Abstract
Background
There is heterogeneity in the pattern of early cognitive deficits in Alzheimer’s disease (AD). However, whether the severity of initial cognitive deficits relate to different clinical trajectories of AD progression is unclear.
Objective
To determine if deficits in specific cognitive domains at initial visit relate to rate of progression in clinical trajectories of AD dementia.
Methods
68 subjects from the National Alzheimer’s Coordinating Center database who had autopsy confirmed AD as the primary diagnosis and at least 3 serial assessments a year apart, with MMSE >15 and Clinical Dementia Rating scale–Global (CDR-G) ≤1 at initial visit were included. Mixed regression model was used to examine the association between initial neuropsychological performance and rate of change on MMSE and Clinical Dementia Rating scale Sum of Boxes (CDR SB).
Results
Preservation of working memory, but not episodic memory, in the Mild Cognitive Impairment and early dementia stages of AD relates to slower rate of functional decline.
Discussion
These findings are relevant for estimating rate of decline in AD clinical trials and in counseling patients and families. Improving working memory performance as a possible avenue to decrease rate of functional decline in AD dementia warrants closer investigation.
Keywords: Alzheimer’s disease, dementia, cognitive decline, neuropsychology in dementia, working memory, rate of decline
Introduction
There is significant variability in the rate of progression of clinical symptoms in Alzheimer’s disease (AD) [1]. The progression of AD from diagnosis to death is approximately 10 years in most cases, although rapid progression is observed in others [2]. It is often difficult on an individual basis to predict which patients with Mild Cognitive Impairment (MCI) or early dementia will decline faster, needing early additional supportive care. Identifying variables at the initial visit of a patient with MCI or early dementia that could be related to faster or slower clinical trajectories would be valuable for patient and family counseling and planning. This information could help assist in exploring underlying biological factors relating to varying clinical trajectories and would help us develop therapies tailored for individual patients.
It is increasingly recognized that there is substantial heterogeneity among AD patients, with differences in age-of-onset, clinical and behavioral symptoms, in principal brain regions of neuropathologic burden, and speed of clinical progression [3]. Some have noted that clinical features, such as age-at-onset, sex, duration of illness, and the presence or absence of extrapyramidal features are associated with the time to progression as assessed by time to nursing home placement, decline in Mini-Mental State Examination (MMSE) score, or death [3]. Others have noted that the initial stage of disease at the beginning of the observation period (“how far”) is an important predictor of subsequent decline (“how fast”) [3,4]. Some staging measures fail to document linear decline over the course of AD, and both “bilinear” and “trilinear” models of decline have been proposed [5]. Predictive models of progression have been developed and validated for AD [6]. A large literature in AD also documents predictors of conversion from normal cognition to amnestic MCI and to AD dementia [7-11].
Multiple models [5, 6, 12, 13] and a meta-analysis of AD progression models [14] document clinical predictors of rates of decline. One longitudinal study of neuropsychological predictors of decline has noted that short-term memory deficits, temporospatial disorientation, and constructive apraxia predict more rapid dependency in AD patients [15]. Lower memory scores and executive dysfunction at baseline have been shown to predict greater likelihood of future decline [16, 17]. But previous studies have a few limitations. Most studies predicting rate of decline in AD do not have conclusive biomarkers or autopsy-confirmed diagnosis of AD pathology. This is a significant drawback, as the sensitivity and specificity of a probable and possible AD diagnosis without the aid of biomarkers was only between 70.9% - 87.3% for sensitivity and 44.3% −70.8% for specificity in one large analysis [18]. Further, when a non-AD dementia was diagnosed, 39% of these individuals were found to have AD pathology at autopsy [18]. This adds significant error to previous analyses of rates of decline of AD without autopsy confirmation.
There is significant variability in initial symptoms among clinical variants of AD and patients do not have the same degree of cognitive impairments across cognitive domains. The rate of clinical progression in terms of MMSE scores has been noted to be different between neuropathological subtypes of AD, with fastest rate in hippocampal sparing AD and the slowest rate in limbic-predominant AD [19]. However, the highly verbal nature of the MMSE could overestimate AD severity in patients with predominant language deficits [20]. Performance in each of the cognitive domains is not independent and there is significant interaction among cognitive domains. For example, executive functioning is a mediator between other cognitive domains and daily functioning and may contribute to rate of decline [21]. We therefore undertook an analysis of autopsy-confirmed AD dementia subjects in a large national database to investigate how clinical and neuropsychological markers at initial visit in the MCI and early dementia stage of AD relate to rate of cognitive and functional decline.
Materials and methods
The National Alzheimer’s Coordinating Center (NACC) maintains a database of participant information collected from 34 past and present Alzheimer’s Disease Centers funded by the National Institute on Aging. Data from the uniform data set (UDS) maintained by NACC between September 2005 and May 2012 used for the present analysis. Details on data collection and curating are well documented [22]. The Clinical Dementia Rating (CDR-Global) Scale assesses the participant’s current cognitive and functional status. The level of impairment in the domains or ‘boxes’ of memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care are rated. The CDR-Global ratings are calculated using a complex algorithm and range from 0, (no dementia) to 3 (severe dementia) [23]. All subjects included in the analysis had an initial global CDR-Global score of 0.5 or 1 and final CDR-Global score >1 meeting clinical criteria for a diagnosis of dementia. The primary diagnosis of AD dementia was confirmed by neuropathology analysis. The CDR Sum of Boxes (CDR-SB) is an operationalized measure of cognitive and functional ability. CDR-SB scores are calculated by simply adding the ‘box scores’, and range from 0 to 18 (higher scores indicate more impairment). The CDR-SB mean scores decline nearly linearly across different severities of AD in the 2-year Alzheimer’s Disease Neuroimaging Initiative (ADNI) study [24]. CDR-SB provides a global assessment of cognitive and functional ability and is significantly associated with the severity of amyloid burden [25] and metabolic deficits in patients with AD [26]. All subjects also had a minimum score >15 on the MMSE [27] at initial visit. The mean time from last visit to death was 0.43 years (range 1-2 years). The subjects included in the analysis had a minimum of 3 longitudinal visits separated by one year. Presence of any behavioral symptom at initial visit (delusions, hallucinations, agitation, depression or dysphoria, anxiety, elation or euphoria, apathy, irritability or lability and disinhibition: yes or no) were evaluated for their predictive role in future cognitive decline, in addition to each subject’s Hachinski ischemic scores. Patient characteristics are described in Table 1.
Table 1.
Demographic characteristics
| Variable | n | Mean | Std Dev | Range |
|---|---|---|---|---|
| Age (years) | 67 | 73.8 | 9.6 | 47-94 |
| Education (years) | 68 | 15.7 | 2.7 | 9-23 |
| Female (%) | 42(62%) | |||
| Initial MMSE | 68 | 22.7 | 3.5 | 16-30 |
| Final MMSE | 56 | 11.6 | 5.6 | 0-22 |
| Initial CDR-G | 68 | 0.8 | 0.2 | 0.5-1 |
| Final CDR-G | 68 | 2.3 | 0.5 | 2-3 |
| Initial CDR-SB | 68 | 4.7 | 2.3 | 0.5-9 |
| Final CDR-SB | 68 | 13.6 | 2.7 | 10-18 |
| Follow up (years) | 68 | 3.9 | 0.89 | 3-6 |
| Braak stage | 68 | |||
| 3 | 1 (1%) | |||
| 4 | 7 (10%) | |||
| 5 | 32 (47%) | |||
| 6 | 28 (41%) | |||
| APOE 4 | 68 | |||
| No APOE 4 allele | 39 (57%) | |||
| 1 APOE 4 allele | 22 (32%) | |||
| 2 APOE 4 alleles | 7 (10%) | |||
Neuropsychological Measures
A core battery of neuropsychological measures was administered to all participants at each visit [28]. All four cognitive domains documented in the UDS were evaluated: attention, executive functioning, language, and memory. Attention was assessed using the Digit Span subtest (Digits Forward) from the Wechsler Adult Intelligence Scale (WAIS) [29] and the Trail-Making Test (TMT) Part A [30]. Executive functioning was quantified using WAIS Digit Span (Digits Backwards) [29], Trail Making Test Part B, and the Digit Symbol-Coding subtest from the WAIS [29, 30]. Digits Backward Length (i.e., number of digits correctly repeated in reverse order) was also included as a variable of interest. Object naming was assessed using the 30-item version of the Boston Naming Test (BNT) [31]. The evaluation of memory included measures of verbal episodic memory (Wechsler Memory Scale, Logical Memory subtest) [29, 32] and semantic memory (animal names generated in 60 seconds) [33]. Table 2 notes the detailed neuropsychological characteristics of the subjects at initial visit.
Table 2.
Neuropsychological characteristics at initial visit
| Test | n | Mean | Std Dev | Range |
|---|---|---|---|---|
| Logical Memory | 68 | 4.8 | 3.4 | 0-16 |
| Digit Span - Forward |
68 | 6.9 | 2.2 | 2-12 |
| Digit Span – Forward Length |
68 | 5.8 | 1.3 | 3-8 |
| Digit Span - Backward |
68 | 4.5 | 1.6 | 0-10 |
| Digit Span – Backward Length |
68 | 3.5 | 1.1 | 0-7 |
| Semantic Fluency (animals) |
68 | 11.6 | 5.1 | 2-29 |
| Semantic Fluency (vegetables) |
68 | 7.3 | 3.8 | 0-20 |
| Trail Making Test – Part A |
68 | 74.4 | 40.0 | 23-150 |
| Trail Making Test – Part B |
49 | 219.3 | 85.6 | 54-300 |
| Digit Symbol Coding |
51 | 24.4 | 12.0 | 0-55 |
| Boston Naming Test | 66 | 21.0 | 5.5 | 4-29 |
Statistical analysis
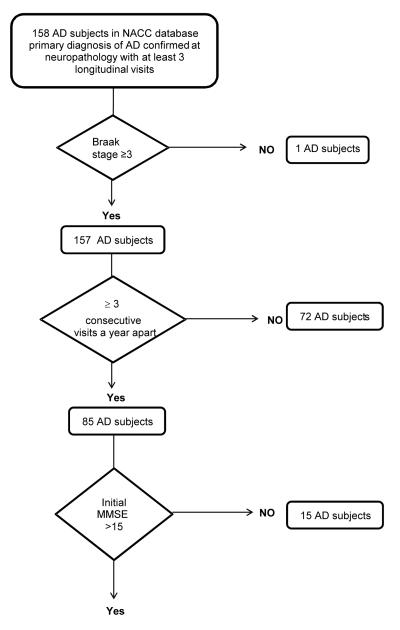
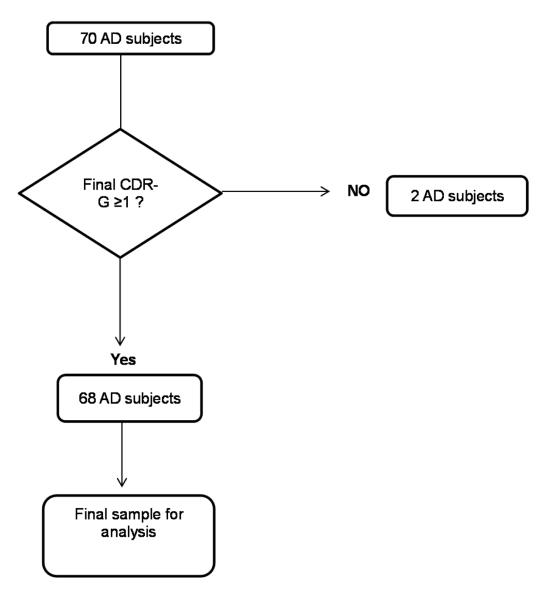
Of the total of 158 subjects that had a primary diagnosis of AD dementia confirmed by neuropathology. Flow chart for subject selection is noted in Figure 1. Rationale for subject inclusion/exclusion criteria was to reduce the potential effect of the large variability of initial cognitive deficits on the rate of decline analysis. A total of 68 subjects were used for further analysis. Given the ordinal nature of APOE and Braak stage, they were included in the models as continuous variables. All the analyses were done with JMP Pro 10.0 (SAS Institute) and SAS 9.2 (SAS Institute).
Figure 1.
Flow chart for AD sample selection for present analysis from the NACC database
Mixed models for repeated measurements were used to determine which measures were associated with rate of change of MMSE and CDR-SB after adjustment for demographic covariates. In each model, age, sex, education, and APOE 4status were included as covariates and all the initial performance measures included as possible predictors. For Braak stage a multivariate regression model was built using a mixed strategy of backward elimination and forward selection. Only terms with significance p ≤ 0.05 were included (except for the covariates). In the final models, interactions between initial neuropsychological measures were explored and were not significant.
Role of age at diagnosis, duration of clinical follow up, presence of initial behavioral symptoms, and Hachinski score at first visit on rate of decline of MMSE and CDR-SB were explored. The role of final neuropathology burden, noted by Braak stage, as an independent predictor of rate of decline on MMSE and CDR-SB was analyzed in a secondary analysis.
Results
Rate of change of MMSE
Age at diagnosis (b = 0.14, p = 0.0006), initial MMSE (b = 0.37, p = 0.001), initial Digit span backwards trials correct scores (b = 0.67, p = 0.004), and initial BNT scores (b = 0.23, p = 0. 007) were all associated with lower rate of decline in MMSE. (Table 3)
Table 3.
Mixed models (repeated measures) to predict MMSE and CDRSB decline and multiple regression model for Braak Stage
| Baseline Variable |
MMSE* | CDRSB* | Braak Stage* |
|---|---|---|---|
| Age at Diagnosis |
0.14 (0.0006) | −0.008 (0.70) | −0.02 (0.01) |
| Education | −0.17 (0.19) | 0.22 (0.004) | −0.043 (0.22) |
| Gender (Male) | 0.29 (0.70) | −0.02 (0.97) | 0.09 (.31) |
| APOE | 0.56 (0.27) | −0.29 (0.32) | |
| Initial MMSE | .37 (0.001) | ||
| Digit Backwards trials correct |
0.67 (0.004) | −0.29 (0.03) | |
| Boston Naming Test |
0.23 (0.007) | ||
| Initial CDR SB | 0.80 (<0.0001) | ||
| Digit backwards length |
−0.15 (0.05) | ||
| 1st Behavioral Symptom |
0.76 (0.059) |
Beta (p – value). Interactions were explored and were non-significant.
Rate of change of CDR-SB
Higher education (b = 0.22, p = 0.004) and initial CDR-SB (0.80, p < 0.0001) were associated with an increase in rate of decline on CDR-SB. A higher Digit Span backwards trials correct scores (b = −0.29, p = 0.03) related to slower rate of decline on CDR-SB. Presence of a behavioral symptom at initial visit (b=0.79, p=0.059) reached close to significance cut off and related to an increase in rate of decline in CDR-SB. (Table 3)
In our supplementary analysis, longer duration of clinical follow-up was not related to slower rates of decline on MMSE and CDR-SB (p = 0.63, and p = 0.90, respectively)). We further noted that the initial Hachinski score was not related to rate of decline on MMSE and CDR-SB (p = 0.81, and p = 0.92, respectively). Braak stage was not associated with the slope of MMSE or CDR-SB in a univariate model. In a regression model, age at diagnosis (b = −0.02, p = 0.05) and initial Digit backwards length (b = −0.19, p = 0.02) were associated with lower final Braak stage but not logical memory scores.
Discussion
In the current study, we found that rate of cognitive and functional decline could be predicted based on baseline performance on measures of working memory and naming, along with initial behavioral symptoms and demographic factors, among subjects with autopsy-confirmed AD dementia in a longitudinal cohort. Notably, performance on the episodic memory measure did not predict rate of decline in either MMSE or CDR-SB. Braak stage at autopsy did not explain the rate of decline in MMSE or CDR-SB after accounting for age, sex, and education, further supporting the notion that additional factors (apart from neuropathology) contribute to longitudinal changes in AD dementia. Higher education and high initial digit backwards performance were related to lower Braak stage at autopsy.
Perhaps our most clinically significant finding relates to the association between initial neuropsychological performance and longitudinal changes in global functional status (CDR-SB). Better initial performance on digit span backwards task was related to a slower rate of functional decline over time. This measure taps overlapping but distinctive cognitive skills, including working memory, complex attention, and speed of information processing, and can be categorized as a measure of attention and executive functioning.
A large body of empirical work has found relationships between executive functioning and performance of instrumental activities of daily living (IADL’s) in healthy older adults [34], as well as among individuals with MCI and mild AD [10,16,17,35]. A very recent study found that performance on tasks requiring working memory and complex attention were related to real-world task performance [36]. Executive functioning also has been noted as a mediator between other cognitive domains and daily functioning [21] and associated with higher number of falls and gait speed slowing [37]. Others have posited that executive functioning explains at least 3 times as much variance in IADL performance as memory [38] This has relevance for our results, as we found no association between memory performance and CDR-SB change over time. As such, those with more intact executive and attentional skills at baseline may be able to more effectively compensate for memory impairment, at least temporarily. Deficits in attentional control and working memory are also increasingly recognized as early cognitive changes in the AD prodrome [39]. Alternatively, better function in these measures may be a marker of more robust cognitive reserve [40].
We also note significant associations between performance on two measures (i.e., BNT and Digits Backwards) and longitudinal changes in MMSE score. The highly verbal nature of the MMSE has been noted to overestimate dementia severity in patients with primary language disorders [20], and MMSE scores correlate with BNT scores and baseline higher intellectual performance. Our results support this close correspondence. These results are consistent with the CDR-SB results in supporting the role for relative preservation of working memory skills at baseline to help compensate for cognitive decline, although it should be noted that the MMSE does not thoroughly assess executive functions.
Taken together, these results suggest that slower rate of decline in MMSE and global functioning are both related to relatively preserved frontal lobe functions of attention and working memory. Episodic memory impairment, though a hallmark of AD phenomenology, does not appear to influence the rate of decline of these measures. There are several potential explanations for this observation. Episodic memory could be uniformly impaired across all subjects and would, therefore, not significantly contribute to the model (i.e., floor effect). This was supported in our post-hoc analysis, in which we found that the variance in episodic memory scores was lower than in other cognitive domains.
Multiple studies have assessed CSF biomarkers (Abeta/Tau) and MRI measures of hippocampal atrophy in predicting AD progression. Our analysis suggests that variations in cognitive profiles of patients, in addition to subject demographics, duration of symptoms, and early progression rate, impact later clinical measures of progression in AD dementia. This could be related to differences in regional neuropathology burden and/or differences in biological factors underlying disease progression or due to specific test features of MMSE and CDR-SB in capturing some cognitive domains better than others and need to be considered as measures that reflect clinical disease progression in AD.
Among covariates there was a consistent influence of education on rates of decline in MMSE and CDR-SB. Although highly educated subjects often have a slower rate of decline prior to dementia, faster cognitive decline has been well described in the same subjects following dementia onset [41,42]. Our results are consistent with this and may be attributed to effects of cognitive reserve [40]. Age at diagnosis was noted to have a discrepant influence, with higher age of diagnosis related to slower rate of decline on MMSE but not CDR-SB. One possible reason could be that medical co-morbidities, including physical disability from illness, interval surgical interventions, or uncontrolled vascular risk factors (e.g., diabetes), may influence global ability (and CDR scores) but could not completely be accounted for in our analysis.
Our results have important implications. Currently, the drug vs. placebo arm comparisons in clinical trials evaluate cognitive function from the start of the study using MMSE to establish initial disease severity. As pre-progression rates and differing neuropsychological profiles of subjects lead to differences in rates of progression, accurate evaluation of drug vs. placebo effect might be best captured by more careful matching of subjects across both executive function and episodic memory deficits than is currently practiced. Further, some studies support the effectiveness and durability of the cognitive training interventions in improving targeted cognitive abilities in the elderly [43] but reports of transfer of benefits to other cognitive domains following cognitive training in the older population are few [44,45]. Our results suggest that targeting working memory performance could be a fruitful avenue to improve functional task performance and slow rate of progression to AD dementia and warrants further investigation.
Study limitations include the nature of our sample of convenience with potential bias in participant recruitment and in their agreeing for final autopsy confirmation of diagnosis. Having autopsy confirmation leads to a smaller number of subjects in our analysis compared to previously published AD progression studies, even as it adds to the strength of this analysis in that all the subjects have a definitive AD dementia diagnosis. All subjects included in the analysis had a minimum of 3 visits. When we estimate that the AD pathology progresses over almost two decades in dominantly-inherited AD, a 3-year analysis of rate of progression is limiting. This study therefore best captures rate of decline among AD subjects with early dementia or MCI at initial visit. The number of cognitive measures included in the study was also less than would be found in most standard neuropsychological batteries used for clinical evaluation of dementia, which may have limited our ability to detect other early cognitive predictors of decline. A detailed analysis of neuropsychologial measures predicting conversion from MCI to dementia could not be undertaken in the present dataset due to the smaller number of subjects in the MCI range at initial visit (39.7%).
Previous staging measures have proposed a non-linear decline in cognition over the course of AD [5, 41], but decline is often modeled to occur at a constant linear rate after the onset of dementia [41, 42]. The CDR-SB mean scores have been noted to decline nearly linearly across different severities of AD in the 2-year ADNI study [24]. Our analysis therefore tracks clinical progression of AD only in stages of dementia and assumes a linear rate of decline. Future studies on a larger sample with a longer longitudinal follow-up are needed to confirm the present results.
Our results are best used to understand predictors of clinical deterioration and neuropsychological variables of interest at the time of first clinical visit in individuals with MCI or early dementia. Most sporadic AD patients are expected to meet a health care provider at this stage and are often in need for counseling as to what to expect regarding rate of symptom progression. These findings are relevant to developing better cognitive markers for estimating rate of clinical progression in prospective AD clinical trials and in counseling patients and families. Improving working memory performance as a possible avenue to decrease rate of functional decline in AD dementia warrants closer investigation.
Acknowledgements
We thank the NACC publication review committee for their suggestions and comments.
Grant Number: The NACC database is funded by NIA Grant U01 AG016976.
Sponsor: None
Footnotes
Manuscript Contributions: JAP: Concept, design of study, analysis/interpretation of data, drafting/revising the manuscript for content.
ABJ: Design of study, analysis/interpretation of data, drafting/revising the manuscript for content.
EW: Design of study, analysis/interpretation of data, drafting/revising the manuscript for content.
LM: Analysis of data.
JLC: Interpretation of data, drafting/revising the manuscript for content.
Disclosures: Drs. Pillai, Bonner-Jackson, Walker and Ms. Mourany have no disclosures.
Dr. Cummings has the following disclosures:
a. Consultation for Pharmaceutical Companies:
Acadia, ADAMAS, Anavex, Avanir, Baxter, Boehinger-Ingelheim, Bristol-Myers Squibb, Eisai, EnVivo, Genentech, GlaxoSmithKline, Lilly, Lundbeck, Merck, Novartis, Orion, Otsuka, Pfizer, Prana, QR Pharma, Resverlogix, Roche, Sonexa, Suven, Takeda, and Toyoma pharmaceutical companies.
b. Consultation for Assessment Companies:
GE Healthcare and MedAvante.
c. Stock:
ADAMAS, Prana, Sonexa, MedAvante, Neurotrax, and Neurokos
d. Other:
Dr. Cummings owns the copyright of the Neuropsychiatric Inventory
e. Expert witness/legal consultation:
Dr. Cummings has provided expert witness consultation regarding olanzapine and ropinerol.
References
- 1.Cummings JL. Cognitive and behavioral heterogeneity in Alzheimer’s disease: seeking the neurobiological basis. Neurobiol Aging. 2000;21(6):845–861. doi: 10.1016/s0197-4580(00)00183-4. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt C, Wolff M, Weitz M, Bartlau T, Korth C, Zerr I. Rapidly progressive Alzheimer disease. Arch Neurol. 2011;68(9):1124–1130. doi: 10.1001/archneurol.2011.189. [DOI] [PubMed] [Google Scholar]
- 3.Drachman DA, O’Donnell BF, Lew RA, Swearer JM. The prognosis in Alzheimer’s disease. ’How far’ rather than ’how fast’ best predicts the course. Arch Neurol. 1990;47(8):851–856. doi: 10.1001/archneur.1990.00530080033007. [DOI] [PubMed] [Google Scholar]
- 4.Kraemer HC, Tinklenberg J, Yesavage JA. ’How far’ vs ’how fast’ in Alzheimer’s disease. The question revisited. Arch Neurol. 1994;51(3):275–279. doi: 10.1001/archneur.1994.00540150069019. [DOI] [PubMed] [Google Scholar]
- 5.Doody RS, Massman P, Dunn JK. A method for estimating progression rates in Alzheimer disease. Arch Neurol. 2001;58(3):449–454. doi: 10.1001/archneur.58.3.449. [DOI] [PubMed] [Google Scholar]
- 6.Doody RS, Pavlik V, Massman P, Rountree S, Darby E, Chan W. Predicting progression of Alzheimer’s disease. Alzheimers Res Ther. 2010;2:2–9. doi: 10.1186/alzrt25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen P, Ratcliff G, Belle SH, Cauley JA, DeKosky ST, Ganguli M. Patterns of cognitive decline in presymptomatic Alzheimer disease: a prospective community study. Arch Gen Psychiatry. 2001;58(9):853–858. doi: 10.1001/archpsyc.58.9.853. [DOI] [PubMed] [Google Scholar]
- 8.Tabert MH, Manly JJ, Liu X, Pelton GH, Rosenblum S, Jacobs M, Zamora D, Goodkind M, Bell K, Stern Y, Devanand DP. Neuropsychological prediction of conversion to Alzheimer disease in patients with mild cognitive impairment. Arch Gen Psychiatry. 2006;63(8):916–924. doi: 10.1001/archpsyc.63.8.916. [DOI] [PubMed] [Google Scholar]
- 9.Fleisher AS, Sowell BB, Taylor C, Gamst AC, Petersen RC, Thal LJ. Alzheimer’s Disease Cooperative Study. Clinical predictors of progression to Alzheimer disease in amnestic mild cognitive impairment. Neurology. 2007;68(19):1588–1595. doi: 10.1212/01.wnl.0000258542.58725.4c. [DOI] [PubMed] [Google Scholar]
- 10.Blacker D, Lee H, Muzikansky A, Martin EC, Tanzi R, McArdle JJ, Moss M, Albert M. Neuropsychological measures in normal individuals that predict subsequent cognitive decline. Arch Neurol. 2007;64(6):862–871. doi: 10.1001/archneur.64.6.862. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed S, Mitchell J, Arnold R, Nestor PJ, Hodges JR. Predicting rapid clinical progression in amnestic mild cognitive impairment. Dement Geriatr Cogn Disord. 2008;25(2):170–177. doi: 10.1159/000113014. [DOI] [PubMed] [Google Scholar]
- 12.Stallard E, Kinosian B, Zbrozek AS, Yashin AI, Glick HA, Stern Y. Estimation and validation of a multiattribute model of Alzheimer disease progression. Med Decis Making. 2010;30(6):625–638. doi: 10.1177/0272989X10363479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito K, Corrigan B, Zhao Q, French J, Miller R, Soares H, Katz E, Nicholas T, Billing B, Anziano R, Fullerton T. Alzheimer’s Disease Neuroimaging Initiative. Disease progression model for cognitive deterioration from Alzheimer’s Disease Neuroimaging Initiative database. Alzheimers Dement. 2011;7(2):151–160. doi: 10.1016/j.jalz.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 14.Ito K, Ahadieh S, Corrigan B, French J, Fullerton T, Tensfeldt T. Alzheimer’s Disease Working Group. Disease progression meta-analysis model in Alzheimer’s disease. Alzheimers Dement. 2010;6(1):39–53. doi: 10.1016/j.jalz.2009.05.665. [DOI] [PubMed] [Google Scholar]
- 15.Sarazin M, Stern Y, Berr C, Riba A, Albert M, Brandt J, Dubois B. Neuropsychological predictors of dependency in patients with Alzheimer disease. Neurology. 2005;64(6):1027–1031. doi: 10.1212/01.WNL.0000154529.53488.30. [DOI] [PubMed] [Google Scholar]
- 16.Albert MS, Moss MB, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. J Int Neuropsychol Soc. 2001;7(5):631–639. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- 17.Dickerson BC, Sperling RA, Hyman BT, Albert MS, Blacker D. Clinical prediction of Alzheimer disease dementia across the spectrum of mild cognitive impairment. Arch Gen Psychiatry. 2007;64(12):1443–1250. doi: 10.1001/archpsyc.64.12.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005- 2010. J Neuropathol Exp Neurol. 2012;71(4):266–273. doi: 10.1097/NEN.0b013e31824b211b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW. Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 2011;10(9):785–796. doi: 10.1016/S1474-4422(11)70156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osher J, Wicklund A, Rademaker A, Johnson N, Weintraub S. The Mini-Mental State Examination in behavioral variant frontotemporal dementia and primary progressive aphasia. Am J Alzheimers Dis Other Demen. 2007;22(6):468–473. doi: 10.1177/1533317507307173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Bryant SE, Falkowski J, Hobson V, Johnson L, Hall J, Schrimsher GW, Win O, Ngo B, Dentino A. Executive functioning mediates the link between other neuropsychological domains and daily functioning: a Project FRONTIER study. Int Psychogeriatr. 2011;23(1):107–113. doi: 10.1017/S1041610210000967. [DOI] [PubMed] [Google Scholar]
- 22.Beekly DL, Ramos EM, Lee WW, Deitrich WD, Jacka ME, Wu J, Hubbard JL, Koepsell TD, Morris JC, Kukull WA. NIA Alzheimer’s Disease Centers. The National Alzheimer’s Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord. 2007;21(3):249–258. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- 23.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 24.Hernandez C, Cedarbaum JM, Jaros M, Hernandez C, Coley N, Andrieu S, Grundman M, Vellas B. Alzheimer’s Disease Neuroimaging Initiative.Rationale for use of the Clinical Dementia Rating Sum of Boxes as a primary outcome measure for Alzheimer’s disease clinical trials. Alzheimers Dement. 2013;9(1S):S45–S55. doi: 10.1016/j.jalz.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Doraiswamy PM, Sperling RA, Coleman RE, Johnson KA, Reiman EM, Davis MD, Grundman M, Sabbagh MN, Sadowsky CH, Fleisher AS, Carpenter A, Clark CM, Joshi AD, Mintun MA, Skovronsky DM, Pontecorvo MJ. AV45-A11 Study Group. Amyloid-b assessed by florbetapir F 18 PET and 18-month cognitive decline: a multicenter study. Neurology. 2012;79(16):1636–1644. doi: 10.1212/WNL.0b013e3182661f74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perneczky R, Hartmann J, Grimmer T, Drzezga A, Kurz A. Cerebral metabolic correlates of the clinical dementia rating scale in mild cognitive impairment. J Geriatr Psychiatry Neurol. 2007;20(2):84–88. doi: 10.1177/0891988706297093. [DOI] [PubMed] [Google Scholar]
- 27.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 28.Weintraub S, Salmon D, Mercaldo, Ferris S, Graff-Radford NR, Chui H, Cummings J, DeCarli C, Foster NL, Galasko D, Peskind E, Dietrich W, Beekly DL, Kukull WA, Morris JC, et al. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): The neuropsychologic test battery. Alz Dis Assoc Disord. 2009;23(2):91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wechsler D. The Wechsler Adult Intelligence Scale – Revised. The Psychological Corporation; San Antonio, TX: 1981. [Google Scholar]
- 30.Army Individual Test Battery . Department of Health and Human Services. War Department, Adjutant General’s Office; Washington, DC: 1944. [Google Scholar]
- 31.Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Lea & Febiger; Philadelphia, PA: 1983. [Google Scholar]
- 32.Wechsler D. Wechsler Memory Scale—Revised. The Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
- 33.Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 3rd Ed Oxford University Press; New York: 2006. [Google Scholar]
- 34.Vaughan L, Giovanello K. Executive function in daily life: Age-related influences of executive processes on instrumental activities of daily living. Psychology and Aging. 2010;25(2):343–355. doi: 10.1037/a0017729. [DOI] [PubMed] [Google Scholar]
- 35.Marshall GA, Rentz DM, Frey MT, Locascio JJ, Johnson KA, Sperling RA, the Alzheimer’s Disease Neuroimaging Initiative Executive function and instrumental activities of daily living in mild cognitive impairment and Alzheimer’s disease. Alzheimers Dement. 2011;7(3):300–308. doi: 10.1016/j.jalz.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miloyan BH, Razani J, Larco A, Avila J, Chung J. Aspects of attention predict real-world task performance in Alzheimer’s disease. Applied Neuropsychology: Adult. 2013 doi: 10.1080/09084282.2012.685133. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kearney FC, Harwood RH, Gladman JR, Lincoln N, Masud T. The relationship between executive function and falls and gait abnormalities in older adults: a systematic review. Dement Geriatr Cogn Disord. 2013;36(1-2):20–35. doi: 10.1159/000350031. [DOI] [PubMed] [Google Scholar]
- 38.Royall DR, Lauterbach EC, Kaufer D, Malloy P, Coburn KL, Black KJ, the Committee on Research of the American Neuropsychiatric Association The cognitive correlates of functional status: A review from the Committee on Research of the American Neuropsychiatric Association. Journal of Neuropsychiatry and Clinical Neuroscience. 2007;19(3):249–265. doi: 10.1176/jnp.2007.19.3.249. [DOI] [PubMed] [Google Scholar]
- 39.Summers MJ, Saunders NLJ. Neuropsychological measures predict decline to Alzheimer’s dementia from Mild Cognitive Impairment. Neuropsychology. 2012;26(4):498–508. doi: 10.1037/a0028576. [DOI] [PubMed] [Google Scholar]
- 40.Stern Y. Cognitive reserve. Neuropsychologia. 2009;47(10):2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hall CB, Derby C, LeValley A, Katz MJ, Verghese J, Lipton RB. Education delays accelerated decline on a memory test in persons who develop dementia. Neurology. 2007;69(17):1657–1664. doi: 10.1212/01.wnl.0000278163.82636.30. [DOI] [PubMed] [Google Scholar]
- 42.Pillai JA, Hall CB, Dickson DW, Buschke H, Lipton RB, Verghese J. Association of crossword puzzle participation with memory decline in persons who develop dementia. J Int Neuropsychol Soc. 2011;(6):1006–13. doi: 10.1017/S1355617711001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, Morris JN, Rebok GW, Smith DM, Tennstedt SL, Unverzagt FW, Willis SL. Advanced Cognitive Training for Independent and Vital Elderly Study Group. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288(18):2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zelinski EM. Far transfer in cognitive training of older adults. Restor. Neurol. Neurosc. 2009;27:455–471. doi: 10.3233/RNN-2009-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anguera JA, Boccanfuso J, Rintoul JL, Al-Hashimi O, Faraji F, Janowich J, Kong E, Larraburo Y, Rolle C, Johnston E, Gazzaley A. Video game training enhances cognitive control in older adults. Nature. 2013;501(7465):97–101. doi: 10.1038/nature12486. [DOI] [PMC free article] [PubMed] [Google Scholar]