Abstract
A significant percentage of breast cancer victims will suffer from metastases indicating that new approaches to preventing breast cancer metastasis are thus needed. Dietary stearate and chemotherapy have been shown to reduce breast cancer metastasis. We tested the complementary use of dietary stearate with a taxol-based chemotherapy which work through separate mechanisms to reduce breast cancer metastasis. We therefore carried out a prevention study in which diets were initiated prior to human MDA-MB-435 cancer cells being injected into the host and a treatment study in which diets were combined with paclitaxel (PTX). Using an orthotopic athymic nude mouse model and three diets (corn oil control diet/CO, low fat /LF or stearate/ST) the prevention study demonstrated that the ST diet decreased the incidence of lung metastasis by 50% compared to both the LF and CO diets. The ST diet also reduced the number and size of metastatic lung nodules compared to the LF diet. Results of the treatment study indicated that both the CO and ST diets decreased the number of mice with lung metastasis compared to the LF diet. Both CO and ST also decreased the number of lung metastases per mouse compared to the LF diet however only the ST diet cohort was significant. Histomorphometric analysis of the lung tumor tissue indicated that the ST diet plus PTX decreased angiogenesis compared to the LF diet plus PTX. In conclusion these results support combining diet with chemotherapy in both treatment and prevention settings.
Keywords: stearate, breast cancer, metastasis, paclitaxel, fatty acids, angiogenesis
Introduction
Breast cancer is the most frequently diagnosed solid tumor and the leading cause of cancer death in women worldwide [1]. Chemotherapy with paclitaxel (PTX) and other taxanes is a common treatment for breast cancer post surgical intervention. Studies have demonstrated clinical benefits with use of such taxanes for lymph node-positive, early stage or metastatic breast cancer [2]. The primary mechanism of action of the taxanes is to stabilize intracellular microtubules and prevent their disassembly. Since the reorganization of microtubules is essential to cell division, disruption of this dynamic causes the cells to arrest in the G2 phase of the cell cycle [3]. Even with chemotherapy ninety percent of cancer deaths are caused by metastases [4]. Approximately 1/3 of breast cancer patients have metastases to regional lymph nodes detected at initial diagnosis [5]. Breast cancer patients with metastases at the time of diagnosis have a median survival time of approximately 2–4 years. Also, 24–30% of lymph node-negative and 50–60% of patients diagnosed as lymph node-positive will suffer from either a local or distant relapse, indicating that a significant percentage of breast cancer victims will suffer from metastases [6]. Thus there is a need to develop new approaches for prevention and treatment of breast cancer metastasis.
Stearic acid, or stearate, is an 18-carbon saturated fatty acid found in many foods in the western diet including beef, chocolate, and milk fats. In contrast to other comparable saturated fatty acids, such as palmitate (C16:0) as well as trans-fatty acids, stearate does not increase plasma low density lipoprotein cholesterol (LDLc) concentrations [7]. Importantly LDLc is the primary target for cardiovascular disease risk reduction. One study concluded that the replacement of trans-fatty acids with stearate in foods that require solid fats would have a beneficial effect on LDLc [8]. Interestingly, stearate has been found to have ‘anti-breast cancer properties’ both in vitro and in vivo, inhibiting proliferation, migration, carcinogenesis, tumor invasion, and metastatic tumor burden [9–17]. The mechanism(s) whereby dietary stearate inhibits breast cancer progression likely include signaling through a diacylglycerol/protein kinase C pathway to cause apoptosis as well as inhibition of rho which is a member of the ras superfamily of small GTPases and a known oncogene [15, 17]. These studies suggested the possibility of dietary stearate as a preventative agent and/or an adjuvant therapeutic strategy for breast cancer.
Metastasis is a complex process by which cancer spreads from a primary to a secondary site. Cancer cell migration, angiogenesis, and proliferation are all important components of this process [18, 19]. We hypothesized that since dietary stearate and PTX have different mechanisms of action that they would be more effective when combined against breast cancer metastasis.
In these studies we explored the roles of dietary stearate as a complementary dietary agent to PTX for both the treatment and prevention of breast cancer lung metastasis in an animal model.
Materials and methods
Animals and diets
Female athymic (nude) mice, 3–4 weeks old, were purchased from Harlan Sprague Dawley, Inc. (Indianapolis, IN) and maintained in microisolater cages in our institutional pathogen-free facilities. Three diets were used for both studies: a low fat diet (LF, 5% corn oil), a corn oil rich control diet (CO, 17% corn oil) and a stearate rich diet (ST, 17% stearate). The diets were prepared by Harlan-Teklad (Madison, WI). The ST and LF diets have been described previously [16] while the CO diet is the same as the ST diet except corn oil replaced stearate. The dietary form of stearate was Hystrene 9718 (a food grade product containing ~97% stearate and ~3% palmitate) obtained from Chemtura. Middlebury, CT. The animals were fed ad libitum and the amount of food consumed was recorded. Mice were anesthetized by inhalation of 3% isoflurane (Vet One, Meridian, ID) in 2.5% O2 in an induction box and weighed weekly. All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC), University of Alabama at Birmingham (UAB).
Cell Culture
MDA-MB-435 human breast cancer cells (obtained from Dr. Dan Welch; UAB, now at the University of Kansas, Cancer Center) were grown and maintained in DMEM:F12 supplemented with 5% FBS, 2 mM glutamine, 1 mM sodium pyruvate, 0.2× non-essential amino acids and 1% penicillin/streptomycin (5% CO2). Cells were grown to 80–90% confluence prior to preparation for injection. To detach cells from the plates, cells were washed with PBS and then treated with 3 mM Versene (Invitrogen, Grand Island, NY). Cells were pelleted by centrifugation at 1000 RPM for 5 minutes at room temperature and resuspended in Hank’s buffered saline solution (HBSS). Cells were diluted to 107 cells/mL and kept on ice until the time of injection to prevent clumping.
Experimental design
Prevention Studies
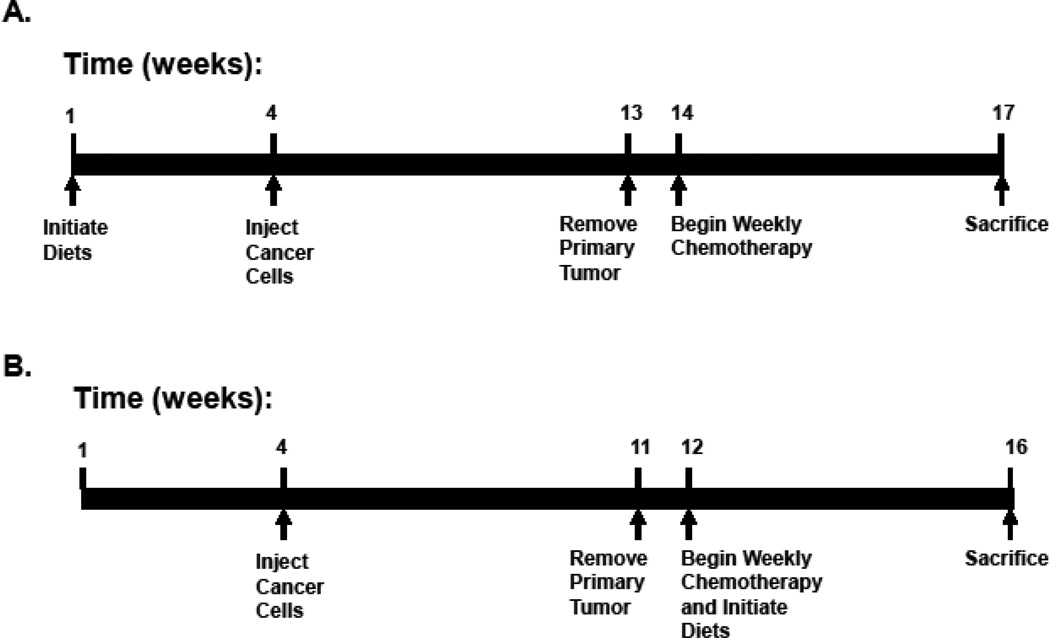
Our previous work indicated that dietary stearate per se reduced metastatic tumor burden but had no effect on the incidence of metastasis [16]. The first experiment was designed to determine whether dietary stearate initiated prior to the introduction of human cancer cells into the animal host would be more effective at preventing the incidence of breast cancer lung metastasis when combined with PTX treatment. Animals were divided randomly into one of three groups—a LF diet group, a CO diet group and a ST diet group with each group having 25–30 mice per group. All mice were placed on their respective diets 3 weeks prior to injection of breast cancer cells and these diets were continued until the end of the experiment. The primary tumors were removed at 9 weeks post-cancer cell injection. Chemotherapy using 20 mg/kg PTX once a week for three weeks was started one week after surgical excision of primary tumors. Mice were sacrificed and the lungs collected one week after the last PTX dose (Fig.1A).
Figure 1.
Experimental Timetables. (A) Prevention Study: Nude mice were placed on either a low fat (LF) diet, a corn oil (CO) diet, or a stearate (ST) diet 3 weeks prior to injection of cancer cells and remained on separate diets throughout this study. The tumors were allowed to reach an approximate mean tumor diameter of 10–12 mm (253.6–904.8 mm3) at which time the primary tumors were surgically removed (9 weeks postinjection). Chemotherapy with 20 mg/kg paclitaxel, once a week for 3 weeks, was started 1 week after the surgery. The animals were allowed to develop metastases for 3 weeks, sacrificed and the lungs were collected. (B) Treatment Study: All mice were kept on the LF diet until one week after the primary tumors were surgically excised. At this time the corn oil (CO) and stearate (ST) diets were started concomitantly with chemotherapy as described in part A. The resulting dietary groups were: a LF diet group, a CO diet group, a ST diet group, and these same diets plus chemotherapy.
Treatment Studies
The second set of experiments was designed to test the efficacy of PTX combined with the ST diet as a treatment for breast cancer metastasis. This set of experiments was done similarly to the previous set except all mice were kept on the same LF diet until after the primary tumor was removed. At that time the mice were divided into six groups of 25–30 mice per group and CO and ST diets were initiated. Three of dietary groups (LF, CO and ST) were treated with diet alone while the other three were treated concomitantly with the same diets plus PTX. Again, all studies were started one week after the primary tumor was surgically excised. As in the first set of experiments 3 rounds of 20mg/kg PTX, once per week for three weeks, were given and the mice sacrificed one week after the last PTX treatment (Fig. 1B).
Mammary fat pad injections
Animals were anesthetized by inhalation with 3% isoflurane plus 2.5% O2 in an induction box. The right chest skin was cleaned with a betadine solution (10% povidoneiodine). A small incision was made between the right 2nd and 3rd mammary fat pads and 106 MDA-MB-435 cells suspended in HBSS were injected into the 2nd mammary fat pad using a 27 mm gauge needle (final volume of 100 µl). A single wound clip was used to close the incision and removed the following week.
Chemotherapeutic intraperitoneal injections
The animals were anesthetized as detailed above. The abdominal skin was cleaned with a betadine solution. Paclitaxel (PTX) from LC Laboratories (Woburn, MA) was dissolved in Cremophor EL:ethanol (1:1, v:v) and then diluted with sterile physiologic saline to a final concentration of 0.5 mg/ml. The drug dosage for both experiments was 20 mg/kg, delivered in a final volume of 1 ml, injected intraperitoneally.
Tumor measurement
After the breast cancer cell injections, mice were monitored weekly for the development of primary tumor masses. Once the tumors became visible (1–2 weeks post-injection), they were measured using a digital caliper. The tumor volume was estimated using the equation for a prolate ellipsoid where volume = π (4/3) (length/2) (width/2) [(length+width)/4].
Tumor excision
The animals were anesthetized as described above. The skin overlying the mammary tumor area was cleaned with a betadine solution and an incision was made circumferentially around the tumor down to its base. The tumor was removed and the wound was closed using wound clips which were removed 1 week later.
Necropsy
At the end of all experiments, mice were anesthetized with a combination of ketamine (100 µg) and xylazine (20 µg) and then decapitated. The lungs were dissected from the mice and stored in 10% buffered formalin prior to counting all visible tumors on all surface of the lungs. Two blinded examiners did the counting separately and the combined mean of data from both examiners was used for analysis.
Lung metastatic tumor size measurement
Lungs were placed under the dissecting microscope (Fisher Scientific, Hanover Park, IL) for measurement of metastatic tumor size. Tumor size was expressed as the mean of the longest and shortest diameter so that if the first measurement was 0.04 cm and the second 0.02 cm, the size was recorded as 0.03 cm. The tumors were split into three groups according to their sizes (<0.1cm, small tumor; 0.1–0.2cm, medium tumor; >0.2cm, large tumor), and the number of tumors per mouse were counted separately.
Immunohistochemistry
Paraffin sections of the lung tumors were prepared as previously described (21). Five micrometer thick sections were cut from the formalin fixed, paraffin embedded tissue blocks and floated onto charged glass slides (Super-Frost Plus, Fisher Scientific, Pittsburgh, PA) and dried overnight at 60° C. A hemotoxylin and eosin stained section was obtained from each tissue block. All sections for immunohistochemistry were deparaffinized and hydrated using graded concentrations of ethanol to deionized water.
CD31 immunostaining was performed as previously described (21). Briefly, the tissue sections were pretreated with 0.5 M tris buffer (pH 10). All sections were washed gently in deionized water, then transferred in to 0.05 M Tris-based solution in 0.15M NaCl with 0.1% v/v Triton-X-100, pH 7.6 (TBST). Endogenous peroxidase was blocked with 3% hydrogen peroxide for 10 min. To reduce further nonspecific background staining, slides were incubated with avidin (Jackson ImmunoResearch, West Grove, PA) and biotin blocking solutions (Sigma, St. Louis, MO) for 15 min each, and 3% normal goat serum (Sigma, St. Louis, MO) for 20 min. Slides were then incubated at 4°C overnight with rabbit polyclonal antibody against CD31 (1:200 dilution; Abcam, Cambridge, MA). After washing with TBST, biotinylated goat anti-rabbit IgG (1:1000; Jackson ImmunoResearch, West Grove, PA) was applied to the sections for 30 min at room temperature. Sections were then incubated with Strepavidin-HRP (Sigma, St. Louis, MO), for 30 min, at room temperature. Diaminobenzidine (DAB; Scy Tek Laboratories, Logan, UT) was used as the chromagen and hematoxylin (Richard-Allen Scientific, Kalamazoo, MI) as the counterstain.
Ki67 and caspase-3 immunostaining were carried out according to the protocol from the manufacturer (Cell Signaling, Danver, MA). Briefly, the tissue sections were subjected to pretreatment with 0.01 M sodium citrate buffer (pH 6). All sections were washed gently in deionized water, and then transferred in to TBST. Endogenous peroxidase was blocked with 3% hydrogen peroxide for 10 min. To reduce further nonspecific background staining, slides were incubated with 3% normal goat serum for 1 hour. All slides were then incubated at 4°C overnight with rabbit monoclonal antibody against cleaved caspase-3 (1:200 dilution) or rabbit polyclonal antibody to Ki67 (1:200, Abcam, Cambridge, MA). After washing with TBST, Signal Stain Boost IHC Detection Reagent (Cell Signaling, Danvers, MA) was applied to the sections, for 30 min, at room temperature. Diaminobenzidine was used as the chromagen and hematoxylin as the counterstain. A negative control was produced by eliminating the primary antibody from the diluents.
Bioquant® Image Analysis software (Rtm Biometrics, Nashville, TN) was used to quantitate the immunostaining. For Ki67 and caspase-3 immunostaining, three hot spots (1µM2) were first selected at 4× magnification. The numbers of positive and negative cells in these hot spots were then counted and averaged at 40× magnification. The percentage of cells stained with Ki67 or caspase-3 was subsequently calculated. For CD31 immunostaining, the MVD was measured based on Weidner’s method [20]. Briefly, three hot spots (4µM2) were selected at 10× magnification. The number of microvessels in these hot spots was counted at magnification ×40, and the density then calculated. Each positive endothelial cell cluster of immunoreactivity was counted as an individual vessel in addition to the morphologically identifiable vessels with a lumen.
Statistical Analysis
Data were presented as the mean ± SEM. SigmaStat 3.1® software program was used for statistics. The statistical comparisons of the number of lung metastases were performed by one-way analysis of variance (ANOVA) supplemented with the Holm-Sidak test. Two-way ANOVA was used to examine the interaction between the PTX chemotherapy and stearate diet therapy. We used Chi-square to evaluate the incidence of lung metastasis. The significant differences were set as p < 0.05.
Results
Food intake and weight gain for both studies
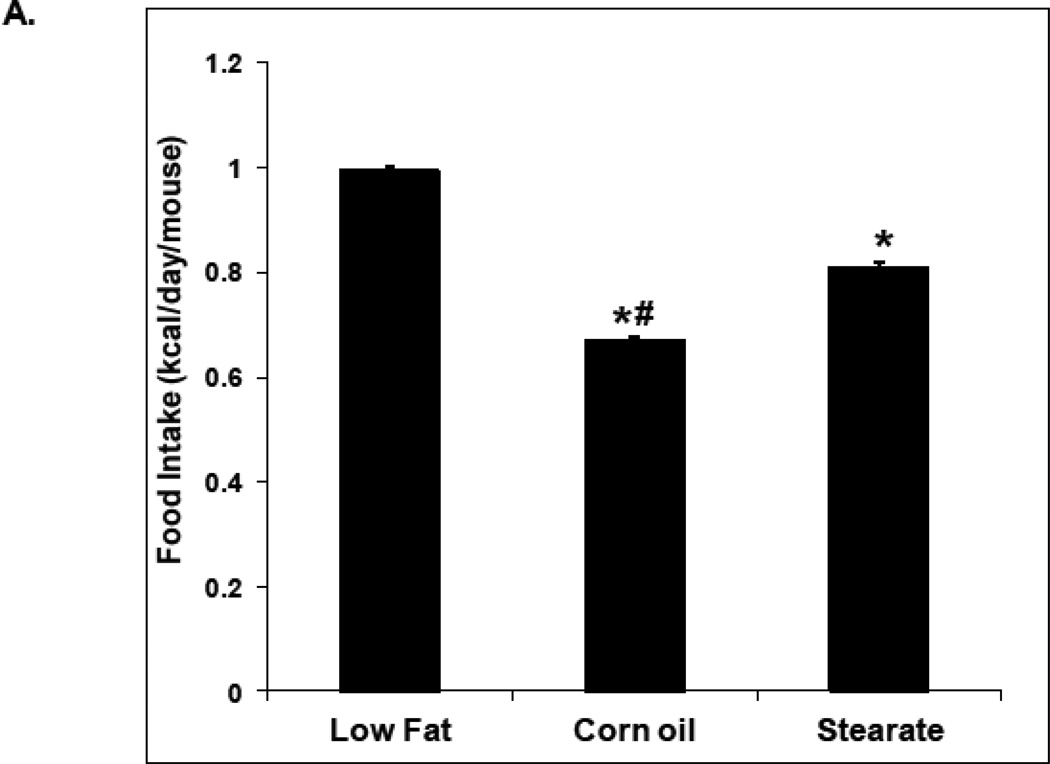
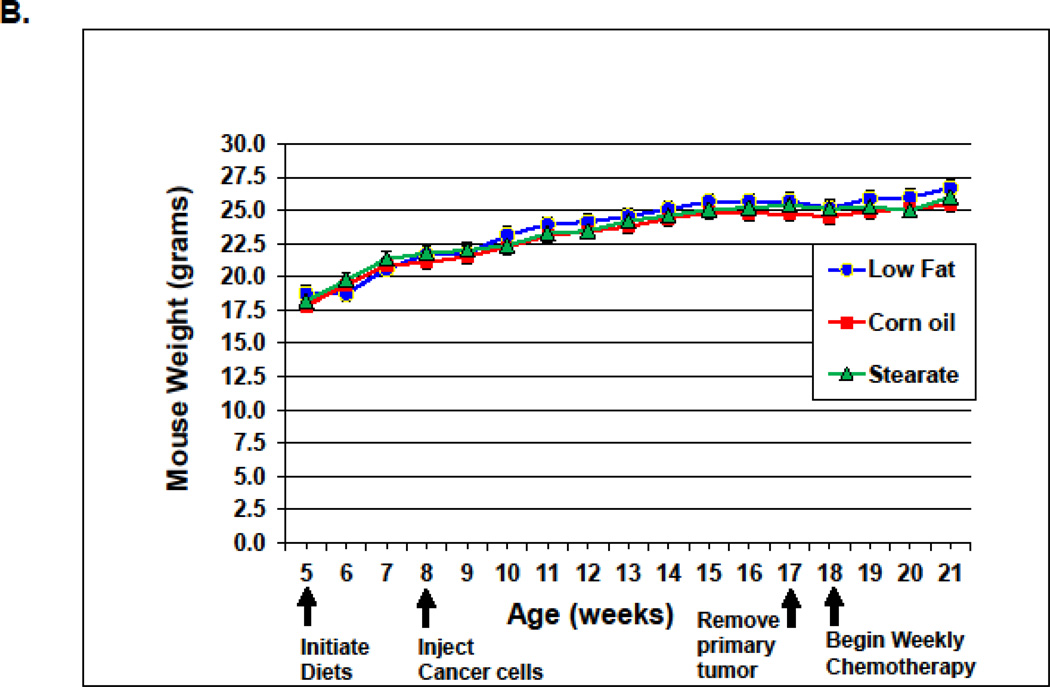
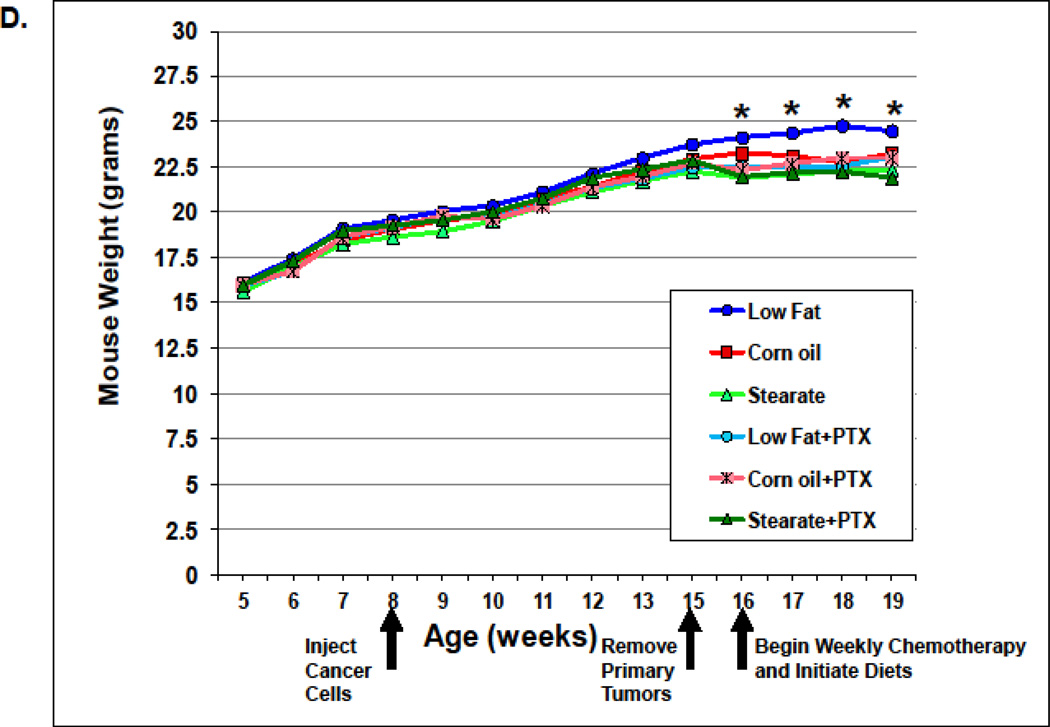
Diets were the same for both studies. The low fat diet (LF) was not isocaloric with the corn oil (CO) or stearate (ST) diets; however, the CO and ST diets were isocaloric. Therefore we monitored food consumption and weight gain. In the first (prevention) study, the LF diet mice consumed the most kilocalories/day (0.99 kcal/day), followed by the ST diet animals (0.81 kcal/day), and then the CO diet mice (0.67 kcal/day) (Fig. 2 A). Despite these differences in food intake, there was no significant difference in weight gain among the diet groups (Fig. 2 B). It is unclear why mice with lung metastasis did not lose weight, especially after receiving chemotherapy. One possible answer is that since the metastases numbers were not large the mice may have tolerated chemotherapy well and did not experience cachexia.
Figure 2.
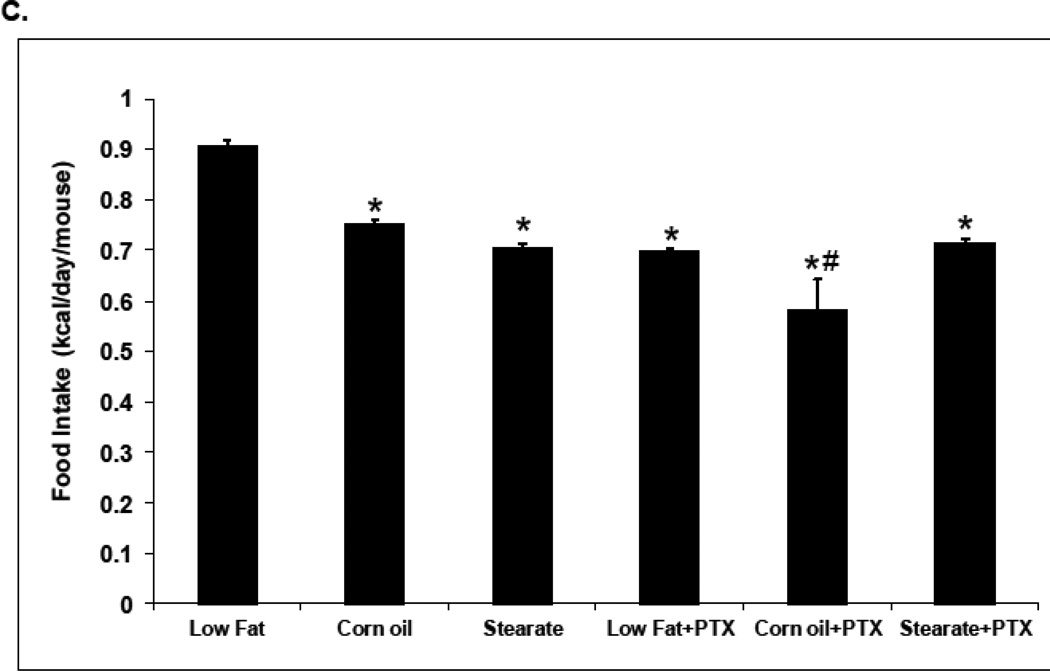
Food intake and weight gain. (A) In the prevention experiment, the LF diet fed mice consumed the most kilocalories/day (0.99 kcal/day), followed by the ST diet mice (0.81 kcal/day), and then the CO diet ingesting mice (0.67 kcal/day) (* p<0.01, LFvs. other diet groups; # p<0.01, CO group vs. ST diet group). (B) Despite differences in food intake, there was no difference in weight gain among the diet groups. (C) In the treatment study, the LF diet mice consumed the most (0.91 kcal/day) as we saw in the prevention trial, while the mice on the CO diet plus chemotherapy (PTX) consumed the least (0.58 kcal/day). The mice in the other groups consumed 0.70–0.75 kcal/day. (* p<0.01, LF vs. other diet groups; # p<0.01, CO plus PTX vs. CO diet only group). (D) In the treatment study the weight of the mice from the LF group was higher than the mice from the other groups after diet therapy started (* p<0.01, LF vs. other diet groups).
n the second (treatment) study LF diet mice, as we observed in the first study, consumed the most (0.91 kcal/day), while the mice on the CO diet plus PTX consumed the least (0.58 kcal/day). Mice in the ST group consumed 0.75 kcal/day (Fig. 2C). There was no difference in the weight of the PTX plus diet groups, in fact, there was no difference between the CO or ST diets alone and among all of the PTX plus diet groups (5 groups). However, the LF diet alone group did show a significant gain in weight at week 16 which was maintained until the end of the experiment (Fig. 2 D). This increase may have been due to the increased metastatic tumor burden seen with the LF diet (see discussion).
Study #1 (prevention)
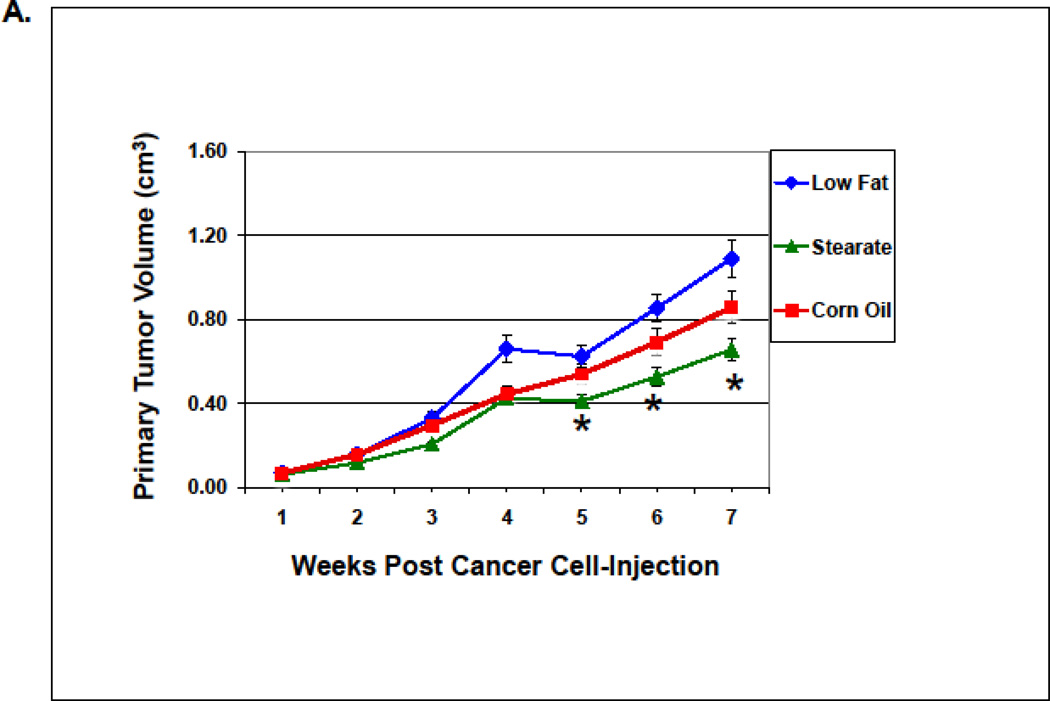
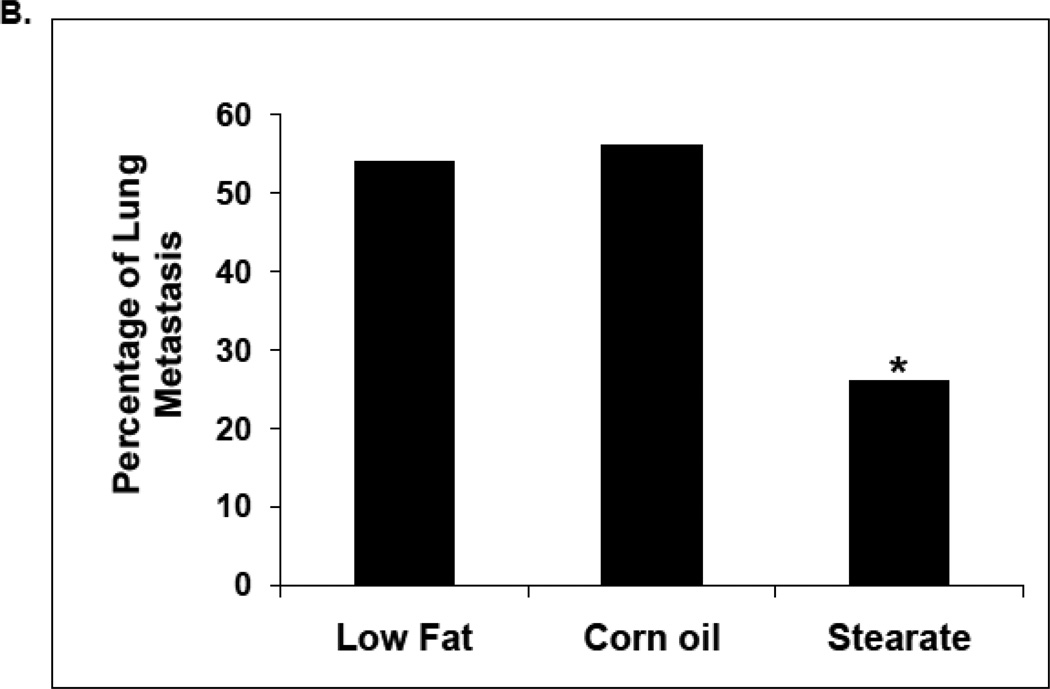
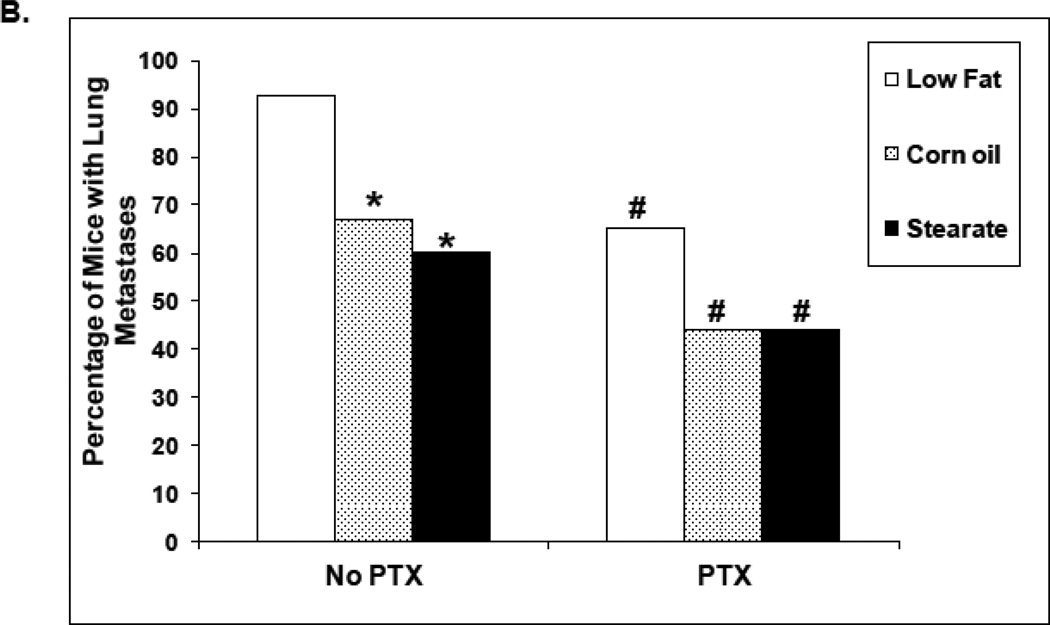
When dietary stearate was initiated before the introduction of cancer cells, the primary tumor size was reduced compared to LF diet (Fig. 3A). We have observed this same effect previously however the incidence of breast cancer lung metastasis was not affected by the ST diet or any other diet tested per se [16]. Thus, our primary end point for this study was the incidence of lung metastasis when combining diet and chemotherapy. We found that PTX with the LF diet reduced the incidence of lung metastasis by approximately 50%. In previous studies by us using the same LF diet, breast cancer cells and mice showed that 90% of mice developed metastases (16). As shown in Fig. 3 B, when all diets were complementary to PTX, mice on the ST diet had ~50% fewer animals with lung metastasis as compared to the LF or CO diet groups (p<0.05).
Figure 3.
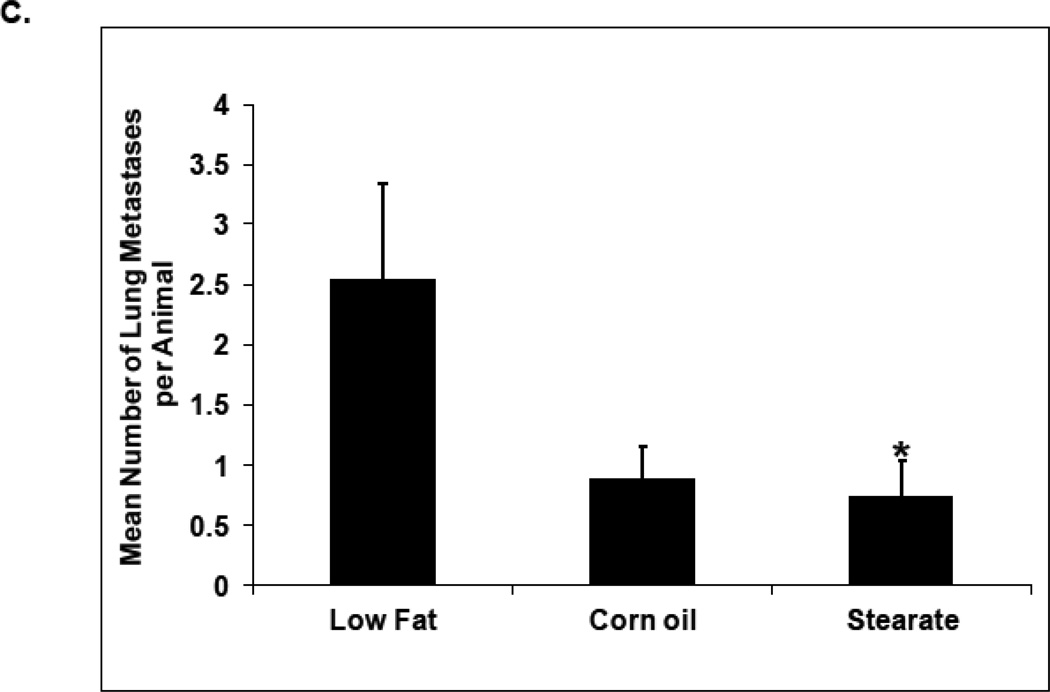
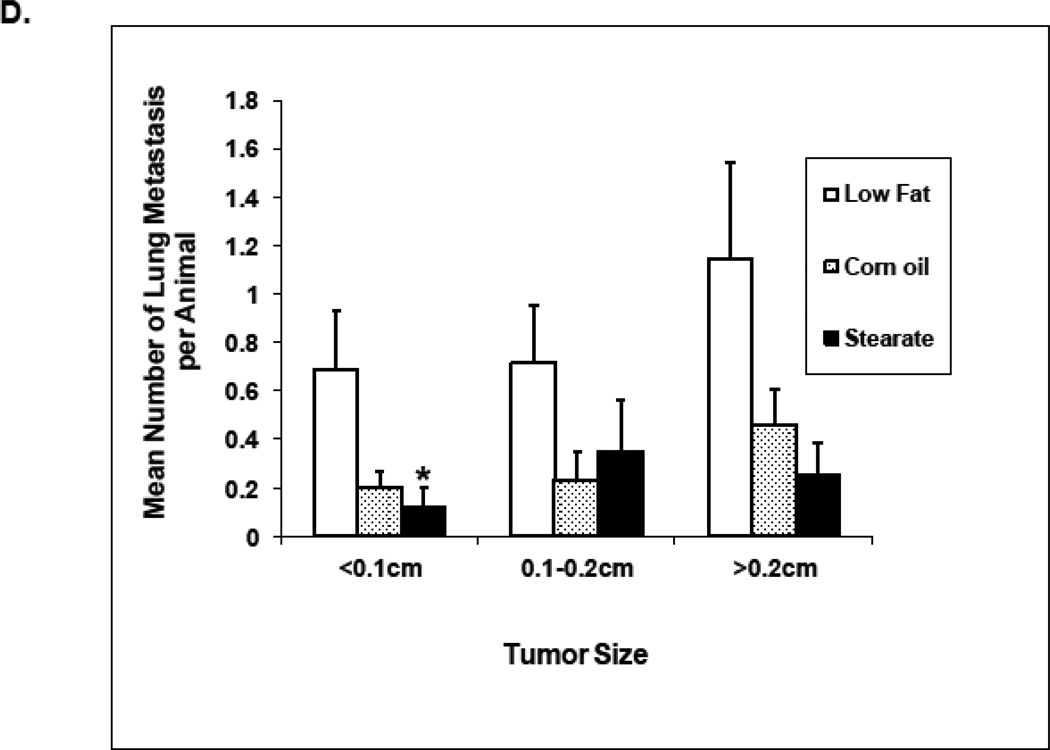
Effects of diets plus chemotherapy on lung metastasis incidence and tumor burden in the prevention study. (A) There was no difference in the time to development of measurable tumors between the diet groups. The ST diet mice had smaller primary tumors beginning at 5 weeks post injection compared to the LF diet mice and remained smaller until they were removed (n=25–30 animals per diet; *, p<0.05). (B) The number of mice with lung metastases was counted following necropsy, and the incidence of lung metastasis determined. Mice on the ST diet had a significantly decreased incidence of lung metastasis as compared to the LF or CO diet groups. (n=25–30 animals per diet; *, p<0.05). Percentages are compared to the LF control without chemotherapy (see Fig. 4B). (C) The number of lung metastatic tumors per animal was compared following necropsy. Mice from the ST diet group had a significantly decreased number of lung metastases compared to those from the LF diet group (n=25–30 animals per diet; *, p<0.01). Although mice on the CO diet had a lower number of metastases, significance was not reached. (D) The size of lung metastatic tumors was measured with a dissecting microscope and tumors were stratified into three groups based on size (diameter <0.1cm, small size; 0.1–0.2cm, medium size; >0.2cm, large size). Mice on the ST and CO diets had fewer tumors in each size group however the only significant difference was the ST diet had fewer small size lung metastasis compared to the LF group (n=25–30 animals per diet; *, p<0.01).
When considering the entire cohort of mice both the ST and CO diets plus PTX had a reduced number of lung metastases when compared to mice on the LF diet, however, only the ST diet plus PTX reached significance (Fig. 3 C). When only mice that developed tumors in each diet group are considered the average number of tumors per mouse was 6.4 (LF diet plus PTX), 4.8 (ST diet plus PTX), and 2.1 (CO diet plus PTX). The only significant difference in these numbers is that CO diet plus PTX is significantly less than the LF diet plus PTX (p<0.0063). Mice with tumors on the ST and CO diets plus PTX also had fewer lung metastases of every size when compared to LF diet plus PTX (Fig. 3 D). However, only the ST diet plus chemotherapy reached statistical significance, having fewer small size tumors than the LF diet plus PTX. One possible reason for the lack of a significant effect of CO diet plus PTX is that the response to this diet is more variable than what is seen with the ST diet plus PTX.
Summarizing, in terms of prevention, the ST diet dramatically reduced the incidence of lung metastasis compared to the other two diet groups when PTX was used for treatment. In addition, both the ST and CO diets both reduce metastatic tumor burden compared to the LF diet.
Study #2 (treatment)
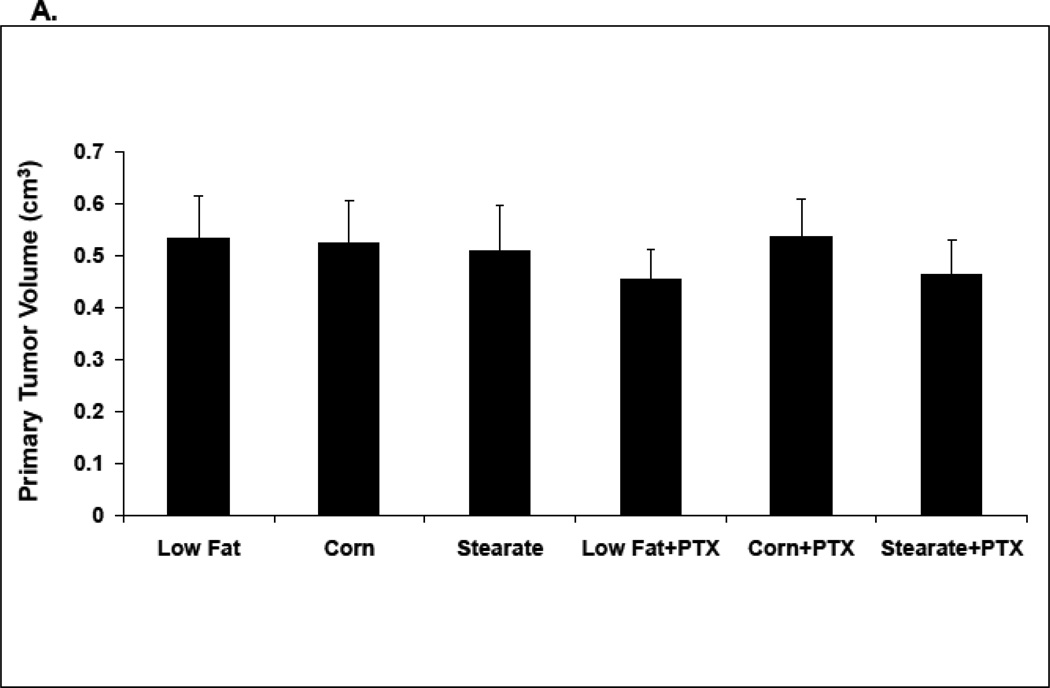
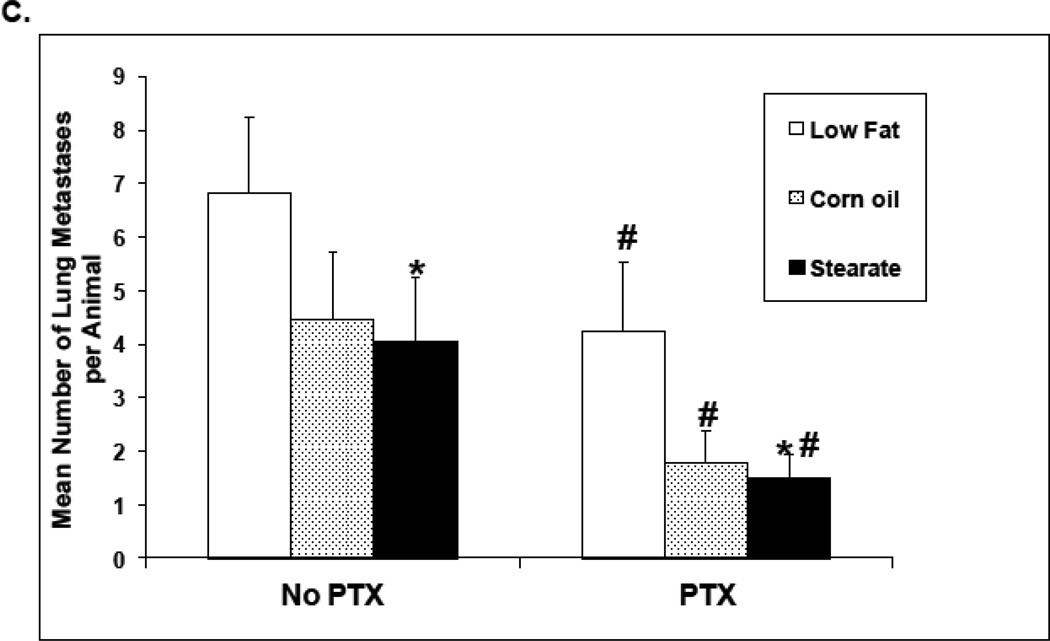
In the second set of experiments all mice were kept on the LF diet until the unique diets were offered with PTX one week after the primary tumor was removed. This is similar to the timing of chemotherapy that occurs in many women with breast cancer. Importantly, all groups were shown to have no significant differences in the volume of primary tumors prior to the diet changes plus chemotherapy (4A). As shown in Fig. 4 B, mice receiving no chemotherapy but switching to both the ST and CO diets alone had a significantly reduced incidence of lung metastases when compared to the LF diet group alone. Switching diets and adding chemotherapy yielded a further constant lowering of the incidence of lung metastases regardless of the diet (Fig. 4B). Importantly there was no significant difference between CO and ST with or without PTX in terms of percentage of mice with metastasis. When the number of metastatic tumors per animal was compared as a cohort, chemotherapy significantly reduced the number of lung metastases for all diets (Fig. 4C). Also as shown in Figure 4C the ST + PTX was statistically different from the LF + PTX. When considering only mice that developed metastasis again there was no difference between CO and ST diets with or without PTX; 4.1 tumors per mouse ST vs 4.5 CO and 3.4 lung metastases per mouse CO + PTX vs 2.8 ST + PTX (p>0.1). Nor were there differences between LF + PTX (4.8) and either CO + PTX (3.4) or ST + PTX (2.8).
Figure 4.
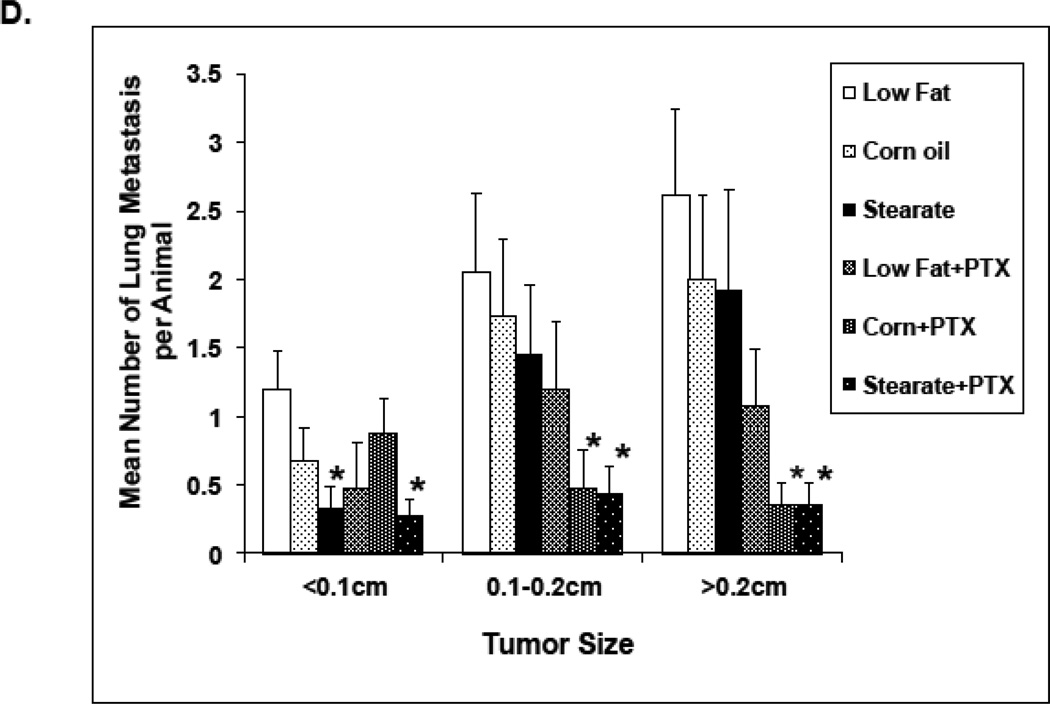
The effects of diet and chemotherapy on lung metastasis in the treatment study. (A) All mice were kept on the LF diet until the primary tumor was removed. There was no significant differences in primary tumor volumes prior to diet changes and chemotherapy(25–30 animals per diet; *, p=0.095). (B) Mice on both the ST and CO diets alone had a significantly reduced incidence of lung metastases compared to LF diet group. (n=25–30 animals per diet; *, p<0.01). Mice receiving chemotherapy (PTX) had a significantly lower incidence of lung metastases under all diet conditions (#, p<0.01, LF diet plus PTX group vs. LF diet alone, corn oil diet plus PTX group vs. corn oil diet alone, and stearate diet plus PTX group vs. stearate diet alone). (C) Both chemotherapy and the ST diet significantly reduced the number of lung metastases (PTX vs. no PTX group, p<0.01; ST vs. LF diet group, * p<0.05). Although the number of metastases was also decreased in the CO diet groups, significance was not reached. (D) Mice on CO diet plus PTX and mice on the ST diet plus PTX had significantly decreased numbers of medium and large size lung metastases compared to the LF diet mice. The number of small sized tumors was also significantly decreased in the ST diet alone and ST diet plus PTX groups compared to LF diet. (*, p<0.05)
As shown in Fig. 4 D, mice on the ST diet plus PTX had reduced numbers of small, medium and large lung metastases compared to LF diet animals without PTX. The ST diet per se also resulted in a reduced number of lung metastases compared to LF diet alone animals. Interestingly the CO diet plus PTX mice had significantly decreased numbers of medium and large size lung metastases compared to the LF diet without PTX mice.
Thus, in the adjuvant treatment setting, the CO and ST diets were both effective at reducing the incidence and tumor burden and the diets were significantly more effective with chemotherapy. In order to investigate possible mechanisms whereby diets were inhibiting lung metastasis, we examined the effect of diet and PTX on angiogenesis, cell proliferation and apoptosis.
The effect of PTX and diet on angiogenesis
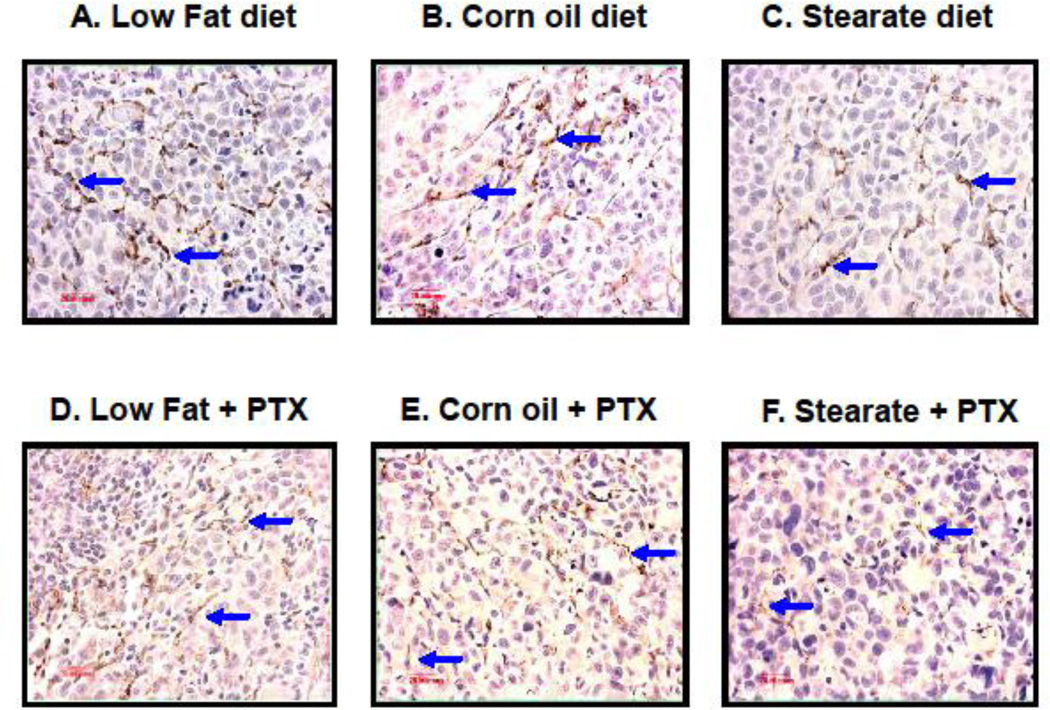
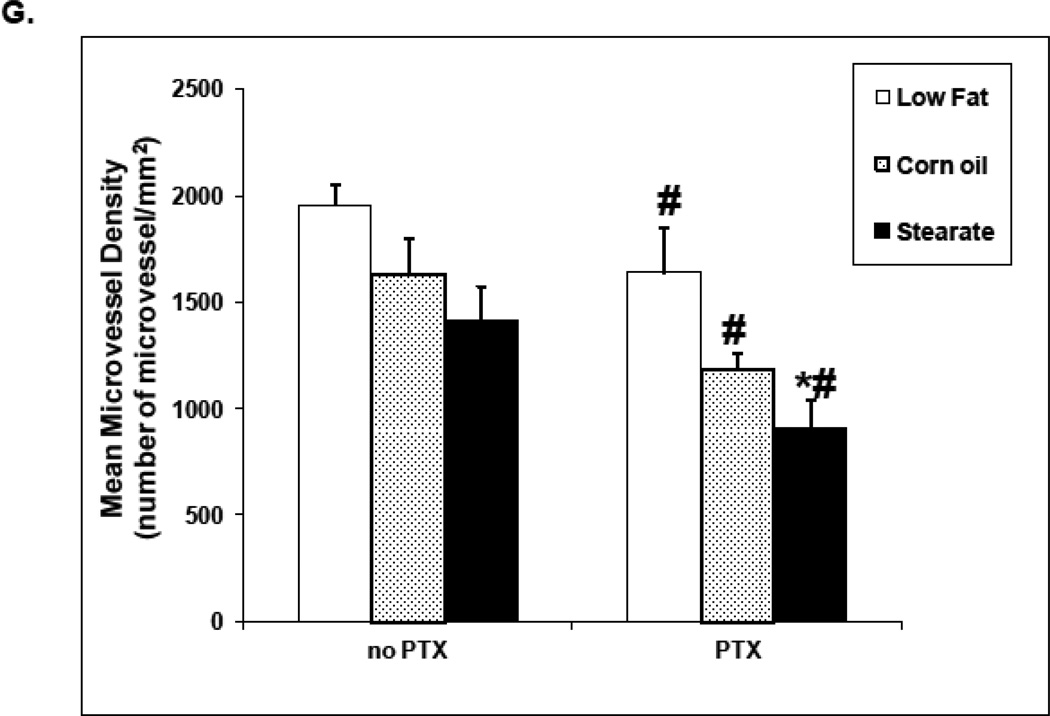
CD31 immunostaining was used to quantify metastatic lung tumor angiogenesis from mice in study #2. As shown in Fig. 5 A–F, tumors from the ST diet and the ST diet plus PTX mice have reduced numbers of microvessels when compared to the other dietary groups. The diets per se did not affect microvessel density (MVD). Although both CO and ST diets decreased MVD, neither was significant (Fig. 5 G). As a group, PTX treated mice had decreased MVD regardless of the diet and when combined with the ST diet further reduced MVD as compared to the LF diet plus PTX.
Figure 5.
ST diet and chemotherapy (PTX) reduce angiogenesis in lung tumors from treatment experiment. Paraffin sections were prepared from the lung metastatic tumors in all 6 cohorts, followed by CD31 immunostaining. (A–F) are representative images of CD31 immunostaining in each of the different experimental groups. Tumors from the ST diet only group and all chemotherapy groups have reduced numbers of microvessels when compared to the LF and CO only groups. (G) When microvessel density (MVD) was measured and compared by two-way ANOVA, the data again showed that in the presence of PTX chemotherapy, lung tumors from the mice fed with ST diet had significantly reduced MVD when compared to the PTX group fed with LF diet (*, p<0.01). In the absence of chemotherapy, although the MVD was decreased in both ST and CO diet groups, significant difference was not observed.
The effect of PTX plus diet on proliferation
As shown in Fig. 6 A–F, lung tumors from mice receiving chemotherapy, as a group, had fewer Ki67 positive cells, while diets alone did not influence proliferation in a statistically significant manner (Fig. 6 G).
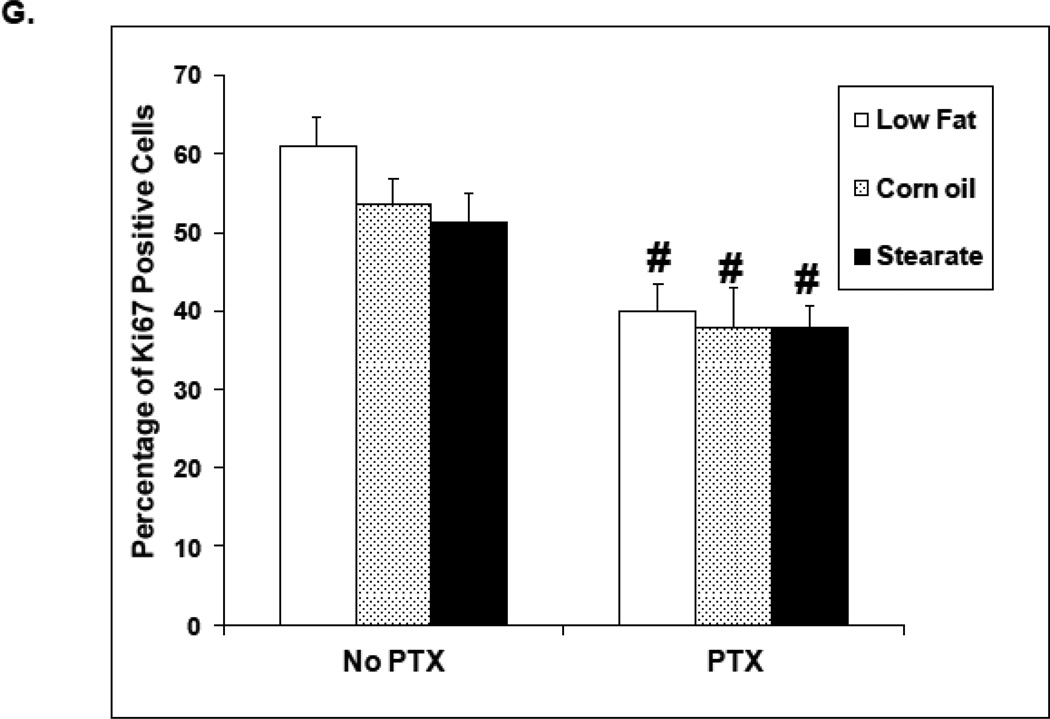
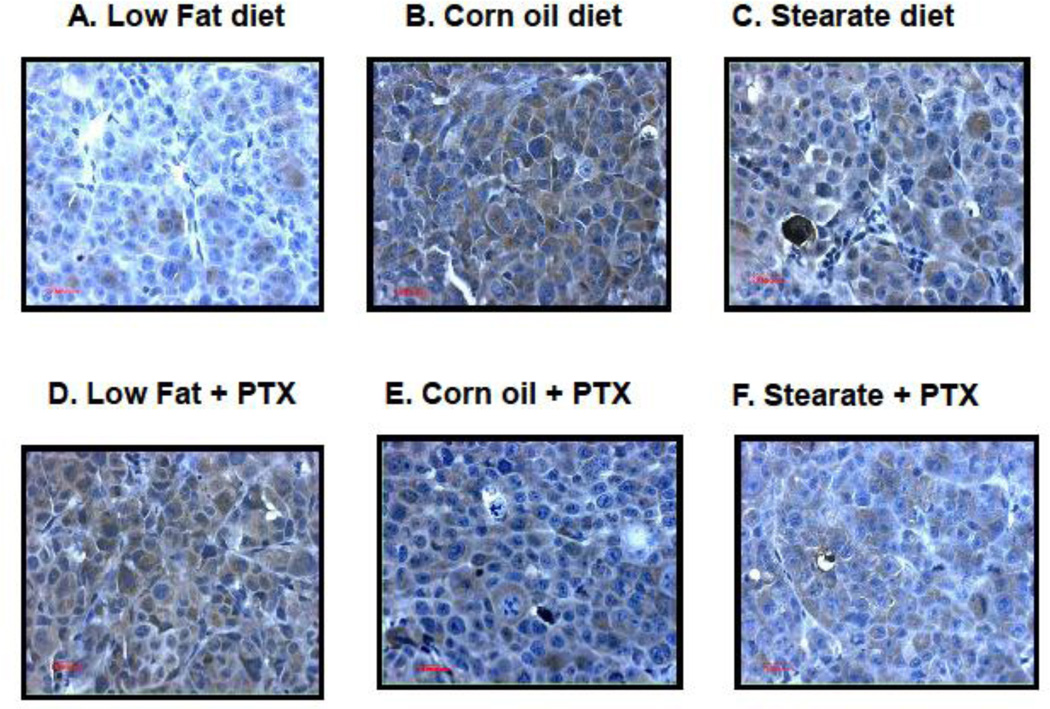
Figure 6.
Chemotherapy but not diet affects lung tumor cell proliferation in treatment experiment. Ki67 immunostaining was performed on lung metastatic tumor paraffin sections. (A–F) are representatives of Ki67 staining in each of the different groups. Tumors from the mice receiving chemotherapy had significantly reduced number of Ki67 positive cells as compared to mice receiving only diet manipulation. (G) When the number and percentage of Ki67 positive cells were counted and calculated, two-way ANOVA analysis showed that chemotherapy significantly inhibited the proliferation (PTX group vs. no PTX group, p<0.01).
The effect of chemotherapy and diet therapy on apoptosis
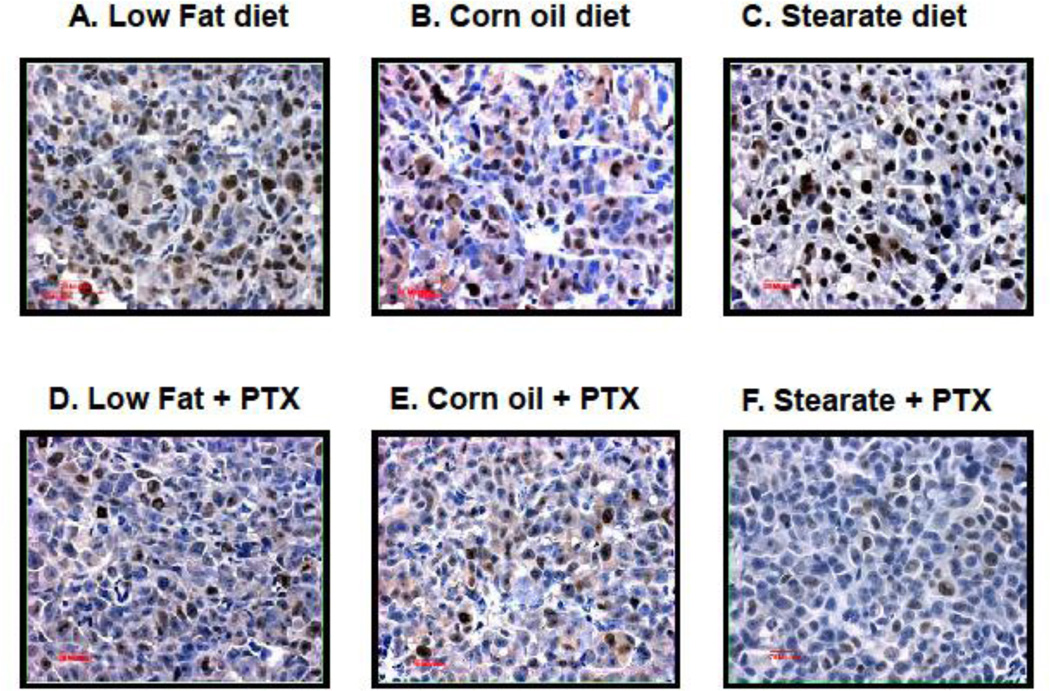
Caspase-3 immunostaining was performed to evaluate apoptosis in metastatic lung tumors. As shown in Fig. 7 A–G, tumors from both the ST and CO diet only groups had significantly more caspase-3 positive cells compared to the LFdiet. However, in the presence of chemotherapy, no difference was observed among these three diet groups although PTX exposure did increase caspase-3 positive cells when compared to LF diet alone.
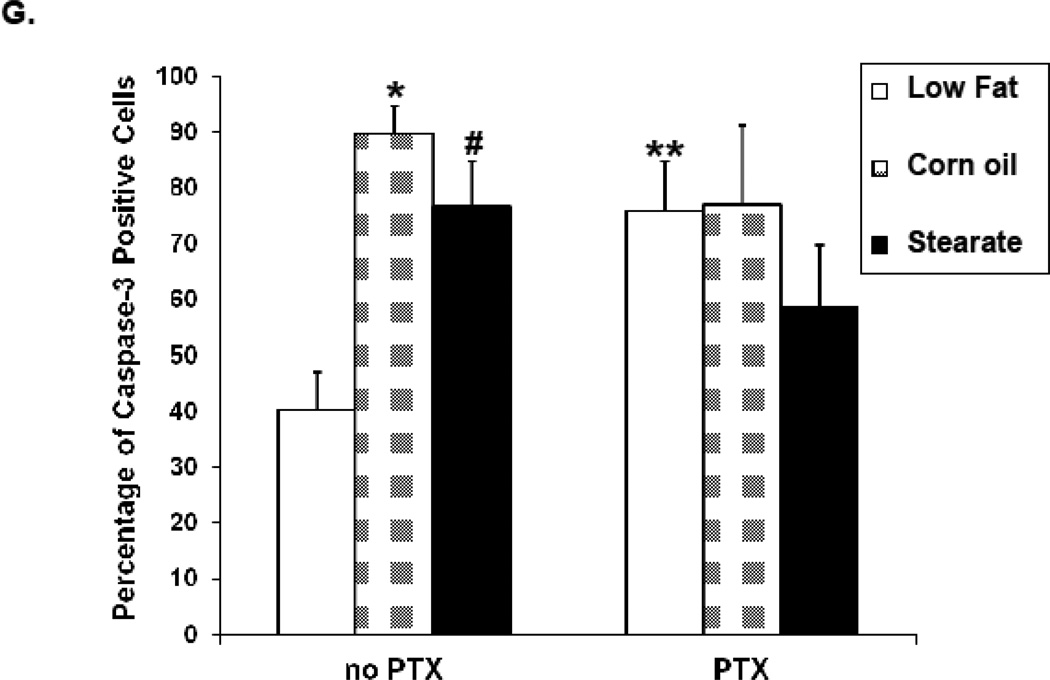
Figure 7.
Diet and chemotherapy both affect lung tumor cell apoptosis in the treatment experiment. Caspase-3 immunostaining was performed on metastatic tumor paraffin sections. (A–F) are representatives of caspase-3 staining from each of the 6 experimental groups. The tumors from the LF diet group had the least number of caspase-3 positive cells. (G) When the number and percentage of caspase-3 positive cells were calculated, tumors from both the ST and CO diet groups had more caspase-3 positive cells (*, p<0.01, CO vs. LF diet group; #, p<0.05, ST vs. LF diet group). However, in the presence of chemotherapy, this difference was not statistically significant. Tumors from the mice receiving the LF diet plus PTX had significantly increased caspase-3 positive cells when compared to the LF diet only group (**, p<0.05).
In summary CO and ST diets used as an adjuvant treatment appear to be decreasing the incidence and/or tumor burden associated with lung metastasis by increasing the apopotosis and decreasing angiogenesis of metastatic lung tumor cells.
Discussion
Stearate has been found to inhibit breast tumor cell proliferation and invasion as well as selectively inducing apoptosis. In animal models dietary stearate has been shown to prevent carcinogenesis as well as reduce both breast cancer and its metastastic tumor burden [9,10,16,17]. The present investigation was designed to extend these studies by examining dietary stearate as a potential complementary agent when used with breast cancer metastasis chemotherapy (paclitaxel/PTX). Specifically, two sets of experiments were designed to test for complementary dietary prevention and treatment roles.
The treatment study indicated that both high fat diets reduced the incidence of metastasis compared to the low fat (LF) diet either with or without concomitant PTX treatment. This was a surprising finding since none of our previously tested diets including the ST diet reduced the incidence of metastasis in this model. The difference in this study is that the high fat diets were given after removal of the primary tumor and all mice were fed the low fat diet up to that point. Thus, the CO and ST diets were acting on cancer cells that had developed into tumors while being exposed to the LF diet as were all host cells. The low fat diet may have selected for a metastatic cancer or “seed” that was not well adapted for a high fat diet. Alternatively the stromal cells or “soil” may have developed in such a way that the high fat diet treatments were less able to support metastasis. Mechanistically, it is possible that the LF diet favors cell survival or angiogenesis compared to the high fat diets. Consistent with the former possibility, both high fat diets increased lung tumor apoptosis compared to the LF diet. This result is consistent with our in vitro studies indicating that stearate specifically induces apoptosis of breast cancer cells [15]. The latter possibility is also supported for the ST diet as we found reduced angiogenesis in the lung metastasis of ST diet mice. Stearate inhibition of angiogenesis is also supported by our previous studies which found that stearate reduced Rho activity and expression in human breast cancer cells as well as mammary cancer cell expression in a rat model of carcinogenesis [17]. Rho may act as an oncogene and tumor endothelial cells have increased Rho activity compared to non-cancer derived endothelial cells. Rho further, may regulate anchorage independent growth and the tumor microenvironment by regulating shape dependent growth [21]. Rho has also been linked to tumor cell migration and invasion [22] as well as angiogenesis [23]. In fact, intratumoral injections of anti-RhoA and anti-RhoC siRNAs virtually eliminated breast cancer cell growth and angiogenesis in a nude mouse model [24]. It is not clear what the precise mechanism is whereby the CO diet induced apoptosis of lung-associated tumor cells since generally corn oil has been found to be tumorigenic in rodent models [25] and associated with increased tumor burden and metastasis [26]. One possible explanation is that the tumors developed in a high carbohydrate diet environment and, when switched from a high carbohydrate diet to a high fat diet, either ST or CO mice, were unable to adapt to this change possibly due to the switch in major energy source. The connection between carbohydrates and cancer is well documented, all the way back to the experiments of Otto Warburg et al. in the 1920’s (27) His experiments demonstrated that tumors, unlike normal tissues, converted large amounts of glucose to lactate via aerobic glycolysis. The reasons often given for why tumors prefer glycolysis over oxidation include: a) being able to grow in a hypoxic environment, b) glycolysis avoids the reactive oxygen species produced by oxidation, c) glycolytic metabolites can be processed via the pentose phosphate pathway into nucleic acids and lipids, d) glycolysis does not depend on the mitochondria and therefore tumor cells may evade mitochondrial induced apoptosis, e) the genes and pathways involved in a highly active glycolytic process are also anti-apoptotic, and f) the lactate produced by glycolysis creates an acidic environment which promotes tumor aggressiveness via metastasis. The idea of carbohydrate restriction for the treatment and prevention of cancer has been discussed in the literature (28) and may account for the beneficial effects of CO as well as ST in our studies.
In general, these dietary studies support a strong role for the ability of diet to influence metastasis whether it is a “pre-cancer” diet, a diet change when cancer is removed, or whether the diet change when cancer is removed is accompanied by chemotherapy.
Unlike the diets alone, PTX, as expected, inhibited breast tumor cell proliferation in the lungs irrespective of diet which is consistent with its mechanism of action. Importantly, when the high fat diets were combined with PTX, they had an additive effect reducing the incidence of lung metastasis from 90% to less than 50%. This suggests that the diets are likely working through a different mechanism than PTX. Whereas PTX acts through destabilization of microtubules, the ST and CO diets appear to be working via inhibition of angiogenesis and increasing tumor cell apoptosis. Overall, these results indicate that both high fat diets increased the effectiveness of chemotherapy when given concomitantly in the setting where cancer has developed with a low fat diet.
This study also raises the question of whether establishing a high fat diet prior to cancer development will also enhance chemotherapy or whether the benefit will be lost as cancer cells might develop preferentially in the presence of such a high fat diet. A previous study that took this approach demonstrated that the ST diet was able to reduce the metastatic lung tumor burden but not the incidence of metastasis itself, although this study did not use chemotherapy [16].
Interestingly, the results of the prevention study herein, which initiated the high fat diets prior to injecting cancer cells, indicate that the ST diet but not the CO diet has a dramatic effect with PTX in reducing the incidence of mice with lung metastasis to ~25%. This is a remarkable reduction since the treatment study indicated that the overall incidence is 93% for mice on the LF diet. Since we know that the ST diet per se does not alter the incidence of lung metastasis in this model [16] and that PTX treatment alone results in a 40–50% decrease in the incidence of lung metastasis, these results indicate chemotherapy and diet can be more effective than either alone. One consideration is that in the prevention study the primary tumor size, as expected, was reduced with the ST diet compared to both the LF and CO diets. In the treatment study this was not an issue as all mice grew primary tumors to the same size on the same LF diet. Thus the reduced incidence of mice with lung metastasis seen with only the high fat diets cannot be attributed to primary tumor size. Since this effect is not seen when the high fat diets are given prior to the introduction of cancer cells without chemotherapy, it is possible that there is a dietary effect on the “soil” in which the primary tumor develops that is an important factor for regulating metastasis. Perhaps the inhibition of angiogenesis is accounting for the results in both the treatment and prevention experiments.
The significance of these studies comes from the potential for diet to alter breast cancer metastasis either alone or more importantly with chemotherapy. Our results suggest that diet plus chemotherapy may be more effective than chemotherapy alone. While the high fat diets used in these experiments are ~40% of calories (~20% by weight), this is not unlike the western diet. Since the ST diet was particularly effective in the prevention study it may be worthwhile exploring the possibility of a long term ST diet. Unlike other saturated fatty acids, such as palmitate (C16:0), stearate does not increase plasma low density lipoprotein cholesterol (LDLc) concentrations [7]. A recent review of epidemiologic and clinical studies evaluated the relation between stearate and cardiovascular disease risk factors to determine the feasibility of replacing trans-fatty acids (TFA) with stearate. They found that in foods that require solid fats the replacement of TFA with stearate would have a beneficial effect on LDLc which is the primary target for CVD risk reduction [8]. While this sounds promising caution should be taken. Future studies need to address the optimum effective treatment concentration and safety of any such ST diet. Nevertheless epidemiological and animal studies have demonstrated a neutral or protective effect of stearate with respect to the risk of breast cancer. A cohort study of post-menopausal women showed that both stearate alone and the stearate/oleate ratio were negatively associated with breast cancer risk [29]. A more recent study using dietary stearate in the NMU rat breast cancer carcinogen model found that stearate reduced the incidence of NMU induced mammary cancer (i.e. carcinogenesis) and the overall tumor burden [17].
The mechanisms by which the combination of PTX and stearate generate their combined effect in reducing the incidence of mice with lung metastasis are unclear. This work and one of our previous studies have shown that without paclitaxel chemotherapy, the anti-metastatic effect of stearate may be due, at least in part, to the ability of stearate to induce apoptosis in human breast cancer cells [15]. However, in the presence of PTX, the stearate effect on apoptosis becomes insignificant. Importantly the levels of apoptosis are quite high in these tumors with only the high fat diets suggesting the possibility of a maximum amount of caspase activity being reached with diet alone. Although inhibition of cancer cell proliferation was found to be an important effect of stearate in vitro [13], neither of the high fat diets enhanced the ability of chemotherapy to inhibit proliferation indicating that this is not one of properties that improved chemotherapy effectiveness.
Our present study showed that inhibition of angiogenesis may be an important mechanism for both high fat diets although the microvessel density in lung tumors was found to be significantly decreased only with chemotherapy. One of the critical steps of angiogenesis is the proliferation of vascular endothelial cells [30], and it has been shown that angiogenesis may be inhibited by selective induction of apoptosis of proliferating endothelial cells [31, 32]. Others have already demonstrated that stearate increased endothelial cell apoptosis in a time and concentration dependent manner [33, 34].
We refer to the model used herein as a model for breast cancer metastasis however it should be noted that there is an ongoing debate as to whether the MDA-MB-435 cell line used in this study is a breast cancer cell line (35, 36) or one that is derived from the M14 melanoma cell line (37, 38). Although this may be considered a limitation of the present study, regardless of cell type, most consider this cell line to be useful as a model of metastasis, and as such, combining diet and chemotherapy to more effectively reduce metastasis is a significant finding.
Another possible limitation of these studies is that athymic nude mice were used. Although they are necessary for studies involving metastatic human cancer cells they have a deficient immune system. Since fatty acids are known to affect the immune system and the immune system can affect tumor development and metastasis it is possible that mice with an intact immune system would have different results.
In summary, both CO and ST diets reduced the breast cancer lung metastatic tumor burden in association with paclitaxel chemotherapy and the ST diet also reduced the incidence of lung metastasis in the prevention study. The ST and CO diets were also both effective at decreasing metastatic tumor burden and incidence of lung metastases in the treatment study. This is likely due to the high fat diets causing apoptosis of tumor cells and reducing angiogenesis. These results provide “proof of principle” that diet can be combined with chemotherapy in either a treatment or prevention setting.
Supplementary Material
Acknowledgements
This study was funded by the UAB SPORE in Breast Cancer 5P50CA089019. We express our appreciation to the UAB Metabolic Bone Disease Histomorphometry Core Laboratory for processing the formalin-fixed paraffin-embedded sections, and carrying out the immunostaining and histomorphometry.
Abbreviations
- LF
low fat diet
- CO
corn oil diet
- ST
stearate diet
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Saloustros E, Mavroudis D, Georgoulias V. Paclitaxel and docetaxel in the treatment of breast cancer. Expert Opin Pharmacother. 2008;9:2603–2616. doi: 10.1517/14656566.9.15.2603. [DOI] [PubMed] [Google Scholar]
- 3.Pienta K. Preclinical mechanisms of action of docetaxel and docetaxel combinations in prostate cancer. Semin Oncol. 2001;28(4) Suppl 15:3–7. doi: 10.1016/s0093-7754(01)90148-4. [DOI] [PubMed] [Google Scholar]
- 4.Bogenrieder T, Herlyn M. Axis of evil: molecular mechanisms of cancer metastasis. Oncogene. 2003;22:6524–6536. doi: 10.1038/sj.onc.1206757. [DOI] [PubMed] [Google Scholar]
- 5.Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer Statistics, 2001. CA Cancer J Clin. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 6.Chung CT, Carlson RW. Goals and objectives in the management of metastatic breast cancer. Oncologist. 2003;8:514–520. doi: 10.1634/theoncologist.8-6-514. [DOI] [PubMed] [Google Scholar]
- 7.Grundy SM. Influence of stearic acid on cholesterol metabolism relative to other long-chain fatty acids. Am J Clin Nutr. 1994;60:986S–990S. doi: 10.1093/ajcn/60.6.986S. [DOI] [PubMed] [Google Scholar]
- 8.Hunter JE, Zhang J, Kris-Etherton PM. Cardiovascular disease risk of dietary stearic acid compared with trans, other saturated, and unsaturated fatty acids: a systematic review. Am J Clin Nutr. 2010;91:46–63. doi: 10.3945/ajcn.2009.27661. [DOI] [PubMed] [Google Scholar]
- 9.Tinsley IJ, Schmitz JA, Pierce DA. Influence of dietary fatty acids on the incidence of mammary tumors in the C3H mouse. Cancer Res. 1981;41:1460–1465. [PubMed] [Google Scholar]
- 10.Bennett AS. Effect of dietary stearic acid on the genesis of spontaneous mammary adenocarcinomas in strain A/ST mice. Int J Cancer. 1984;34:529–533. doi: 10.1002/ijc.2910340416. [DOI] [PubMed] [Google Scholar]
- 11.Habib NA, Wood CB, Apostolov K, Barker W, Hershman MJ, Aslam M, Heinemann D, Fermor B, Williamson RC, Jenkins WE, et al. Stearic acid and carcinogenesis. Br J Cancer. 1987;56:455–458. doi: 10.1038/bjc.1987.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh RK, Hardy RW, Wang MH, Williford J, Gladson CL, McDonald JM, Siegal GP. Stearate inhibits human tumor cell invasion. Invasion & Metastasis. 1995;15:144–155. [PubMed] [Google Scholar]
- 13.Wickramasinghe NS, Jo H, McDonald JM, Hardy RW. Stearate inhibition of breast cancer cell proliferation. A mechanism involving epidermal growth factor receptor and G-proteins. Am J Pathol. 1996;148:987–995. [PMC free article] [PubMed] [Google Scholar]
- 14.Hardy S, El-Assaad W, Przybytkowski E, Joly E, Prentki M, Langelier Y. Saturated fatty acid-induced apoptosis in MDA-MB-231 breast cancer cells. A role for cardiolipin. J Biol Chem. 2003;278:31861–31870. doi: 10.1074/jbc.M300190200. [DOI] [PubMed] [Google Scholar]
- 15.Evans LM, Cowey SL, Siegal GP, Hardy RW. Stearate preferentially induces apoptosis in human breast cancer cells. Nutr Cancer. 2009;61:746–753. doi: 10.1080/01635580902825597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans LM, Toline EC, Desmond R, Siegal GP, Hashim AI, Hardy RW. Dietary stearate reduces human breast cancer metastasis burden in athymic nude mice. Clin Exp Metastasis. 2009;26:415–424. doi: 10.1007/s10585-009-9239-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li C, Zhao X, Toline E, Siegal GP, Evans LM, Ibrahim-Hashim A, Desmond R, Hardy RW. Prevention of carcinogenesis and inhibition of breast cancer tumor burden by dietary stearate. Carcinogenesis. 2011;32:1251–1258. doi: 10.1093/carcin/bgr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Townson JL, Naumov GN, Chambers AF. The role of apoptosis in tumor progression and metastasis. Curr Mol Med. 2003;3:631–642. doi: 10.2174/1566524033479483. [DOI] [PubMed] [Google Scholar]
- 19.Boedefeld WM, 2nd, Bland KI, Heslin MJ. Recent insights into angiogenesis, apoptosis, invasion, and metastasis in colorectal carcinoma. Ann Surg Oncol. 2003;10:839–851. doi: 10.1245/aso.2003.02.021. [DOI] [PubMed] [Google Scholar]
- 20.Weidner N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat. 1995;36:169–180. doi: 10.1007/BF00666038. [DOI] [PubMed] [Google Scholar]
- 21.Ingber DE. Can cancer be reversed by engineering the tumor microenvironment? Semin Cancer Biol. 2008;18:356–364. doi: 10.1016/j.semcancer.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitz AA, Govek EE, Böttner B, Van Aelst L. Rho GTPases: signaling, migration, and invasion. Exp Cell Res. 2000;25:1–12. doi: 10.1006/excr.2000.5049. [DOI] [PubMed] [Google Scholar]
- 23.Merajver SD, Usmani SZ. Multifaceted role of Rho proteins in angiogenesis. J Mammary Gland Biol Neoplasia. 2005;10:291–298. doi: 10.1007/s10911-006-9002-8. [DOI] [PubMed] [Google Scholar]
- 24.Pillé JY, Denoyelle C, Varet J, et al. Anti-RhoA and anti-RhoC siRNAs inhibit the proliferation and invasiveness of MDA-MB-231 breast cancer cells in vitro and in vivo. Mol Ther. 2005;11:267–274. doi: 10.1016/j.ymthe.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 25.Welsch CW. Dietary fat, calories, and mammary gland tumorigenesis. Adv Exp Med Biol. 1992;322:203–222. doi: 10.1007/978-1-4684-7953-9_16. [DOI] [PubMed] [Google Scholar]
- 26.Rose DP, Connolly JM. Influence of dietary fat intake on local recurrence and progression of metastases arising from MDA-MB-435 human breast cancer cells in nude mice after excision of the primary tumor. Nutr Cancer. 1992;18:113–122. doi: 10.1080/01635589209514211. [DOI] [PubMed] [Google Scholar]
- 27.Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klement RJ, Kammerer U. Is there a role for carbohydrate restriction in the treatment and prevention of cancer? Nutrition & Metabolism. 2011;8:75. doi: 10.1186/1743-7075-8-75. (open access) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saadatian-Elahi M, Norat T, Goudable J, Riboli E. Biomarkers of dietary fatty acid intake and the risk of breast cancer: a meta-analysis. Int J Cancer. 2004;111:584–591. doi: 10.1002/ijc.20284. [DOI] [PubMed] [Google Scholar]
- 30.Munaron L. Intracellular calcium, endothelial cells and angiogenesis. Recent Pat Anticancer Drug Discov. 2006;1:105–119. doi: 10.2174/157489206775246502. [DOI] [PubMed] [Google Scholar]
- 31.Naumova E, Ubezio P, Garofalo A, et al. The vascular targeting property of paclitaxel is enhanced by SU6668, a receptor tyrosine kinase inhibitor, causing apoptosis of endothelial cells and inhibition of angiogenesis. Clin Cancer Res. 2006;12:1839–1849. doi: 10.1158/1078-0432.CCR-05-1615. [DOI] [PubMed] [Google Scholar]
- 32.Dong LF, Swettenham E, Eliasson J, et al. Vitamin E analogues inhibit angiogenesis by selective induction of apoptosis in proliferating endothelial cells: the role of oxidative stress. Cancer Res. 2007;67:11906–11913. doi: 10.1158/0008-5472.CAN-07-3034. [DOI] [PubMed] [Google Scholar]
- 33.Artwohl M, Roden M, Waldhäusl W, et al. Free fatty acids trigger apoptosis and inhibit cell cycle progression in human vascular endothelial cells. FASEB J. 2004;18:146–158. doi: 10.1096/fj.03-0301fje. [DOI] [PubMed] [Google Scholar]
- 34.Artwohl M, Lindenmair A, Sexl V, et al. Different mechanisms of saturated versus polyunsaturated FFA-induced apoptosis in human endothelial cells. J Lipid Res. 2008;49:2627–2640. doi: 10.1194/jlr.M800393-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Chambers AF. MDA-MB-435 and M14 cell lines: identical but not M14 melanoma? CancerRes. 2009;69:5292–5293. doi: 10.1158/0008-5472.CAN-09-1528. [DOI] [PubMed] [Google Scholar]
- 36.Nerlich AG, Bachmeier BE. Density-dependent lineage instability of MDAMB-435 breast cancer cells. Oncol Lett. 2013;5:1370–1374. doi: 10.3892/ol.2013.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rae JM, Creighton CJ, Meck JM, et al. MDA-MB-435 cells are derived from M14 melanoma cells--a loss for breast cancer, but a boon for melanoma research. Breast Cancer Res Treat. 2007;104:13–19. doi: 10.1007/s10549-006-9392-8. [DOI] [PubMed] [Google Scholar]
- 38.Christgen M, Lehmann U. MDA-MB-435: the questionable use of a melanoma cell line as a model for human breast cancer is ongoing. Cancer Biol Ther. 2007;6:1355–1357. doi: 10.4161/cbt.6.9.4624. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.