Abstract
In eukaryotic cells, complex membrane structures called organelles are highly developed to exert specialized functions. Mitochondria are one of such organelles consisting of the outer and inner membranes with characteristic protein and phospholipid compositions. Maintaining proper phospholipid compositions of the membranes is crucial for mitochondrial integrity, thereby contributing to normal cell activities. Since cellular locations for phospholipid synthesis are restricted to specific compartments such as the endoplasmic reticulum (ER) membrane and the mitochondrial inner membrane, newly synthesized phospholipids have to be transported and distributed properly from the ER or mitochondria to other cellular membranes. Although understanding of molecular mechanisms of phospholipid transport are much behind those of protein transport, recent studies using yeast as a model system began to provide intriguing insights into phospholipid exchange between the ER and mitochondria as well as between the mitochondrial outer and inner membranes. In this review, we summarize the latest findings of phospholipid transport via mitochondria and discuss the implicated molecular mechanisms.
Keywords: mitochondria, phospholipid, membrane contact site, flippase, scramblase, cardiolipin, Ups protein, ERMES
Phospholipid synthesis requires phospholipid transport among the ER and mitochondrial membranes
Most glycerophospholipids are synthesized via two synthetic pathways called the Kennedy pathway and the CDP-diacylglycerol (DAG) pathway in yeast (1, 2). In the Kennedy pathway, soluble high-energy intermediate such as CDP-ethanolamine and CDP-choline react with DAG to produce phoshatidylethanolamine (PE) and phoshatidylcholine (PC), respectively (3). In the CDP-DAG pathway, phospholipids are produced from a high-energy intermediate phospholipid CDP-DAG (1, 2). Besides in the CDP-DAG pathway, phospholipid synthesis strongly depends on phospholipid transport between the ER and mitochondria as well as between the mitochondrial outer and inner membranes (OM and IM) (4–6). Here we summarize the phospholipid synthetic reactions in the CDP-DAG pathway in yeast to highlight the importance of the phospholipid transport in normal operation of this pathway.
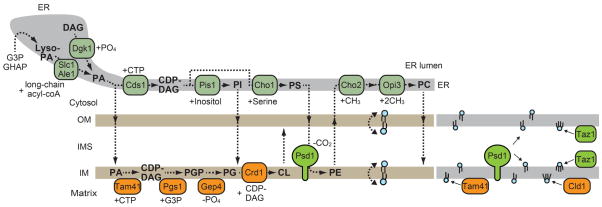
In the CDP-DAG pathway, phophatidic acid (PA) is the starting material for the synthesis of all other phospholipids. PA is mainly produced in two ways: First, glycerol 3-phosphate (G3P) or dihydroacetone phosphate (DHAP) is acylated to form lyso-PA or acyl-DHAP, which is also converted to lyso-PA. Lyso-PA is further acylated by an ER and lipid particle-resident enzyme Slc1 or an ER-resident enzyme Ale1 to produce PA (7–9). Second, DAG is phosphorylated to form PA by the DAG kinase, Dgk1 in the ER (10). The location of Slc1, Ale1 and Dgk1 in the ER indicates that the ER plays a central role in the PA synthesis. PA is then converted to CDP-DAG by the ER-resident PA cytidylyltransferase Cds1 (11, 12). CDP-DAG reacts with serine or inositol, rendering its CMP moiety substituted to serine or inositol to form phoshatidylserine (PS) or phosphatidylinositol (PI), respectively (13, 14). Importantly, the synthesis of PS and PI also occurs in the ER membrane since both PS-synthase Cho1 and PI-synthase Pis1 are localized in the ER (8, 15, 16). Thus, PA, PS and PI need to be supplied from the ER to mitochondria (Figure 1).
Figure 1. Phospholipid biosynthetic pathways in the ER and mitochchondria.
(Left) Phospholipid synthesis requires inter- and intra-organelle phospholipid transport. Broken lines indicate phospholipid movements. (Right) Tam41 and Cld1 exert their catalytic functions for PA and CL present on the matrix side but not the IMS side of the IM. Meanwhile, when located on the IMS side of the OM and IM, PS and MLCL can be used as substrates for Psd1 and Taz1, respectively.
In mitochondria, PA is used as a precursor for the synthesis of the mitochondrial signature phospholipid cardiolipin (CL) while PS is used to produce another important lipid PE. A series of enzymes involved in the CL synthesis are located mainly in the matrix while a PE-synthase (PS-decarboxylase) Psd1 is anchored to the IM by its N-terminal transmembrane (TM) segment, leaving the C-terminal catalytic domain exposed to the intermembrane space (IMS) (17) (Figure 1). Therefore, PA has to be delivered to the inner leaflet of the IM for CL synthesis (Figure 1). Similary, PS needs to be shifted to the inner leaflet of the OM or the outer leaflet of the IM for PE synthesis (Figure 1). Once PA reaches the matrix side of the IM, it is converted to CDP-DAG. We have recently identified a matrix-resident protein, Tam41, as a mitochondrial PA cytidylyltransferase (CDP-DAG synthase), which mediates the formation of CDP-DAG from PA (12). CDP-DAG is combined with glycerol 3-phosphate to form phoshatidylglycerol phosphate (PGP) by PGP-synthase Pgs1 (18). PGP is then dephosphorylated to produce phosphatidylglycerol (PG) by the PGP phosphatase Gep4 (19). Finally, CL is synthesized from CDP-DAG and PG by the CL synthase Crd1 (20, 21) (Figure 1). For further maturation of CL, CL is deacylated by the cardiolipin-sepecific phospholipase A Cld1, which is peripherally associated with the inner leaflet of the IM (22). The resulting monolyso-CL (MLCL) is transported to the IMS-side of the IM and then reacylated by acyltransferase Taz1 to generate mature CL with unsaturated acyl chains (23–25) (Figure 1). Taz1 is reported to be peripherally associated with the IMS-side of both the OM and IM (23). Although CL is mainly found in the IM, a fraction of CL is also present in the OM (26). CL in the OM seems to be important for functions of the TOM40 complex, the mitochondrial protein translocator complex in the OM. Besides, in neuronal cells, CL is reportedly exposed to the cytosolic surface of the OM upon stimulation of mitophagy by adding pro-mitophagy stimuli such as rotenone (27, 28). Thus, CL translocates from the IM to OM and this step may be regulated by mitochondrial stress.
The most abundant phospholipid, phosphatidylcholine (PC), is generated by three successive methylations of PE in addition to the Kennedy pathway. Since Cho2 and Opi3, the enzymes responsible for the methylation of PE, are exclusively localized in the ER (8), PE generated in mitochondria has to be exported from mitochondria to the ER membrane. Moreover, PC must be transported back to mitochondria as mitochondria cannot produce PC (Figure 1).
Phospholipid transport from the ER to mitochondria: The molecular tether between the ER and mitochondria
Since the ER is the central site of the phospholipid synthesis, a large amount of phospholipid molecules should be delivered from the ER to other cellular membranes to maintain their characteristic phospholipid compositions (29, 30). Since organelles of the endomembrane system such as the Golgi apparatus, endosomes, vacuoles (lysosomes) and the plasma membrane are all connected with the ER membrane through the vesicle trafficking (31–33), phospholipids of these organelles can be directly derived from the ER by lipid vesicles. In contrast, mitochondria are thought to receive phospholipids from the ER by the non-vesicular transport mechanisms because vesicle transport has not been observed so far between these organelles. Since phospholipid exchange between distinct membranes across an aqueous layer is a very slow reaction that rarely occurs spontaneously, close proximity of mitochondria to the ER may be advantageous for enhancement of the phospholipid transport between these organelles (34–36). The possible contact between the ER and mitochondria, which is a part of the ER called MAM (mitochondria-associated membrane), has been suggested in yeast and mammals as a membrane sub-domain functioning possibly for phospholipid and/or calcium transport (15, 36–39). In yeast, the molecular identity of the tethering structures between the ER and OM termed the ERMES (ER-Mitochondria Encounter Structure) complex was recently revealed (40). The ERMES complex consists of an ER-resident protein Mmm1, three mitochondrial OM-integral proteins, Mmm2, Mdm10 and Gem1, and a peripherally associated OM protein Mdm12 (40, 41) (Figure 2A). These proteins were originally identified as factors involved in maintenance of the normal mitochondrial morphology (42–47). When one of the ERMES components is missing, mitochondrial morphology is significantly altered to ball-like or aggregate structures (42–47) (Figure 2B). Microscopic analyses have revealed that the ERMES complexes are not evenly distributed on the ER membrane or OM, but forms several discrete foci between the ER and OM in a cell (47, 48) (Figure 2C). Among the ERMES subunits, Mmm1, Mmm2, Mdm10 and Mdm12 appear to be core components of the complex since depletion of any of these components leads to destabilization of the ERMES complex (40). On the other hand, Gem1 may function as a regulator of the number and size of the ERMES complex (49). Tom7, a subunit of the mitochondrial OM translocator complex, the TOM40 complex, was suggested to regulate transfer of Mdm10 between the ERMES complex and the TOB/SAM complex, which is another OM translocator complex for β-barrel proteins, and to associate transiently or partially with the ERMES complex as well (50).
Figure 2. The ERMES complex physically connects the ER to the OM.
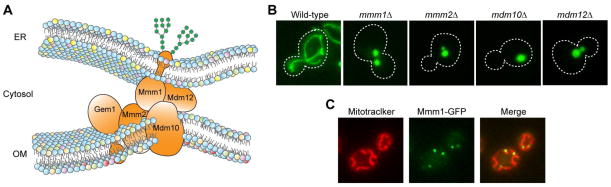
(A) Schematic of the tethering between the ER and OM by the ERMES complex. Mmm1 is N-glycosylated. (B) Mitochondria-targeted GFP is expressed in wild-type or mutant cells lacking ERMES components (mmm1Δ, mmm2Δ, mdm10Δ and mdm12Δ) and observed by fluorescence microscopy. (C) Mitochondria in cells expressing C-terminally GFP-tagged Mmm1 are stained with Mitotracker and observed under a fluorescence microscope.
An attractive idea is that the ERMES mediates phospholipid transport between the ER membrane and the mitochondrial OM. The close apposition of two membranes could enhance the phospholipid exchange by providing a shortcut of hydrophobic lipid transport without passing through the aqueous environments. Kornmann et al. examined the conversion rate of PS to PC, which depends on the phospholipid exchange between the ER and mitochondria, by pulse-chase experiments (Figure 3). They showed that the PS to PC conversion rate is retarded when one of the ERMES core components is absent (40). Consistently, a bioinformatics analyses suggested that Mmm1, Mmm2 and Mdm12 contain an SMP (synaptotagmin-like, mitochondrial and lipid-binding proteins) domain, which belongs to the TULIP (tubular lipid-binding protein) domain super family with a hydrophobic pocket for hydrophobic lipid binding (51, 52). However, this idea is argued by the observations that the PS to PE conversion rates in vivo and in vitro do not differ between in the absence and presence of ERMES components (53, 54). It is thus plausible that there is an ERMES-independent lipid exchange route between the ER and mitochondria.
Figure 3. Pulse-chase experiments with 14C-serine to analyze phospholipid transport between the ER and mitochondria.
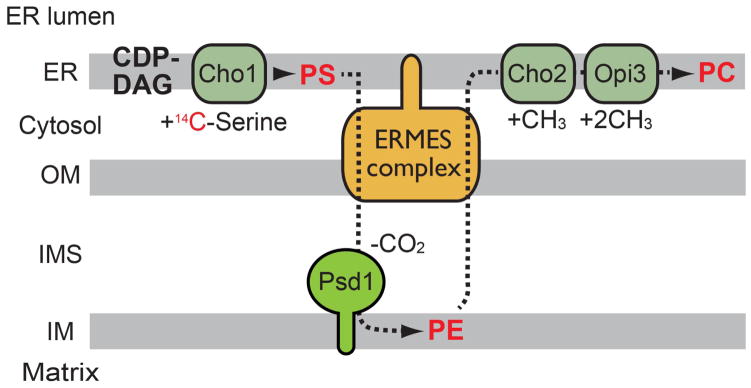
PS is pulse-labeled with 14C-serine and then the fate of PS is chased. PS synthesized in the ER is transported to mitochondria and decarboxylated by Psd1 to form PE. PE is then transported back to the ER and methylated by Cho2 and Opi3 to become PC.
Expressions of an artificial tethering protein between the ER and OM consisting of GFP protein flanked by mitochondrial OM-targeting signal at the N-terminus and ER-membrane targeting signal at the C-terminus named ChiMERA restores defects in PS to PE or PS to PC conversion in the absence of the ERMES (40, 54). This may suggest that physical tethering of the ER and OM is sufficient to promote phospholipid exchange between them because the ChiMERA does not have any functions that actively transport phospholipids. Alternative interpretation is that normal morphologies and proper distributions of mitochondria and the ER in cells are important for phospholipid transport between the ER and mitochondria since expression of the ChiMERA also restored the tubular mitochondria morphology in ERMES-deficient cells (40, 54). Supporting this idea, lack of ER-shaping proteins such as Rtn1, Yop1 and Sey1 together with the ERMES components slows phospholipid transport and causes synthetic growth defects (54). Therefore, the primary function of the ERMES as the phospholipid transport machinery is still under debate although its membrane tethering function is now widely recognized.
Phospholipid transport within mitochondria. A novel PA transfer protein, Ups1
Once PS and PA are supplied from the ER to the OM in mitochondria, they need to be further transported to the IM, where phospholipid synthetic enzymes for PE and CL are located. However, little was known about how phospholipids are transported within mitochondria until Ups1, a conserved IMS protein, was found to mediate lipid transport from the OM to IM (55). We have originally identified Ups1 as a factor important for biogenesis of Mgm1, a mitochondrial fusion protein (56). Mgm1 is synthesized as a precursor protein with an N-terminal cleavable presequence followed by two alternative TM segments, both of which could function as a stop-transfer signal for its integration into the IM via the TIM23 translocator complex (57). Upon crossing the IM, about 50% of Mgm1 is arrested at the first TM segment in the TIM23 channel and laterally released into the IM to generate an N-anchor IM protein, a large form of Mgm1 (l-Mgm1) (57). On the other hand, translocation of the remaining 50% of Mgm1 continues with the aid of the mitochondrial Hsp70-associated motor and chaperone (MMC) proteins of the TIM23 complex until lateral release of the second TM segment into the IM takes place. Subsequently, the rhomboid protease Pcp1/Ugo2 in the IM cleaves the second TM segment in Mgm1 to release a short form of Mgm1 into the IMS (s-Mgm1) as a soluble protein (58–60). The balance between the levels of l-Mgm1 and s-Mgm1 is important for the maintenance of the normal mitochondrial morphology (56–59). For example, lack of Ups1 leads to the loss of s-Mgm1 as well as aberrant mitochondrial morphology (56). However, loss of Ups1 was also found to be associated with a decrease in the CL level in mitochondria (61, 62), which may raise the possibility that the primary function of Ups1 can be related to the lipid composition. For example, the decreased level of CL could lead to dissociations of MMC proteins from the TIM23 channel, which will result in defective import activities of the TIM23 complex to generate a lower level of the s-Mgm1 form (61).
An interesting twist of the above observation of the decreased level of CL by the absence of Ups1 was the finding of the Ups1 function as the transporter of CL precursor PA from the OM to the IM (55). Connerth et al. purified a functional recombinant protein complex comprising Ups1 and its stoichiometric partner protein Mdm35 (63, 64) from E. coli cells and tested its binding and transport activities for phospholipids. They showed that the Ups1-Mdm35 complex is able to bind to liposomes containing negatively charged phospholipids such as PA, CL, PG, CDP-DAG, PS and PI while Mdm35 alone does not bind to liposomes with any lipid compositions (55). Despite the broad specificity in binding to the negatively charged phospholipids, the Ups1-Mdm35 complex was found to specifically promote PA transfer between liposomes in vitro (55). This suggests that the binding to and transport of phospholipids are functionally distinct events. Moreover, they found that PA transport is accelerated when acceptor liposomes contain low amounts (10%) of negatively charged phospholipids such as PA, PG, CDP-DAG or CL as compared with liposomes consisting of only PC and PE. This stimulatory effect is the highest when PA is present in the acceptor liposomes. In contrast, increasing amounts of CL in acceptor liposomes lead to stable interactions of Ups1 with the liposomes, which results in inefficient PA transport. This inhibitory effect by increasing amounts of CL in the target membrane could function as a negative -feedback mechanism that regulates the flux of the CL synthetic pathway to maintain the optimum level of CL. When the CL level is low, Ups1 may be present in the IMS as a soluble protein to mediate PA transfer. When the CL level is high, Ups1 may stay associated with the IM, thereby undergoing less shuttling between the IM and OM and perhaps enhancing degradation of Ups1 itself by IM-localized proteases (Figure 4). Indeed, it was shown that Ups1 is readily degraded by such IM-localized proteases as Yme1 and Atp23 and is thus maintained at a relatively low level under a normal culturing condition (64) (Figure 4). Although the Ups1-Mdm35 complex mediates PA transport bi-directionally in the reconstituted system, the conversion of PA to CL in the IM may be more favorable for uni-directional PA transport with the possibly suppressed backward PA transport.
Figure 4. Functional model of the Ups1-Mdm35 complex.
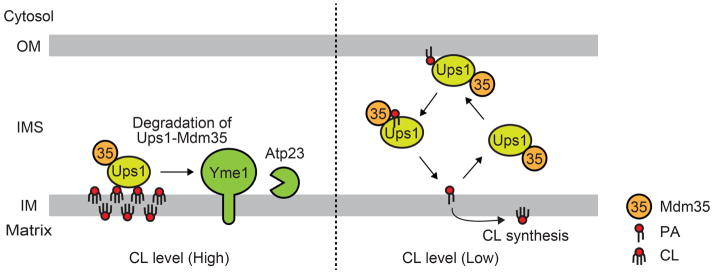
(Left) High concentration of CL in the IM keeps the Ups1-Mdm35 complex close to the IM, promoting the Ups1-Mdm35 degradation by the IM-located proteases, Yme1 and Atp23. (Right) The Ups1-Mdm35 complex shuttles between the OM and IM to mediate the PA transport due to its low affinity to the IM when CL concentration is low in the IM.
Possible alternative routes for PA transport and CL synthesis in mitochondria
Although the PA transport activity of the Ups1-Mdm35 complex was demonstrated in vitro, Ups1 appears dispensable for the PA transport or CL synthesis in vivo. In yeast, Ups1 is homologous to two other proteins named Ups2 and Ups3, both of which also form a complex with Mdm35 (63, 64). Studies have shown the antagonistic roles of Ups1 and Ups2 in the CL metabolism: the decreased CL level and growth defects in the ups1Δ cells are restored by the simultaneous deletion of the UPS2 gene (61, 62). A possible explanation for this is that Ups2 is a negative regulator for an alternative PA transport route and/or a CL synthesis route, which are inactive or minor in the presence of Ups1, and the loss of Ups2 may trigger activation of these routes (Figure 5). Another surprising finding also suggests the alternative CL synthetic pathway, i.e. deletion of UPS1 restores the defects in growth and CL accumulation in the cells lacking Tam41 and/or Pgs1, which were thought to be essential for the CL synthesis (12, 18, 65). These observations evidently indicate that the loss of Ups1 renders tam41Δ, pgs1Δ or tam41Δpgs1Δ cells capable of synthesizing CL by unknown mechanisms. Thus, it is likely that there is a yet-to-be identified alternative CL synthetic pathway that is activated by the loss of Ups1 together with the depletion of Ups2, Tam41 or Pgs1 (Figure 5).
Figure 5. Possible unidentified PA transport and CL synthetic routes.
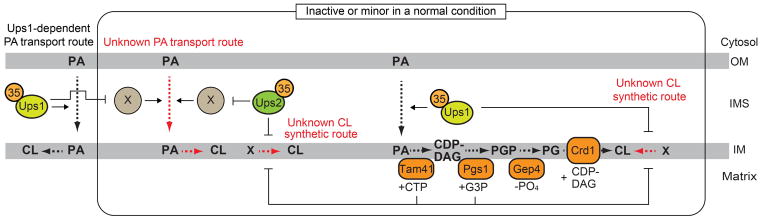
(Left) At the normal condition, which Ups1, Tam41 and Pgs1 are present, PA transported by the Ups1-Mdm35 appears to be predominantly used to produce CL. (Right) The simultaneous loss of Ups1 with either Ups2, Tam41 or Pgs1 may activate alternative PA transport and/or CL synthetic routes, resulting in restored CL accumulation. Red broken lines indicated speculative PA transport and CL synthetic routes.
Kuroda et al. found that an IM protein, Fmp30, which is a yeast homolog of mammalian N-acylPE-specific phospholipase D (NAPE-PLD), is important for cell growths in the absence of the mitochondrial PE synthase Psd1 (66). Since loss of CL synthase Crd1 is synthetic lethal with the PSD1 deletion (67), Fmp30 may well be involved in the CL metabolism. Upon deletion of UPS1, Fmp30 may be functionally activated and become essential for the CL synthesis although its function in the CL synthesis remains minor when Ups1 is present. Indeed, the loss of Fmp30 causes a slight decrease in the CL level and shows synthetic lethality with the deletion of UPS1 (66). Clearly, further analyses are requited to test the possible involvement of Fmp30 in the alternative, still elusive CL synthetic pathway.
Regulation of PE export from mitochondria to the ER
Regardless of the presence of Ups1, loss of Ups2 alone leads to a decrease in PE in mitochondria (61, 62). This could suggest that import of PS from the ER to the IM, where PS decarboxylase (PE synthase) is present, and/or PE synthesis itself is compromised when Ups2 is absent in mitochondria. However, this was not the case; both PS transport and PE synthesis are normal even in the absence of Ups2 (62, 68). Instead, our pulse-chase experiments with 14C-serine revealed that the loss of Ups1 or Ups2 leads to decelerated or accelerated PE to PC conversions, respectively (69), suggesting that Ups1 and Ups2 regulate the PE export from mitochondria to the ER, in a positive or negative manner, respectively (Figure 6A). In addition to the decreased PE level, ups2Δ mitochondria show a slightly increased level of PC, which is synthesized by methylation of PE in the ER (61) (Figure 6B, ups2Δ). On the other hand, ups1Δ mitochondria contain a comparable level of PE although deletion of UPS1 apparently hampers the PE export. This could be explained by the decreased steady state level of Psd1 in ups1Δ cells that were cultivated in fermentable media for the pulse-chase experiment (68, 69) (Figure 6C, ups1Δ, Fermentable). It is likely that Ups1 is required for both maintenance of the Psd1 level and the PE export from the IM to the ER under fermentable conditions. Then the compromised PE production and its export from mitochondria due to the loss of Ups1 would compensate each other to recover the PE level to be normal. It should be also noted that under non-fermentable conditions, the normal level of Psd1 is maintained probably by the restored membrane potential in ups1Δ mitochondria (68) (Figure 6D, ups1Δ, Non-fermentable). Under this growth condition, the PE level of ups1Δ mitochondria increases (61), suggesting a positive role of Ups1 in the PE export from mitochondria to the ER.
Figure 6. Regulation of PE export from mitochondria to the ER.
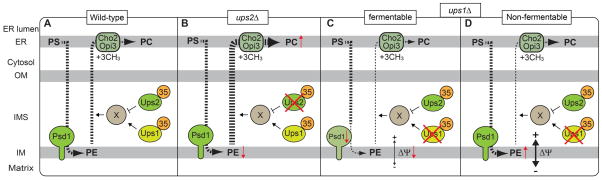
Newly synthesized PS is imported from the ER to mitochondria, converted to PE and then exported back to the ER. The export of PE seems to be regulated by Ups1 and Ups2. X indicates a putative PE transfer protein. Broken lines indicate movements of phospholipids. Bold and thin broken lines depict accelerated and decelerated phospholipid transports, respectively. Our pulse chase experiments have suggested that (B) loss of Ups2 accelerates PE export from mitochondria to the ER, leading to decreased levels of PE and increased levels of PC (71). (C) In contrast, loss of Ups1 appears to slow the PE export. The loss of Ups1 also leads to decreased steady state levels of Psd1 probably due to low membrane potential (ΔΨ) across the IM under the fermentable condition (70, 71). (D) The decreased ΔΨ in the absence of Ups1 is restored under the non-fermentable condition, which restores ΔΨ (61).
Since the Ups1-Mdm35 complex transports PA from the OM to IM, it may also directly enhance the PE export from the mitochondria to the ER. However, the PE level is decreased in not only ups2Δ but also ups1Δups2Δ mitochondria. This indicates that the decrease in the PE level in ups2Δ mitochondria does not depend on Ups1. Thus, we propose that Ups1 and Ups2 function as regulators for the unidentified PE transfer protein/mechanism and antagonistically affect the PE export.
Mitochondrial contact sites between the OM and IM
Although the Ups1-Mdm35 complex is shown to function at the OM-IM contact site (55), it is still unclear whether the close contact between the OM and IM is essential for the phospholipid transport. Accumulated evidence shows that there are a number of physical interactions between proteins in the OM and IM. For example, Tom22 of the TOM40 complex, a protein translocator in the OM, and Tim23 and Tim50 in the TIM23 complex, a protein translocator in the IM, can be chemically crosslinked, revealing the physical contact among these proteins that could achieve efficient coupling of protein translocation across the OM and IM (70, 71) (Figure 7). Consistently, the TIM23 complex is located in the inner boundary membrane, which is a sub-compartment of the IM close to the OM (72). It would be interesting to assume that the TOM-TIM interactions may contribute to efficient transport of not only mitochondrial proteins but also phospholipids within mitochondria.
Figure 7. Schematic of previously reported protein-protein interactions in the OM and IM.
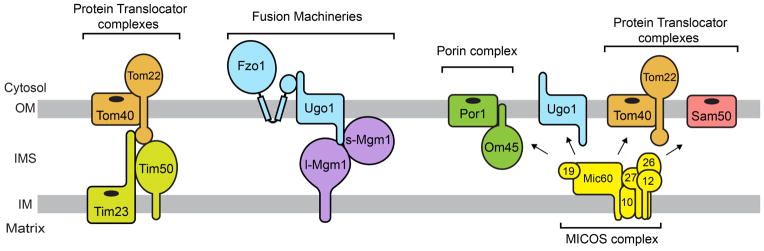
Arrows indicate physical interactions between the MICOS complex and proteins in the OM.
Mitochondrial fusion proteins Fzo1, Ugo1 and Mgm1 also physically interact with each other and thought to couple OM fusion to IM fusion (73, 74). While Fzo1 and Ugo1 are OM-resident proteins, Mgm1 is located in both the OM and IM (75) (Figure 7). Thus, in addition to the organelle fusion activity, Mgm1 may contribute to OM-IM interactions, if any, to mix phospholipids between the OM and IM.
Recently, the MICOS (mitochondrial contact site) complex consisting of Mic60, Mic10, Mic12, Mic19, Mic26 and Mic27 was identified as an IM protein complex that is enriched in the OM-IM contact sites in yeast (76–79). The MICOS complex is reported to interact with various proteins in the OM, Sam50, Ugo1, Om45, Por1 and the TOM40 complex (76–78) (Figure 7). The genetic interaction map focusing on genes responsible for mitochondrial functions (the MITO-MAP) has revealed that the MICOS genes genetically interact with the ERMES genes (MMM1, MMM2, MDM10, MDM12 and GEM1) and the ones involved in CL synthesis (GEP4, CRD1) (78), suggesting that the MICOS may play an important role in the phospholipid metabolism, as well.
Other candidate proteins for phospholipid transfer mediator/regulator
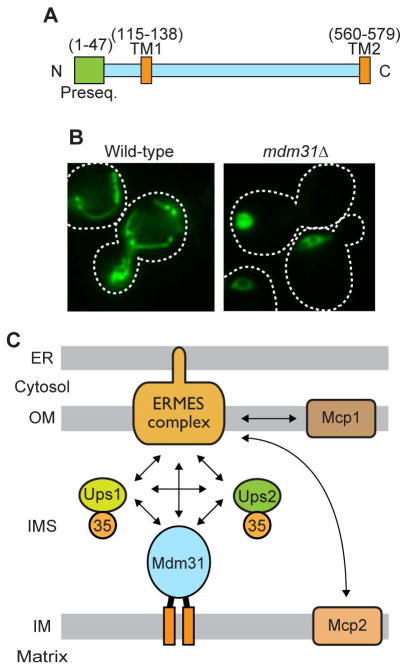
An IM protein Mdm31 is a candidate for a phospholipid transfer mediator/regulator between the OM and IM. Mdm31 contains two TM segments in its N- and C-termini and is integrated into the IM via its TM segments from the IMS (69) (Figure 8A). Mdm31 was originally identified as a factor required for maintenance of normal mitochondrial morphology and stability of mitochondrial DNA (45, 80). Mitochondrial morphology is altered to ball-like structure in cells lacking Mdm31 (45, 69, 80) (Figure 8B). In addition, it was shown that loss of Mdm31 causes synthetic lethality with any of the ERMES genes (82). Later, we found that MDM31 also genetically interacts with UPS1, UPS2 and MDM35 (69), i.e. deletion of MDM31 exhibits synthetic growth defects with deletion of UPS1, UPS2 or MDM35 (69) (Figure 8C). In turn, overexpression of Mdm31 partially restores the defects in growth, CL accumulation and mitochondrial morphology in cells lacking ERMES and Ups1 while overexpression of Ups2 but not Ups1 partially rescues defects in growth, the CL level and mitochondrial morphology in mdm31Δ cells (69). These findings suggest that Mdm31 possesses overlapping functions with the ERMES complex, Ups1 and Ups2 although its precise functions remain to be experimentally determined by future studies.
Figure 8. Mdm31 is a potential phospholipid transfer mediator/regulator in mitochondria.
(A) Schematic of primary sequence of Mdm31. Preseq., presequence; TM, transmembrane segment; N, N-terminus; C, C-terminus. (B) Mitochondria-targeted GFP is expressed in wild-type and mdm31Δ cells and observed under a fluorescence microscope. (C) The ERMES, UPS1, UPS2, MDM35 and MDM31 genes genetically interact each other. MCP1 and MCP2 are multicopy supressors for the ERMES-deficient cells. Arrows indicate the genetic interactions.
Screening for multi-copy suppressors of defective growths of cells lacking Mdm10, a component of the ERMES complex, identified two novel mitochondrial proteins named Mcp1 and Mcp2 (81). Mcp1 and Mcp2 are OM and IM-integrated proteins, respectively (Figure 8). Overexpression of Mcp1 or Mcp2 partially restores the defects in cell growth, mitochondrial morphology and phospholipid composition of mdm10Δ cells. In addition, combination of mcp1Δ or mcp2Δ mutation and deletion of any ERMES component results in synthetic lethality or severe growth defects. Thus, Mcp1 and Mcp2 are also candidates for factors involved in phospholipid transport.
Summary and Future Directions
How newly synthesized phospholipids are distributed properly to biological membranes has been recognized as a fundamental problem in biology but has not been resolved well. Recent identification of the ERMES complex as a molecular tether between the ER and mitochondria and the Ups1/Ups2-Mdm35 complex as a phospholipid transfer machinery/regulator in the IMS have begun to shed light on the mechanisms underlying mitochondrial phospholipid transport (40, 55). However at the same time, these findings brought up many new questions on lipid transport.
First, the primary function of the ERMES complex remains to be determined. It is conceivable that the close proximity of distinct biological membranes by their tethering accelerates phospholipid exchange between these membranes. However, whether or not the ERMES possesses a catalytic activity as phospholipid transfer proteins remains to be analyzed. Since some of the ERMES components are predicted to contain TULIP domain with a hydrophobic pocket, which could function as a lipid binding domain (51, 52), structural analyses of the ERMES components should be essential to reveal their molecular functions. Second, identification of the ERMES complex as a physical tether between the ER and mitochondria raised a question of whether mitochondria are connected with other organelles in addition to the ER. Indeed, identification of the molecular tether between mitochondria and the plasma membrane in yeast was reported (82). It is exciting to search for novel tethering sites between mitochondria and other organelles such as the vacuole/lysosome, Golgi, endosome, and peroxisome. Third, although it has been revealed that the Ups1-Mdm35 complex can mediate PA transfer between liposomes (55), other phospholipids such as PC, PS and PI must be supplied from the ER to mitochondria while PE and CL have to be transported from the IM to OM and the ER (Figure 1). It is completely unknown what proteins and what mechanisms are responsible for the transport of these phospholipids to or from mitochondria. Forth, how phospholipids efficiently move from the one leaflet to the other leaflet of the mitochondrial membranes is poorly understood. In mitochondrial membranes, phospholipids appear to flip quite efficiently. For example, our pulse-chase experiments using 14C-serine have clearly shown that newly synthesized radio-labeled PS is rapidly converted to PC in a short period of time (~15 min) (69). This PS to PC conversion involves a round trip of PS from the ER to the IM, where it is converted to PE (Figure 1 and 3). Furthermore, in order for not only PS and PE but also PA, PI and PC synthesized in the ER to reach the inner leaflet (matrix side) of the IM, they must flip in the OM and IM. CL, which is synthesized on the matrix side of the IM, also translocates across the IM to reach the IMS side, where CL acyltransferase is located (Figure 1). Although phospholipid flippases, which belong to the type IV P-type ATPase family, are found in the Golgi, endosome and plasma membranes (83) in yeast and a mitochondrial phospholipid scramblase is found in mammal (84), such enzymes have not been identified so far in mitochondria in yeast. Identification of mitochondrial flippase/scramblase, if any, should be important in future studies.
To identify novel factors involved in phospholipid transport in mitochondria and to elucidate their functions, development of a reliable in vitro assay system must be essential. Until now, various in vitro assay systems using isolated mitochondria, isolated MAM, and semi-intact cells have been employed to analyze phospholipid transport between different biological membranes including mitochondria (68, 85–90), however their application to various phospholipid transport processes is still limited. Techniques to follow lipid transport among different membranes in living cells, which should bring about a break-through in this field, remain to be developed, too. Identification and functional characterization of novel factors involved in phospholipid transport via mitochondria will provide valuable insight into the profound mechanisms underlying mitochondrial functions, including not only the phospholipid metabolism but also mitochondrial morphogenesis, apoptosis, mitophagy, aging and so on.
Acknowledgments
We acknowledge support of this work by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT) and the grant for CREST from Japan Science and Technology (JST) Agency to T. E and Y.T and NIH (GM089853) to H. S.
References
- 1.Henry SA, Kohlwein SD, Carman GM. Metabolism and regulation of glycerolipids in the yeast Saccharomyces cerevisiae. Genetics. 2012;190:317–349. doi: 10.1534/genetics.111.130286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horvath SE, Daum G. Lipids of mitochondria. Prog Lipid Res. 2013;52:590–614. doi: 10.1016/j.plipres.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Gibellini F, Smith TK. The Kennedy pathway-De novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB life. 2010;62:414–428. doi: 10.1002/iub.337. [DOI] [PubMed] [Google Scholar]
- 4.Osman C, Voelker DR, Langer T. Making heads or tails of phospholipids in mitochondria. J Cell Biol. 2011;192:7–16. doi: 10.1083/jcb.201006159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scharwey M, Tatsuta T, Langer T. Mitochondrial lipid transport at a glance. J Cell Sci. 2013;126:5317–5323. doi: 10.1242/jcs.134130. [DOI] [PubMed] [Google Scholar]
- 6.Tatsuta T, Scharwey M, Langer T. Mitochondrial lipid trafficking. Trends Cell Biol. 2014;24:44–52. doi: 10.1016/j.tcb.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Athenstaedt K, Daum G. Biosynthesis of phosphatidic acid in lipid particles and endoplasmic reticulum of Saccharomyces cerevisiae. J Bacteriol. 1997;179:7611–7616. doi: 10.1128/jb.179.24.7611-7616.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huh WK, Falvo JV, Gerke LC, Carroll AS, Howson RW, Weissman JS, O’Shea EK. Global analysis of protein localization in budding yeast. Nature. 2003;425:686–691. doi: 10.1038/nature02026. [DOI] [PubMed] [Google Scholar]
- 9.Jain S, Stanford N, Bhagwat N, Seiler B, Costanzo M, Boone C, Oelkers P. Identification of a novel lysophospholipid acyltransferase in Saccharomyces cerevisiae. J Biol Chem. 2007;282:30562–30569. doi: 10.1074/jbc.M706326200. [DOI] [PubMed] [Google Scholar]
- 10.Han GS, O’Hara L, Carman GM, Siniossoglou S. An unconventional diacylglycerol kinase that regulates phospholipid synthesis and nuclear membrane growth. J Biol Chem. 2008;283:20433–20442. doi: 10.1074/jbc.M802903200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen H, Heacock PN, Clancey CJ, Dowhan W. The CDS1 gene encoding CDP-diacylglycerol synthase in Saccharomyces cerevisiae is essential for cell growth. J Biol Chem. 1996;27:789–795. doi: 10.1074/jbc.271.2.789. [DOI] [PubMed] [Google Scholar]
- 12.Tamura Y, Harada Y, Nishikawa S, Yamano K, Kamiya M, Shiota T, Kuroda T, Kuge O, Sesaki H, Imai K, Tomii K, Endo T. Tam41 is a CDP-diacylglycerol synthase required for cardiolipin biosynthesis in mitochondria. Cell Metab. 2013;17:709–718. doi: 10.1016/j.cmet.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Letts VA, Klig LS, Bae-Lee M, Carman GM, Henry SA. Isolation of the yeast structural gene for the membrane-associated enzyme phosphatidylserine synthase. Proc Natl Acad Sci U S A. 1983;80:7279–7283. doi: 10.1073/pnas.80.23.7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nikawa J, Kodaki T, Yamashita S. Primary structure and disruption of the phosphatidylinositol synthase gene of Saccharomyces cerevisiae. J Biol Chem. 1987;262:4876–4881. [PubMed] [Google Scholar]
- 15.Gaigg B, Simbeni R, Hrastnik C, Paltauf F, Daum G. Characterization of a microsomal subfraction associated with mitochondria of the yeast, Saccharomyces cerevisiae. Involvement in synthesis and import of phospholipids into mitochondria. Biochim Biophys Acta. 1995;1234:214–220. doi: 10.1016/0005-2736(94)00287-y. [DOI] [PubMed] [Google Scholar]
- 16.Fischl AS, Carman GM. Phosphatidylinositol biosynthesis in Saccharomyces cerevisiae: purification and properties of microsome-associated phosphatidylinositol synthase. J Bacteriol. 1983;154:304–311. doi: 10.1128/jb.154.1.304-311.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horvath SE, Bottinger L, Vogtle FN, Wiedemann N, Meisinger C, Becker T, Daum G. Processing and topology of the yeast mitochondrial phosphatidylserine decarboxylase 1. J Biol Chem. 2012;287:36744–36755. doi: 10.1074/jbc.M112.398107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang SC, Heacock PN, Clancey CJ, Dowhan W. The PEL1 gene (renamed PGS1) encodes the phosphatidylglycero-phosphate synthase of Saccharomyces cerevisiae. J Biol Chem. 1998;273:9829–9836. doi: 10.1074/jbc.273.16.9829. [DOI] [PubMed] [Google Scholar]
- 19.Osman C, Haag M, Wieland FT, Brugger B, Langer T. A mitochondrial phosphatase required for cardiolipin biosynthesis: the PGP phosphatase Gep4. EMBO J. 2010;29:1976–1987. doi: 10.1038/emboj.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tuller G, Hrastnik C, Achleitner G, Schiefthaler U, Klein F, Daum G. YDL142c encodes cardiolipin synthase (Cls1p) and is non-essential for aerobic growth of Saccharomyces cerevisiae. FEBS lett. 1998;421:15–18. doi: 10.1016/s0014-5793(97)01525-1. [DOI] [PubMed] [Google Scholar]
- 21.Chang SC, Heacock PN, Mileykovskaya E, Voelker DR, Dowhan W. Isolation and characterization of the gene (CLS1) encoding cardiolipin synthase in Saccharomyces cerevisiae. J Biol Chem. 1998;273:14933–14941. doi: 10.1074/jbc.273.24.14933. [DOI] [PubMed] [Google Scholar]
- 22.Baile MG, Whited K, Claypool SM. Deacylation on the matrix side of the mitochondrial inner membrane regulates cardiolipin remodeling. Mol Biol Cell. 2013;24:2008–2020. doi: 10.1091/mbc.E13-03-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Claypool SM, McCaffery JM, Koehler CM. Mitochondrial mislocalization and altered assembly of a cluster of Barth syndrome mutant tafazzins. J Cell Biol. 2006;174:379–390. doi: 10.1083/jcb.200605043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joshi AS, Zhou J, Gohil VM, Chen S, Greenberg ML. Cellular functions of cardiolipin in yeast. Biochim Biophys Acta. 2009;1793:212–218. doi: 10.1016/j.bbamcr.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Claypool SM, Koehler CM. The complexity of cardiolipin in health and disease. Trends Biochem Sci. 2012;37:32–41. doi: 10.1016/j.tibs.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gebert N, Joshi AS, Kutik S, Becker T, McKenzie M, Guan XL, Mooga VP, Stroud DA, Kulkarni G, Wenk MR, Rehling P, Meisinger C, Ryan MT, Wiedemann N, Greenberg ML, et al. Mitochondrial cardiolipin involved in outer-membrane protein biogenesis: implications for Barth syndrome. Curr Biol. 2009;19:2133–2139. doi: 10.1016/j.cub.2009.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu CT, Ji J, Dagda RK, Jiang JF, Tyurina YY, Kapralov AA, Tyurin VA, Yanamala N, Shrivastava IH, Mohammadyani D, Qiang Wang KZ, Zhu J, Klein-Seetharaman J, Balasubramanian K, Amoscato AA, et al. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat Cell Biol. 2013;15:1197–1205. doi: 10.1038/ncb2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chu CT, Bayir H, Kagan VE. LC3 binds externalized cardiolipin on injured mitochondria to signal mitophagy in neurons: implications for Parkinson disease. Autophagy. 2014;10:376–378. doi: 10.4161/auto.27191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zinser E, Sperka-Gottlieb CD, Fasch EV, Kohlwein SD, Paltauf F, Daum G. Phospholipid synthesis and lipid composition of subcellular membranes in the unicellular eukaryote Saccharomyces cerevisiae. J Bacteriol. 1991;173:2026–2034. doi: 10.1128/jb.173.6.2026-2034.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Kroon AI, Dolis D, Mayer A, Lill R, de Kruijff B. Phospholipid composition of highly purified mitochondrial outer membranes of rat liver and Neurospora crassa. Is cardiolipin present in the mitochondrial outer membrane? Biochim Biophys Acta. 1997;1325:108–116. doi: 10.1016/s0005-2736(96)00240-4. [DOI] [PubMed] [Google Scholar]
- 31.Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975;189:867. doi: 10.1126/science.189.4206.867-b. [DOI] [PubMed] [Google Scholar]
- 32.Whyte JR, Munro S. Vesicle tethering complexes in membrane traffic. J Cell Sci. 2002;115:2627–2637. doi: 10.1242/jcs.115.13.2627. [DOI] [PubMed] [Google Scholar]
- 33.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 34.Lev S. Non-vesicular lipid transport by lipid-transfer proteins and beyond. Nat Rev Mol Cell Biol. 2010;11:739–750. doi: 10.1038/nrm2971. [DOI] [PubMed] [Google Scholar]
- 35.Toulmay A, Prinz WA. Lipid transfer and signaling at organelle contact sites: the tip of the iceberg. Curr Opin Cell Biol. 2011;23:458–463. doi: 10.1016/j.ceb.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Helle SC, Kanfer G, Kolar K, Lang A, Michel AH, Kornmann B. Organization and function of membrane contact sites. Biochim Biophys Acta. 2013;1833:2526–2541. doi: 10.1016/j.bbamcr.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 37.Voelker DR. New perspectives on the regulation of intermembrane glycerophospholipid traffic. J Lipid Res. 2003;44:441–449. doi: 10.1194/jlr.R200020-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Hayashi T, Rizzuto R, Hajnoczky G, Su TP. MAM: more than just a housekeeper. Trends Cell Biol. 2009;19:81–88. doi: 10.1016/j.tcb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vance JE. MAM (mitochondria-associated membranes) in mammalian cells: Lipids and beyond. Biochim Biophys Acta. 2014;1841:595–609. doi: 10.1016/j.bbalip.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 40.Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, Weissman JS, Walter P. An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science. 2009;325:477–481. doi: 10.1126/science.1175088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stroud DA, Oeljeklaus S, Wiese S, Bohnert M, Lewandrowski U, Sickmann A, Guiard B, van der Laan M, Warscheid B, Wiedemann N. Composition and topology of the endoplasmic reticulum-mitochondria encounter structure. J Mol Biol. 2011;413:743–750. doi: 10.1016/j.jmb.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 42.Burgess SM, Delannoy M, Jensen RE. MMM1 encodes a mitochondrial outer membrane protein essential for establishing and maintaining the structure of yeast mitochondria. J Cell Biol. 1994;126:1375–1391. doi: 10.1083/jcb.126.6.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sogo LF, Yaffe MP. Regulation of mitochondrial morphology and inheritance by Mdm10p, a protein of the mitochondrial outer membrane. J Cell Biol. 1994;126:1361–1373. doi: 10.1083/jcb.126.6.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berger KH, Sogo LF, Yaffe MP. Mdm12p, a component required for mitochondrial inheritance that is conserved between budding and fission yeast. J Cell Biol. 1997;136:545–553. doi: 10.1083/jcb.136.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dimmer KS, Fritz S, Fuchs F, Messerschmitt M, Weinbach N, Neupert W, Westermann B. Genetic basis of mitochondrial function and morphology in Saccharomyces cerevisiae. Mol Biol Cell. 2002;13:847–853. doi: 10.1091/mbc.01-12-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frederick RL, Okamoto K, Shaw JM. Multiple pathways influence mitochondrial inheritance in budding yeast. Genetics. 2008;178:825–837. doi: 10.1534/genetics.107.083055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Youngman MJ, Hobbs AE, Burgess SM, Srinivasan M, Jensen RE. Mmm2p, a mitochondrial outer membrane protein required for yeast mitochondrial shape and maintenance of mtDNA nucleoids. J Cell Biol. 2004;164:677–688. doi: 10.1083/jcb.200308012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hobbs AE, Srinivasan M, McCaffery JM, Jensen RE. Mmm1p, a mitochondrial outer membrane protein, is connected to mitochondrial DNA (mtDNA) nucleoids and required for mtDNA stability. J Cell Biol. 2001;152:401–410. doi: 10.1083/jcb.152.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kornmann B, Osman C, Walter P. The conserved GTPase Gem1 regulates endoplasmic reticulum-mitochondria connections. Proc Natl Acad Sci U S A. 2011;108:14151–14156. doi: 10.1073/pnas.1111314108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamano K, Tanaka-Yamano S, Endo T. Tom7 regulates Mdm10-mediated assembly of the mitochondrial import channel protein Tom40. The Journal of biological chemistry. 2010;285:41222–41231. doi: 10.1074/jbc.M110.163238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kopec KO, Alva V, Lupas AN. Homology of SMP domains to the TULIP superfamily of lipid-binding proteins provides a structural basis for lipid exchange between ER and mitochondria. Bioinformatics. 2010;26:1927–1931. doi: 10.1093/bioinformatics/btq326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kopec KO, Alva V, Lupas AN. Bioinformatics of the TULIP domain superfamily. Biochem Soc Trans. 2011;39:1033–1038. doi: 10.1042/BST0391033. [DOI] [PubMed] [Google Scholar]
- 53.Nguyen TT, Lewandowska A, Choi JY, Markgraf DF, Junker M, Bilgin M, Ejsing CS, Voelker DR, Rapoport TA, Shaw JM. Gem1 and ERMES do not directly affect phosphatidylserine transport from ER to mitochondria or mitochondrial inheritance. Traffic. 2012;13:880–890. doi: 10.1111/j.1600-0854.2012.01352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Voss C, Lahiri S, Young BP, Loewen CJ, Prinz WA. ER-shaping proteins facilitate lipid exchange between the ER and mitochondria in S. cerevisiae. J Cell Sci. 2012;125:4791–4799. doi: 10.1242/jcs.105635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Connerth M, Tatsuta T, Haag M, Klecker T, Westermann B, Langer T. Intramitochondrial transport of phosphatidic acid in yeast by a lipid transfer protein. Science. 2012;338:815–818. doi: 10.1126/science.1225625. [DOI] [PubMed] [Google Scholar]
- 56.Sesaki H, Dunn CD, Iijima M, Shepard KA, Yaffe MP, Machamer CE, Jensen RE. Ups1p, a conserved intermembrane space protein, regulates mitochondrial shape and alternative topogenesis of Mgm1p. J Cell Biol. 2006;173:651–658. doi: 10.1083/jcb.200603092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herlan M, Bornhovd C, Hell K, Neupert W, Reichert AS. Alternative topogenesis of Mgm1 and mitochondrial morphology depend on ATP and a functional import motor. J Cell Biol. 2004;165:167–173. doi: 10.1083/jcb.200403022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herlan M, Vogel F, Bornhovd C, Neupert W, Reichert AS. Processing of Mgm1 by the rhomboid-type protease Pcp1 is required for maintenance of mitochondrial morphology and of mitochondrial DNA. J Biol Chem. 2003;278:27781–27788. doi: 10.1074/jbc.M211311200. [DOI] [PubMed] [Google Scholar]
- 59.McQuibban GA, Saurya S, Freeman M. Mitochondrial membrane remodelling regulated by a conserved rhomboid protease. Nature. 2003;423:537–541. doi: 10.1038/nature01633. [DOI] [PubMed] [Google Scholar]
- 60.Sesaki H, Southard SM, Hobbs AE, Jensen RE. Cells lacking Pcp1p/Ugo2p, a rhomboid-like protease required for Mgm1p processing, lose mtDNA and mitochondrial structure in a Dnm1p-dependent manner, but remain competent for mitochondrial fusion. Biochem Biophys Res Comm. 2003;308:276–283. doi: 10.1016/s0006-291x(03)01348-2. [DOI] [PubMed] [Google Scholar]
- 61.Tamura Y, Endo T, Iijima M, Sesaki H. Ups1p and Ups2p antagonistically regulate cardiolipin metabolism in mitochondria. J Cell Biol. 2009;185:1029–1045. doi: 10.1083/jcb.200812018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Osman C, Haag M, Potting C, Rodenfels J, Dip PV, Wieland FT, Brugger B, Westermann B, Langer T. The genetic interactome of prohibitins: coordinated control of cardiolipin and phosphatidylethanolamine by conserved regulators in mitochondria. J Cell Biol. 2009;184:583–596. doi: 10.1083/jcb.200810189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tamura Y, Iijima M, Sesaki H. Mdm35p imports Ups proteins into the mitochondrial intermembrane space by functional complex formation. EMBO J. 2010;29:2875–2887. doi: 10.1038/emboj.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Potting C, Wilmes C, Engmann T, Osman C, Langer T. Regulation of mitochondrial phospholipids by Ups1/PRELI-like proteins depends on proteolysis and Mdm35. EMBO J. 2010;29:2888–2898. doi: 10.1038/emboj.2010.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kutik S, Rissler M, Guan XL, Guiard B, Shui G, Gebert N, Heacock PN, Rehling P, Dowhan W, Wenk MR, Pfanner N, Wiedemann N. The translocator maintenance protein Tam41 is required for mitochondrial cardiolipin biosynthesis. J Cell Biol. 2008;183:1213–1221. doi: 10.1083/jcb.200806048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuroda T, Tani M, Moriguchi A, Tokunaga S, Higuchi T, Kitada S, Kuge O. FMP30 is required for the maintenance of a normal cardiolipin level and mitochondrial morphology in the absence of mitochondrial phosphatidylethanolamine synthesis. Mol Microbiol. 2011;80:248–265. doi: 10.1111/j.1365-2958.2011.07569.x. [DOI] [PubMed] [Google Scholar]
- 67.Joshi AS, Thompson MN, Fei N, Huttemann M, Greenberg ML. Cardiolipin and mitochondrial phosphatidylethanolamine have overlapping functions in mitochondrial fusion in Saccharomyces cerevisiae. J Biol Chem. 2012;287:17589–17597. doi: 10.1074/jbc.M111.330167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tamura Y, Onguka O, Itoh K, Endo T, Iijima M, Claypool SM, Sesaki H. Phosphatidylethanolamine biosynthesis in mitochondria: phosphatidylserine (PS) trafficking is independent of a PS decarboxylase and intermembrane space proteins UPS1P and UPS2P. J Biol Chem. 2012;287:43961–43971. doi: 10.1074/jbc.M112.390997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tamura Y, Onguka O, Hobbs AE, Jensen RE, Iijima M, Claypool SM, Sesaki H. Role for two conserved intermembrane space proteins, Ups1p and Ups2p, in intra-mitochondrial phospholipid trafficking. J Biol Chem. 2012;287:15205–15218. doi: 10.1074/jbc.M111.338665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tamura Y, Harada Y, Shiota T, Yamano K, Watanabe K, Yokota M, Yamamoto H, Sesaki H, Endo T. Tim23-Tim50 pair coordinates functions of translocators and motor proteins in mitochondrial protein import. J Cell Biol. 2009;184:129–141. doi: 10.1083/jcb.200808068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shiota T, Mabuchi H, Tanaka-Yamano S, Yamano K, Endo T. In vivo protein-interaction mapping of a mitochondrial translocator protein Tom22 at work. Proc Natl Acad Sci U S A. 2011;108:15179–15183. doi: 10.1073/pnas.1105921108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wurm CA, Jakobs S. Differential protein distributions define two sub-compartments of the mitochondrial inner membrane in yeast. FEBS lett. 2006;580:5628–5634. doi: 10.1016/j.febslet.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 73.Wong ED, Wagner JA, Scott SV, Okreglak V, Holewinske TJ, Cassidy-Stone A, Nunnari J. The intramitochondrial dynamin-related GTPase, Mgm1p, is a component of a protein complex that mediates mitochondrial fusion. J Cell Biol. 2003;160:303–311. doi: 10.1083/jcb.200209015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sesaki H, Jensen RE. Ugo1p links the Fzo1p and Mgm1p GTPases for mitochondrial fusion. J Biol Chem. 2004;279:28298–28303. doi: 10.1074/jbc.M401363200. [DOI] [PubMed] [Google Scholar]
- 75.Sesaki H, Southard SM, Yaffe MP, Jensen RE. Mgm1p, a dynamin-related GTPase, is essential for fusion of the mitochondrial outer membrane. Mol Biol Cell. 2003;14:2342–2356. doi: 10.1091/mbc.E02-12-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Harner M, Korner C, Walther D, Mokranjac D, Kaesmacher J, Welsch U, Griffith J, Mann M, Reggiori F, Neupert W. The mitochondrial contact site complex, a determinant of mitochondrial architecture. EMBO J. 2011;30:4356–4370. doi: 10.1038/emboj.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.von der Malsburg K, Muller JM, Bohnert M, Oeljeklaus S, Kwiatkowska P, Becker T, Loniewska-Lwowska A, Wiese S, Rao S, Milenkovic D, Hutu DP, Zerbes RM, Schulze-Specking A, Meyer HE, Martinou JC, et al. Dual role of mitofilin in mitochondrial membrane organization and protein biogenesis. Dev Cell. 2011;21:694–707. doi: 10.1016/j.devcel.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 78.Hoppins S, Collins SR, Cassidy-Stone A, Hummel E, Devay RM, Lackner LL, Westermann B, Schuldiner M, Weissman JS, Nunnari J. A mitochondrial-focused genetic interaction map reveals a scaffold-like complex required for inner membrane organization in mitochondria. J Cell Biol. 2011;195:323–340. doi: 10.1083/jcb.201107053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pfanner N, van der Laan M, Amati P, Capaldi RA, Caudy AA, Chacinska A, Darshi M, Deckers M, Hoppins S, Icho T, Jakobs S, Ji J, Kozjak-Pavlovic V, Meisinger C, Odgren PR, et al. Uniform nomenclature for the mitochondrial contact site and cristae organizing system. J Cell Biol. 2014;204:1083–1086. doi: 10.1083/jcb.201401006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dimmer KS, Jakobs S, Vogel F, Altmann K, Westermann B. Mdm31 and Mdm32 are inner membrane proteins required for maintenance of mitochondrial shape and stability of mitochondrial DNA nucleoids in yeast. J Cell Biol. 2005;168:103–115. doi: 10.1083/jcb.200410030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tan T, Ozbalci C, Brugger B, Rapaport D, Dimmer KS. Mcp1 and Mcp2, two novel proteins involved in mitochondrial lipid homeostasis. J Cell Sci. 2013;126:3563–3574. doi: 10.1242/jcs.121244. [DOI] [PubMed] [Google Scholar]
- 82.Lackner LL, Ping H, Graef M, Murley A, Nunnari J. Endoplasmic reticulum-associated mitochondria-cortex tether functions in the distribution and inheritance of mitochondria. Proc Natl Acad Sci U S A. 2013;110:E458–467. doi: 10.1073/pnas.1215232110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sebastian TT, Baldridge RD, Xu P, Graham TR. Phospholipid flippases: building asymmetric membranes and transport vesicles. Biochim Biophys Acta. 2012;1821:1068–1077. doi: 10.1016/j.bbalip.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu J, Epand RF, Durrant D, Grossman D, Chi NW, Epand RM, Lee RM. Role of phospholipid scramblase 3 in the regulation of tumor necrosis factor-alpha-induced apoptosis. Biochemistry. 2008;47:4518–4529. doi: 10.1021/bi701962c. [DOI] [PubMed] [Google Scholar]
- 85.Voelker DR. Reconstitution of phosphatidylserine import into rat liver mitochondria. J Biol Chem. 1989;264:8019–8025. [PubMed] [Google Scholar]
- 86.Simbeni R, Tangemann K, Schmidt M, Ceolotto C, Paltauf F, Daum G. Import of phosphatidylserine into isolated yeast mitochondria. Biochim Biophys Acta. 1993;1145:1–7. doi: 10.1016/0005-2736(93)90374-9. [DOI] [PubMed] [Google Scholar]
- 87.Achleitner G, Zweytick D, Trotter PJ, Voelker DR, Daum G. Synthesis and intracellular transport of aminoglycerophospholipids in permeabilized cells of the yeast, Saccharomyces cerevisiae. J Biol Chem. 1995;270:29836–29842. doi: 10.1074/jbc.270.50.29836. [DOI] [PubMed] [Google Scholar]
- 88.Shiao YJ, Balcerzak B, Vance JE. A mitochondrial membrane protein is required for translocation of phosphatidylserine from mitochondria-associated membranes to mitochondria. Biochem J. 1998;331:217–223. doi: 10.1042/bj3310217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu WI, Voelker DR. Characterization of phosphatidylserine transport to the locus of phosphatidylserine decarboxylase 2 in permeabilized yeast. J Biol Chem. 2001;276:7114–7121. doi: 10.1074/jbc.M010278200. [DOI] [PubMed] [Google Scholar]
- 90.Wu WI, Voelker DR. Reconstitution of phosphatidylserine transport from chemically defined donor membranes to phosphatidylserine decarboxylase 2 implicates specific lipid domains in the process. J Biol Chem. 2004;279:6635–6642. doi: 10.1074/jbc.M311570200. [DOI] [PubMed] [Google Scholar]