Abstract
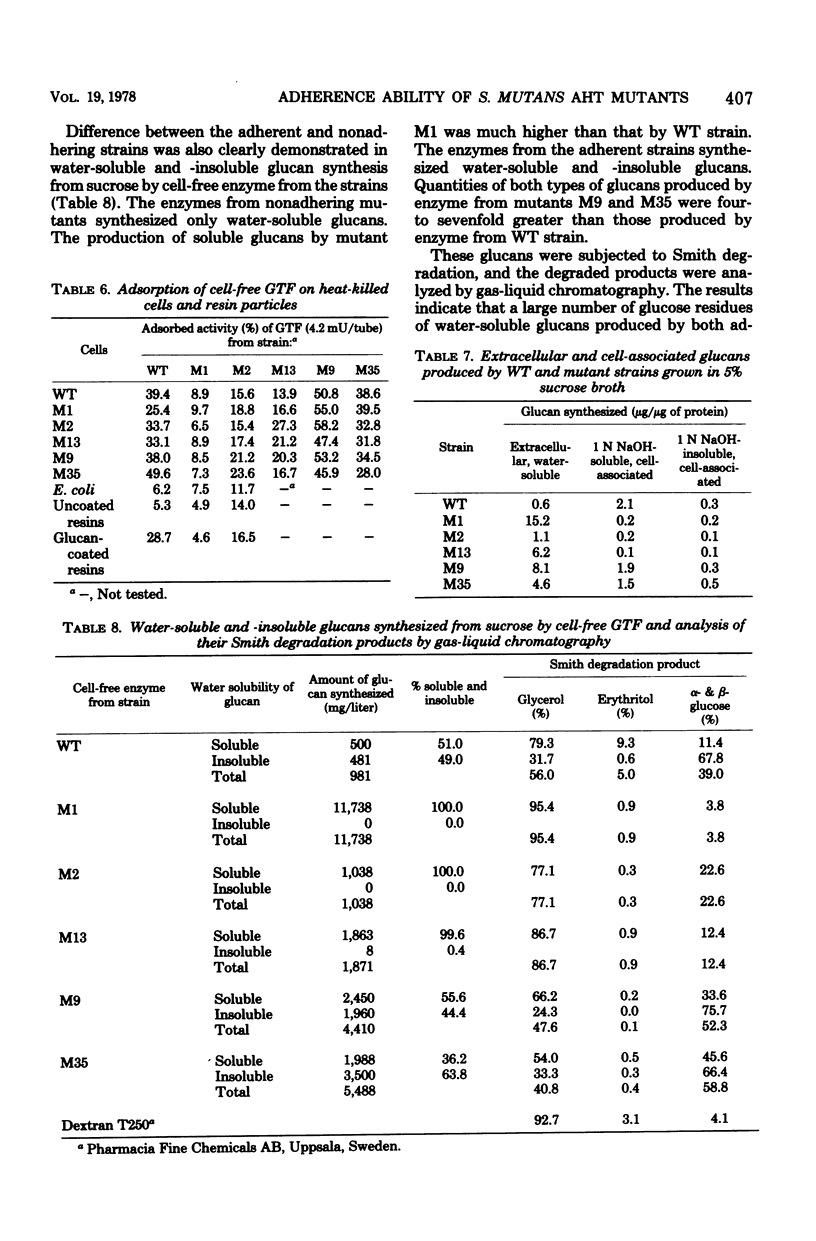
Streptococcus mutans AHT mutants M1, M2, and M13 failed to adhere to a glass surface, whereas mutants M9 and M35 exhibited decreased and increased adherence, respectively, as compared with the parent strain, when grown in sucrose broth. Extracellular glucosyltransferase prepared from glucose-grown cultures of the adherent strains (wild type, M9, and M35) induced adherence of heat-killed cells of the homologous and heterologous streptococcal strains as well as of Escherichia coli K-12 and uncoated resin particles. The glucosyltransferase was adsorbed on all the streptococcal cells and glucan-coated resins, but not on E. coli cells and the uncoated resins. Glucosyltransferase from the nonadhering mutants (M1, M2, M13) neither was significantly adsorbed on nor induced adherence of any of the cells and resins. Cell-free enzymes from the glucose-grown adherent strains produced water-soluble and water-insoluble glucans, whereas those from the nonadhering mutants produced only water-soluble glucans. Small amounts of alkali-soluble, cell-associated glucan were recovered from the sucrose-grown nonadhering mutants. Thus, the relative proportions of glucosyltransferase isozymes elaborated by the S. mutans mutants, insofar as they affect the physico-chemical properties of the glucans produced, seem to determine the adherence abilities of the cells. The adsorption of glucosyltransferase on glucan molecules on the cell surface is not required for the adherence of S. mutans, but de novo glucan synthesis is important in the adherence process.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowen W. H. The induction of rampant dental caries in monkeys (Macaca irus). Caries Res. 1969;3(3):227–237. doi: 10.1159/000259597. [DOI] [PubMed] [Google Scholar]
- Ceska M., Granath K., Norrman B., Guggenheim B. Structural and enzymatic studies on glucans synthesized with glucosyltransferases of some strains of oral streptococci. Acta Chem Scand. 1972;26(6):2223–2230. doi: 10.3891/acta.chem.scand.26-2223. [DOI] [PubMed] [Google Scholar]
- Chludzinski A. M., Germaine G. R., Schachtele C. F. Purification and properties of dextransucrase from Streptococcus mutans. J Bacteriol. 1974 Apr;118(1):1–7. doi: 10.1128/jb.118.1.1-7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stoppelaar J. D., Van Houte J., Backer Dirks O. The relationship between extracellular polysaccharide-producing streptococci and smooth surface caries in 13-year-old children. Caries Res. 1969;3(2):190–199. doi: 10.1159/000259582. [DOI] [PubMed] [Google Scholar]
- Ebisu S., Misaki A., Kato K., Kotani S. The structure of water-insoluble glucans of cariogenic Streptococcus mutans, formed in the absence and presence of dextranase. Carbohydr Res. 1974 Dec;38:374–381. doi: 10.1016/s0008-6215(00)82375-7. [DOI] [PubMed] [Google Scholar]
- Edwardsson S. The caries-inducing property of variants of Streptococcus mutans. Odontol Revy. 1970;21(2):153–157. [PubMed] [Google Scholar]
- FITZGERALD R. J., JORDAN H. V., STANLEY H. R. Experimental caries and gingival pathologic changes in the gnotobiotic rat. J Dent Res. 1960 Sep-Oct;39:923–935. doi: 10.1177/00220345600390052701. [DOI] [PubMed] [Google Scholar]
- FITZGERALD R. J., KEYES P. H. Demonstration of the etiologic role of streptococci in experimental caries in the hamster. J Am Dent Assoc. 1960 Jul;61:9–19. doi: 10.14219/jada.archive.1960.0138. [DOI] [PubMed] [Google Scholar]
- Freedman M. L., Tanzer J. M. Dissociation of plaque formation from glucan-induced agglutination in mutants of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):189–196. doi: 10.1128/iai.10.1.189-196.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui K., Fukui Y., Moriyama T. Purification and properties of dextransucrase and invertase from Streptococcus mutans. J Bacteriol. 1974 Jun;118(3):796–804. doi: 10.1128/jb.118.3.796-804.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Schachtele C. F. Streptococcus mutans dextransucrase: mode of interaction with high-molecular-weight dextran and role in cellular aggregation. Infect Immun. 1976 Feb;13(2):365–372. doi: 10.1128/iai.13.2.365-372.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Berman K. S., Knoettner P., Kapsimalis B. Dental caries and alveolar bone loss in gnotobiotic rats infected with capsule forming streptococci of human origin. Arch Oral Biol. 1966 Jun;11(6):549–560. doi: 10.1016/0003-9969(66)90220-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Synthesis of insoluble dextran and its significance in the formation of gelatinous deposits by plaque-forming streptococci. Arch Oral Biol. 1968 Oct;13(10):1249–1262. doi: 10.1016/0003-9969(68)90081-2. [DOI] [PubMed] [Google Scholar]
- Guggenheim B. Enzymatic hydrolysis and structure of water-insoluble glucan produced by glucosyltransferases from a strain of streptococcus mutans. Helv Odontol Acta. 1970 Nov;14(Suppl):89+–89+. [PubMed] [Google Scholar]
- Guggenheim B., Newbrun E. Extracellular glucosyltransferase activity of an HS strain of Streptococcus mutans. Helv Odontol Acta. 1969 Oct;13(2):84–97. [PubMed] [Google Scholar]
- Guggenheim B., Schroeder H. E. Biochemical and morphological aspects of extracellular polysaccharides produced by cariogenic streptococci. Helv Odontol Acta. 1967 Oct;11(2):131–152. [PubMed] [Google Scholar]
- Higuchi M., Endo K., Hoshino E., Araya S. Preferential induction of rough variants in Streptococcus mutans by ethidium bromide. J Dent Res. 1973 Sep-Oct;52(5):1070–1075. doi: 10.1177/00220345730520051401. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Sandham H. J. Prevalence of Streptococcus mutans on various tooth surfaces in Negro children. Arch Oral Biol. 1971 Oct;16(10):1237–1240. doi: 10.1016/0003-9969(71)90053-7. [DOI] [PubMed] [Google Scholar]
- Inoeu M., Egami T., Takehara T., Osugi T., Morioka T. [Partial purification and properties of extracellular sucrases from S. mutans strain AHT]. Koku Eisei Gakkai Zasshi. 1974 Mar;24(1):6–18. doi: 10.5834/jdh.24.6. [DOI] [PubMed] [Google Scholar]
- Johnson M. C., Bozzola J. J., Shechmeister I. L. Morphological study of Streptococcus mutans and two extracellular polysaccharide mutants. J Bacteriol. 1974 Apr;118(1):304–311. doi: 10.1128/jb.118.1.304-311.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. C., Bozzola J. J., Shechmeister I. L., Shklair I. L. Biochemical study of the relationship of extracellular glucan to adherence and cariogenicity in Streptococcus mutans and an extracellular polysaccharide mutant. J Bacteriol. 1977 Jan;129(1):351–357. doi: 10.1128/jb.129.1.351-357.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasse B. Human streptococci and experimental caries in hamsters. Arch Oral Biol. 1966 Apr;11(4):429–436. doi: 10.1016/0003-9969(66)90107-5. [DOI] [PubMed] [Google Scholar]
- Kuramitsu H. K. Adherence of Streptococcus mutans to dextran synthesized in the presence of extracellular dextransucrase. Infect Immun. 1974 Apr;9(4):764–765. doi: 10.1128/iai.9.4.764-765.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K. Characterization of extracellular glucosyltransferase activity of Steptococcus mutans. Infect Immun. 1975 Oct;12(4):738–749. doi: 10.1128/iai.12.4.738-749.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K. Properties of a mutant of Streptococcus mutans altered in glucosyltransferase activity. Infect Immun. 1976 Feb;13(2):345–353. doi: 10.1128/iai.13.2.345-353.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liljemark W. F., Schauer S. V. Studies on the bacterial components which bind Streptococcus sanguis and Streptococcus mutans to hydroxyapatite. Arch Oral Biol. 1975 Sep;20(9):609–615. doi: 10.1016/0003-9969(75)90082-5. [DOI] [PubMed] [Google Scholar]
- Littleton N. W., Kakehashi S., Fitzgerald R. J. Recovery of specific "caries-inducing" streptococci from carious lesions in the teeth of children. Arch Oral Biol. 1970 May;15(5):461–463. doi: 10.1016/0003-9969(70)90073-7. [DOI] [PubMed] [Google Scholar]
- McCabe M. M., Smith E. E. Origin of the cell-associated dextransucrase of Streptococcus mutans. Infect Immun. 1973 Jun;7(6):829–838. doi: 10.1128/iai.7.6.829-838.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek S. M., McGhee J. R., Shiota T., Devenyns D. Virulence of Streptococcus mutans: cariogenicity of S. mutans in adult gnotobiotic rats. Infect Immun. 1977 Feb;15(2):466–471. doi: 10.1128/iai.15.2.466-471.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek S. M., Shiota T., Ikeda T., Navia J. M., McGhee J. R. Virulence of Streptococcus mutans: biochemical and pathogenic characteristics of mutant isolates. Proc Soc Exp Biol Med. 1975 Nov;150(2):498–502. doi: 10.3181/00379727-150-39064. [DOI] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. I. Roles of insoluble dextran-levan synthetase enzymes and cell wall polysaccharide antigen in plaque formation. Infect Immun. 1973 Oct;8(4):555–562. doi: 10.1128/iai.8.4.555-562.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. II. Nature of the binding site and the adsorption of dextran-levan synthetase enzymes on the cell-wall surface of the streptococcus. Infect Immun. 1974 Feb;9(2):419–429. doi: 10.1128/iai.9.2.419-429.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of the Adherence of Streptococcus mutans to Smooth Surfaces III. Purification and Properties of the Enzyme Complex Responsible for Adherence. Infect Immun. 1974 Nov;10(5):1135–1145. doi: 10.1128/iai.10.5.1135-1145.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbrun E. Extracellular polysaccharides synthesized by glucosyltransferases of oral streptococci. Composition and susceptibility to hydrolysis. Caries Res. 1972;6(2):132–147. doi: 10.1159/000259785. [DOI] [PubMed] [Google Scholar]
- Robrish S. A., Reid W., Krichevsky M. I. Distribution of enzymes forming polysaccharide from sucrose and the composition of extracellular polysaccharide synthesized by Streptococcus mutans. Appl Microbiol. 1972 Aug;24(2):184–190. doi: 10.1128/am.24.2.184-190.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F., Germaine G. R., Harlander S. K. Production of elevated levels of dextransucrase by a mutant of Streptococcus mutans. Infect Immun. 1975 Oct;12(4):934–937. doi: 10.1128/iai.12.4.934-937.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F., Staat R. H., Harlander S. K. Dextranases from oral bacteria: inhibition of water-insoluble glucan production and adherence to smooth surfaces by Streptococcus mutans. Infect Immun. 1975 Aug;12(2):309–317. doi: 10.1128/iai.12.2.309-317.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shklair I. L., Keene H. J., Cullen P. The distribution of Streptococcus mutans on the teeth of two groups of naval recruits. Arch Oral Biol. 1974 Feb;19(2):199–202. doi: 10.1016/0003-9969(74)90214-3. [DOI] [PubMed] [Google Scholar]
- Spinell D. M., Gibbons R. J. Influence of culture medium on the glucosyl transferase- and dextran-binding capacity of Streptococcus mutans 6715 cells. Infect Immun. 1974 Dec;10(6):1448–1451. doi: 10.1128/iai.10.6.1448-1451.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., HARRISON J. S. Studies on yeast metabolism. 7. Yeast carbohydrate fractions. Separation from nucleic acid, analysis, and behaviour during anaerobic fermentation. Biochem J. 1956 May;63(1):23–33. doi: 10.1042/bj0630023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M., Freedman M. L., Fitzgerald R. J., Larson R. H. Diminished virulence of glucan synthesis-defective mutants of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):197–203. doi: 10.1128/iai.10.1.197-203.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houte J., Upeslacis V. N. Studies of the mechanism of sucrose-associated colonization of Streptococcus mutans on teeth of conventional rats. J Dent Res. 1976 Mar-Apr;55(2):216–222. doi: 10.1177/00220345760550020901. [DOI] [PubMed] [Google Scholar]
- ZINNER D. D., JABLON J. M., ARAN A. P., SASLAW M. S. EXPERIMENTAL CARIES INDUCED IN ANIMALS BY STREPTOCOCCI OF HUMAN ORIGIN. Proc Soc Exp Biol Med. 1965 Mar;118:766–770. doi: 10.3181/00379727-118-29964. [DOI] [PubMed] [Google Scholar]
- de Stoppelaar J. D., König K. G., Plasschaert A. J., van der Hoeven J. S. Decreased cariogenicity of a mutant of Streptococcus mutans. Arch Oral Biol. 1971 Aug;16(8):971–975. doi: 10.1016/0003-9969(71)90186-5. [DOI] [PubMed] [Google Scholar]


