Abstract
The average of overweight individual can have differential fat depots in target-organs or specific compartments of the body. This ectopic fat distribution may be more of a predictive factor for cardiovascular risk than obesity. Abdominal visceral obesity, a representative ectopic fat, is robustly associated with insulin resistance and cardiovascular risk. Fat depots in the liver and muscle tissue cause adverse cardiometabolic risk by affecting glucose and lipid metabolism. Pericardial fat and perivascular fat affect coronary atherosclerosis, cardiac function, and hemodynamics. Fat around the neck is associated with systemic vascular resistance. Fat around the kidney may increase blood pressure and induce albuminuria. Fat accumulation in or around the pancreas alters glucose metabolism, conferring cardiovascular risk.
Ectopic fat may act as an active endocrine and paracrine organ that releases various bioactive mediators that influence insulin resistance, glucose and lipid metabolism, coagulation, and inflammation, which all contribute to cardiovascular risk. As both obese and apparently lean individuals can have ectopic fat, regional fat distribution may play an important role in the development of cardiovascular diseases in both non-obese and obese people.
Keywords: ectopic fat, cardiovascular risk, mechanism, imaging
Introduction
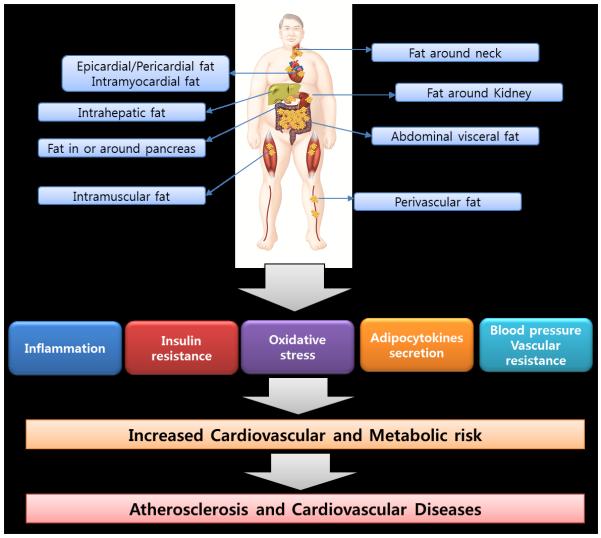
Obesity and its complications have been increasing worldwide and are becoming a huge burden to both human health and healthcare costs.1-3 In addition to overall obesity, ectopic fat has been found to contribute to cardiovascular disease (CVD). Ectopic fat is fat accumulation in or around specific organs or compartments of the body as the omentum, liver, muscle, and pancreas. Fat may accumulate around the heart, blood vessels, kidney, and neck. Fat depots in the liver or muscle tissue can increase cardiovascular risk by affecting lipid and glucose metabolism, particularly when accompanied by inflammation. Pericardial fat, perivascular fat, pericoronary fat, and myocardial fat may exert their harmful effects on the heart and blood vessels by direct lipotoxicity and by indirect cytokine secretion. Renal sinus fat is a unique fat depot that may confer additional cardiovascular risk such as microalbuminuria and high blood pressure. Recently, researchers highlighted the concept of fat accumulation in the pancreas (“fatty pancreas”) associated with altered glucose metabolism and insulin resistance, which may also contribute to cardiovascular risk. Collectively, the unique concept of ectopic fat storage in key target-organs contributes to understanding of a pathogenic role of ectopic fat in cardiovascular risk (Figure 1).
Figure 1.
Ectopic fat storage in key target-organs or compartments contributing to atherosclerosis and cardiovascular risk
This review summarizes the clinical implications of ectopic fat depots from a cardiovascular perspective. We focused on the underlying mechanisms between ectopic fat depots and CVD derived mostly from human studies. Subcutaneous adipose tissue was excluded from this review due to the uncertainty of its relation with vascular diseases in humans.
Fat in the abdomen
Increased abdominal visceral adipose tissue (VAT) is associated with a clustering of metabolic risk factors as well as insulin resistance.4-7 A number of cross-sectional studies have shown that patients with subclinical atherosclerosis or overt coronary artery disease (CAD) have greater VAT than those without, even after adjusting for body mass index (BMI).5,8-10 In stark contrast, relatively small numbers of studies reported an independent role of VAT in cardiovascular events.11,12 A 10-year follow up study with Japanese-Americans showed that VAT was an independent risk factor for incident CAD.11 Similarly, a study with participants from the Framingham Heart Study found that visceral adiposity was associated with incident CVD after adjustments for clinical risk factors and overall adiposity.12 Abdominal VAT accumulation was positively associated with the progression of coronary noncalcified plaque.13
Several mechanisms have been suggested to explain the association of VAT with cardiovascular risk.14,15 One such mechanism is that VAT has increased lipolytic activity and, therefore, increases delivery of free fatty acids (FFAs) to the liver, ultimately leading to insulin resistance.16 Low-grade inflammation often presents in increased VAT. This inflamed fat can affect immunity.17 Inflamed fat depots produce atherosclerosis-prone cytokines, the circulating concentrations of which are modulated by metabolic or inflammatory processes.18,19 Indeed, in the Framingham Heart Study, VAT was positively related to inflammation and oxidative stress.20
Obesity in humans is associated with increases in oxidative stress markers such as isoprostanes and protein-bound carbonyls in adipose tissue.21,22 The increased reactive oxygen species (ROS) in large VAT is accompanied by an increase in mRNA expression levels of NADPH oxidase subunits, an enzyme complex that generates ROS, and a decrease in mRNA expression levels and activities of antioxidant enzymes such as glutathione peroxidase (GPX) and Cu/Zn superoxide dismutase (Cu/Zn SOD).23 Several animal studies suggest other mechanisms. Activity of methionine sulphoxide reductase A, an anti-oxidant defense enzyme, was reduced by ~25% in the visceral adipose of Zucker diabetic fatty rats.24 S-glutathionylation was decreased in the adipose tissue of obese rats.25 These data provide that VAT alters the redox system, resulting in an increase of ROS production, which might be involved in the pathogenesis of insulin resistance in obesity.
Hypertrophied adipose tissue is characterized by an infiltration of macrophages and is a major source of proinflammatory cytokines including interleukin (IL)-6 and tumor necrosis factor (TNF)-α.26 In obese patients with high VAT accumulation, the systemic renin angiotensin system (RAS) is impaired.27 There are several possible physiological and molecular mechanisms that link VAT with the RAS: 1) increased secretion of angiotensinogen from adipocytes, 2) interaction between hyperinsulinemia induced by increased VAT and activation of angiotensin type 1-receptors, 3) influence of adipocytokines (TNFα and IL-6) released from VAT on RAS system.28,29 The increased activity of the RAS in subjects with high VAT volume may play a key role in hypertension and its cardiovascular complications. For instance, VAT volume was significantly associated with the prevalence of microalbuminuria in low-risk, non-diabetic subjects.30 VAT is also associated with increased levels of plasminogen activator inhibitor-1 (PAI-1), reflecting depressed fibrinolysis with a greater tendency towards clinically relevant thrombosis.31 Bioactive mediators released from VAT combined with inflammation, oxidative stress, and activated RAS all lead to endothelial dysfunction and atherosclerosis.2,32,33 The elderly, compared to the young with the same BMI, had increased VAT, which linked to atherosclerotic biomarkers and subclinical atherosclerosis.34
Computed tomography (CT), magnetic resonance image (MRI), and new dual-energy X-ray absorptiometry (DXA) techniques have been used to measure abdominal fat, differentially from subcutaneous adipose tissue (SAT).5,35,36 Ultrasonography is also a method used to estimate abdominal VAT.37
Nonalcoholic fatty liver disease
There is substantial evidence supporting a relation between nonalcoholic fatty liver disease and CVDs. In the Framingham Heart Study, fatty liver was associated with a clustering of cardiovascular risk after adjustment for other fat depots including VAT.38 Another study reported that intrahepatic lipid depots were more closely linked with cardiovascular complications than with VAT.39 More directly, nonalcoholic fatty liver disease has been found to be an independent predictor of incident cardiovascular events in patients with type 2 diabetes mellitus (T2DM) as well as normal subjects.40-42
Several mechanisms have been proposed for the association of fatty liver with cardiovascular risk.38 The liver plays a central role in lipid metabolism and storage. In addition to the critical role in TG synthesis and storage, hepatocytes contain lipid droplets in the lumen of the endoplasmic reticulum where very low density lipoprotein (VLDL) is assembled.43 Thus, the liver secretes TG into the blood in apolipoprotein B (apoB)-containing VLDL particles. Fatty liver leads to hepatic insulin resistance, which induces hepatic VLDL production via changes in the rate of apoB synthesis and degradation or de novo lipogenesis.44
Furthermore, fatty liver stimulates the production of inflammatory cytokines.45 In particular, nonalcoholic steatohepatitis may instigate the pathogenesis of CVD through the systemic release of several inflammatory, hemostatic, and oxidative-stress mediators.46 Fatty liver may also play a part in cardiac dysfunction. Hepatic triglyceride content in T2DM was associated with decreased myocardial perfusion.47 Another study also showed that high triglyceride content in the liver was associated with myocardial dysfunction in T2DM.48 These results support that fat accumulation in the liver plays an important role in the atherosclerotic milieu by promoting insulin resistance and systemic inflammation.
Many studies have tried to measure fat accumulation in the liver non-invasively. Classically, ultrasonography has been used to define fatty liver, but it has the limitation of quantification.41 Recently, CT has been used to measure fat accumulation in the liver, but CT has the risk associated with radiation exposure.49 The noninvasive volume-localized 1H-magnetic resonance spectroscopy (MRS) has been used experimentally.50 This method has the unique advantage of measuring intrahepatic lipid content accurately. However, due to the high cost of the specialized machinery, it is not widely used.
Intramuscular fat
In patients with diabetes, muscle density assessed by quantitative CT (representation of higher fat infiltration) was observed to be lower than that in patients without diabetes, even after adjusting for BMI.51 Individuals with insulin-resistance had greater intramuscular fat deposits than those with insulin sensitivity, showing that fat depots in muscle tissue contribute to impaired glucose uptake.52 Several studies with MRS have demonstrated that the accumulation of intramyocellular triglycerides induces the development of insulin resistance in various populations.53,54 Furthermore, patients with diabetes who had more fat in muscle tissue had greater carotid intima-media thickness than their healthy counterparts.55 These results provide evidence that intramuscular fat depot is associated with atherosclerosis via increasing insulin resistance independent of overall obesity.
Skeletal muscle tissue is the main destination for insulin-stimulated glucose disposal and fatty acid metabolism, which are principal determinants of systemic insulin resistance.56 The defects in fatty acid metabolism in people who have excessive intramuscular fat support that fat accumulation in the muscle is closely associated with insulin resistance.57 A defect in mitochondrial oxidative phosphorylation and fat accumulation in muscle was found to be related to insulin resistance in humans.58,59 Thus, mitochondrial dysfunction in the muscle with impairments in fatty acid and triglyceride metabolism is a key in the etiology of insulin resistance, leading to cardiometabolic abnormalities.60
There are several methods to measure fat accumulation in the muscle tissue. CT has been used to measure fat deposition in muscle. However, CT scan is an indirect method that estimates the fat content by measuring the Hounsfield unit (HU).61 DXA is also frequently used.62 The advantage that DXA offers is its ability to measure upper and lower extremity fat amounts separately with a single scan image.
Volume-localized 1H-MRS can also be used in fat measurement in the muscle. This method has the unique advantage differentiating between intramyocellular and extramyocellular fat deposits.50
Fat in the neck
Fat in the neck, a proxy for upper-body fat, is a unique ectopic fat depot that contributes to additional cardiovascular risk.63 Several studies have shown that fat in the neck is positively correlated with visceral fat, insulin resistance, and metabolic syndrome.63-65 Increased fat deposition around the neck is associated with sleep apnea syndrome,66 which increases cardiovascular risk through hemodynamic and hematologic changes.67 Altered endothelial permeability and diminished peripheral blood flow that accompanies a larger neck circumference68 may promote insulin resistance in metabolically active tissues. In addition, repeated hypoxia and reoxygenation during sleep by airway obstruction provoked by a large fat accumulation around neck may increase production of reactive oxygen species, which also play an important role in the development of CVD.69 However, whether and how fat in the neck is associated with cardiometabolic risk factors independently of VAT should be further elucidated in mechanistic studies.
CT and MRI techniques can measure fat accumulation in the neck with the advantage of distinguishing deep fat from subcutaneous fat.70,71
Fat depots around the heart and coronary arteries or in the myocardium
Fat around the heart has been reported to be associated with coronary calcification, atheromatous plaques, and CAD with or without VAT.72-74 Fat located around the heart is divided into two types: 1) pericardial fat, which is located between the external surface of the parietal pericardium and the internal border of the mediastinum and 2) epicardial fat, which is located within the pericardial sac, mainly in the atrioventricular and interventricular grooves and around the atria and ventricles.75
In the Framingham Heart Study, the amount of pericardial fat was positively associated with coronary artery calcification independent of conventional risk factors.76 Furthermore, individuals with more pericardial fat had reduced left ventricular function or increased risk of atrial fibrillation.74,77 These findings suggest the potential locally toxic effect of pericardial fat on cardiac function and conduction. Thus, pericardial fat has a pathogenic effect on either the structural or functional aspects of cardiovascular risk.
Fat depots around the heart and coronary arteries can be classified into pericardial fat, epicardial fat, and pericoronary fat.78 Pericardial fat has direct effects on the development of coronary atherosclerosis in a paracrine manner.76
In addition, epicardial fat releases many cytokines such as leptin, resistin, TNFα, IL-6, visfatin, and chemerin.79 Leptin was found to exacerbate endothelial dysfunction via a protein kinase C-β-dependent pathway.80 It has been recently reported that pericoronary adipocytes have the potential to modulate the inflammatory process between endothelial and inflammatory cells.81,82 An animal study has shown that perivascular fat may act differently according to anatomic location.83 These data suggest that factors released from coronary adipose tissue and the location of fat around vessels may affect vascular functions differentially in insulin resistant milieu.
Fat depots around the heart and coronary arteries seem to stimulate the progression of atherosclerosis via ‘outside-to-inside signaling’.84 Fat around the heart is known to be associated with oxidative stress, activation of the coagulation cascade, disturbances in the RAS, and enhanced lipid oxidation, which generates oxidized LDL.85
Excessive fat can also accumulate in the myocardium. The ectopic fat depot inside the heart may induce cardiomyopathy, and subsequently heart failure. Histological evaluation of patients with heart failure and severe metabolic dysregulation revealed an overload of intramyocardial triglycerides.86 Accumulation of large amounts of lipid in the myocardium may induce apoptosis of cardiomyocytes and cardiac dysfunction. More specifically, increased levels of fatty acid transfer proteins in the myocardium may lead to cardiomyopathy.87
Epicardial fat thickness can be measured on the free wall of the right ventricle using echocardiography.88 A recently developed multidetector-row CT technique offers volumetric assessment of fat depots around the heart with high reproducibility.74 MRI using a three-dimensional approach can also measure the amount of fat around the heart.89 Lipid content in cardiac muscle can be quantified using 1H-MRS.90
Fat around kidney
Fat accumulation in and around the kidney seems to play an important role in maintaining blood pressure and kidney function.91,92 In a study with middle-aged subjects at risk for cardiovascular events, renal sinus fat was associated with high blood pressure after accounting for conventional risk factors for hypertension and abdominal fat depots.91 A recent study with individuals at diabetic risk reported that fat depots in the renal sinus area was independently associated with microalbuminuria, which is a robust risk factor for future CVDs.92 Estimated glomerular filtration rate and uricemia were associated with para- and perirenal fat, even after adjusting for BMI in patients with T2DM.93 In the Framingham Heart Study, renal sinus fat area was associated with hypertension after accounting for VAT.94 These data suggest that fat accumulation in the renal sinus may contribute to development of CVD via vascular tone and albuminuria.
We can deduce the underlying mechanisms connecting renal sinus fat and CVD from animal studies showing that the elevation of compressive forces by fat depots in the renal sinus constricted low pressure conduits such as renal veins and ureters.95,96 Fat accumulation in the kidneys was associated with glomerulosclerosis and proteinuria with increased expression of sterol regulatory element binding protein.97 Thus, renal sinus fat or fatty kidney may induce structural and functional damage in the kidney and subsequently increase systemic vascular resistance and cardiovascular risk.
By using ultrasonography, fat thickness in the pararenal and perirenal area can be measured from the inner side of the abdominal musculature to the surface of the kidney.93 Fat accumulation around kidney can also be measured from a single slice of the kidney by using multidetector-row CT.98
Fatty pancreas or fat accumulation in and around the pancreas
Fat can accumulate in and around the pancreas. Several studies showed that intrapancreatic fat amount was associated with pancreatic β-cell dysfunction.99-101 Old age, high BMI, low physical activity, and dyslipidemia may induce fat accumulation in the pancreas,99,102 with ensuing pancreatic dysfunction.
Although there have been limited data linking fat depots in the pancreas with cardiovascular risk directly, pancreatic fat accumulation may contribute to cardiovascular risk by inducing insulin resistance and aggravating glucose metabolism.
The measurement of pancreatic fat accumulation in humans is challenging because the pancreas is located in the retroperitoneum. Variable shape and vague boundaries make the pancreas difficult to measure. Compared to fatty liver, the assessment of pancreatic fat by imaging techniques has not received much attention. Previously, a few empirical studies measured fat accumulation in the pancreas using CT and MRI techniques.99,103 Concerns with these studies include using a machine with low resolution, small number of study subjects, and the lack of detail in measurement methodologies. A recent study reported that CT attenuation indexes can be used to quantitate pancreatic fat, which was validated by histologic assessment of pancreatic fat fraction.104 Our group also showed that pancreas fat was quantitatively measured by multidetector-low CT with HU range, which was associated with impaired glucose metabolism.105 In another recent study with 685 healthy volunteers, the subjects who had both fatty pancreas measured by fat-water MRI and fatty liver by MRS had a higher insulin resistance index than those who had either condition alone.106
Summary and conclusions
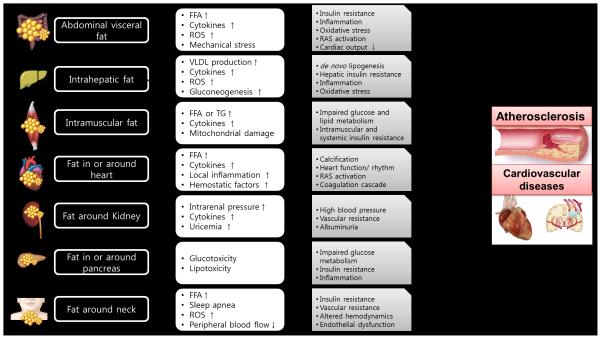
This article summarized various types of ectopic fat and their potential mechanisms for their associated cardiovascular risk (Figure 2). While the mechanisms of ectopic fat depots have not been well studied, a growing body of evidence supports an independent contribution of ectopic fat deposition to cardiovascular risk beyond those implicated in general obesity. Based on many studies, ectopic fat seems to act as an active endocrine and paracrine organ, secreting a number of cytokines that influence insulin resistance and diverse processes of atherosclerosis.107
Figure 2.
Mechanisms of various ectopic fats related with atherosclerosis and cardiovascular diseases (FFA, free fatty acid, ROS, reactive oxygen species, VLDL, very low density lipoprotein, TG, triglyceride, RAS, renin-angiotensin system)
The impact of ectopic fat on CVD progression may be limited in the initial stages but its long-term effects can be substantial because it has both local effects on key target-organs and systemic effects on the cardiovascular system. Longitudinal studies investigating independent roles of ectopic fat depot on future cardiovascular events are warranted. Interventional studies to prove the additional benefits of targeting fat depots in specific organs or areas in individuals with cardiovascular risk may also be required for definitive proof.
Acknowledgments
None.
Sources of Funding JBM is supported by K24 DK080140.
NONSTANDARD ABBREVIATIONS AND ACRONYMS
- CVD
cardiovascular disease
- VAT
visceral adipose tissue
- CAD
coronary artery disease
- BMI
body mass index
- FFA
free fatty acid
- ROS
reactive oxygen species
- IL-6
interleukin-6
- TNF-α
tumor necrosis factor-α
- RAS
renin-angiotensin system
- CT
computed tomography
- MRI
magnetic resonance image
- DXA
dual-energy X-ray absorptiometry
- SAT
subcutaneous adipose tissue
- T2DM
type 2 diabetes mellitus
- VLDL
very low density lipoprotein
- MRS
magnetic resonance spectroscopy
Footnotes
Disclosures Nothing to declare.
Contributor Information
Soo Lim, Department of Internal Medicine, Seoul National University College of Medicine, Seoul National University Bundang Hospital, Seoul, Korea.
James B. Meigs, General Medicine Division, Department of Medicine, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
References
- 1.Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 2.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 3.Lim S, Despres JP, Koh KK. Prevention of atherosclerosis in overweight/obese patients. - In need of novel multi-targeted approaches- Circ J. 2011;75:1019–1027. doi: 10.1253/circj.cj-10-1240. [DOI] [PubMed] [Google Scholar]
- 4.Despres JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, Rodes-Cabau J, Bertrand OF, Poirier P. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039–1049. doi: 10.1161/ATVBAHA.107.159228. [DOI] [PubMed] [Google Scholar]
- 5.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D’Agostino RB, Sr., O’Donnell CJ. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 6.Yamashita S, Nakamura T, Shimomura I, Nishida M, Yoshida S, Kotani K, Kameda-Takemuara K, Tokunaga K, Matsuzawa Y. Insulin resistance and body fat distribution. Diabetes Care. 1996;19:287–291. doi: 10.2337/diacare.19.3.287. [DOI] [PubMed] [Google Scholar]
- 7.Kuk JL, Church TS, Blair SN, Ross R. Does measurement site for visceral and abdominal subcutaneous adipose tissue alter associations with the metabolic syndrome? Diabetes Care. 2006;29:679–684. doi: 10.2337/diacare.29.03.06.dc05-1500. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez-Ono A, Monter-Carreola G, Zamora-Gonzalez J, Cardoso-Saldana G, Posadas-Sanchez R, Torres-Tamayo M, Posadas-Romero C. Association of visceral fat with coronary risk factors in a population-based sample of postmenopausal women. Int J Obes Relat Metab Disord. 2002;26:33–39. doi: 10.1038/sj.ijo.0801842. [DOI] [PubMed] [Google Scholar]
- 9.Hegazi RA, Sutton-Tyrrell K, Evans RW, Kuller LH, Belle S, Yamamoto M, Edmundowicz D, Kelley DE. Relationship of adiposity to subclinical atherosclerosis in obese patients with type 2 diabetes. Obes Res. 2003;11:1597–1605. doi: 10.1038/oby.2003.212. [DOI] [PubMed] [Google Scholar]
- 10.Marques MD, Santos RD, Parga JR, Rocha-Filho JA, Quaglia LA, Miname MH, Avila LF. Relation between visceral fat and coronary artery disease evaluated by multidetector computed tomography. Atherosclerosis. 2010;209:481–486. doi: 10.1016/j.atherosclerosis.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 11.Fujimoto WY, Bergstrom RW, Boyko EJ, Chen KW, Leonetti DL, Newell-Morris L, Shofer JB, Wahl PW. Visceral adiposity and incident coronary heart disease in Japanese-American men. The 10-year follow-up results of the Seattle Japanese-American Community Diabetes Study. Diabetes Care. 1999;22:1808–1812. doi: 10.2337/diacare.22.11.1808. [DOI] [PubMed] [Google Scholar]
- 12.Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol. 2013;62:921–925. doi: 10.1016/j.jacc.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imai A, Komatsu S, Ohara T, Kamata T, Yoshida J, Miyaji K, Takewa M, Kodama K. Visceral abdominal fat accumulation predicts the progression of noncalcified coronary plaque. Atherosclerosis. 2012;222:524–529. doi: 10.1016/j.atherosclerosis.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 14.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 15.Cornier MA, Despres JP, Davis N, Grossniklaus DA, Klein S, Lamarche B, Lopez-Jimenez F, Rao G, St-Onge MP, Towfighi A, Poirier P. Assessing adiposity: a scientific statement from the american heart association. Circulation. 2011;124:1996–2019. doi: 10.1161/CIR.0b013e318233bc6a. [DOI] [PubMed] [Google Scholar]
- 16.Kabir M, Catalano KJ, Ananthnarayan S, Kim SP, Van Citters GW, Dea MK, Bergman RN. Molecular evidence supporting the portal theory: a causative link between visceral adiposity and hepatic insulin resistance. Am J Physiol Endocrinol Metab. 2005;288:E454–E461. doi: 10.1152/ajpendo.00203.2004. [DOI] [PubMed] [Google Scholar]
- 17.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Couillard C, Mauriege P, Prud’homme D, Nadeau A, Tremblay A, Bouchard C, Despres JP. Plasma leptin concentrations: gender differences and associations with metabolic risk factors for cardiovascular disease. Diabetologia. 1997;40:1178–1184. doi: 10.1007/s001250050804. [DOI] [PubMed] [Google Scholar]
- 19.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 20.Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, Keaney JF, Jr., Meigs JB, Lipinska I, Kathiresan S, Murabito JM, O’Donnell CJ, Benjamin EJ, Fox CS. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116:1234–1241. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi H, Matsuda M, Fukuhara A, Komuro R, Shimomura I. Dysregulated glutathione metabolism links to impaired insulin action in adipocytes. Am J Physiol Endocrinol Metab. 2009;296:E1326–E1334. doi: 10.1152/ajpendo.90921.2008. [DOI] [PubMed] [Google Scholar]
- 22.Brown LA, Kerr CJ, Whiting P, Finer N, McEneny J, Ashton T. Oxidant stress in healthy normal-weight, overweight, and obese individuals. Obesity (Silver Spring) 2009;17:460–466. doi: 10.1038/oby.2008.590. [DOI] [PubMed] [Google Scholar]
- 23.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uthus EO, Picklo MJ., Sr Obesity reduces methionine sulphoxide reductase activity in visceral adipose tissue. Free Radic Res. 2011;45:1052–1060. doi: 10.3109/10715762.2011.591793. [DOI] [PubMed] [Google Scholar]
- 25.Picklo MJ, Sr., Idso JP, Jackson MI. S-Glutathionylation of hepatic and visceral adipose proteins decreases in obese rats. Obesity (Silver Spring) 2013;21:297–305. doi: 10.1002/oby.20002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 27.Aneja A, El-Atat F, McFarlane SI, Sowers JR. Hypertension and obesity. Recent Prog Horm Res. 2004;59:169–205. doi: 10.1210/rp.59.1.169. [DOI] [PubMed] [Google Scholar]
- 28.Engeli S. Role of the renin-angiotensin- aldosterone system in the metabolic syndrome. Contrib Nephrol. 2006;151:122–134. doi: 10.1159/000095324. [DOI] [PubMed] [Google Scholar]
- 29.Engeli S, Schling P, Gorzelniak K, Boschmann M, Janke J, Ailhaud G, Teboul M, Massiera F, Sharma AM. The adipose-tissue renin-angiotensin-aldosterone system: role in the metabolic syndrome? Int J Biochem Cell Biol. 2003;35:807–825. doi: 10.1016/s1357-2725(02)00311-4. [DOI] [PubMed] [Google Scholar]
- 30.Kim H, Kim HJ, Shin N, Han M, Park H, Kim M, Kwon H, Choi SY, Heo NJ. Visceral obesity is associated with microalbuminuria in nondiabetic Asians. Hypertens Res. 2014 doi: 10.1038/hr.2014.47. [DOI] [PubMed] [Google Scholar]
- 31.Skurk T, Hauner H. Obesity and impaired fibrinolysis: role of adipose production of plasminogen activator inhibitor-1. Int J Obes Relat Metab Disord. 2004;28:1357–1364. doi: 10.1038/sj.ijo.0802778. [DOI] [PubMed] [Google Scholar]
- 32.Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93:359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 33.Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 34.Lee Y, Shin H, Vassy JL, Kim JT, Cho SI, Kang SM, Choi SH, Kim KW, Park KS, Jang HC, Lim S. Comparison of regional body composition and its relation with cardiometabolic risk between BMI-matched young and old subjects. Atherosclerosis. 2012;224:258–265. doi: 10.1016/j.atherosclerosis.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 35.Hu HH, Nayak KS, Goran MI. Assessment of abdominal adipose tissue and organ fat content by magnetic resonance imaging. Obes Rev. 2011;12:e504–e515. doi: 10.1111/j.1467-789X.2010.00824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rothney MP, Catapano AL, Xia J, Wacker WK, Tidone C, Grigore L, Xia Y, Ergun DL. Abdominal visceral fat measurement using dual-energy X-ray: association with cardiometabolic risk factors. Obesity (Silver Spring) 2013;21:1798–1802. doi: 10.1002/oby.20223. [DOI] [PubMed] [Google Scholar]
- 37.Faber DR, van der GY, Westerink J, Visseren FL. Increased visceral adipose tissue mass is associated with increased C-reactive protein in patients with manifest vascular diseases. Atherosclerosis. 2010;212:274–280. doi: 10.1016/j.atherosclerosis.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 38.Speliotes EK, Massaro JM, Hoffmann U, Vasan RS, Meigs JB, Sahani DV, Hirschhorn JN, O’Donnell CJ, Fox CS. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: the Framingham Heart Study. Hepatology. 2010;51:1979–1987. doi: 10.1002/hep.23593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, Okunade A, Klein S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A. 2009;106:15430–15435. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamaguchi M, Kojima T, Takeda N, Nagata C, Takeda J, Sarui H, Kawahito Y, Yoshida N, Suetsugu A, Kato T, Okuda J, Ida K, Yoshikawa T. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol. 2007;13:1579–1584. doi: 10.3748/wjg.v13.i10.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Targher G, Bertolini L, Poli F, Rodella S, Scala L, Tessari R, Zenari L, Falezza G. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes. 2005;54:3541–3546. doi: 10.2337/diabetes.54.12.3541. [DOI] [PubMed] [Google Scholar]
- 42.Targher G, Bertolini L, Rodella S, Tessari R, Zenari L, Lippi G, Arcaro G. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2007;30:2119–2121. doi: 10.2337/dc07-0349. [DOI] [PubMed] [Google Scholar]
- 43.Wang H, Quiroga AD, Lehner R. Analysis of lipid droplets in hepatocytes. Methods Cell Biol. 2013;116:107–127. doi: 10.1016/B978-0-12-408051-5.00007-3. [DOI] [PubMed] [Google Scholar]
- 44.Meshkani R, Adeli K. Hepatic insulin resistance, metabolic syndrome and cardiovascular disease. Clin Biochem. 2009;42:1331–1346. doi: 10.1016/j.clinbiochem.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 45.Lo L, McLennan SV, Williams PF, Bonner J, Chowdhury S, McCaughan GW, Gorrell MD, Yue DK, Twigg SM. Diabetes is a progression factor for hepatic fibrosis in a high fat fed mouse obesity model of non-alcoholic steatohepatitis. J Hepatol. 2011;55:435–444. doi: 10.1016/j.jhep.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 46.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341–1350. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 47.Rijzewijk LJ, Jonker JT, van der Meer RW, et al. Effects of hepatic triglyceride content on myocardial metabolism in type 2 diabetes. J Am Coll Cardiol. 2010;56:225–233. doi: 10.1016/j.jacc.2010.02.049. [DOI] [PubMed] [Google Scholar]
- 48.Bonapace S, Perseghin G, Molon G, Canali G, Bertolini L, Zoppini G, Barbieri E, Targher G. Nonalcoholic fatty liver disease is associated with left ventricular diastolic dysfunction in patients with type 2 diabetes. Diabetes Care. 2012;35:389–395. doi: 10.2337/dc11-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qayyum A, Chen DM, Breiman RS, Westphalen AC, Yeh BM, Jones KD, Lu Y, Coakley FV, Callen PW. Evaluation of diffuse liver steatosis by ultrasound, computed tomography, and magnetic resonance imaging: which modality is best? Clin Imaging. 2009;33:110–115. doi: 10.1016/j.clinimag.2008.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miljkovic-Gacic I, Wang X, Kammerer CM, Gordon CL, Bunker CH, Kuller LH, Patrick AL, Wheeler VW, Evans RW, Zmuda JM. Fat infiltration in muscle: new evidence for familial clustering and associations with diabetes. Obesity (Silver Spring) 2008;16:1854–1860. doi: 10.1038/oby.2008.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jacob S, Machann J, Rett K, Brechtel K, Volk A, Renn W, Maerker E, Matthaei S, Schick F, Claussen CD, Haring HU. Association of increased intramyocellular lipid content with insulin resistance in lean nondiabetic offspring of type 2 diabetic subjects. Diabetes. 1999;48:1113–1119. doi: 10.2337/diabetes.48.5.1113. [DOI] [PubMed] [Google Scholar]
- 53.Kautzky-Willer A, Krssak M, Winzer C, Pacini G, Tura A, Farhan S, Wagner O, Brabant G, Horn R, Stingl H, Schneider B, Waldhausl W, Roden M. Increased intramyocellular lipid concentration identifies impaired glucose metabolism in women with previous gestational diabetes. Diabetes. 2003;52:244–251. doi: 10.2337/diabetes.52.2.244. [DOI] [PubMed] [Google Scholar]
- 54.Weiss R, Dufour S, Taksali SE, et al. Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet. 2003;362:951–957. doi: 10.1016/S0140-6736(03)14364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim SK, Park SW, Hwang IJ, Lee YK, Cho YW. High fat stores in ectopic compartments in men with newly diagnosed type 2 diabetes: an anthropometric determinant of carotid atherosclerosis and insulin resistance. Int J Obes (Lond) 2010;34:105–110. doi: 10.1038/ijo.2009.210. [DOI] [PubMed] [Google Scholar]
- 56.Phillips DI, Caddy S, Ilic V, Fielding BA, Frayn KN, Borthwick AC, Taylor R. Intramuscular triglyceride and muscle insulin sensitivity: evidence for a relationship in nondiabetic subjects. Metabolism. 1996;45:947–950. doi: 10.1016/s0026-0495(96)90260-7. [DOI] [PubMed] [Google Scholar]
- 57.Kelley DE, Goodpaster BH. Skeletal muscle triglyceride. An aspect of regional adiposity and insulin resistance. Diabetes Care. 2001;24:933–941. doi: 10.2337/diacare.24.5.933. [DOI] [PubMed] [Google Scholar]
- 58.Abdul-Ghani MA, DeFronzo RA. Mitochondrial dysfunction, insulin resistance, and type 2 diabetes mellitus. Curr Diab Rep. 2008;8:173–178. doi: 10.1007/s11892-008-0030-1. [DOI] [PubMed] [Google Scholar]
- 59.Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muoio DM, Neufer PD. Lipid-induced mitochondrial stress and insulin action in muscle. Cell Metab. 2012;15:595–605. doi: 10.1016/j.cmet.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71:885–892. doi: 10.1093/ajcn/71.4.885. [DOI] [PubMed] [Google Scholar]
- 62.Hansen RD, Williamson DA, Finnegan TP, et al. Estimation of thigh muscle cross-sectional area by dual-energy X-ray absorptiometry in frail elderly patients. Am J Clin Nutr. 2007;86:952–958. doi: 10.1093/ajcn/86.4.952. [DOI] [PubMed] [Google Scholar]
- 63.Ben-Noun L, Laor A. Relationship of neck circumference to cardiovascular risk factors. Obes Res. 2003;11:226–231. doi: 10.1038/oby.2003.35. [DOI] [PubMed] [Google Scholar]
- 64.Laakso M, Matilainen V, Keinanen-Kiukaanniemi S. Association of neck circumference with insulin resistance-related factors. Int J Obes Relat Metab Disord. 2002;26:873–875. doi: 10.1038/sj.ijo.0802002. [DOI] [PubMed] [Google Scholar]
- 65.Preis SR, Massaro JM, Hoffmann U, D’Agostino RB, Sr., Levy D, Robins SJ, Meigs JB, Vasan RS, O’Donnell CJ, Fox CS. Neck circumference as a novel measure of cardiometabolic risk: the Framingham Heart study. J Clin Endocrinol Metab. 2010;95:3701–3710. doi: 10.1210/jc.2009-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Strohl KP, Redline S. Recognition of obstructive sleep apnea. Am J Respir Crit Care Med. 1996;154:279–289. doi: 10.1164/ajrccm.154.2.8756795. [DOI] [PubMed] [Google Scholar]
- 67.Mohsenin V. Sleep-related breathing disorders and risk of stroke. Stroke. 2001;32:1271–1278. doi: 10.1161/01.str.32.6.1271. [DOI] [PubMed] [Google Scholar]
- 68.Chung S, Yoon IY, Shin YK, Lee CH, Kim JW, Lee T, Choi DJ, Ahn HJ. Endothelial dysfunction and C-reactive protein in relation with the severity of obstructive sleep apnea syndrome. Sleep. 2007;30:997–1001. doi: 10.1093/sleep/30.8.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sugamura K, Keaney JF., Jr. Reactive oxygen species in cardiovascular disease. Free Radic Biol Med. 2011;51:978–992. doi: 10.1016/j.freeradbiomed.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mortimore IL, Marshall I, Wraith PK, Sellar RJ, Douglas NJ. Neck and total body fat deposition in nonobese and obese patients with sleep apnea compared with that in control subjects. Am J Respir Crit Care Med. 1998;157:280–283. doi: 10.1164/ajrccm.157.1.9703018. [DOI] [PubMed] [Google Scholar]
- 71.Shigeta Y, Enciso R, Ogawa T, Ikawa T, Clark GT. Cervical CT derived neck fat tissue distribution differences in Japanese males and females and its effect on retroglossal and retropalatal airway volume. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:275–284. doi: 10.1016/j.tripleo.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fox CS, Gona P, Hoffmann U, Porter SA, Salton CJ, Massaro JM, Levy D, Larson MG, D’Agostino RB, Sr., O’Donnell CJ, Manning WJ. Pericardial fat, intrathoracic fat, and measures of left ventricular structure and function: the Framingham Heart Study. Circulation. 2009;119:1586–1591. doi: 10.1161/CIRCULATIONAHA.108.828970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Iacobellis G, Lonn E, Lamy A, Singh N, Sharma AM. Epicardial fat thickness and coronary artery disease correlate independently of obesity. Int J Cardiol. 2011;146:452–454. doi: 10.1016/j.ijcard.2010.10.117. [DOI] [PubMed] [Google Scholar]
- 74.Kim TH, Yu SH, Choi SH, Yoon JW, Kang SM, Chun EJ, Choi SI, Shin H, Lee HK, Park KS, Jang HC, Lim S. Pericardial fat amount is an independent risk factor of coronary artery stenosis assessed by multidetector-row computed tomography: the Korean Atherosclerosis Study 2. Obesity (Silver Spring) 2011;19:1028–1034. doi: 10.1038/oby.2010.246. [DOI] [PubMed] [Google Scholar]
- 75.Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J. 2007;153:907–917. doi: 10.1016/j.ahj.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 76.Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, O’Donnell CJ, Fox CS. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 77.Thanassoulis G, Massaro JM, O’Donnell CJ, Hoffmann U, Levy D, Ellinor PT, Wang TJ, Schnabel RB, Vasan RS, Fox CS, Benjamin EJ. Pericardial fat is associated with prevalent atrial fibrillation: the Framingham Heart Study. Circ Arrhythm Electrophysiol. 2010;3:345–350. doi: 10.1161/CIRCEP.109.912055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lim S, Meigs JB. Ectopic fat and cardiometabolic and vascular risk. Int J Cardiol. 2013;169:166–176. doi: 10.1016/j.ijcard.2013.08.077. [DOI] [PubMed] [Google Scholar]
- 79.Payne GA, Kohr MC, Tune JD. Epicardial perivascular adipose tissue as a therapeutic target in obesity-related coronary artery disease. Br J Pharmacol. 2012;165:659–669. doi: 10.1111/j.1476-5381.2011.01370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Payne GA, Borbouse L, Kumar S, Neeb Z, Alloosh M, Sturek M, Tune JD. Epicardial perivascular adipose-derived leptin exacerbates coronary endothelial dysfunction in metabolic syndrome via a protein kinase C-beta pathway. Arterioscler Thromb Vasc Biol. 2010;30:1711–1717. doi: 10.1161/ATVBAHA.110.210070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chatterjee TK, Idelman G, Blanco V, et al. Histone deacetylase 9 is a negative regulator of adipogenic differentiation. J Biol Chem. 2011;286:27836–27847. doi: 10.1074/jbc.M111.262964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chatterjee TK, Aronow BJ, Tong WS, Manka D, Tang Y, Bogdanov VY, Unruh D, Blomkalns AL, Piegore MG, Jr., Weintraub DS, Rudich SM, Kuhel DG, Hui DY, Weintraub NL. Human coronary artery perivascular adipocytes overexpress genes responsible for regulating vascular morphology, inflammation, and hemostasis. Physiol Genomics. 2013;45:697–709. doi: 10.1152/physiolgenomics.00042.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Owen MK, Witzmann FA, McKenney ML, Lai X, Berwick ZC, Moberly SP, Alloosh M, Sturek M, Tune JD. Perivascular adipose tissue potentiates contraction of coronary vascular smooth muscle: influence of obesity. Circulation. 2013;128:9–18. doi: 10.1161/CIRCULATIONAHA.112.001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yudkin JS, Eringa E, Stehouwer CD. “Vasocrine“ signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet. 2005;365:1817–1820. doi: 10.1016/S0140-6736(05)66585-3. [DOI] [PubMed] [Google Scholar]
- 85.McGavock JM, Victor RG, Unger RH, Szczepaniak LS. Adiposity of the heart, revisited. Ann Intern Med. 2006;144:517–524. doi: 10.7326/0003-4819-144-7-200604040-00011. [DOI] [PubMed] [Google Scholar]
- 86.Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, Noon GP, Frazier OH, Taegtmeyer H. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004;18:1692–1700. doi: 10.1096/fj.04-2263com. [DOI] [PubMed] [Google Scholar]
- 87.Nielsen LB, Perko M, Arendrup H, Andersen CB. Microsomal triglyceride transfer protein gene expression and triglyceride accumulation in hypoxic human hearts. Arterioscler Thromb Vasc Biol. 2002;22:1489–1494. doi: 10.1161/01.atv.0000030199.06252.26. [DOI] [PubMed] [Google Scholar]
- 88.Iacobellis G, Assael F, Ribaudo MC, Zappaterreno A, Alessi G, Di Mario U, Leonetti F. Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction. Obes Res. 2003;11:304–310. doi: 10.1038/oby.2003.45. [DOI] [PubMed] [Google Scholar]
- 89.Fluchter S, Haghi D, Dinter D, Heberlein W, Kuhl HP, Neff W, Sueselbeck T, Borggrefe M, Papavassiliu T. Volumetric assessment of epicardial adipose tissue with cardiovascular magnetic resonance imaging. Obesity (Silver Spring) 2007;15:870–878. doi: 10.1038/oby.2007.591. [DOI] [PubMed] [Google Scholar]
- 90.McGavock JM, Lingvay I, Zib I, Tillery T, Salas N, Unger R, Levine BD, Raskin P, Victor RG, Szczepaniak LS. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation. 2007;116:1170–1175. doi: 10.1161/CIRCULATIONAHA.106.645614. [DOI] [PubMed] [Google Scholar]
- 91.Chughtai HL, Morgan TM, Rocco M, Stacey B, Brinkley TE, Ding J, Nicklas B, Hamilton C, Hundley WG. Renal sinus fat and poor blood pressure control in middle-aged and elderly individuals at risk for cardiovascular events. Hypertension. 2010;56:901–906. doi: 10.1161/HYPERTENSIONAHA.110.157370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wagner R, Machann J, Lehmann R, Rittig K, Schick F, Lenhart J, Artunc F, Linder K, Claussen CD, Schleicher E, Fritsche A, Haring HU, Weyrich P. Exercise-induced albuminuria is associated with perivascular renal sinus fat in individuals at increased risk of type 2 diabetes. Diabetologia. 2012;55:2054–2058. doi: 10.1007/s00125-012-2551-z. [DOI] [PubMed] [Google Scholar]
- 93.Lamacchia O, Nicastro V, Camarchio D, Valente U, Grisorio R, Gesualdo L, Cignarelli M. Para- and perirenal fat thickness is an independent predictor of chronic kidney disease, increased renal resistance index and hyperuricaemia in type-2 diabetic patients. Nephrol Dial Transplant. 2011;26:892–898. doi: 10.1093/ndt/gfq522. [DOI] [PubMed] [Google Scholar]
- 94.Foster MC, Hwang SJ, Porter SA, Massaro JM, Hoffmann U, Fox CS. Fatty kidney, hypertension, and chronic kidney disease: the framingham heart study. Hypertension. 2011;58:784–790. doi: 10.1161/HYPERTENSIONAHA.111.175315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dwyer TM, Mizelle HL, Cockrell K, Buhner P. Renal sinus lipomatosis and body composition in hypertensive, obese rabbits. Int J Obes Relat Metab Disord. 1995;19:869–874. [PubMed] [Google Scholar]
- 96.Dwyer TM, Bigler SA, Moore NA, Carroll JF, Hall JE. The altered structure of renal papillary outflow tracts in obesity. Ultrastruct Pathol. 2000;24:251–257. doi: 10.1080/01913120050176707. [DOI] [PubMed] [Google Scholar]
- 97.Guebre-Egziabher F, Alix PM, Koppe L, Pelletier CC, Kalbacher E, Fouque D, Soulage CO. Ectopic lipid accumulation: A potential cause for metabolic disturbances and a contributor to the alteration of kidney function. Biochimie. 2013;95:1971–1979. doi: 10.1016/j.biochi.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 98.Foster MC, Hwang SJ, Porter SA, Massaro JM, Hoffmann U, Fox CS. Development and reproducibility of a computed tomography-based measurement of renal sinus fat. BMC Nephrol. 2011;12:52. doi: 10.1186/1471-2369-12-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Heni M, Machann J, Staiger H, Schwenzer NF, Peter A, Schick F, Claussen CD, Stefan N, Haring HU, Fritsche A. Pancreatic fat is negatively associated with insulin secretion in individuals with impaired fasting glucose and/or impaired glucose tolerance: a nuclear magnetic resonance study. Diabetes Metab Res Rev. 2010;26:200–205. doi: 10.1002/dmrr.1073. [DOI] [PubMed] [Google Scholar]
- 100.Tushuizen ME, Bunck MC, Pouwels PJ, Bontemps S, van Waesberghe JH, Schindhelm RK, Mari A, Heine RJ, Diamant M. Pancreatic fat content and beta-cell function in men with and without type 2 diabetes. Diabetes Care. 2007;30:2916–2921. doi: 10.2337/dc07-0326. [DOI] [PubMed] [Google Scholar]
- 101.Pinnick KE, Collins SC, Londos C, Gauguier D, Clark A, Fielding BA. Pancreatic ectopic fat is characterized by adipocyte infiltration and altered lipid composition. Obesity (Silver Spring) 2008;16:522–530. doi: 10.1038/oby.2007.110. [DOI] [PubMed] [Google Scholar]
- 102.van der Zijl NJ, Goossens GH, Moors CC, van Raalte DH, Muskiet MH, Pouwels PJ, Blaak EE, Diamant M. Ectopic fat storage in the pancreas, liver, and abdominal fat depots: impact on beta-cell function in individuals with impaired glucose metabolism. J Clin Endocrinol Metab. 2011;96:459–467. doi: 10.1210/jc.2010-1722. [DOI] [PubMed] [Google Scholar]
- 103.Saisho Y, Butler AE, Meier JJ, Monchamp T, len-Auerbach M, Rizza RA, Butler PC. Pancreas volumes in humans from birth to age one hundred taking into account sex, obesity, and presence of type-2 diabetes. Clin Anat. 2007;20:933–942. doi: 10.1002/ca.20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim SY, Kim H, Cho JY, Lim S, Cha K, Lee KH, Kim YH, Kim JH, Yoon YS, Han HS, Kang HS. Quantitative Assessment of Pancreatic Fat by Using Unenhanced CT: Pathologic Correlation and Clinical Implications. Radiology. 2014;271:104–112. doi: 10.1148/radiol.13122883. [DOI] [PubMed] [Google Scholar]
- 105.Lim S, Bae JH, Chun EJ, Kim H, Kim SY, Kim KM, Choi SH, Park KS, Florez JC, Jang HC. Differences in pancreatic volume, fat content, and fat density measured by multidetector-row computed tomography according to the duration of diabetes. Acta Diabetol. 2014 doi: 10.1007/s00592-014-0581-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 106.Wong VW, Wong GL, Yeung DK, Abrigo JM, Kong AP, Chan RS, Chim AM, Shen J, Ho CS, Woo J, Chu WC, Chan HL. Fatty Pancreas, Insulin Resistance, and beta-Cell Function: A Population Study Using Fat-Water Magnetic Resonance Imaging. Am J Gastroenterol. 2014;109:589–597. doi: 10.1038/ajg.2014.1. [DOI] [PubMed] [Google Scholar]
- 107.Bluher M. Clinical relevance of adipokines. Diabetes Metab J. 2012;36:317–327. doi: 10.4093/dmj.2012.36.5.317. [DOI] [PMC free article] [PubMed] [Google Scholar]