Abstract
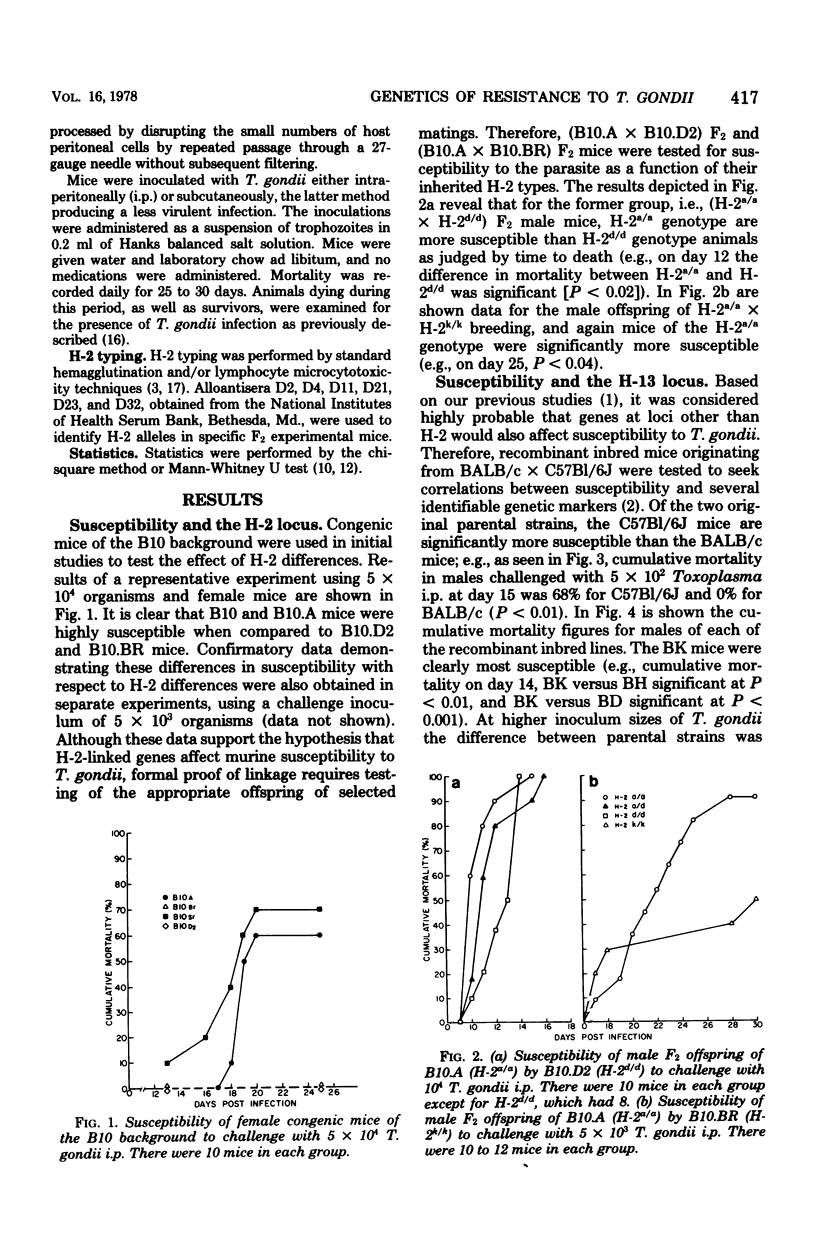
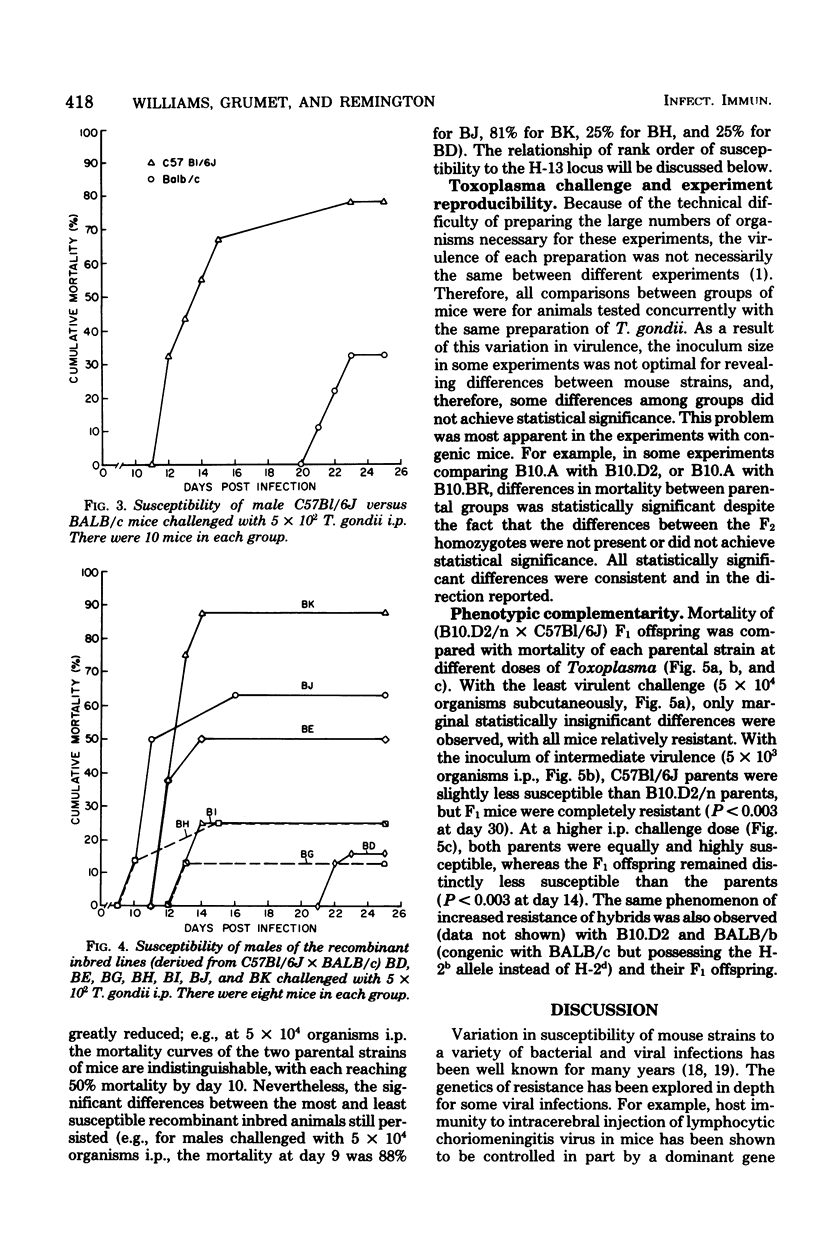
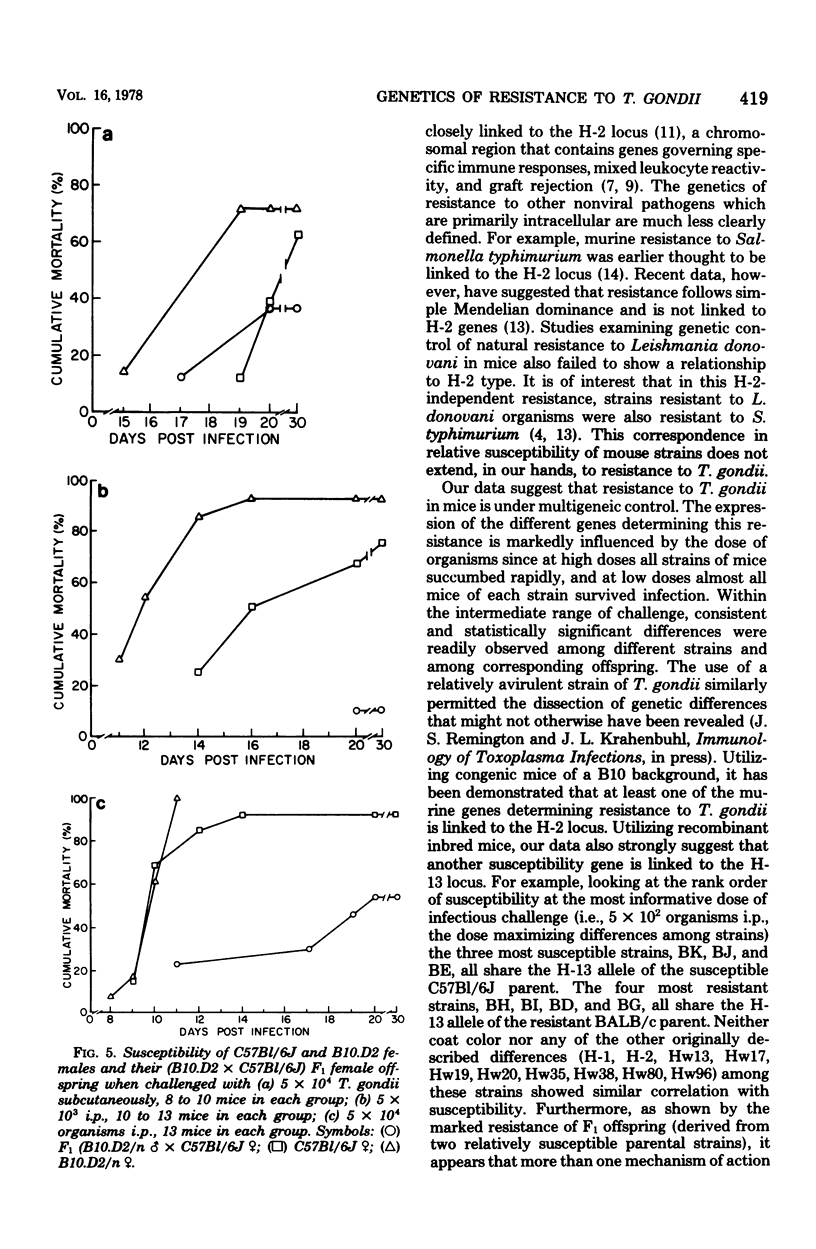
The genetics of murine susceptibility to Toxoplasma gondii was investigated in inbred mice and their F1 and F2 offspring. Among four strains of congenic mice of the B10 background, those with H-2a/a and H-2b/b genotypes were more susceptible than were those with H-2d/d and H-2k/k genotypes. Breeding studies utilizing three of these strains demonstrated linkage between the H-2a allele and greater susceptibility. These data suggest the existence of an H-2-linked gene affecting susceptibility to T. gondii. In challenge of recombinant inbred mice derived from C57Bl/6J (high susceptibility) and BALB/c (low susceptibility) strains, lines BE, BJ, and BK were more susceptible than lines BD, BG, BH, and BI. These data are consistent with the existence of a second disease susceptibility gene linked to the H-13 locus. F1 offspring of the C57B1/6J X B10.D2 mice were significantly less susceptible than either parent. This phenotypic complementary suggests the presence of more than one genetic mechanism of resistance to T. gondii. From these combined data, we conclude that (i) susceptibility to T. gondii in mice is affected by at least two genes, (ii) one of the genes is linked to the H-2 and one to the H-13 locus, and (iii) more than a single mechanism of resistance must be considered to explain the observed genetic controls of susceptibility.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araujo F. G., Williams D. M., Grumet F. C., Remington J. S. Strain-dependent differences in murine susceptibility to toxoplasma. Infect Immun. 1976 May;13(5):1528–1530. doi: 10.1128/iai.13.5.1528-1530.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey D. W. Recombinant-inbred strains. An aid to finding identity, linkage, and function of histocompatibility and other genes. Transplantation. 1971 Mar;11(3):325–327. doi: 10.1097/00007890-197103000-00013. [DOI] [PubMed] [Google Scholar]
- Bradley D. J. Letter: Genetic control of natural resistance to Leishmania donovani. Nature. 1974 Jul 26;250(464):353–354. doi: 10.1038/250353a0. [DOI] [PubMed] [Google Scholar]
- FRENKEL J. K., JACOBS L. Ocular toxoplasmosis; pathogenesis, diagnosis and treatment. AMA Arch Ophthalmol. 1958 Feb;59(2):260–279. [PubMed] [Google Scholar]
- Kamei K., Sato K., Tsunematsu Y. A strain of mouse highly susceptible to Toxoplasma. J Parasitol. 1976 Oct;62(5):714–714. [PubMed] [Google Scholar]
- Klein J. The H-2 system: past and present. Transplant Proc. 1973 Mar;5(1):11–21. [PubMed] [Google Scholar]
- Krahenbuhl J. L., Ruskin J., Remington J. S. The use of killed vaccines in immunization against an intracellular parasite: Toxoplasma gondii. J Immunol. 1972 Feb;108(2):425–431. [PubMed] [Google Scholar]
- McDevitt H. O., Bechtol K. B., Hämmerling G. J. Histocompatibility-linked genetic control of specific immune responses. Soc Gen Physiol Ser. 1974;29:101–120. [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J., Mitchell G. F., McDevitt H. O. Histocompatibility-linked genetic control of disease susceptibility. Murine lymphocytic choriomeningitis virus infection. J Exp Med. 1973 May 1;137(5):1201–1212. doi: 10.1084/jem.137.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Genetics of resistance to infection with Salmonella typhimurium in mice. J Infect Dis. 1976 Jan;133(1):72–78. doi: 10.1093/infdis/133.1.72. [DOI] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Natural resistance to Salmonella infection, delayed hypersensitivity and Ir genes in different strains of mice. Nature. 1974 Mar 22;248(446):345–347. doi: 10.1038/248345a0. [DOI] [PubMed] [Google Scholar]
- REMINGTON J. S., JACOBS L., KAUFMAN H. E. Studies on chronic toxoplasmosis; the relation of infective dose to residual infection and to the possibility of congenital transmission. Am J Ophthalmol. 1958 Nov;46(5 Pt 2):261–268. [PubMed] [Google Scholar]
- Remington J. S., Bloomfield M. M., Russell E., Jr, Robinson W. S. The RNA of toxoplasma gondii. Proc Soc Exp Biol Med. 1970 Feb;133(2):623–626. doi: 10.3181/00379727-133-34531. [DOI] [PubMed] [Google Scholar]
- STIMPFLING J. H., BOYSE E. A., MISHELL R. PREPARATION OF ISOANTISERA IN LABORATORY MICE. Methods Med Res. 1964;10:18–21. [PubMed] [Google Scholar]



