Sorting Test, which had a sensitivity and specificity of 61.2% and 84.4%, respectively. The other NP tests had much lower sensitivity but higher specificity.
Table 4. Diagnostic value of neuropsychological test for diagnosing HAND in Korean HIV-infected individuals.
| Cognitive Domain and Neuropsychological Test | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|
| Speed of Information Processing | |||||
| K-WAIS Digit Symbol Subtest | 1.96(0.00-5.77) | 99.30(97.93-100.00) | 50.00(0.00-100.00) | 73.96(67.75-80.17) | 73.71(67.52-79.91) |
| Trail Making Test, Part A | 0.00(0.00-0.00) | 100.00(100.00-100.00) | - | 73.71(67.52-79.91) | 73.71(67.52-79.91) |
| Memory (learning and recall) | |||||
| K-Auditory Verbal Learning Test (K-AVLT) | 6.00(0.00-12.58) | 99.30(97.93-100.00) | 75.00(32.56-100.00) | 75.13(68.97-81.29) | 75.13(69.03-81.23) |
| K-Complex Figure Test (K-CFT) | 9.80(1.64-17.97) | 99.30(97.93-100.00) | 83.33(53.51-100.00) | 75.53(69.39-81.68) | 75.77(69.74-81.80) |
| Abstraction/Executive | |||||
| Wisconsin Card Sorting Test (128-item) | 61.22(47.58-74.87) | 84.40(78.41-90.39) | 57.69(44.26-71.12) | 86.23(80.48-91.98) | 78.42(72.57-84.27) |
| K-WAIS Similarity Subtest | 4.00(0.00-9.43) | 100.00(100.00-100.00) | 100.00(100.00-100.00) | 74.74(68.56-80.92) | 75.00(68.88-81.13) |
| Trail Making Test, Part B | 32.00(19.07-44.93) | 95.10(91.57-98.64) | 69.57(50.76-88.37) | 80.00(73.99-86.01) | 78.76(72.99-84.53) |
| Attention/Working Memory | |||||
| K-WAIS Digit Span Subtest | 5.88(0.00-12.34) | 99.30(97.93-100.00) | 75.00(32.56-100.00) | 74.74(68.56-80.92) | 74.74(68.63-80.86) |
| Sensory perceptual/motor skills | |||||
| Grooved Pegboard Test (Dominant and Non-dominant Test) | 90.20(82.03-98.36) | 72.03(64.67-79.39) | 53.49(42.95-64.03) | 95.37(91.41-99.33) | 76.80(70.86-82.74) |
| Verbal/language | |||||
| K-WAIS Vocabulary Subtest | 3.92(0.00-9.25) | 100.00(100.00-100.00) | 100.00(100.00-100.00) | 74.48(68.31-80.65) | 74.74(68.63-80.86) |
HAND, HIV-associated neurocognitive disorder; K-WAIS, K-Wechsler Adult Intelligence Scale
Discussion
This is the first study of the prevalence of HAND in HIV-infected patients in Korea, and we found that approximately a quarter of our patients had HAND (26.3%). Of these individuals 52.9% had ANI and 47.1% had MND. Of those with HAND, 82% were receiving cART with suppressed viral loads, suggesting that suppressing HIV replication might be not sufficient to treat HAND. These results are similar to reports from Western countries, with the reported prevalence of HAND of 16∼38% in the era of cART [9-13].
Our study also found lower Hb levels and use of a current PI-based cART regimen to be independent risk factors for HAND. Although low Hb levels may represent underlying chronic disease, the specific mechanism of the effect of low Hb on the occurrence of HAND is unclear, although previous studies have also noted low Hb level as a risk factor for HAND [29] and HAD [30]. The reason behind the observed significant correlation between the current use of a PI-based cART regimen and HAND is unclear, but it may represent the possibility of clinician's effort for treatment of HAND rather than independent factor of it. In other words, we think that cART might have been switched to a PI-based regimen because of memory impairment of HIV-infected patients. This is because NNRTI such as efavirenz were reported risk factors of HAND [31] and PIs have mostly high CPE scores [32]. Thus, in our study, HIV-infected patients with HAND seemed to have more PI-based regimen than them without HAND. Another reason for the association between PI and HAND would be that perhaps clinicians tend to prescribe PI-based cART regimens for the more ill patients rather than NNRTI-based regimen.
Some studies of Western cohorts have reported that nadir CD4+ T cell counts were an independent risk factor of HAND [33-35], but this was not observed in our cohort. In our study, interquartile ranges of nadir CD4+ T cell counts of all participants were 69-277 cells/mm3, and especially, 25% quartile was higher than that of other study [33]. This might be because cART had a tendency to be started earlier in Korea; thus, we think that nadir CD4+ T cell count was not significantly associated with HAND because of the overall higher CD4+ counts in our population. Since the prevalence of HAND in our population was similar to these Western reports, this finding may provide insight that nadir CD4+ count may in fact be a confounder with and not a predictor of HAND.
In our study, the IHDS and MoCA-K measures used as screening tools for HAND had better performance than the developed ‘four key questions’. Since IHDS is widely used as a screening tool for HAD[21], it has the potential to be a useful screening instrument for HAND. For our study, participants who were classified with neurocognitive impairment on the IHDS also performed significantly lower on other tests of cognitive function, especially processing speed and verbal learning/memory [36]. Alternatively, MoCA is a brief cognitive screening tool with high sensitivity and specificity for detecting mild cognitive impairment or dementia [22,37], and in our study, a cut-off value of 26 (scores of 25 or below indicate impairment) yielded the best balance between sensitivity and specificity for the HAND. Since clinicians often depend on patients to self-report memory complaints, especially in the early stages of cognitive impairment, we attempted to develop a screening tool with four questions, but this tool was not found to be reliable. We think that this was because enrolled patients might not have answered the questions frankly.
Beyond screening tools, we also evaluated which NP tests had the best test characteristics for diagnosing HAND. We found that the Grooved Pegboard Test and Wisconsin Card Sorting Test had the best sensitivity and specificity in our study population. Specifically, the Grooved Pegboard Test is commonly used measure of psychomotor slowing used with HIV-positive populations [38], and it has been reported that it can significantly differentiate demented from non-demented HIV-infected patients [39]. Therefore, the Grooved Pegboard Test might be useful in diagnosing HAND in HIV infected Koreans.
Our study had some limitations. First, although there were 194 well-characterized participants in the study, the sample size of those with HAND was only 51. The small sample size has the possibility to influence results due to an unequal distribution of impairment and to determine risk factors for HAND. Second, there was no HAD in this study. This might represent a selection bias, i.e. those with HAD would not have been able to find their way to consent and participate. Third, our results were not applicable to females with HIV because 93.8% of enrolled patients were men; however, the study sample was representative of the Korean HIV-infected population[40]. Fourth, this study was conducted among HIV-infected patients in an urban outpatient clinic in Korean, which may not be representative of HIV-infected individuals in community and rural setting or to the general Korean population. Lastly, the presence of peripheral neuropathy in HAND influencing NP tests, especially the Grooved Pegboard was not excluded by neurologic examination.
In conclusion, HAND is a prevalent comorbidity among HIV-infected Koreans. Active screening and diagnosis with useful tools like IHDS, MoCA-K and Grooved Pegboard Test likely should be performed not to overlook this important complication.
Supplementary Material
Acknowledgments
Source of Funding: This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2013R1A1A2005412 and NRF-2011-220-E00015), a grant of Chronic Infectious Disease Cohort (4800-4859-304-260) from Korea Centers for Disease Control and Prevention, and a grant of the Korean Society for AIDS. This research was also supported by the US National Institute of Health (NIH) AI100665, AI36214, MH097520, MH83552, and MH62512. Statistical advice was reviewed by the statistician Hye Sun Lee, M.S., at the Biostatistics Collaboration Unit, Yonsei University College of Medicine, Seoul, Korea.
Footnotes
Conflicts of interest: There are no conflicts of interest.
References
- 1.Sonnerborg AB, Ehrnst AC, Bergdahl SK, Pehrson PO, Skoldenberg BR, Strannegard OO. HIV isolation from cerebrospinal fluid in relation to immunological deficiency and neurological symptoms. AIDS. 1988;2:89–93. doi: 10.1097/00002030-198804000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Chang L. In vivo magnetic resonance spectroscopy in HIV and HIV-related brain diseases. Rev Neurosci. 1995;6:365–378. doi: 10.1515/revneuro.1995.6.4.365. [DOI] [PubMed] [Google Scholar]
- 3.Chang L, Ernst T, Leonido-Yee M, Walot I, Singer E. Cerebral metabolite abnormalities correlate with clinical severity of HIV-1 cognitive motor complex. Neurology. 1999;52:100–108. doi: 10.1212/wnl.52.1.100. [DOI] [PubMed] [Google Scholar]
- 4.Masliah E, Achim CL, Ge N, DeTeresa R, Terry RD, Wiley CA. Spectrum of human immunodeficiency virus-associated neocortical damage. Ann Neurol. 1992;32:321–329. doi: 10.1002/ana.410320304. [DOI] [PubMed] [Google Scholar]
- 5.Masliah E, Ge N, Morey M, DeTeresa R, Terry RD, Wiley CA. Cortical dendritic pathology in human immunodeficiency virus encephalitis. Lab Invest. 1992;66:285–291. [PubMed] [Google Scholar]
- 6.Heaton RK, Marcotte TD, Mindt MR, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10:317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- 7.Ellis RJ, Hsia K, Spector SA, et al. Cerebrospinal fluid human immunodeficiency virus type 1 RNA levels are elevated in neurocognitively impaired individuals with acquired immunodeficiency syndrome. HIV Neurobehavioral Research Center Group. Ann Neurol. 1997;42:679–688. doi: 10.1002/ana.410420503. [DOI] [PubMed] [Google Scholar]
- 8.Letendre SL, McCutchan JA, Childers ME, et al. Enhancing antiretroviral therapy for human immunodeficiency virus cognitive disorders. Ann Neurol. 2004;56:416–423. doi: 10.1002/ana.20198. [DOI] [PubMed] [Google Scholar]
- 9.Fernandes Filho SM, de Melo HR. Frequency and risk factors for HIV-associated neurocognitive disorder and depression in older individuals with HIV in northeastern Brazil. Int Psychogeriatr. 2012;24:1648–1655. doi: 10.1017/S1041610212000944. [DOI] [PubMed] [Google Scholar]
- 10.Robertson KR, Smurzynski M, Parsons TD, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007;21:1915–1921. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- 11.Cysique LA, Brew BJ. Prevalence of non-confounded HIV-associated neurocognitive impairment in the context of plasma HIV RNA suppression. J Neurovirol. 2011;17:176–183. doi: 10.1007/s13365-011-0021-x. [DOI] [PubMed] [Google Scholar]
- 12.Simioni S, Cavassini M, Annoni JM, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24:1243–1250. doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- 13.White DA, Heaton RK, Monsch AU. Neuropsychological studies of asymptomatic human immunodeficiency virus-type-1 infected individuals. The HNRC Group. HIV Neurobehavioral Research Center. J Int Neuropsychol Soc. 1995;1:304–315. doi: 10.1017/s1355617700000308. [DOI] [PubMed] [Google Scholar]
- 14.Heaton RK, Clifford DB, Franklin DR, Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smurzynski M, Wu K, Letendre S, et al. Effects of central nervous system antiretroviral penetration on cognitive functioning in the ALLRT cohort. AIDS. 2011;25:357–365. doi: 10.1097/QAD.0b013e32834171f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zung WW. A Self-Rating Depression Scale. Arch Gen Psychiatry. 1965;12:63–70. doi: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]
- 18.Mor V, Laliberte L, Morris JN, Wiemann M. The Karnofsky Performance Status Scale. An examination of its reliability and validity in a research setting. Cancer. 1984;53:2002–2007. doi: 10.1002/1097-0142(19840501)53:9<2002::aid-cncr2820530933>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 19.1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 20.Amico KR, Fisher WA, Cornman DH, et al. Visual analog scale of ART adherence: association with 3-day self-report and adherence barriers. J Acquir Immune Defic Syndr. 2006;42:455–459. doi: 10.1097/01.qai.0000225020.73760.c2. [DOI] [PubMed] [Google Scholar]
- 21.Sacktor NC, Wong M, Nakasujja N, et al. The International HIV Dementia Scale: a new rapid screening test for HIV dementia. AIDS. 2005;19:1367–1374. [PubMed] [Google Scholar]
- 22.Lee JY, Dong Woo L, Cho SJ, et al. Brief screening for mild cognitive impairment in elderly outpatient clinic: validation of the Korean version of the Montreal Cognitive Assessment. J Geriatr Psychiatry Neurol. 2008;21:104–110. doi: 10.1177/0891988708316855. [DOI] [PubMed] [Google Scholar]
- 23.Yeom TH, Park YS, Oh KJ, Lee YH. Korean version Wechsler adult intelligence scale. Seoul: Korea Guidance; 1992. [Google Scholar]
- 24.Kim M, Hyun MH. Relationships between Trail Making Test (A, B, B-A, B/A) scores and age, education, comparison of performance head injury patient and psychiatric patient. The Korean Journal of Clinical Psychology. 2004;23:353–366. [Google Scholar]
- 25.Kim HK. Rey-Kim Memory Test. Seoul, Korea: Publishing Neuropsychology; 1999. [Google Scholar]
- 26.Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Test Manual, Revised and Expanded. Florida: Psychological Assessment Resources, Inc.; 1993. [Google Scholar]
- 27.Lee TY. Normative values for the Grooved Pegboard Test in Adult. Phys Ther Kor. 2001;8:87–94. [Google Scholar]
- 28.Woods SP, Rippeth JD, Frol AB, et al. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol. 2004;26:759–778. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]
- 29.Njamnshi AK, Bissek AC, Ongolo-Zogo P, et al. Risk factors for HIV-associated neurocognitive disorders (HAND) in sub-Saharan Africa: the case of Yaounde-Cameroon. J Neurol Sci. 2009;285:149–153. doi: 10.1016/j.jns.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McArthur JC, Hoover DR, Bacellar H, et al. Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS Cohort Study. Neurology. 1993;43:2245–2252. doi: 10.1212/wnl.43.11.2245. [DOI] [PubMed] [Google Scholar]
- 31.Ciccarelli N, Fabbiani M, Di Giambenedetto S, et al. Efavirenz associated with cognitive disorders in otherwise asymptomatic HIV-infected patients. Neurology. 2011;76:1403–1409. doi: 10.1212/WNL.0b013e31821670fb. [DOI] [PubMed] [Google Scholar]
- 32.Letendre S, Marquie-Beck J, Capparelli E, et al. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65:65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellis RJ, Badiee J, Vaida F, et al. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS. 2011;25:1747–1751. doi: 10.1097/QAD.0b013e32834a40cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valcour V, Yee P, Williams AE, et al. Lowest ever CD4 lymphocyte count (CD4 nadir) as a predictor of current cognitive and neurological status in human immunodeficiency virus type 1 infection--The Hawaii Aging with HIV Cohort. J Neurovirol. 2006;12:387–391. doi: 10.1080/13550280600915339. [DOI] [PubMed] [Google Scholar]
- 35.McCombe JA, Vivithanaporn P, Gill MJ, Power C. Predictors of symptomatic HIV-associated neurocognitive disorders in universal health care. HIV Med. 2013;14:99–107. doi: 10.1111/j.1468-1293.2012.01043.x. [DOI] [PubMed] [Google Scholar]
- 36.Lawler K, Mosepele M, Ratcliffe S, et al. Neurocognitive impairment among HIV-positive individuals in Botswana: a pilot study. J Int AIDS Soc. 2010;13:15. doi: 10.1186/1758-2652-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 38.Bryden PJ, Roy EA. A new method of administering the Grooved Pegboard Test: performance as a function of handedness and sex. Brain Cogn. 2005;58:258–268. doi: 10.1016/j.bandc.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Davis HF, Skolasky RL, Jr, Selnes OA, Burgess DM, McArthur JC. Assessing HIV-associated dementia: modified HIV dementia scale versus the Grooved Pegboard. AIDS Read. 2002;12:29–31. 38. [PubMed] [Google Scholar]
- 40.2011 Annual Report on the Notified HIV/AIDS in Korea. PHWR. 2012;5:11–14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.