Abstract
It is well known that the protective capacity of the collateral circulation falls short in many individuals with ischemic disease of the heart, brain and lower extremities. In the past fifteen years, opportunities created by molecular and genetic tools, together with disappointing outcomes in many “angiogenic” trials, has led to a significant increase in the number of studies that focus on: 1) understanding the basic biology of the collateral circulation; 2) identifying the mechanisms that limit the collateral circulation’s capacity in many individuals; 3) devising methods to measure collateral extent, which has been found to vary widely among individuals; and 4) developing treatments to increase collateral blood flow in obstructive disease. Unfortunately, accompanying this increase in reports has been a proliferation of vague terms used to describe the disposition and behavior of this unique circulation, as well as the increasing miss-use of well-ensconced ones by new (and old) students of the collateral circulation. With this in mind, we provide a brief glossary of readily understandable terms to denote the formation, adaptive growth, and mal-adaptive rarefaction of the collateral circulation. We also propose terminology for several newly discovered processes that occur in the collateral circulation. Finally, we include terms used to describe vessels that are sometimes confused with collaterals, as well as terms describing processes active in the general arterial-venous circulation when ischemic conditions engage the collateral circulation. We hope this brief review will help unify the terminology used in collateral research.
Keywords: collateral circulation, ischemic heart disease, ischemic stroke, peripheral artery disease, angiogenesis
The collateral circulation is a network of specialized “endogenous bypass vessels” that is present in most tissues and provides protection against ischemic injury caused by ischemic stroke, coronary atherosclerosis, peripheral artery disease and other conditions and diseases. Unfortunately, this protection falls far short in a large fraction of individuals due to differences in the number or diameter of these vessels present before—and/or their ability to undergo adaptive growth after—the onset of occlusive disease. Not surprising then, are the increasing number of reports in the literature that focus on: 1) understanding the basic biology of the collateral circulation; 2) identifying the genetic, cellular and environmental mechanisms that limit the collateral circulation’s capacity in many individuals to provide alternative routes of flow; 3) devising methods to measure collateral extent, which varies widely among individuals; and 4) developing treatments to increase collateral blood flow in obstructive disease. Indeed, it is becoming evident that collaterals differ so much from arteries, capillaries and veins in phenotypic and functional properties that they might well be considered a “third circulation” along with the general arterial-venous and lymphatic circulations. However, much less is known about the collateral circulation.
Accompanying the increased number of papers studying the collateral circulation in tissues is a mushroom-like proliferation of vague terms used to describe the disposition and behavior of these peculiar vessels. Webster’s Dictionary defines collateral as “coinciding in tendency or effect.” Accordingly, a collateral vessel would be one capable of supplying flow to an area normally supplied by another vessel, ie, coinciding in effect. However, the term has other common meanings, as shown by the many miss-hits returned by a Pubmed or Google search on “collateral”, when the searcher’s interest is the collateral circulation, rather than arteriovenous shunts, recurrent nerves, ligaments, roadways or real estate purchases. Moreover, in addition to the proliferation of ambiguous terms is the increasing miss-use of well-ensconced ones by new (and old!) students of the collateral circulation.
To help “collaterologists” better communicate with each other and with the outsider looking in, we offer here a brief glossary of, hopefully, readily understandable terms that are often used to denote the formation, adaptive growth, and mal-adaptive rarefaction of the collateral circulation. We also include terms used to describe vessels that are sometimes confused with collaterals, or processes active in the general arterial-venous circulation that accompany ischemic conditions that engage the collateral circulation. We hope this modest attempt at unifying the terminology used in study of the collateral circulation encourages practitioners to send us feedback and additional terms that we may have missed. Where needed, a term and its definition is followed in brackets by terms, both old and new, that have been used to mean the same thing but are either less clear and we suggest better avoided or used with caution, or are/have been used in other areas of vascular research to mean something different thus creating an opportunity for confusion. Lastly, given the availability of many reviews addressing the collateral circulation and the general vascular terms discussed below, as well as the nature and limited-space format of this commentary, we provide a limited number of representative references when the issue at hand is less widely known, not addressed in the reviews, or has only recently been described.
Collaterals — naturally occurring artery-to-artery or arteriole-to-arteriole anastomoses present in healthy tissues that increase their anatomic diameter, ie, outwardly remodel, in obstructive disease (Figure). Because they cross-connect two feed-arteries or the crowns of adjacent arterial trees, respectively, blood flow along their length comes from opposite directions in the healthy tissue at baseline.1–3 This results in a “to and fro” flow that prevents hemostatic thrombosis, resulting in little or no net-flow at a point generally near the midpoint of the collateral. Thus, collaterals reside in a unique hemodynamic environment of low and oscillatory shear stress. Collaterals should not be confused with arteriovenous anastomoses (shunt vessels) which have long been called collaterals in some clinical areas (eg, lung4). Two types of collaterals are distinguished:
Collateral arteries — artery-to-artery anastomoses that tend to be present in similar locations among humans and other mammalian species. Usually carry explicit names in human, eg: superior ulnar collateral artery, genicular artery and other anastomotic arteries around elbows, knees and other articulations, palmar and plantar arch collaterals, ileolumbar-superior epigastric communicating artery, bronchial-to-pulmonary vein arteries, other collateral arteries in the abdomen and thorax, anterior and posterior communicating arteries/collaterals of the circle of Willis. Compared to microvascular collaterals (defined below), collateral arteries in healthy young adults generally exhibit minimal or no tortuosity, undergo considerably less anatomic lumen enlargement on a percentage basis (“remodeling”) in response to a chronic increase in shear stress in obstructive disease,5,6 form during embryogenesis by a different process (discussed below).
Microvascular collaterals — arteriole-to-arteriole anastomoses that cross-connect a small fraction (generally < 0.05%) of the arterioles in the crowns of adjacent arterial trees. Average less than 100 microns diameter in most healthy species including human (Figure). Present in most but not all tissues (eg, absent in the retinal circulation and non-capsular kidney except in rare circumstances7). Examples: pial (leptomenigeal) collaterals of the brain and spinal cord, coronary collaterals, collaterals in skeletal muscle and skin. Depending on species and tissue, several collaterals may have artery-size calibers (greater than ~150 µm, the diameter generally used to distinguish arterioles from arteries8) eg, in the healthy heart9–11 and between the crowns of the superior and inferior epigastric and other thoraco-abdominal artery trees. Characteristics of microvascular collaterals: significant tortuosity even in young adults, outward remodeling of their lumen diameters generally by 5-to-10-fold in humans with occlusive disease,10–12 and at least in mouse strains, large genetic background-dependent variability in their number, diameter and remodeling.2,13 Since many collateral arteries carry explicit names (eg, as given above), use of the term “collaterals”, alone, will usually imply the population of microvascular collaterals in a given tissue.
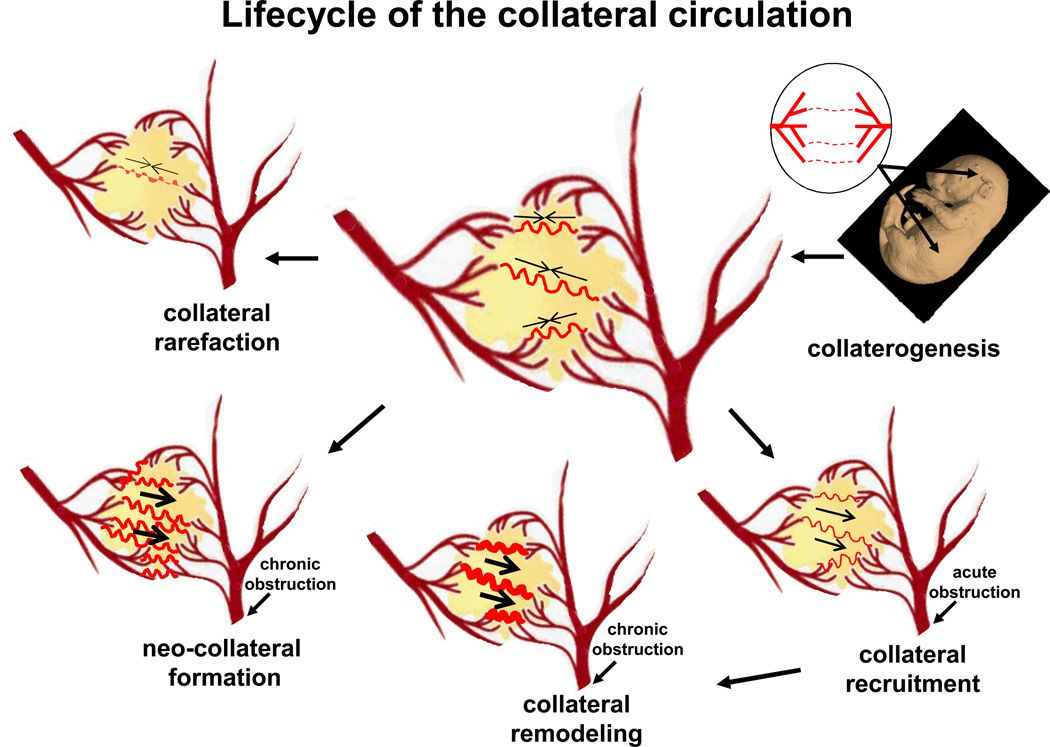
Figure.
Major aspects of the vascular biology of the collateral circulation. Embryonic formation of native collaterals (collaterogenesis), which is depicted in the upper right diagram as several collaterals forming between two trees, begins at ~E14 in mouse and determines collateral extent in the adult (center diagram with several collaterals shown connecting two arterial trees).37 Acute obstruction induces flow across the collateral network (recruitment), followed by remodeling and, potentially, formation of additional collaterals in chronic obstructive disease (neo-collateral formation). Loss of native collaterals (rarefaction) can be caused by aging and other risk factors. The two arterial trees in the center of figure are modification of the logo for the Fifth International Angiogenesis Meeting, Amsterdam, 2014. The mouse embryo image (E14) is from: http://www.emouseatlas.org/emap/ema/home.html.
Collateral arteries and microvascular collaterals usually have one or more side-branches. Thus, besides presumably helping to optimize regional metabolic control of oxygen delivery in healthy tissues, collaterals also serve as scaffolds for delivery of blood flow to parenchymal tissue within the “collateral zone” (watershed) between adjacent artery trees.13
The above collateral vessels are further distinguished from other types of arterial anastomoses that are collateral-like but are not denoted as collaterals, per se. There are two types:
Intra-tree anastomoses — arteriole-to-arteriole anastomoses that, where present, connect adjacent branches within a given tree. Present in many tissues, eg, in heart,9 skeletal muscle,14,15 intestinal mesentery,8 and the cerebral cortex of many species (eg, rat16 but not mouse2). Like collaterals, they can have opposing flow at their mid-point.8 They also serve the same “endogenous bypass function” of collaterals if an adjacent branch becomes obstructed.17 However, since they protect much less tissue, do not interconnect separate arterial trees, are much shorter and have little or no tortuosity, they are denoted with a unique term.
Arcade arteries — arteries that often take an arching course and may or may not, depending on the individual, anastomose with another artery, for example in human: gastric and omental arcade arteries, marginal mesenteric arteries, pancreaticoduodenal arcade, gastroepiploic artery, internal thoracic artery, collateral intercostal artery, vasa vasorum of the aorta, and arteries supplying long axial structures (eg, bile duct, trachea, spinal cord).18
Native collaterals — collateral arteries and microvascular collaterals that are present in healthy tissues, ie, free of arterial obstruction (Figure). A healthy tissue at rest has little or no pressure drop across its collaterals, thus little or no net collateral flow between the interconnected trees.2–3 This causes even high resolution flow-based imaging methods (eg, laser speckle contrast) to be “blind” to the presence of native collaterals until obstruction is induced.19 Also, microvascular collaterals in healthy tissues are generally below the resolution of conventional in vivo angiographic methods. Thus, the functional importance of native collaterals in many tissues was doubted for many years.10,11 However, improvements in vascular casting and other anatomic techniques (eg, 3D reconstruction with micro-CT or cryomicrotome9), Doppler and other flow-based imaging methods, and indirect methods of measuring collateral-dependent flow,20,21 allow detection in experimental animals and patients, although limits to resolution for non-casting methods do not allow quantification of collateral extent below certain diameters. [Synonym: pre-existing collaterals].
Collateral extent — collective term to denote the combination of number and average diameter of native, pre-existing collaterals in a tissue without obstructed flow. Collateral extent varies widely among and within the same species—at least in the case of mice—primarily due to variation at a single genetic locus.23 It has also been suggested to vary widely in the coronary circulation of healthy pound dogs, based on a 10-fold variation in wedge pressure measured from the circumflex artery.22 And likewise in patients with acute stroke20 or without coronary artery disease,21 where differences in collateral remodeling (ie, arteriogenesis, defined below) secondary to chronic arterial obstruction are not present to confound the interpretation vis-à-vis native collateral extent. “Environmental” factors such as aging and chronic endothelial dysfunction have been shown to reduce collateral extent in mice.24 [Avoid: collateralization.]
Collateral recruitment — induction of flow from an adjacent arterial tree(s) to the obstructed tree across a collateral(s) (Figure). Caused by sudden or chronic obstruction, which creates a pressure drop across the collateral network. [Avoid: active/patent/open collaterals, since native collaterals have not been observed to reside in a constricted, closed state.]
Arteriogenesis — anatomic increase in lumen area and wall thickness of a collateral vessel. Induced by a shear stress-mediated process10–12 and in certain tissues from factors released in the ischemic environment.25 Induced by periodic or sustained, or partial or complete, obstruction of flow in one of the adjacent arterial trees, which causes unidirectional flow across the collateral(s). Requires days-to-weeks for the final new diameter to be achieved, depending on tissue, species and amount of remodeling. Usually accompanied by an increase in collateral length, resulting in increased tortuosity. Synonym: collateral remodeling (Figure). [Avoid collateral: -dilation, since dilation denotes a sudden increase in vessel diameter due to relaxation of smooth muscle; -dilatation or -expansion, since these terms are variably used to mean either anatomic lumen enlargement or dilation; -development or -formation, since these terms are also used to denote de-novo vessel formation in the embryo or after birth; -growth, since this is often used as a synonym for arteriogenesis but does not distinguish it from formation of new collaterals (see “collaterogenesis”, below). Avoid: collateralization.]
Of note, the term arteriogenesis with its above definition was introduced nearly two decades ago26 and since then has been used in the majority of papers studying the collateral circulation to denote the above process. However, the term has also acquired several different meanings: 1) Arteriogenesis was used in several earlier reports to describe the outgrowth of surrounding arterioles into prolactinomas in the anterior pituitary27 and into surgically placed plastic meshes.28 2) It is also used in developmental biology to indicate the formation of artery trees in the embryo by remodeling of the primary embryonic capillary plexus (termed “tree formation” or “arterial morphogenesis”, discussed below). 3) Furthermore, arteriogenesis has been used to denote an increase in the size (territory) of an arterial tree during post-natal tissue growth or due to other conditions or experimental interventions29 (termed “tree growth”, discussed below).
Other notes: Arteriogenesis has often been used to denote the remodeling of native microvascular collateral arterioles into arteries, sometimes accompanied by the statement that the former have little or no importance until they remodel into “functional arteries”. However, it is becoming more widely appreciated that, depending on their extent, native microvascular collaterals can provide significant functional flow that substantially lessens ischemic tissue injury after sudden arterial obstruction before they remodel.13,22–24,30 Improved (or impaired) arteriogenesis/collateral remodeling is often invoked as the sole mechanism underlying an experimental intervention that results in an increase (or decrease) in recovery of blood flow to a tissue over time after arterial obstruction, even though other mechanisms may be involved (albeit generally with less impact) that are capable of causing or contributing to this, eg, angiogenesis (increased capillary number), or changes in pressure and resistance above or below the collateral network—the latter from vasoactive, edematous or leukocyte-platelet adhesive mechanisms.
Collateral regression — the process of reverse-remodeling that occurs after removal of an obstruction (eg, after stenting),31 or in the setting of sudden obstruction wherein several native collaterals sometimes remodel more and thus become “dominant” over others that regress back toward their native diameters.30,32 If the aforementioned scenarios lead to loss of collaterals that were formerly present, rather than regression below threshold for detection, this would constitute collateral regression (see below), although such has not been described in the literature.
Collateral rarefaction — loss of native collateral number and/or a decrease in collateral diameter from that present in the healthy adult (Figure). Studies in mice have found that a decrease in the number and diameter of microvascular collaterals occurs with aging, endothelial dysfunction, and the presence of certain other cardiovascular risk factors and diseases24—findings supported by recent studies in humans.33,34 [Avoid “pruning” in this context since it is better used to denote the process during normal development of removal of a portion of the branches from a nascent capillary plexus, eg, in the developing retinal vasculature35 and the removal during the first few weeks after birth of a fraction of the nascent arteriole anastomoses that form during collaterogenesis in the mouse embryo.2]
Collaterogenesis — formation of collaterals during embryonic and postnatal development to yield the collateral extent present in the healthy adult tissue. There are two types:
Arterial collaterogenesis: Based mostly on studies of the origin of the collateral arteries of the circle of Willis where it has been studied in detail,eg, 36 arterial collaterals present in the adult may arise by either retension of a vessel(s) present early in embryonic development (embryonic remnant) or by a different collateral connection that replaces the early vessel later in development. This results in the variable presence, diameter and location of arterial collaterals among adults (eg, presence in the adult of the “embryonic” (remnant) versus “adult” morphology/pattern of the posterior communicating collateral arteries of the circle of Willis).
Microvascular collaterogenesis: Based on studies in mouse brain were the process can be studied with fidelity, collaterals form during gestation relatively late, after the arterial trees have formed (Figure).2,37 The nascent collaterals then become invested with smooth muscle cells, increase their diameter, and reduce their number by a pruning process. This process of maturation of the collateral circulation is completed during the first several weeks after birth, resulting in the collateral number and diameter present in the adult. Coronary collaterogenesis in humans also occurs relatively late (19-39 weeks of gestation),38 after coronary artery tree formation (weeks 8-16).10
Neo-collateral formation — de novo formation of additional or “new” microvascular collaterals after arterial obstruction in the adult (Figure). Whether this occurs has long been debated,10,11 since previous studies were unable to exclude the possibility that newly detected collaterals in the setting of arterial obstruction were native collaterals that had escaped detection until they had remodeled. However, neo-collateral formation has recently been reported after acute arterial occlusion in murine brain13 and skeletal muscle.39 Supporting the concept that neo-collaterals can form in humans is the well-known detection of an apparent profusion of ectopic microvascular collaterals in patients with moyamoya syndrome,40 the salutory effect of the encephaloduro-arteriosynangiosis surgical procedure used in certain types of cerebral artery occlusion not amenable to thrombolysis or thrombectomy41 and the Vineberg procedure that antedated coronary artery bypass grafting.42
The following are terms describing processes often active in the general arterial-venous circulation when ischemic conditions engage the collateral circulation. They are also sometimes used to include or even denote collateral remodeling/arteriogenesis or neocollateral formation, which can result in confusion.
Arterialization — increase in number and/or length of the distal-most arterioles of an arterial tree, ie, an increase in territory of an artery tree in the adult. Caused by chronic exercise or muscle loading,43 chronic dilation-induced increase in wall stress within a tree,44,45 and in certain experimental settings showing that arterialization depends on a distinct signaling cascade.46 Evidence supports involvement of muralization by smooth muscle cells of pre-existing and/or newly formed capillaries, followed by lumen enlargement and wall thickening, a process that has also been associated with neo-collateral formation.39 A related process, distal muscularization, occurs in pulmonary hypertension, where small precapillary arterioles that normally lack smooth muscle cells become invested with smooth muscle actin-expressing cells.47 Note, arterialization is also used to denote the changes in thickness of a donor vein after arterial bypass grafting or pathological conditions such as in the portal vein in acute liver failure.
Tree growth — the process during gestation, postnatal tissue growth, vascular hypertrophy or other conditions, of an increase in anatomic territory of an artery or vein tree. Often quantified as an increase in the diameter of its trunk and branches, branch number, branch length, number of branching orders, and number of branches each order has. Synonym: arterialization when referring to arterial trees in the adult.
Angiogenesis — formation of capillaries from pre-existing capillaries. Two variations are distinguished, based on time of occurrence:
Embryonic angiogenesis — formation of additional capillaries in the embryonic plexus and certain elongated capillary vessels (eg, intersomitic vessels sprouting from the dorsal aortae) due to sprouting from existing capillaries and intussusception of pre-existing capillaries. [Confusion may arise with use of the term, angiogenesis, to include the subsequent embryonic remodeling of the plexus into artery and vein trees—a process that is also denoted arterial and venous morphogenesis.]
Postnatal/adult angiogenesis —increase in capillary number or length caused by sprouting or intussusception that occurs in a tissue during growth to adulthood, adaptive hypertrophy, or changes in metabolism (eg, skeletal muscle fiber type switching). Also accompanies repair and pathological processes (eg, tissue injury, ischemia, inflammation, tumor growth). In certain tissues the capillary plexus forms after birth and then undergoes remodeling into artery and vein trees (eg, mouse retina).
Importantly, the term angiogenesis is sometimes used as a general term to denote the de novo formation or increase in diameter, length or branching of any type of vessel in any type of physiological, pathological or therapeutic setting during gestation and thereafter. However, this general use of the term is subject to confusion.
Therapeutic angiogenesis — general term used to denote a pharmacologic (or other therapeutic) stimulation of “vessel growth” or new vessel formation, resulting in an increase in tissue blood flow at baseline or at maximal dilation after acute or chronic arterial obstruction. It is attributable to one or more of the following in response to a treatment administered before or after onset of arterial obstruction: 1) increased number of capillaries (angiogenesis), 2) remodeling of native collaterals, 3) neo-collateral formation, and 4) arterialization. However, in addition to potential contributions of these mechanisms, such increases in flow can also be caused by an increase in perfusion pressure or decrease in resistance above or below a collateral network whose extent is unchanged. Given the above, and since the term does not denote an underlying mechanism(s), its meaning can be misapplied or misinterpreted if the context is not made clear. Thus far, no therapeutics that augment angiogenesis or collateral remodeling in patients have been approved, although several are under investigation. [Synonym: therapeutic revascularization, which usually refers to abolishment or lessening of the deficiency of perfusion in obstructive conditions by one or more of the above mechanisms. However, a number of different endovascular and surgical arterial procedures are also termed revascularization.]
Neovascularization — a synonym for “therapeutic angiogenesis”. However, since it can also mean physiological or pathological (eg, inflammatory) “vessel growth”, per the above mechanisms, its use is subject to the same limitations as therapeutic angiogenesis. [Synonym: neo-vessel formation]
Acknowledgements
We thank the members of our labs for thoughtful comments during the preparation of this manuscript.
Sources of Funding
National Institutes of Health grants RO1 HL111070, NS083633 (JEF), HL083366, HL115114, Fibus Family Foundation (WMC), Fritz Bender Stiftung (ED), R01 HL 084619, P01 HL107205 and Leducq Foundation ARTERMIS Network (MS).
Footnotes
Disclosures
None.
References
- 1.Trzeciakowski J, Chilian WM. Chaotic behavior of the coronary circulation. Med Biol Eng Comput. 2008;46:433–442. doi: 10.1007/s11517-008-0329-8. [DOI] [PubMed] [Google Scholar]
- 2.Chalothorn D, Faber JE. Formation and maturation or the murine native cerebral collateral circulation. J Molec Cell Cardiol. 2010;49:251–259. doi: 10.1016/j.yjmcc.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toriumi H, Tatarishvili J, Tomita M, Tomita Y, Unekawa M, Suzuki N. Dually supplied T-junctions in arteriolo-arteriolar anastomosis in mice: key to local hemodynamic homeostasis in normal and ischemic states? Stroke. 2009;40:3378–3383. doi: 10.1161/STROKEAHA.109.558577. [DOI] [PubMed] [Google Scholar]
- 4.Porres DV, Morenza OP, Pallisa E, Roque A, Andreu J, Martínez M. Learning from the pulmonary veins. Radiographics. 2013;33:999–1022. doi: 10.1148/rg.334125043. [DOI] [PubMed] [Google Scholar]
- 5.Hillmeister P, Gatzke N, Dülsner A, Bader M, Schadock I, Hoefer I, Hamann I, Infante-Duarte C, Jung G, Troidl K, Urban D, Stawowy P, Frentsch M, Li M, Nagorka S, Wang H, Shi Y, le Noble F, Buschmann I. Arteriogenesis is modulated by bradykinin receptor signaling. Circ Res. 2011;109:524–533. doi: 10.1161/CIRCRESAHA.111.240986. [DOI] [PubMed] [Google Scholar]
- 6.Hecht N, He J, Kremenetskaia I, Nieminen M, Vajkoczy P, Woitzik J. Cerebral hemodynamic reserve and vascular remodeling in C57/BL6 mice are influenced by age. Stroke. 2012;43:3052–3062. doi: 10.1161/STROKEAHA.112.653204. [DOI] [PubMed] [Google Scholar]
- 7.Shoja MM, Tubbs RS, Loukas M, Shokouhi G, Ghabili K, Agutter PS. The sub-peritoneal arterial plexus of Sir William Turner. Ann Anat. 2010;192:194–198. doi: 10.1016/j.aanat.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Zweifach BW. Microcirculation. Annu Rev Physiol. 1973;35:117–150. doi: 10.1146/annurev.ph.35.030173.001001. [DOI] [PubMed] [Google Scholar]
- 9.van Horssen P, Siebes M, Spaan JA, Hoefer IE, van den Wijngaard JP. Innate collateral segments are predominantly present in the subendocardium without preferential connectivity within the left ventricular wall. J Physiol. 2014;592(Pt 5):1047–1060. doi: 10.1113/jphysiol.2013.258855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomanek RJ. Coronary Vasculature. New York: Springer; 2013. [Google Scholar]
- 11.Schaper W. Collateral circulation: Past and present. Basic Res Cardiol. 2009;104:5–21. doi: 10.1007/s00395-008-0760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Royen N, Piek JJ, Schaper W, Fulton WF. A critical review of clinical arteriogenesis research. J Am Coll Cardiol. 2009;55:17–25. doi: 10.1016/j.jacc.2009.06.058. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Prabhakar P, Sealock RW, Faber JE. Wide genetic variation in the native pial collateral circulation is a major determinant of variation in severity of stroke. J Cerebral Blood Flow Metab. 2010;30:923–934. doi: 10.1038/jcbfm.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engelson ET, Schmid-Schönbein GW, Zweifach BW. The microvasculature in skeletal muscle. II. Arteriolar network anatomy in normotensive and spontaneously hypertensive rats. Microvasc Res. 1986;31:356–374. doi: 10.1016/0026-2862(86)90024-5. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson E, Germann G, Mathur A. Microcirculation in muscle. Ann Plast Surg. 1986;17:13–16. doi: 10.1097/00000637-198607000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Coyle P. Diameter and length changes in cerebral collaterals after middle cerebral artery occlusion in the young rat. Anat Rec. 1984;210:357–364. doi: 10.1002/ar.1092100211. [DOI] [PubMed] [Google Scholar]
- 17.Wei L, Erinjeri JP, Rovainen CM, Woolsey TA. Collateral growth and angiogenesis around cortical stroke. Stroke. 2001;32:2179–2184. doi: 10.1161/hs0901.094282. [DOI] [PubMed] [Google Scholar]
- 18.Uflacker R. Atlas of Vascular Anatomy. An Angiographic Approach. 2nd edition. Philadelphia, PA: Lippincott Williams and Wilkins; 2007. [Google Scholar]
- 19.Winship IR, Armitage GA, Ramakrishnan G, Dong B, Todd KG, Shuaib A. Augmenting collateral blood flow during ischemic stroke via transient aortic occlusion. J Cereb Blood Flow Metab. 2014;34:61–71. doi: 10.1038/jcbfm.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shuaib A, Butcher K, Mohammad AA, Saqqur M, Liebeskind DS. Collateral blood vessels in acute ischaemic stroke: a potential therapeutic target. Lancet Neurol. 2011;10:909–921. doi: 10.1016/S1474-4422(11)70195-8. [DOI] [PubMed] [Google Scholar]
- 21.Seiler C, Engler R, Berner L, Stoller M, Meier P, Steck H, Traupe T. Prognostic relevance of coronary collateral function: confounded or causal relationship? Heart. 2013;99:1408–1414. doi: 10.1136/heartjnl-2013-304369. [DOI] [PubMed] [Google Scholar]
- 22.Bloor CM. Functional significance of the coronary collateral circulation. A review. Am J Pathol. 1974;76:561–588. [PMC free article] [PubMed] [Google Scholar]
- 23.Sealock R, Zhang, Lucitti J, Moore S, Faber J. Congenic fine-mapping identifies a major causal locus for variation in the native collateral circulation and ischemic injury in brain and lower extremity. Circ Res. 2014;114:660–671. doi: 10.1161/CIRCRESAHA.114.302931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faber JE, Zhang H, Lassance-Soares RM, Prabhakar P, Najafi AH, Burnett MS, Epstein SE. Aging causes collateral rarefaction and increased severity of ischemic injury in multiple tissues. Arterioscler Thromb Vasc Biol. 2011;31:1748–1756. doi: 10.1161/ATVBAHA.111.227314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chilian WM, Mass HJ, Williams SE, Layne SM, Smith EE, Scheel KW. Microvascular occlusions promote coronary collateral growth. Am J Physiol. 1990;258:H1103–H1111. doi: 10.1152/ajpheart.1990.258.4.H1103. [DOI] [PubMed] [Google Scholar]
- 26.Schaper W. On arteriogenesis--a reply. Basic Res Cardiol. 2003;98:183–184. doi: 10.1007/s00395-003-0403-1. [DOI] [PubMed] [Google Scholar]
- 27.Elias KA, Weiner RI. Inhibition of estrogen-induced anterior pituitary enlargement and arteriogenesis by bromocriptine in Fischer 344 rats. Endocrinology. 1987;120:617–621. doi: 10.1210/endo-120-2-617. [DOI] [PubMed] [Google Scholar]
- 28.Herrmann LG, Bollack C. Arteriogenesis induced by tubes of plastic mesh. Surgery. 1955;38:993–998. [PubMed] [Google Scholar]
- 29.Moraes F, Paye J, Mac Gabhann F, Zhuang ZW, Zhang J, Lanahan AA, Simons M. Endothelial cell-dependent regulation of arteriogenesis. Circ Res. 2013;113:1076–1086. doi: 10.1161/CIRCRESAHA.113.301340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deindl E, Schaper W. Editorial: avenue to arteriogenesis. Curr Vasc Pharmacol. 2013;11:2–4. doi: 10.2174/157016113804547647. [DOI] [PubMed] [Google Scholar]
- 31.Werner GS, Emig U, Mutschke O, Schwarz G, Bahrmann P, Figulla HR. Regression of collateral function after recanalization of chronic total coronary occlusions: a serial assessment by intracoronary pressure and Doppler recordings. Circulation. 2003;108:2877–2882. doi: 10.1161/01.CIR.0000100724.44398.01. [DOI] [PubMed] [Google Scholar]
- 32.Ziegler MA, Distasi MR, Bills RG, Miller SJ, Alloosh M, Murphy MP, Akingba AG, Sturek M, Dalsing MC, Unthank JL. Marvels, mysteries, and misconceptions of vascular compensation to peripheral artery occlusion. Microcirculation. 2010;17:3–20. doi: 10.1111/j.1549-8719.2010.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menon BK, Smith EE, Coutts SB, Welsh DG, Faber JE, Damani Z, Goyal M, Hill MD, Demchuk AM, Hee Cho K-H, Chang H-W, Hong J-H, Sohn SI. Leptomeningeal collaterals are associated with modifiable metabolic risk factors. Ann Neurol. 2013 Mar 28; doi: 10.1002/ana.23906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Marchi SF, Gloekler S, Meier P, Traupe T, Steck H, Cook S, Vogel R, Seiler C. Determinants of preformed collateral vessels in the human heart without coronary artery disease. Cardiology. 2011;118:198–206. doi: 10.1159/000328648. [DOI] [PubMed] [Google Scholar]
- 35.Cheng C, Haasdijk R, Tempel D, van de Kamp EH, Herpers R, Bos F, Den Dekker WK, Blonden LA, de Jong R, Bürgisser PE, Chrifi I, Biessen EA, Dimmeler S, Schulte-Merker S, Duckers HJ. Endothelial cell-specific FGD5 involvement in vascular pruning defines neovessel fate in mice. Circulation. 2012;125:3142–3158. doi: 10.1161/CIRCULATIONAHA.111.064030. [DOI] [PubMed] [Google Scholar]
- 36.Van Overbeeke JJ, Hillen B, Tulleken CA. A comparative study of the circle of Willis in fetal and adult life. The configuration of the posterior bifurcation of the posterior communicating artery. J Anat. 1991;176:45–54. [PMC free article] [PubMed] [Google Scholar]
- 37.Lucitti JL, Mackey J, Morrison JC, Haigh JJ, Adams RH, Faber JE. Formation of the collateral circulation is regulated by vascular endothelial growth factor-A and A Disintegrin and Metalloprotease Family Members 10 and 17. Circ Res. 2012;111:1539–1550. doi: 10.1161/CIRCRESAHA.112.279109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cortis BS, Serratto M. The collateral coronary circulation in the human fetus: angiographic findings. Cardiologia. 1998;43:77–81. [PubMed] [Google Scholar]
- 39.Mac Gabhann F, Peirce SM. Collateral capillary arterialization following arteriolar ligation in murine skeletal muscle. Microcirculation. 2010;17:333–347. doi: 10.1111/j.1549-8719.2010.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith ER, Scott RM. Spontaneous occlusion of the circle of Willis in children: pediatric moyamoya summary with proposed evidence-based practice guidelines. A review. J Neurosurg Pediatr. 2012;9:353–360. doi: 10.3171/2011.12.PEDS1172. [DOI] [PubMed] [Google Scholar]
- 41.Matsushima T, Inoue K, Kawashima M, Inoue T. History of the development of surgical treatments for moyamoya disease. Neurol Med Chir (Tokyo) 2012;52:278–286. doi: 10.2176/nmc.52.278. [DOI] [PubMed] [Google Scholar]
- 42.Vineberg A, Munro DD, Cohen H, Buller W. Four years' clinical experience with internal mammary artery implantation in the treatment of human coronary artery insufficiency including additional experimental studies. J Thorac Surg. 1955;29:1–32. discussion, 32-6. [PubMed] [Google Scholar]
- 43.Hansen-Smith F, Egginton S, Zhou AL, Hudlicka O. Growth of arterioles precedes that of capillaries in stretch-induced angiogenesis in skeletal muscle. Microvasc Res. 2001;62:1–14. doi: 10.1006/mvre.2001.2308. [DOI] [PubMed] [Google Scholar]
- 44.Van Gieson EJ, Murfee WL, Skalak TC, Price RJ. Enhanced smooth muscle cell coverage of microvessels exposed to increased hemodynamic stresses in vivo. Circ Res. 2003;92:929–936. doi: 10.1161/01.RES.0000068377.01063.79. [DOI] [PubMed] [Google Scholar]
- 45.Price RJ, Skalak TC. Chronic alpha 1-adrenergic blockade stimulates terminal and arcade arteriolar development. Am J Physiol. 1996;271(2 Pt 2):H752–H759. doi: 10.1152/ajpheart.1996.271.2.H752. [DOI] [PubMed] [Google Scholar]
- 46.Lanahan A, Zhang X, Fantin A, Zhuang Z, Rivera-Molina F, Speichinger K, Prahst C, Zhang J, Wang Y, Davis G, Toomre D, Ruhrberg C, Simons M. The neuropilin 1 cytoplasmic domain is required for VEGF-A-dependent arteriogenesis. Dev Cell. 2013;25:156–168. doi: 10.1016/j.devcel.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tuder RM, Archer SL, Dorfmüller P, Erzurum SC, Guignabert C, Michelakis E, Rabinovitch M, Schermuly R, Stenmark KR, Morrell NW. Relevant issues in the pathology and pathobiology of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D4–D12. doi: 10.1016/j.jacc.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]