ABSTRACT
Background
Adherence to dual antiplatelet therapy (DAPT) is critical after coronary stenting. Although adherence rates are frequently assessed in clinical trials, adherence rates in the unselected population recommended for treatment but beyond clinical trials are largely unknown. Therefore, we performed a systematic review of published observational studies to describe rates of DAPT adherence, trends in DAPT use over time, and patient‐level factors associated with nonadherence.
Hypothesis
DAPT adherence declines with increasing time after drug‐eluting stent implantation.
Methods
PubMed, Cumulative Index to Nursing and Allied Health Literature, Embase, and Web of Knowledge were searched through November 20, 2012 for studies including patients receiving 1 or more drug‐eluting stents and reporting the use of aspirin and/or thienopyridines, or assessing factors associated with nonadherence to DAPT after bare metal or drug‐eluting stent placement.
Results
We included 34 studies in the description of DAPT adherence and 11 studies in the description of factors associated with nonadherence. Adherence to DAPT and thienopyridines was high at 1 month but declined by 12 months. Aspirin adherence was at least 90% throughout. Factors associated with nonadherence included bleeding, lower education level, immigrant status, and lack of education regarding DAPT.
Conclusions
DAPT adherence is suboptimal at 12 months, and interventions to increase adherence should focus on reducing bleeding risk and improving communication between patients and physicians.
Introduction
Percutaneous coronary intervention (PCI) is an effective treatment in patients with acute coronary syndromes and stable angina refractory to medical therapy. However, the administration of both aspirin and additional inhibitors of the platelet receptor P2Y12 (ie, dual antiplatelet therapy [DAPT]), in the months following stent placement is necessary to decrease the risk of stent thrombosis.1 In fact, early discontinuation of therapy is the most powerful predictor of stent thrombosis for patients treated with a drug‐eluting stent (DES).2, 3 Therefore, current American College of Cardiology (ACC) and American Heart Association (AHA) guidelines recommend DAPT for a minimum of 12 months after DES placement.4
The necessity of prolonged DAPT after coronary stenting with a DES and the direct relationship of failure to adhere with poor outcomes have focused attention on rates of nonadherence, identifying those at risk for nonadherence and designing interventions to improve adherence rates. Although several studies have attempted to identify various factors associated with nonadherence to address the issue of poor compliance, no integrated assessment of the factors associated with DAPT nonadherence across a broad array of treatment settings and beyond prospective clinical trials has been performed.
We therefore performed a systematic review of published studies of patients receiving a coronary DES to describe rates of DAPT adherence, trends in adherence over time, and patient‐level factors associated with nonadherence to provide insight into the magnitude of the clinical problem of nonadherence to DAPT and to identify potential targets for interventions designed to improve adherence.
Methods
The protocol for this systematic review was prospectively registered in PROSPERO.5
Search Strategy
PubMed, Embase, and Cumulative Index to Nursing and Allied Health Literature were searched on November 20, 2012, and Web of Knowledge was searched on November 23, 2012. Each search consisted of subject headings and free‐text terms related to coronary artery disease, bare‐metal or drug‐eluting stents, and adherence or compliance (see Supporting Methods in the online version of this article). In addition, 1 author performed a manual review of major relevant journals (see Supporting Table 1, in the online version of this article) from January 2012 through January 2013 to identify any articles that may have been in publication during the time period of the database search, as well as a search of the bibliography of any included study identified through the initial database searches.
Study Selection
Only studies reporting primary data were eligible for inclusion. All studies selected for our analysis of DAPT adherence rates included patients receiving at least 1 coronary DES and reported adherence to aspirin, ticlopidine, clopidogrel, or prasugrel at 1, 6, or 12 months postprocedure. Adherence was defined as use of a medication during the prescribed period, without regard for the reason for discontinuation. Studies were excluded if they selected patients based on adherence‐related factors (eg, stent thrombosis) or reported on patients not filling prescriptions rather than directly estimating adherence. Clinical trials, meta‐analyses, and reviews were also excluded. If 2 or more studies reported overlapping data, the study with the most complete data (as assessed by sample size and length of follow‐up) was included, along with any nonoverlapping data points. We attempted to contact the authors of any study that appeared to have collected but not reported adherence rates, but did not seek clarification of studies not in English.
Two authors independently screened each study identified through the database searches. Both authors reviewed the full text of any study thought potentially eligible for inclusion by either, and any disagreement was resolved by consensus. Articles not in English were reviewed by an interpreter and 1 author. Cohen's kappa (κ) was used to quantify inter‐rater agreement on inclusion.
Data Extraction
Data were extracted from each study independently by 2 authors using a standardized form, with discrepancies resolved by consensus. Data from studies not in English were extracted by 1 author with the assistance of an interpreter. If the expected duration of DAPT was not specified in the study methods, we used any mention of the expected duration of DAPT found in the remainder of the article. In the few cases where neither of these strategies was successful, we assumed that the relevant guidelines in the country of origin during the period of data collection governed the expected duration of DAPT. All factors associated with adherence to DAPT after bare metal stent (BMS) or DES in a multivariate model were recorded.
When the total sample size at the time adherence assessment was not available, the sample size was assumed to remain constant throughout the study. Three studies contained adherence data within figures but did not state exact rates.6, 7, 8 Therefore, adherence rates were estimated from the figures with the use of Plot Digitizer (University of South Alabama, Mobile, AL), and the average rate calculated by the 2 authors was then taken to be the actual rate of medication adherence.
Statistical Analyses
Summary adherence rates were estimated by meta‐analysis with a random effects model. An analysis of the influence of the time period of data collection on adherence rates was prespecified in our protocol and was performed by metaregression with a random effects model, with the end date of data collection as the independent variable. A few small studies had use rates of 100%, resulting in a standard error of 0, which could not be included in the metaregression. Therefore, we subtracted 0.0001% from each of these adherence estimates to allow inclusion. All statistical analyses were performed with Stata 12.1 (StataCorp, College Station, TX) and the metan and metareg packages.
Results
Study Selection
The screening and selection of studies is summarized in the Figure. We reviewed the full text of 299 of 1165 (25.7%) unique records identified through our database search. Of these, 20 studies (6.7%) were included in our analysis of DAPT adherence. Inter‐rater agreement was acceptable for the title and abstract (κ = 0.65) and full‐text (κ = 0.73) reviews. Furthermore, our bibliography review and hand search yielded an additional 14 articles, for a total of 34 articles included in our analysis of DAPT adherence.
Study Characteristics
Thirty‐four studies reported adherence rates for aspirin, thienopyridines, or both at 1, 6, or 12 months after DES placement (Table 1). The studies had a mean sample size of 4286, and data collection spanned April 2002 through February 2010. Most studies (31/34) assessed adherence by asking patients or reviewing the medical record; 3 studies utilized a database to determine medication adherence. Seventy‐nine percent of studies (27/34) defined nonadherence as not taking the medication at the time of assessment.
Table 1.
Properties of Studies Included in the Systematic Review of Dual Antiplatelet Therapy Adherence Rates
| First Author | Publication Year | No. | Period of Data Collection | Country | Data Collection | Source of Adherence Information | Definition of Nonadherence |
|---|---|---|---|---|---|---|---|
| Urban15 | 2006 | 15 157 | 4/2002–9/2005 | International | P | PR/MR | NT |
| Trabattoni16 | 2007 | 867 | 4/2002–12/2004 | Italy | P | PR | NT |
| Airoldi17 | 2007 | 3021 | 6/2002–1/2004 | Italy/Germany | P | PR | NT |
| Schulz18 | 2009 | 6816 | 7/2002–12/2006 | Germany | P | PR | NT |
| Spertus2 | 2006 | 500 | 1/2003–6/2004 | USA | P | PR | NT |
| Petersen8 | 2010 | 9256 | 1/2003–8/2006 | USA | R | D | NT |
| Park19 | 2006 | 1911 | 2/2003–10/2004 | South Korea | P | PR/MR | NT |
| Oh20 | 2012 | 2146 | 3/2003–6/2009 | South Korea | P | PR/MR | NT |
| Gaglia21 | 2010 | 5688 | 4/2003–6/2009 | USA | R | PR | NT |
| Kovacic22 | 2012 | 5681 | 5/2003–5/2008 | USA | P | PR | NT |
| Flores‐Rios23 | 2008 | 604 | 6/2003–2/2005 | Spain | P | PR/MR | NT |
| Ko7 | 2009 | 5263 | 12/2003–3/2006 | Canada | R | D | No rx in 14 days |
| Wang24 | 2009 | 4972 | 1/2004–12/2006 | China | R | PR | NT |
| Abbott25 | 2007 | 1460 | 2/2004–5/2004 | USA | P | PR/MR | NT |
| Lasala26 | 2009 | 7492 | 2/2004–7/2008 | USA | P | PR/MR | NT |
| Pallares27 | 2009 | 257 | 3/2004–8/2005 | USA | P | PR | Taking <80%a |
| Yan28 | 2008 | 1630 | 4/2004–10/2006 | Australia | P | PR/MR | NT |
| Win29 | 2007 | 3323 | 7/2004–9/2005 | USA | P | PR | NT |
| Kimura30 | 2009 | 10 778 | 8/2004–11/2006 | Japan | P | PR/MR | NT for ≥2 months |
| Kimura31 | 2012 | 12 812 | 8/2004–11/2006 | Japan | P | PR/MR | NT for ≥2 months |
| Ikari6 | 2009 | 2051 | 9/2004–9/2005 | Japan | P | PR/MR | NT |
| Tada32 | 2012 | 6802 | 1/2005–12/2007 | Japan | P | PR/MR | NT for ≥2 months |
| Musumeci33 | 2012 | 1437 | 6/2005–6/2008 | Italy | P | PR/MR | NT |
| Lotan34 | 2009 | 8314 | 9/2005–10/2007 | International | P | PR/MR | NT |
| Shroff35 | 2009 | 216 | 9/2005–8/2005 | USA | R | D | MPR‖ <80% |
| Blich36 | 2010 | 314 | 2/2006–1/2007 | Israel | P | PR | NT |
| Urban37 | 2011 | 15 147 | 5/2006–4/2008 | International | P | PR/MR | NT |
| Tsukahara38 | 2010 | 184 | 6/2006–6/2008 | Japan | P | PR/MR | NT |
| Poh39 | 2011 | 203 | 1/2007–12/2007 | Singapore | P | PR | NT for ≥1 week |
| Quadros40 | 2011 | 12 | 11/2007–3/2008 | Brazil | P | PR | NT |
| Fath‐Ordoubadi41 | 2012 | 1640 | 1/2008–12/2009 | International | P | PR/MR | NT |
| Ferreira‐Gonzalez42 | 2012 | 1622 | 1/2008–4/2008 | Spain | P | PR | NT |
| Naidu43 | 2012 | 8061 | 7/2008–2/2010 | USA | P | PR | NT |
| Unverdorben9 | 2007 | 97 | — | Germany | P | PR/MR | NT |
Abbreviations: D, database; MPR, medication possession ratio (see text for definition); MR, medical record; NT, not taking; P, prospective cohort; PR, patient report; R, retrospective cohort; rx, medication.
Studies are sorted in chronological order according to the start of data collection. Properties of the studies by Gaglia21 and Quadros40 include a combined bare‐metal and drug‐eluting stent population.
Taking <80% of medication or missed >2 doses/week.
Baseline demographics and comorbidities varied widely across the included studies (see Supporting Table 2 in the online version of this article). In addition, 56% (19/34) of the studies required written informed consent, 3 studies restricted the patient population to those receiving only 1 specific stent, 26% (9/34) included only a specific class or classes of DESs, and 3 either provided medications for free or ensured that they were covered by insurance.
DAPT Adherence
Rates of DAPT adherence in each individual study are presented in Table 2. Aspirin adherence was reported in 10 studies, thienopyridine adherence in 25, and DAPT adherence in 16. Aspirin use was greater than 90% at 1, 6, and 12 months except in 1 small study.9 In contrast, DAPT and thienopyridine adherence were generally high at 1 month, began to decrease by 6 months, and had declined significantly by 12 months.
Table 2.
Individual Study Estimates of DAPT Use After Drug‐Eluting Stent Implantation
| First Author | Expected Adherence, mo | Aspirin Use, % | Thienopyridine Use, % | DAPT Use, % | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 month | 6 months | 12 months | 1 month | 6 months | 12 months | 1 month | 6 months | 12 months | ||
| Urban15 | 3 | 91.5 | 92.5 | 91.4 | 89.3 | 74.2 | 47.2 | 85.6 | 70.3 | 43.0 |
| Trabattoni16 | 3 | — | — | — | — | — | — | 98.3 | — | — |
| Airoldi17 | 3 | — | — | — | — | 81.2 | 42.6 | 97.6 | 80.7 | 42.8 |
| Schulz18 | 1 | — | — | — | — | — | 80.2 | — | — | — |
| Spertus2 | 3 | — | — | — | 86.4 | 86.7 | 87.1 | — | — | — |
| Petersen8 | 12 | — | — | — | 90.7 | 80.3 | 64.1 | — | — | — |
| Park19 | 6 | — | — | — | — | — | — | — | 96.7 | — |
| Oh20 | 6 | — | — | — | — | 87.9 | — | — | — | — |
| Gaglia21 | 6 | — | — | — | 99.0 | 94.9 | 89.3 | — | — | — |
| Kovacic22 | 12 | — | — | 96.8 | — | — | 97.0 | — | — | 94.6 |
| Flores‐Rios23 | 6 | — | — | — | — | — | — | — | 98.8 | — |
| Ko7 | 12 | — | — | — | 89.9 | 71.9 | 34.4 | — | — | — |
| Wang24 | 9 | — | — | 96.1 | — | — | 94.5 | — | — | — |
| Abbott25 | 3 | — | — | 91.0 | — | — | 59.7 | — | — | — |
| Lasala26 | 6 | — | — | — | — | — | — | — | — | 67.6 |
| Pallares27 | 3 | — | — | — | — | 79.8 | — | — | — | — |
| Yan28 | 3 | — | — | — | 92.0 | — | 61.3 | — | — | — |
| Win29 | 3 | — | 94.7 | 95.5 | — | 86.6 | 78.2 | — | 83.2 | 75.8 |
| Kimura30 | 3 | — | — | — | 97.0 | — | — | — | — | — |
| Kimura31 | 3 | — | — | — | — | 74.9 | 63.0 | — | — | — |
| Ikari6 | 3 | — | — | — | 95.4 | 66.7 | 53.9 | — | — | — |
| Tada32 | 3 | — | — | — | — | 81.8 | 69.4 | — | — | — |
| Musumeci33 | 12 | — | — | — | — | — | — | — | — | 86.9 |
| Lotan34 | 3 | — | — | — | — | — | — | 97.9 | 85.0 | 61.0 |
| Shroff35 | 3 | — | — | — | — | 75.6 | — | — | — | — |
| Blich36 | 12 | — | — | — | — | — | 35.7 | — | — | — |
| Urban37 | 6 | 98.3 | 97.4 | 95.9 | 99.3 | 96.6 | 82.2 | 98.0 | 94.6 | 79.4 |
| Tsukahara38 | 12 | — | — | 99.5 | — | — | — | — | — | 96.2 |
| Poh39 | 12 | — | — | 91.6 | — | — | 93.1 | 96.6 | 91.6 | 87.2 |
| Quadros40 | 12 | — | — | — | 100.0 | — | — | — | — | — |
| Fath‐Ordoubadi41 | 6 | — | — | — | — | — | — | 98.2 | 96.8 | 74.2 |
| Ferreira‐Gonzalez42 | 6 | — | — | — | — | — | — | — | 98.0 | 95.7 |
| Naidu43 | 12 | 95.9 | 93.4 | 91.4 | 97.4 | 94.7 | 90.2 | 94.2 | 90.5 | 85.6 |
| Unverdorben9 | 6 | — | — | 81.3 | — | 99.0 | 100.0 | — | — | — |
Abbreviations: DAPT, dual antiplatelet therapy.
Studies are sorted in chronological order according to the start of data collection.
We attempted to determine the rate of adherence at each time point by meta‐analysis, but there was a high degree of between‐study variance. We therefore performed several subgroup analyses according to study properties (Table 1 and text) to investigate possible sources of this heterogeneity. Metaregression according to any of these variables did not result in a significant reduction in the I 2 statistic, though all of these analyses were underpowered.
Figure 1.
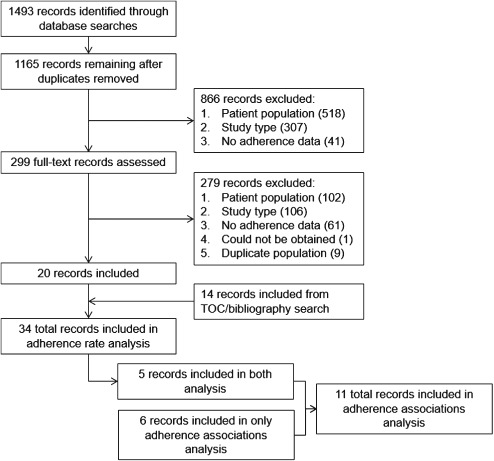
Screening and flow of records through the systematic review. Abbreviations: TOC, table of contents.
As an exploratory analysis, we performed metaregression to predict DAPT use according to the end date of the study to determine the influence of the change in the ACC/AHA guideline4 in 2007 recommending that the duration of DAPT after DES be extended from 3 to 6 months to 12 months. DAPT use at 12 months increased from 53.9% for studies ending in 2004 to 2006 (95% confidence interval [CI]: 33.8%‐73.9%; n = 3) to 82.5% (95% CI: 73.5%‐91.5%; n = 9) for those ending in 2007 to 2009 (P = 0.01, I 2 = 99.9%), whereas thienopyridine use increased from 63.8% (95% CI: 51.8%‐75.8%; n = 12) to 78.1% (95% CI: 68.8%‐87.5%; n = 6) over the same time period (P = 0.02, I 2 = 99.9%).
Factors Associated With Nonadherence to DAPT
A total of 11 studies investigated patient‐level factors associated with nonadherence to DAPT after coronary stenting with either BMS or DES within multivariate models (Table 3). In this analysis, we defined nonadherence as cessation of the course of therapy prior to completion of the originally planned duration. Of the included studies, 1 investigated only bleeding, whereas another focused on factors relevant only to the local population (eg, being non‐Jewish in Israel).
Table 3.
Factors Associated With Nonadherence to DAPT after BMS or DES
| Study | No. | Country | Factor Associated With Nonadherence | OR (95% CI) |
|---|---|---|---|---|
| DAPT | ||||
| Ferreira‐Gonzalez (2010)44 | 1606 | Spain | Oral anticoagulation therapy prescribed at discharge | 3.9 (1.3‐12.0) |
| Immigrant | 3.8 (1.2‐12.0) | |||
| Previous major hemorrhage | 3.8 ( 1.4‐10.0) | |||
| Chronic renal impairment | 2.8 (1.5‐5.3) | |||
| Psychotropic drug consumption | 2.6 (1.3‐5.1) | |||
| Peripheral arterial disease | 1.8 (1.0‐3.2) | |||
| Greater mean number of patients receiving stents | 1.0 (1.0‐1.0) | |||
| Previous myocardial infarction | 0.5 (0.3‐0.8) | |||
| Instructions concerning antiplatelet therapy maintenance administered before discharge (hospital level) | 0.4 (0.2‐0.8) | |||
| Publicly funded hospitals | 0.1 (0.0‐0.6) | |||
| Rossini (2011)45 | 1358 | Italy | In‐hospital major bleeding | 9.0 (3.0‐24.4) |
| Oral anticoagulants at discharge | 8.2 (4.0‐17.0) | |||
| Statin at discharge | 0.4 (0.2‐0.6) | |||
| Kovacic (2012)22 | 5681 | USA | Use of warfarin at discharge | 1.7 (1.3‐2.2) |
| Age (per 1 year increase) | 1.0 (1.0‐1.0) | |||
| Presentation with unstable coronary syndrome or acute myocardial infarction (vs stable presentation) | 0.9 (0.8‐1.0) | |||
| Hypertension | 0.8 (0.7‐1.0) | |||
| Multivessel coronary artery disease | 0.8 (0.7‐0.9) | |||
| Prior myocardial infarction | 0.7 (0.6‐0.9) | |||
| Diabetes | 0.7 (0.6‐0.8) | |||
| Stenting of left main coronary artery | 0.6 (0.4‐0.9) | |||
| Thienopyridines | ||||
| Spertus (2006)2 | 500 | USA | Not completing high school | 1.8 (1.0‐3.1) |
| Ko (2009)7 | 5263 | Canada | Cancer | 1.3 (1.0‐1.8)a |
| Heart failure | 1.2 (1.1‐1.4)a | |||
| Chronic obstructive pulmonary disease | 1.2 (1.0‐1.3)a | |||
| Age | 1.0 (1.0‐1.0)a | |||
| Low income | 0.9 (0.8‐1.0)a | |||
| Blich (2010)36 | 314 | Israel | Non‐Jewish | 19.2 (2.4‐142.0) |
| Lack of explanation of the importance of taking clopidogrel by medical staff at the time of discharge | 10.8 (2.7‐42.9) | |||
| Lack of cardiology follow‐up | 4.7 (1.0‐23.1) | |||
| Armero (2011)46 | 396 | France | Nuisance and internal bleeding | 3.1 (1.0‐9.2) |
| Jura‐Szoltys (2011)47 | 962 | Poland | Liver cirrhosis | 4.8 (1.4‐16.7) |
| Epistaxis | 2.5 (1.4‐4.3) | |||
| History of peptic ulcer disease | 2.1 (1.4‐3.1) | |||
| Bruising | 2.0 (1.4‐3.0) | |||
| Higher‐level education | 0.6 (0.4‐1.0) | |||
| Chronic kidney disease | 0.5 (0.3‐0.9) | |||
| DES implantation | 0.5 (0.3‐0.6) | |||
| Quadros (2011)40 | 400 | Brazil | Salary <2× minimum wage | 8.2 (2.7‐25.0) |
| Lack of health insurance | 4.7 (1.1‐21.0) | |||
| Salary >3× minimum wage | 4.5 (1.3‐16.0) | |||
| Salary 2 × –3× minimum wage | 4.5 (1.3‐16.0) | |||
| Unmarried | 2.5 (1.0‐6.1) | |||
| Acute coronary syndrome | 2.3 (1.3‐4.1) | |||
| Diabetes mellitus | 2.2 (1.0‐4.7) | |||
| Zhu (2011)48 | 9703 | USA | Prior use of clopidogrel (within 12 months) | 1.4 |
| Prior all‐cause hospitalization (within 12 months) | 1.3 | |||
| Did not receive stent during percutaneous coronary intervention | 1.3 | |||
| Chronic obstructive pulmonary disease | 1.3 | |||
| Age <55 years | 1.3 | |||
| Diabetes mellitus | 1.2 | |||
| Prior β‐blocker, statin, ACE inhibitor use (within 12 months) | 0.8 | |||
| Aspirin | ||||
| Cuisset (2011)49 | 308 | France | Immigrant | 8.4 (3.5‐19.8) |
| Treated for diabetes | 4.5 (1.9‐10.9) | |||
| Smoker | 3.1 (1.4‐6.9) | |||
| Older age | 1.0 (1.0‐1.1) |
Abbreviations: ACE, angiotensin‐converting enzyme; CI, confidence interval; DAPT, dual antiplatelet therapy; DES, drug‐eluting stent; OR, odds ratio.
Hazard ratio.
Patient factors associated with nonadherence across multiple studies were limited to the use of oral anticoagulation and previous bleeding (both serious and nuisance). Multiple comorbidities were found to be associated with nonadherence, though only chronic obstructive pulmonary disease (COPD) was associated with nonadherence in more than 1 study. Furthermore, previous myocardial infarction (MI) was found to be associated with improved adherence in 2 studies.
Few studies have comprehensively investigated the effect of socioeconomic factors and healthcare systems factors on DAPT nonadherence. However, lower education level, immigrant status, and the lack of instructions regarding antiplatelet therapy on discharge from the hospital were associated with nonadherence in 2 studies each.
Discussion
Nonadherence to DAPT has long been recognized as an important predictor of poor outcomes after coronary stenting.4 Our systematic review found that rates of DAPT adherence after DES placement are generally high at 1 month, begin to decline by 6 months, and are lower still at 12 months. Furthermore, 12‐month DAPT use after DES increased from 54% in 2004 to 2006 to 83% in 2007 to 2009. These findings demonstrate the effectiveness of clinical guidelines4 in increasing adherence. Although a previous single‐center study reported an increase in DAPT adherence over time,10 this is the first study to comprehensively review diverse, unselected sources of data.
A recent analysis from the PARIS (Patterns of Non‐Adherence to Anti‐Platelet Regimens In Stented Patients) registry suggested that the reason for DAPT cessation influences the risk for major adverse cardiac events, with those stopping therapy due to “disruption” (nonadherence or bleeding) having the highest risk, whereas those discontinuing permanently due to completion of therapy or temporarily due to a need for surgery having a lower risk.11 In this registry, those who discontinued DAPT due to nonadherence or bleeding were older; were less likely to have a previous MI, coronary artery bypass surgery, or diabetes; were more likely to be a smoker, have silent ischemia, or present with an acute coronary syndrome; and were more likely to be on DAPT and oral anticoagulation.11
Our systematic review of patient factors associated with discontinuation of DAPT prior to the prescribed course of therapy (ie, nonadherence) further supports this conclusion. First, previous or in‐hospital bleeding and use of oral anticoagulation were relatively strong predictors of nonadherence, which increases the risk of stent thrombosis. COPD and lack of a previous MI were also associated with nonadherence, though these should be interpreted with caution given the lack of a clear mechanism.
Furthermore, lower education level, immigrant status, and a lack of clearly communicated instructions regarding DAPT prior to discharge from the index hospitalization were associated with nonadherence. These findings suggest that the lack of a clear understanding of DAPT therapy may also contribute to the risk for nonadherence, though few studies of patients with coronary stents have examined nonadherence from the patient's perspective. In 2 small studies, clinicians more frequently identified cost, patient education, and poor transitions in care as common barriers to clopidogrel adherence,12 whereas patients stressed a lack of knowledge and poor communication.13
Therefore, communication between patients and physicians is an important target for interventions aimed at increasing DAPT adherence. In a single‐center study, patients were contacted by telephone at 7 days and at 1, 6, and 9 months post‐DES implantation to encourage DAPT adherence, with resulting “near‐perfect” DAPT adherence.14 Whether these results can be effectively implemented broadly or in the targeted populations at higher risk for nonadherence is worthy of further investigation.
Our study should be interpreted in the context of the available data for analysis. First and foremost, we did not account for the reason for DAPT discontinuation. In addition, our estimates of adherence, especially at 12 months, may be confounded by the time of assessment. For example, 12‐month adherence assessed at 12.5 months may have led to the classification of some who had completed a 12‐month course of DAPT as nonadherent. To minimize this, we specifically excluded any studies that explicitly allowed a range of follow‐up times.
In addition, there was substantial heterogeneity among studies included in this study, specifically in study characteristics, patient populations, data sources, and definitions of adherence. However, this heterogeneity provides some degree of representation of the spectrum of patients receiving coronary stents as well as the diversity across regional medical practices. Furthermore, although the side effect and safety profiles of various P2Y12 inhibitors differ, we were not able to address whether rates of adherence varied according to the type of DAPT prescribed.
Conclusion
We found that adherence to DAPT after DES implantation is high at 1 month, begins to decline by 6 months, and is lower still at 12 months. Efforts to reduce bleeding risk and implement improved direct patient education, possibly targeted to patients with high‐risk features for nonadherence, is an important area of future investigation, with implications for adherence to critical medications more generally.
Supporting information
Search strategies
Supporting References.
List of journals included in manual searches
Properties of studies included in the analysis of DAPT adherence rates.
Acknowledgments
The authors acknowledge Dr. Jose Figueroa and Dr. Daniel Schmidt, both of the Brigham and Women's Hospital and Harvard Medical School in Boston, Massachusetts, and Xinli Hu and Wataru Ebina of Harvard Medical School in Boston, Massachusetts, for their assistance in the review of articles in languages other than English. In addition, the authors acknowledge Julia Whelan of the Francis A. Countway Library of Medicine and Harvard Medical School in Boston, Massachusetts, for her assistance in the development of the literature searches. No compensation was provided for any of these contributions.
Dr. Mauri reports grants to the institution from Abbott, Boston Scientific, Cordis, Medtronic, Eli Lilly, Daiichi‐Sankyo, Bristol‐Myers Squibb, and Sanofi‐Aventis, and consulting for St. Jude Medical and Biotronik. Dr. Yeh is supported by a Career Development Award (1K23HL118138) from the National Heart, Lung, and Blood Institute. None of the remaining authors have any other actual or potential conflicts of interest to report.
References
- 1. Leon MB, Baim DS, Popma JJ, et al. A clinical trial comparing three antithrombotic‐drug regimens after coronary‐artery stenting. N Engl J Med. 1998;339:1665–1671. [DOI] [PubMed] [Google Scholar]
- 2. Spertus JA, Kettelkamp R, Vance C, et al. Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug‐eluting stent placement: results from the PREMIER registry. Circulation. 2006;113:2803–2809. [DOI] [PubMed] [Google Scholar]
- 3. Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug‐eluting stents. JAMA. 2005;293:2126–2130. [DOI] [PubMed] [Google Scholar]
- 4. King SB, Smith SC, Hirshfeld JW, et al. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: 2007 Writing Group to Review New Evidence and Update the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention, writing on behalf of the 2005 Writing Committee. Circulation. 2008;117:261–295. [DOI] [PubMed] [Google Scholar]
- 5.Czarny MJ, Mauri L, Nathan A, et al. Adherence to dual antiplatelet therapy after coronary artery stenting: a systematic review. PROSPERO: International prospective register of systematic reviews. 2012. CRD42012003230. http://www.crd.york.ac.uk/NIHR_PROSPERO/display_record.asp?ID=CRD42012003230.
- 6. Ikari Y, Kotani J, Kozuma K, et al. Assessment of sirolimus‐eluting coronary stent implantation with aspirin plus low dose ticlopidine administration—one year results from CYPHER stent Japan Post‐Marketing Surveillance Registry (J‐PMS). Circ J. 2009;73:1038–1044. [DOI] [PubMed] [Google Scholar]
- 7. Ko DT, Chiu M, Guo H, et al. Patterns of use of thienopyridine therapy after percutaneous coronary interventions with drug‐eluting stents and bare‐metal stents. Am Heart J. 2009;158:592–598. [DOI] [PubMed] [Google Scholar]
- 8. Petersen JL, Barron JJ, Hammill BG, et al. Clopidogrel use and clinical events after drug‐eluting stent implantation: findings from the HealthCore Integrated Research Database. Am Heart J. 2010;159:462–470. [DOI] [PubMed] [Google Scholar]
- 9. Unverdorben M, Degenhardt R, Wiemer M, et al. The paclitaxel‐eluting Coroflex Please stent pilot study (PECOPS I): the one‐year clinical follow‐up. Clin Res Cardiol. 2007;96:803–811. [DOI] [PubMed] [Google Scholar]
- 10. Eisenstein EL, Wojdyla D, Anstrom KJ, et al. Evaluating the impact of public health notification: Duke clopidogrel experience. Circ Cardiovasc Qual Outcomes. 2012;5:767–774. [DOI] [PubMed] [Google Scholar]
- 11. Mehran R, Baber U, Steg PG, et al. Cessation of dual antiplatelet treatment and cardiac events after percutaneous coronary intervention (PARIS): 2 year results from a prospective observational study. Lancet. 2013;382:1714–1722. [DOI] [PubMed] [Google Scholar]
- 12. Decker C, Garavalia L, Garavalia B, et al. Clopidogrel‐taking behavior by drug‐eluting stent patients: discontinuers versus continuers. Patient Prefer Adherence. 2008;2:167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garavalia L, Ho PM, Garavalia B, et al. Clinician‐patient discord: exploring differences in perspectives for discontinuing clopidogrel. Eur J Cardiovasc Nurs. 2011;10:50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rinfret S, Rodes‐Cabau J, Bagur R, et al. Telephone contact to improve adherence to dual antiplatelet therapy after drug‐eluting stent implantation. Heart. 2013;99:562–569. [DOI] [PubMed] [Google Scholar]
- 15. Urban P, Gershlick AH, Guagliumi G, et al. Safety of coronary sirolimus‐eluting stents in daily clinical practice: one‐year follow‐up of the e‐Cypher registry. Circulation. 2006;113:1434–1441. [DOI] [PubMed] [Google Scholar]
- 16. Trabattoni D, Fabbiocchi F, Montorsi P, et al. Stent thrombosis after sirolimus‐ and paclitaxel‐eluting stent implantation in daily clinical practice: analysis of a single center registry. Catheter Cardiovasc Interv. 2007;70:415–421. [DOI] [PubMed] [Google Scholar]
- 17. Airoldi F, Colombo A, Morici N, et al. Incidence and predictors of drug‐eluting stent thrombosis during and after discontinuation of thienopyridine treatment. Circulation. 2007;116:745–754. [DOI] [PubMed] [Google Scholar]
- 18. Schulz S, Schuster T, Mehilli J, et al. Stent thrombosis after drug‐eluting stent implantation: incidence, timing, and relation to discontinuation of clopidogrel therapy over a 4‐year period. Eur Heart J. 2009;30:2714–2721. [DOI] [PubMed] [Google Scholar]
- 19. Park DW, Park SW, Park KH, et al. Frequency of and risk factors for stent thrombosis after drug‐eluting stent implantation during long‐term follow‐up. Am J Cardiol. 2006;98:352–356. [DOI] [PubMed] [Google Scholar]
- 20. Oh IY, Park KW, Kang SH, et al. Association of cytochrome P450 2C19*2 polymorphism with clopidogrel response variability and cardiovascular events in Koreans treated with drug‐eluting stents. Heart. 2012;98:139–144. [DOI] [PubMed] [Google Scholar]
- 21. Gaglia MA Jr, Torguson R, Xue Z, et al. Insurance type influences the use of drug‐eluting stents. JACC Cardiovasc Interv. 2010;3:773–779. [DOI] [PubMed] [Google Scholar]
- 22. Kovacic JC, Lee P, Karajgikar R, et al. Safety of temporary and permanent suspension of antiplatelet therapy after drug eluting stent implantation in contemporary “real‐world” practice. J Interv Cardiol. 2012;25:482–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Flores‐Rios X, Marzoa‐Rivas R, Abugattas‐de Torres JP, et al. Late thrombosis of paclitaxel‐eluting stents: long‐term incidence, clinical consequences, and risk factors in a cohort of 604 patients. Am Heart J. 2008;155:648–653. [DOI] [PubMed] [Google Scholar]
- 24. Wang ZJ, Zhou YJ, Liu YY, et al. Obesity and cardiovascular thrombotic events in patients undergoing percutaneous coronary intervention with drug‐eluting stents. Heart. 2009;95:1587–1592. [DOI] [PubMed] [Google Scholar]
- 25. Abbott JD, Vlachos HA, Selzer F, et al. Gender‐based outcomes in percutaneous coronary intervention with drug‐eluting stents (from the National Heart, Lung, and Blood Institute Dynamic Registry). Am J Cardiol. 2007;99:626–631. [DOI] [PubMed] [Google Scholar]
- 26. Lasala JM, Cox DA, Morris DL, et al. Two‐year results of paclitaxel‐eluting stents in patients with medically treated diabetes mellitus from the TAXUS ARRIVE program. Am J Cardiol. 2009;103:1663–1671. [DOI] [PubMed] [Google Scholar]
- 27. Pallares MJ, Powers ER, Zwerner PL, et al. Barriers to clopidogrel adherence following placement of drug‐eluting stents. Ann Pharmacother. 2009;43:259–267. [DOI] [PubMed] [Google Scholar]
- 28. Yan BP, Duffy SJ, Clark DJ, et al. Rates of stent thrombosis in bare‐metal versus drug‐eluting stents (from a large Australian multicenter registry). Am J Cardiol. 2008;101:1716–1722. [DOI] [PubMed] [Google Scholar]
- 29. Win HK, Caldera AE, Maresh K, et al. Clinical outcomes and stent thrombosis following off‐label use of drug‐eluting stents. JAMA. 2007;297:2001–2009. [DOI] [PubMed] [Google Scholar]
- 30. Kimura T, Morimoto T, Nakagawa Y, et al. Antiplatelet therapy and stent thrombosis after sirolimus‐eluting stent implantation. Circulation. 2009;119:987–995. [DOI] [PubMed] [Google Scholar]
- 31. Kimura T, Morimoto T, Nakagawa Y, et al. Very late stent thrombosis and late target lesion revascularization after sirolimus‐eluting stent implantation: five‐year outcome of the j‐Cypher Registry. Circulation. 2012;125:584–591. [DOI] [PubMed] [Google Scholar]
- 32. Tada T, Natsuaki M, Morimoto T, et al. Duration of dual antiplatelet therapy and long‐term clinical outcome after coronary drug‐eluting stent implantation: landmark analyses from the CREDO‐Kyoto PCI/CABG Registry Cohort‐2. Circ Cardiovasc Interv. 2012;5:381–391. [DOI] [PubMed] [Google Scholar]
- 33. Musumeci G, Rossini R, Lettieri C, et al. Prognostic implications of early and long‐term bleeding events in patients on one‐year dual antiplatelet therapy following drug‐eluting stent implantation. Catheter Cardiovasc Interv. 2012;80:395–405. [DOI] [PubMed] [Google Scholar]
- 34. Lotan C, Meredith IT, Mauri L, et al. Safety and effectiveness of the Endeavor zotarolimus‐eluting stent in real‐world clinical practice: 12‐month data from the E‐Five registry. JACC Cardiovasc Interv. 2009;2:1227–1235. [DOI] [PubMed] [Google Scholar]
- 35. Shroff A, Ali A, Groo VL. Clopidogrel adherence following percutaneous coronary intervention with a drug‐eluting stent in a VA medical center. J Pharm Technol. 2009;25:164–168. [Google Scholar]
- 36. Blich M, Zeidan‐Shwiri T, Petcherski S, et al. Incidence, predictors and outcome of drug‐eluting stent thrombosis in real‐world practice. J Invasive Cardiol. 2010;22:461–464. [PubMed] [Google Scholar]
- 37. Urban P, Abizaid A, Banning A, et al. Stent thrombosis and bleeding complications after implantation of sirolimus‐eluting coronary stents in an unselected worldwide population: a report from the e‐SELECT (Multi‐Center Post‐Market Surveillance) registry. J Am Coll Cardiol. 2011;57:1445–1454. [DOI] [PubMed] [Google Scholar]
- 38. Tsukahara K, Kimura K, Morita S, et al. Impact of high‐responsiveness to dual antiplatelet therapy on bleeding complications in patients receiving drug‐eluting stents. Circ J. 2010;74:679–685. [DOI] [PubMed] [Google Scholar]
- 39. Poh CL, Chan MY, Lau C, et al. Prevalence and predictors of premature discontinuation of dual antiplatelet therapy after drug‐eluting stent implantation: importance of social factors in Asian patients. Intern Med J. 2011;41:623–629. [DOI] [PubMed] [Google Scholar]
- 40. Quadros AS, Welter DI, Camozzatto FO, et al. Identifying patients at risk for premature discontinuation of thienopyridine after coronary stent implantation. Am J Cardiol. 2011;107:685–689. [DOI] [PubMed] [Google Scholar]
- 41. Fath‐Ordoubadi F, Barac Y, Abergel E, et al. Gender impact on prognosis of acute coronary syndrome patients treated with drug‐eluting stents. Am J Cardiol. 2012;110:636–642. [DOI] [PubMed] [Google Scholar]
- 42. Ferreira‐Gonzalez I, Marsal JR, Ribera A, et al. Double antiplatelet therapy after drug‐eluting stent implantation: risk associated with discontinuation within the first year. J Am Coll Cardiol. 2012;60:1333–1339. [DOI] [PubMed] [Google Scholar]
- 43. Naidu SS, Krucoff MW, Rutledge DR, et al. Contemporary incidence and predictors of stent thrombosis and other major adverse cardiac events in the year after XIENCE V implantation: results from the 8,061‐patient XIENCE V United States study. JACC Cardiovasc Interv. 2012;5:626–635. [DOI] [PubMed] [Google Scholar]
- 44. Ferreira‐Gonzalez I, Marsal JR, Ribera A, et al. Background, incidence, and predictors of antiplatelet therapy discontinuation during the first year after drug‐eluting stent implantation. Circulation. 2010;122:1017–1025. [DOI] [PubMed] [Google Scholar]
- 45. Rossini R, Capodanno D, Lettieri C, et al. Prevalence, predictors, and long‐term prognosis of premature discontinuation of oral antiplatelet therapy after drug eluting stent implantation. Am J Cardiol. 2011;107:186–194. [DOI] [PubMed] [Google Scholar]
- 46. Armero S, Bonello L, Berbis J, et al. Rate of nuisance bleedings and impact on compliance to prasugrel in acute coronary syndromes. Am J Cardiol. 2011;108:1710–1713. [DOI] [PubMed] [Google Scholar]
- 47. Jura‐Szoltys E, Chudek J. Epistaxis as the reason for premature discontinuation of clopidogrel after percutaneous coronary angioplasty with stent implantation. Kardiol Pol. 2011;69:817–823. [PubMed] [Google Scholar]
- 48. Zhu B, Zhao Z, McCollam P, et al. Factors associated with clopidogrel use, adherence, and persistence in patients with acute coronary syndromes undergoing percutaneous coronary intervention. Curr Med Res Opin. 2011;27:633–641. [DOI] [PubMed] [Google Scholar]
- 49. Cuisset T, Quilici J, Fugon L, et al. Non‐adherence to aspirin in patients undergoing coronary stenting: negative impact of comorbid conditions and implications for clinical management. Arch Cardiovasc Dis. 2011;104:306–312. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search strategies
Supporting References.
List of journals included in manual searches
Properties of studies included in the analysis of DAPT adherence rates.


