Abstract
Objective
Recently we demonstrated that scavenger receptor type BI (SR-BI), a HDL receptor, was expressed on murine hematopoietic stem/progenitor cells (HSPC) and infusion of reconstituted HDL and purified human apoA-I suppressed HSPC proliferation. We hypothesized that SR-B1 expression is required for the observed anti-proliferative effects of HDL on HSPC.
Approach and Results
SR-BI deficient (SR-BI−/−) mice and wild type (WT) controls were fed on chow or HFD (HFD) for 8–10 weeks. Under chow diet, a significant increase in Lin-Sca1+cKit+ cells (LSK cells, so called HSPC) was found in the BM of SR-BI−/− mice compared with WT mice. HFD induced a further expansion of CD150+CD48− LSK cells (HSCs), HSPCs, and granulocyte monocyte progenitors (GMPs) in SR-BI−/− mice. Injection of reactive oxygen species (ROS) inhibitor N-acetylcysteine attenuated HFD-induced HSPC expansion, leukocytosis and atherosclerosis in SR-BI−/− mice. ApoA-I infusion inhibited HSPC cell proliferation, Akt phosphorylation and ROS production in HSPC and plaque progression in low density lipoprotein receptor knockout (LDLr−/−) apoA-I−/− mice on HFD but had no effect on SR-BI−/− mice on HFD. Transplantation of SR-BI−/− BM cells into irradiated LDLr−/− recipients resulted in enhanced white blood cells (WBC) reconstitution, inflammatory cell production and plaque development. In patients with coronary heart disease, HDL levels were negatively correlated with WBC count and HSPC frequency in the peripheral blood. By flow cytometry, SR-BI expression was detected on human HSPC.
Conclusions
SR-BI plays a critical role in the HDL-mediated regulation HSPC proliferation and differentiation which is associated with atherosclerosis progression.
Key points: HDL, hematopoietic stem/progenitor cells, atherosclerosis
Introduction
High density lipoprotein (HDL) and its major component, apolipoprotein A-I (apoA-I), are negatively correlated with the incidence of coronary heart disease.1 Recently, Yves-Charvet et al., and our group demonstrated that infusion of reconstituted HDL (rHDL) or lipid poor human apoA-I inhibits hematopoietic stem/progenitor cells (HSPCs) proliferation in hypercholesterolemic Abca1−/− Abcg1−/− mice and C57BL/6 mice, therefore, limiting white blood cell expansion in the peripheral blood (PB).2, 3 In addition, we demonstrated that the HDL receptor, scavenger receptor type BI (SR-BI), is expressed on murine Lin-Sca-1+cKit+ (LSK cells, so called HSPC cells),3 which led to the hypothesis that HDL and apoA-I may regulate HSPCs by binding to SR-BI.
HSPCs, responsible for all blood cell generation, reside in a hypoxic bone marrow (BM) niche4, 5 and are largely quiescent. In general, HSPC are defined as Lin-Sca-1+cKit+ (LSK) cells that include both hematopoietic stem and progenitor cells. Within HSPC, the most primitive subpopulation that is capable of repopulating the hematopoietic system is named long term hematopoietic stem cells (LT-HSCs, defined as CD150+CD48-LSK cells). HSCs give rise to multiple progenitor cells (CD150-CD48-MPPs), a mature subset of HSPC, that sequentially generate progenitors, lineage restricted precursors and finally all blood cells. Therefore, LSK cells are widely used in studying HSPC. The potential for self-renewal, proliferation and differentiation of HSPCs is finely balanced by different intrinsic and extrinsic signals, which guarantee the continuous blood supply throughout life. Extrinsic factors, such as cytokines, chemokines, growth factors and extracellular matrix molecules bind to receptors on HSPCs, which then activate downstream signaling pathways, such as phosphoinositide 3-kinase/AKT and MAPK signaling molecules, which regulate cell survival vs. apoptosis, or self-renewal vs. differentiation. The Akt family of serine threonine kinases are activated by phosphorylation of phosphoinositide 3-kinase. Although Akt1−/− or Akt2−/− mice have only mild defects in hematopoiesis, HSPC function, including proliferation and reconstitution capacities, is severely affected in Akt1−/− Akt2−/− mice, suggesting an essential role of Akt in HSPC biology.6, 7 MAPKs are also a family of serine threonine kinases consisting of ERKs, JNKs, and p38MAPKs.8–10 The role of ERKs in HSPC proliferation differentiation and survival is well established, as are the roles of JNKs on erythropoiesis, and p38MPAK activation in the regulation of erythropoiesis and myeloid differentiation. 11
Aside from phospho Akt (pAkt) and MAPK, another group of molecules that critically regulates HSPC proliferation and differentiation are reactive oxygen species (ROS).12, 13 The hypoxic BM microenvironment is responsible for low ROS production in HSPCs, which is important for the maintenance of HSPC quiescence and self-renewal, but not differentiation.11 Comparison between ROShigh HSPCs and ROSlow HSPCs showed that the ROSlow population represents the quiescent HSPC population with self-renew potential, whereas the ROShigh HSPC subpopulation is activated, undergoes differentiation and then exhaustion.12, 13 Moreover, pAkt acts upstream of ROS production,6 whereas, p38MAPK activation is downstream of ROS production in HSPCs. 11
SR-BI, the HDL receptor, is expressed on hepatocytes and facilitates selective cholesterol ester uptake from HDL. In addition, SR-BI also mediates LDL and VLDL clearance.14 Others and we have illustrated the anti-atherosclerotic function of SR-BI in different mouse models.15–18 Different from mice, SR-BI deficiency in humans leads to multiple pathophysiological phenotypes.19, 20 In mice, SR-BI deficiency is associated with impaired HDL function, intracellular cholesterol accumulation and increased oxidative stress.21 We previously demonstrated that SR-BI is expressed on murine HSPC.3 This led us to hypothesize that SR-BI deficiency impairs cholesterol homeostasis, modulates Akt and MAPK phosphorylation and leads to increased ROS production in HSPC, resulting in enhanced HSPC proliferation and differentiation.
We here demonstrate a significant increase in LT-HSC, HSPCs and granulocyte monocyte progenitors (GMP) in the BM of SR-BI−/− mice compared with WT mice on high fat diet (HFD). Infusion of human apoA-I reduced HSPCs proliferation, Akt phosphorylation and ROS production in HSPCs and inhibited plaque progression in LDLr−/− apoA-I−/− mice on HFD but not in SR-BI−/− mice on HFD. Third, transplantation of SR-BI−/− BM cells into irradiated LDLr−/− recipients resulted in enhanced white blood cell reconstitution, inflammatory cell production and plaque development. Fourth, ROS inhibitor treatment reversed leukocytosis and the increased LT-HSC and HSPC frequency and attenuated atherosclerosis progression in SR-BI−/− mice induced by HFD. Finally we tested SR-BI expression on human HSPC and assessed the effect of HDL levels on WBC count as well as HSPC frequency in the blood. As we found in murine models, SR-BI is expressed on human HSPC. In patients with coronary heart disease, HDL levels were negatively correlated with WBC and HSPC frequency in the blood.
Materials and Methods
Human studies
Patients with coronary heart disease were enrolled in this study. Mononuclear cells in the PB (PBMNC) were isolated by Ficoll (GE Heslthcare, Belgium) and stained with an anti-human Lineage cocktail APC (BD), anti-human CD38 APC (eBioscience), anti-human CD34PE (BD) and anti-human CD45RA PerCP-Cy5.5 (eBioscience). The frequency of HSPCs (Lin− CD34+ CD38− CD45RA−/low cells) was determined on a Gallios apparatus (Beckman Coulter).
Murine studies
SR-BI−/− mice and control littermates were fed on HFD (34% fat, 1% cholesterol, Catalog no. D12492 mod, BioServices, the Netherlands) or chow diet for 8–10 weeks. For apoA-I studies, SR-BI−/− and SR-BI+/+ mice, or LDLr−/− apoA-I−/− (DKO) mice, fed with HFD for 11 and 9 weeks respectively, received during the last 3 weeks subcutaneous saline or lipid-free human apoA-I injection (500 μg per injection, 2 injections per week).22 For ROS inhibitor experiments, SR-BI+/+ and SR-BI−/− mice were fed with a HFD and received i.p. injection of saline or N-acetylcysteine (NAC, Sigma-Aldrich, 1 mg/kg, daily) for 12 weeks.
Detailed methods are shown in the online-only Data Supplement.
Results
High fat diet induces leukocytosis, monocytosis, GMP, HSPC and LT-HSC expansion and early onset of atherosclerosis SR-BI−/− mice
We have previously demonstrated that SR-BI, a HDL receptor, is expressed on murine HSPCs.3 As infusion of rHDL or lipid poor human apoA-I inhibits HSPC proliferation in hypercholesterolemic Abca1−/− Abcg1−/− mice and C57BL/6 mice,2, 3 we here investigated if and how SR-BI might be involved in these effects. Eight week-old SR-BI−/− and SR-BI+/+ mice were fed on chow or high fat diet (HFD) (1% cholesterol, 34% fat) for 8–10 weeks. The lipoprotein profiles are shown in supplementary figure I. HFD induced leukocytosis, monocytosis and increased the number of F4/80+ macrophages in the PB of SR-BI−/− mice compared to WT mice on HFD (Figure 1, A–C). In addition, we found more extensive atherosclerotic plaques in the aortic roots in SR-BI−/− compared to WT mice (24517 ± 10625.1 μm2 vs. 6489 ± 1881.3 μm2, n=7 for each, P<0.001).
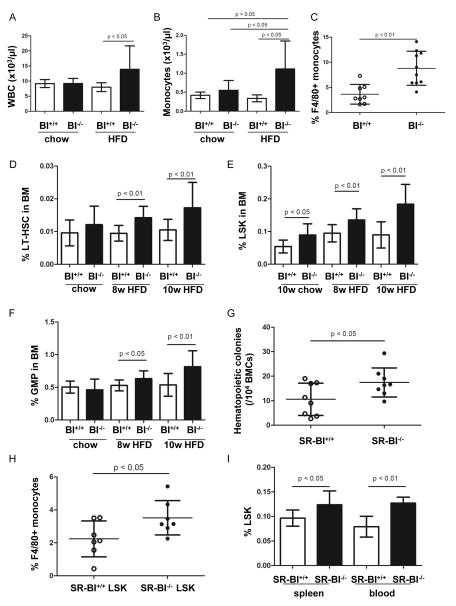
Figure 1. High fat diet promoted leukocytosis, monocytosis in PB and HSPC expansion in BM, which was associated with atherosclerosis development.
SR-BI+/+ and SR-BI−/− mice were placed on chow or high fat diet at the age of 3 months, onward for 8 or 10 weeks. Peripheral white blood cell and monocyte counts were shown in A and B, respectively. n=9–21. (C) To assess the components of monocytes in PB, blood cells of mice on high fat diet were stained with anti-CD11b PE and anti-F4/80 APC-Cy7 and macrophages were quantified by FACS. (D) BMCs were stained with anti-CD150 FITC, anti-CD48 PE and LSK markers and LT-HSC (CD150+ CD48− LSK cells) was quantified by FACS. (E) Quantification of LSK frequency in mice on chow and HFD. (F) Quantification of GMP (CD34+ FcR+ Lin− cKit+ Sca-1 cells) in BMCs. n=8–10. (G) BMCS (1×104) from SR-BI+/+ and SR-BI−/− mice on HFD were seeded on methylcellulose and hematopoietic colonies were numerated at day 10–14. (H) LSK cells of SR-BI+/+ and SR-BI−/− mice on HFD were sorted out by FACS and cultured in vitro for 10 days. Cells were stained with anti-CD11b PE and anti-F4/80 APC-Cy7 to study macrophage production. (I) Quantification of LSK frequency in splenocytes and PBMC. To achieve a comparable analysis of HSPC frequency, 8 of 10 SR-BI+/+ mice on chow diet were females and 8 of 10 SR-BI−/− mice on chow diet were females. The mice on HFD were all males.
To address the role of SR-BI in the effects of HDL on HSPC, we enumerated the frequency of LT-HSC cells (briefly, HSC), LSK cells (HSPC) and granulocyte monocyte progenitors (GMP; CD34+ FcR+ lin− Sca-1− ckit+) in BM of SR-BI−/− and SR-BI+/+ mice on chow and HFD. In animals maintained on chow diet, we found a 1.7-fold increase of the percentage of LSK cells in the BM of SR-BI−/− mice compared with WT controls (LSK%: 0.090% vs. 0.054%; P<0.05; n=8–10). Following HFD, both HSC and LSK frequency was increased in BM of SR-BI−/− compared to WT mice (HSC%: 0.014% vs. 0.009% at 8 weeks of HFD; 0.017% vs. 0.011% at 10 weeks of HFD; n=11 for each, P <0.01; LSK%: 0.135% vs. 0.095% at 8 weeks of HFD; 0.184% vs. 0.090%, n=11 for each, P <0.01) (Figure 1, D–E and Supplementary figure II and VI). Although no difference was seen when mice were maintained on chow diet, the percentage of GMPs in BM cells was 1.2- and 1.5- fold increase in SR-BI−/− mice on HFD after 8 and 10 weeks of HFD, compared to WT mice on HFD (GMP%: 0.633% vs. 0.530% at 8 weeks of HFD; 0.816% vs. 0.537% at 10 weeks of HFD; n=11 for each, P<0.05) (Figure 1F and Supplementary figure VI). Consistent with this, BM cells (BMCs) from SR-BI−/− mice on HFD contained significantly greater numbers of hematopoietic colonies forming cells (CFCs) compared with BMCs from WT mice on HFD (n=8, Figure 1G). In addition, when LSK cells were isolated from mice on HFD and cultured in vitro, SR-BI−/− LSK cells produced more F4/80+ macrophages than SR-BI+/+ LSK cells (n=7, Figure 1H). Finally, the frequency of LSK cells in the spleen and peripheral blood SR-BI−/− mice fed on HFD was significantly higher than in WT mice of HFD (n=4–8, Figure 1I).
Transplanted SR-BI−/− BM caused enhanced atherosclerotic plaques formation, wherein grafted cells could be detected
To further demonstrate the increased frequency of HSPC cells in BM of WT and SR-BI−/− mice on chow diet, we performed limiting dilution competitive repopulation studies. CD45.2+ WT or SR-BI−/− BM cells were mixed with CD45.1 BM cells at ratios of 1:3, or 1:1 or 3:1, and injected in irradiated CD45.1 recipients. SR-BI−/− CD45.2 chimerism at 4 and 16 weeks after transplantation was significantly higher, consistent with increased frequency of progenitors and HSPCs in SR-BI−/− murine BM (n=4–6, Figure 2A).
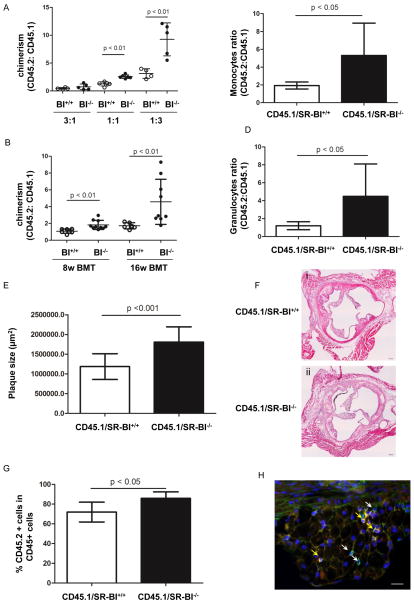
Figure 2. The effect of SR-BI deficiency on BM reconstitution and plaque progression in hypercholesterolemic mice.
(A) To compare the LSK frequency in mice on chow diet, SR-BI+/+ or SR-BI−/− CD45.2 BMCs were mixed with CD45.1 BMCs at ratios of 3:1, 1:1 and 1:3 and injected in irradiated CD45.1 recipients. (B) Equal numbers of SR-BI+/+ or SR-BI−/− BMCs were mixed with CD45.1 BMCs and injected in irradiated LDLr−/− recipients. Following BM transplantation, the recipients were first fed on chow diet for 8 weeks and then switched to high fat diet (HFD) for another 8 weeks. Blood cells were stained with anti-mouse CD45.1 and anti-mouse CD45.2 to assess chimerism by FACS. (C–D) The contribution of SR-BI+/+ and SR-BI−/− HSPC to monocytes and granulocytes is shown in Figure 2C and 2D, respectively. (E) Quantification of atheroma in aortic roots of LDLr−/− mice transplanted with SR-BI+/+ or SR-BI−/− BMCs. (F) Representative H&E pictures of LDLr−/− mice received SR-BI+/+ or SR-BI−/− BMCs. Scale bar: 200 μm. (G) Cryosections were stained with rat anti-mouse CD45 and biotin CD45.2 antibodies overnight and then goat anti-rat Alexa 488 and Streptavidin 555. CD45.1− and CD45.2− derived cells were quantified. Data are expressed as the percentage of CD45.2+CD45+ cells in CD45+ cells. (H) Representative image demonstrating CD45 cells in the plaques. CD45.1-derived cells are indicated by white arrows, whereas CD45.2− derived cells are indicated by yellow arrows. Scale bar: 20 μm. Male donors and recipients were used in both BM transplantation experiments.
We next wished to determine if SR-BI deficient HSC/HSPC play a role in atherosclerotic plaque development under HFD conditions. To address this question, we grafted CD45.2 BM cells of SR-BI+/+ or SR-BI−/− mice mixed with equal numbers of CD45.1 BM cells in irradiated LDLr−/− recipients. After transplantation, recipients were fed on chow diet for the first 2 months and then switched to HFD for another 2 months. Consistent with the limiting dilution analysis, higher chimerism of SR-BI−/− CD45.2 cells was observed at 4 and 16 weeks (n=6–10, Figure 2B). Moreover, after 16 weeks of BMT, significantly greater numbers of CD45.2+ granulocytes and monocytes were observed in recipients transplanted with SR-BI−/− than WT BM cells (n=6–10, Figure 2, C and D). We also found accelerated atherosclerosis in the aortic roots of LDLr−/− recipients transplanted with SR-BI−/− BM cells compared to that WT BM cells (n=6–8, Figure 3, E and F). To further explore the contribution of SR-BI−/−- and SR-BI+/+- derived inflammatory cells to plaque formation, cryosections were stained with biotin-CD45.2 and rat anti-mouse CD45 and then streptavidin 555 and goat anti-rat Alexa 488. The fraction of CD45.2 cells among the CD45+ cells in the plaques was 1.2- fold higher in recipients transplanted with SR-BI−/− BM cells, compared to those transplanted with WT BMCs (n=6–7, Figure 4, G-H and supplementary figure III).
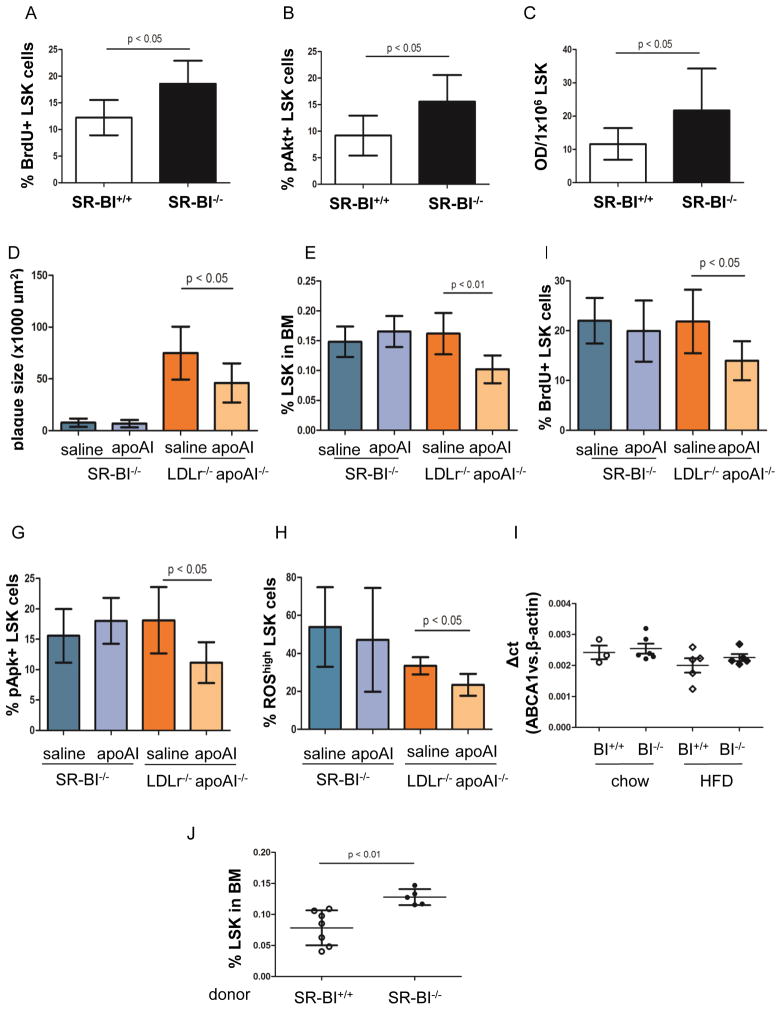
Figure 3. Human apoA-I infusion inhibited HSPC proliferation, reduced ROS production in HSPC and reversed plaque progression.
To quantify the proliferative status of HSPC in vivo, BrdU was injected in mice. BrdU positive LSK cells were quantified by FACS (Figure 4A). Akt phosphorylation (pAkt) in LSK cells was studied by staining BMCs with PE-conjugated anti-pAkt and LSK antibodies for FACS (B) and pAkt level in ex vivo expanded LSK cells were confirmed by ELISA (C). (D) Plaque size in aortic roots of SR-BI−/− and LDLr−/− apoA-I−/− (double knock-out, DKO) mice that were placed on high fat diet (HFD) and received saline or human apoA-I injection. Quantification of LSK frequency (E) and LSK proliferation (F) in BMC of SR-BI−/− and DKO mice that were treated with HFD and injection of saline or apoA-I. (G) The percentage of pAkt+ LSK cells in the entire LSK cell population in mice was measured by FACS. (H) BMCs were stained with LSK antibodies and then incubated with DCF-DA. The percentage of ROShigh LSK cells in the LSK population was quantified by FACS. Only male SR-BI+/+, SR-BI−/− and LDLr−/−apoA-I−/− mice were used in the apoA-I infusion experiments. (I) ABCA1 expression in LSK cells of male SR-BI+/+ and SR-BI−/− mice on chow and HFD. n=3–6. (J) Following apoA-I injection, LSK frequency in LDLr−/− recipients transplanted with SR-BI+/+ or SR-BI−/− BMC. n=5–7. 6 male LDLr−/− and 6 LDLr−/− female recipients were used in the BM transplantation experiment.
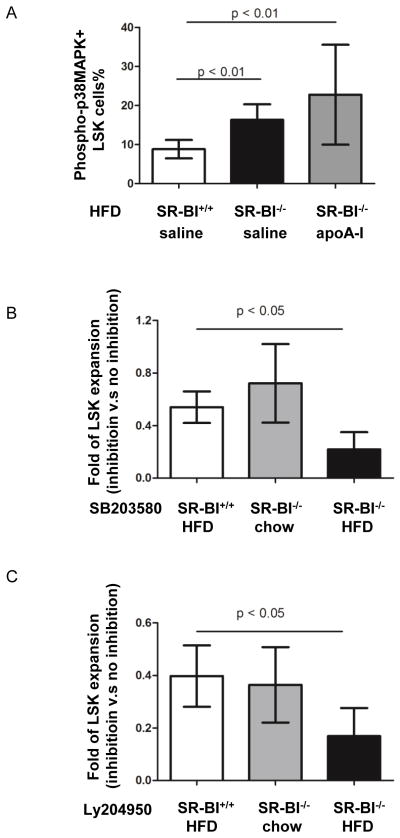
Figure 4. The roles of p38MAPK and Akt phosphorylation on LSK quiescence.
SR-BI+/+ and SR-BI−/− mice were fed on high fat diet (HFD) for 8 weeks and then injected with saline or apoA-I while keeping the mice on HFD. (A) BMCs were stained with PE-conjugated antibody against phospho-p38MAPK and LSK antibodies. The percentage of phospho-p38MAPK+ LSK cells in the LSK population was obtained by FACS. (B–C) LSK cells from SR-BI+/+ on HFD, SR-BI−/− on chow and HFD mice were isolated by FACS and cultured in vitro in the presence or absence of the phospho-p38MAPK inhibitor SB 203580 (B) and pAkt inhibitor Ly 204950 (C). Four days after culture, the cell number was enumerated. Data are expressed as fold reduction when compared to cells cultured without inhibitor. Male SR-BI+/+, SR-BI−/− mice were used in the apoA-I infusion experiments in vivo and HSPC expansion in vitro.
Infusion of lipid free human apoA-I inhibited HFD-induced HSPC proliferation, Akt phosphorylation and plaque progression in LDLr−/− apoA-I−/− mice but had no effect on SR-BI−/− mice
To determine whether the increased HSPC frequency in SR-BI−/− mice on HFD was due to enhanced HSPC proliferation, BrdU was injected i.p. into mice 12 hours before sacrifice and BM cells were stained with anti-LSK and anti-BrdU FITC Abs as described before.3 The percentage of BrdU incorporating LSK cells among LSK population was 12% in WT mice on HFD but increased to 18% to SR-BI−/− mice on HFD (SR-BI+/+: 12.2 ± 3.32% ; SR-BI−/−: 18.6 ± 4.33 ; n=6 for each, P <0.05) (Figure 3A). Apart from enhanced HSPC proliferation, FACS data also demonstrated an increased percentage of pAkt+ LSK cells in SR-BI−/− mice on HFD compared to WT mice (pAkt+ LSK%: 15.5 ± 5.00% v.s 9.2 ± 3.76; n=8 for each, P < 0.05) (Figure 3B). To further assess the pAkt status in HSPC, LSK cells were sorted from BM or SR-BI+/+ and SR-B−/− mice on HFD. After four days of culture in SFEM supplemented with stem cell factor (SCF) and thrombopoietin (TPO), pAkt expression in LSK cells was measured by ELISA (n=11 for each, Figure 3C).
To further explore if SR-BI was required for the HDL-mediated regulation of HSPC, SR-BI−/− and WT mice were placed on HFD for 11 weeks and 500 μg lipid free human apoA-I or saline was injected i.p. into mice twice per week for 3 weeks. In parallel, LDLr−/− apoA-I−/− mice (DKO) mice on HFD for 9 weeks were injected for the last three weeks with saline or apoA-I twice weekly for 3 weeks. Mice with deficiency of LDLr and apoA-I developed hypercholesterolemia and accelerated atherosclerosis when fed on atherogenic diet.22 In addition, SR-BI is expressed in DKO mice. Thus, performing apoA-I infusion on DKO and SR-BI−/− mice would allow us to investigate the effect of SR-BI on cells. Consistent with previous reports,22 apoA-I did not alter cholesterol levels in the blood (data not shown). However, apoA-I infusion reduced plaque size in DKO mice (45985 ± 18951.1 μm2 vs. 74878 ± 25510.1 μm2, n=6–9, P<0.05) (Figure 3, D and supplementary figure IV).22 We found a 1.6- fold reduction in LSK frequency in DKO mice injected with apoA-I compared to saline (n=6–7, Figure 3E). Moreover, the percentage of BrdU+ LSK cells in the whole LSK population was 37% decreased in DKO mice with apoA-I injection, compared with saline group (n=6–7, Figure 3F). In addition, we also found a 39% reduction in pAkt positive LSK cells in mice that received apoA-I infusion compared to control (n=6–7, Figure 3G). Interestingly, apoA-I infusion also reversed the increased ROS content in LSK cells of DKO mice on HFD (% ROShigh LSK cells in LSK population: 33.5 ± 4.50 vs. 23.4 ± 5.78; P < 0.05; n=5–6, Figure 4H). In contrast to DKO mice, apoA-I infusion had no effect on plaque size, LSK cell proliferation or Akt phosphorylation of HSPC in SR-BI−/− mice on HFD (n=4–7, Figure 3, D–H). To further investigate whether regulation of apoA-I on HSPC requires SR-BI, LSK cells were obtained from SR-BI+/+ and SR-BI−/− mice for 8-weeks on chow diet or HFD and transcripts for ABCA1 and β-actin expression measured in LSK cells by qRT-PCR (Figure 3I). We performed another head to head comparison to confirm that SR-BI is required for apoA-I-mediated modulation of HSPC number. LDLr−/− recipients were lethally irradiated and then transplanted with 7 × 106 SR-BI+/+ or SR-BI−/− BMC. Five days after BM transplantation, the recipients were switched from chow diet to HFD for 8 weeks. Starting from 5th weeks of HFD, 500 μg purified human apoA-I was injected subcutaneously to all the recipients twice per week for three weeks. Two days after the last injection, mice were sacrificed and BMC were stained with an Ab cocktail against LSK cells. FACS data demonstrated that LSK frequency in BMC was lower in LDLr−/− recipients transplanted with SR-BI+/+ BMC, compared to that of LDLr−/− recipients transplanted with SR-BI−/− BMC (0.08 ± 0.028% vs. 0.13 ± 0.013%, n=5–7, P<0.05) (Figure 3J). These data indicate that HDL/apoA-I directly regulates HSPC frequency, proliferation, and ROS production, all of which is mediated via HDL receptor, SR-BI.
Inhibition of Akt and p38MAPK phosphorylation maintained HSPC quiescence in SR-BI−/− mice on HFD
As mentioned earlier, p38MAPK phosphorylation is an important mediator of HSPC differentiation. To evaluate whether p38MAPK phosphorylation was involved in the regulation of HSPC by HDL and whether apoA-I infusion had any impact on p38MAPK phosphorylation, SR-BI+/+ and SR-BI−/− mice were fed on HFD and received saline or apoA-I infusion as described above. BMCs of SR-BI+/+ and SR-BI−/− mice were stained with antibodies against LSK markers and phospho-p38MAPK. FACS data demonstrated that the percentage of phospho-p38MAPK+ LSK cells was 1.7- fold higher in SR-BI−/− mice compared to SR-BI+/+ mice (15.2 ± 4.01% vs. 8.8 ± 2.37%, n=8 for each, P < 0.01). Nevertheless, apoA-I infusion did not affect p38MAPK phosphorylation in LSK cells (Figure 4A) or in LSK cells of DKO mice (data not shown).
To further assess the possible roles of pAkt and phospho-p38MAPK in the aberrant behavior of LSK cells from SR-BI−/− mice, LSK cells from SR-BI−/− and WT mice were selected by FACS and cultured in SFEM with stem cell factor, thrombopoietin and IL-3 in the presence or absence of inhibitors against pAkt and phopho-p38MAPK. On day 5 cell numbers were enumerated. LSK expansion was determined by comparing cell number with or without inhibition. As shown in Figure 6B, compared with LSK cells without inhibition, addition of phospho-p38MAPK inhibitor SB203580 led to 46% and 28% reduction of LSK expansion in cells from WT mice on HFD and cells from SR-BI−/− mice on chow diet. However, phospho-p38MAPK inhibitor further inhibited LSK expansion in SR-BI−/− cells on HFD (n=5–6, Figure 4B). Likewise, pAkt inhibition LY 294002 resulted in 60% and 64% decrease in LSK cells in cells from WT mice on HFD and cells from SR-BI−/− mice chow diet, respectively, compared to control. Again, addition of LY 294002 further reduced LSK expansion in SR-BI−/− cells from HFD (n=5–6, Figure 4C).
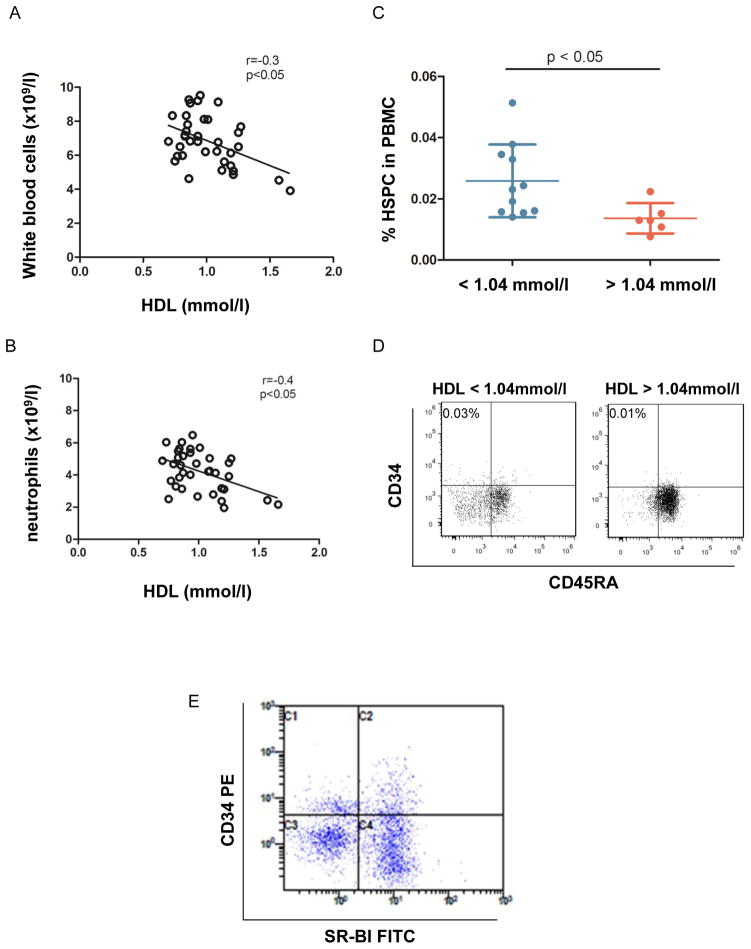
Figure 6. Negative correlation between HDL, WBC and HSPC in PB of human subjects.
In patients with coronary heart disease the correlation between HDL level and WBC and neutrophil count was assessed. Patients whose WBC count exceeded 10 ×109/l or neutrophil frequency exceeding 75% were excluded to avoid the influence of acute inflammation. (A) Correlation between HDL and WBC count. r=−0.339, p=0.04, n=37. (B) Correlation between HDL and neutrophil count. r=−0.356, p=0.031, n=37. (C) When PBMC were stained with anti-CD34, CD38, CD45a and lineage antibodies, and the number of CD34+CD38-CD45A-Lin- cells in PBMC of patients with low (<1.04 mmol/l) and normal HDL (>1.04 mmol/l) was analyzed. (D) Representative dot plots demonstrate CD34+ CD45RA− HSPC when gated on Lin− CD38− cells. HSPC frequency is indicated in the left corner. (E) PBMC were stained with rabbit anti-mouse SR-BI and then goat anti-rabbit Alexa 488 and anti-CD34, CD38, and lineage Abs, SR-BI expression was detected on CD34+CD38-Lin- cells by FACS. The representative dot plots showed SR-BI expression in CD34+ cells when gated on Lin− and CD38− cells.
Inhibition of ROS production limited SR-BI−/− HSPC expansion induced by HFD
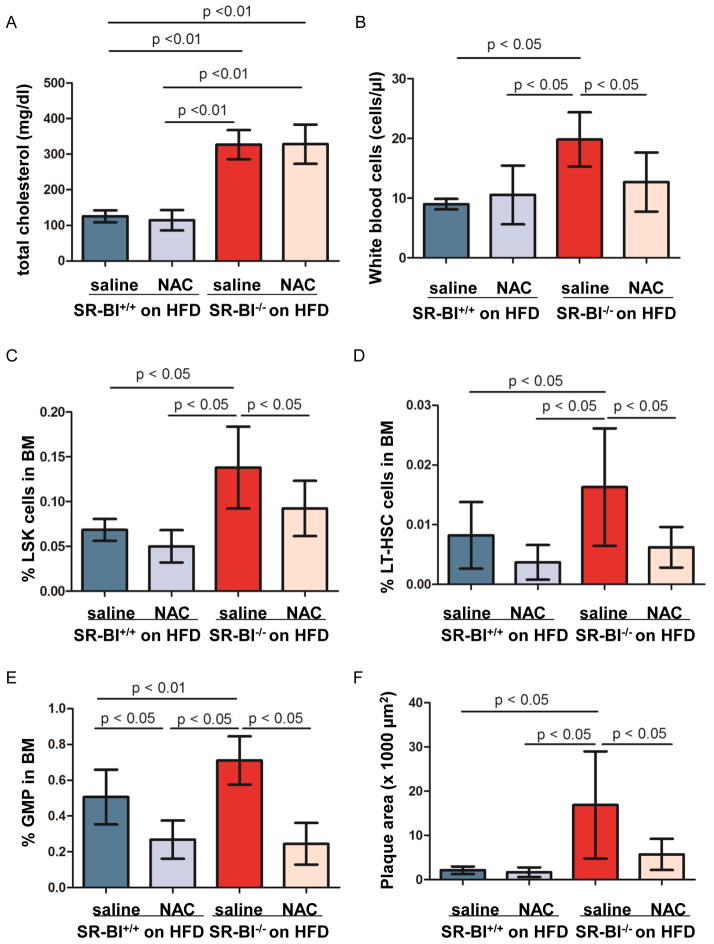
To further determine if the increased ROS levels found in LDLr−/− apoA-I−/− mice on HFD, could also be linked to the SR-B1 receptor, N-acetyl-L-cysteine (NAC) (1 mg/kg daily) was injected in SR-BI+/+ and SR-BI−/− mice placed on HFD at the age of 8 weeks for 12 weeks.23 NAC administration did not alter total cholesterol (n=3–7, Figure 5A) and lipoprotein profiles (data not shown). NAC-treated mice developed body weight loss compared with saline treated mice (data not shown). However, plasma levels of Serum Amyloid A was not different among the groups, indicating that NAC injection did not induce systemic inflammation (SR-BI+/+ mice: 160 ± 13.7 μg/ml on saline group vs. 157 ± 48.7 μg/ml on NAC group; SR-BI−/− mice: 173 ± 9.4 μg/ml vs. 160 ± 57.7 μg/ml on NAC group). Beside it reversed HFD-induced leukocytosis (n=5–8, Figure 5B), NAC treatment led to a significantly decreased frequency of LT-HSC, LSK cells and GMP in the BM of SR-BI−/− mice on HFD (n=7–11, Figure 5, C–E). Furthermore, NAC injection significantly reduced plaque size 3.8- fold in SR-BI−/− mice on HFD, compared to HFD-fed SR-BI−/− mice with saline injection (SR-BI+/+ on HFD: 2112 ± 849.4 μm2 ; SR-BI+/+ on HFD and NAC injection: 1643 ± 1096.5 μm2; SR-BI−/− on HFD: 17237 ± 12131.6 μm2; SR-BI−/− on HFD and NAC treatment: 5691 ± 3538.9 μm2; n=6–11, Figure 5F and supplementary figure V).
Figure 5. The effect of ROS inhibition on HSPC frequency and plaque progression.
SR-BI+/+ and SR-BI−/− mice were fed on high fat diet (HFD), without or with N-acetyl-L-cysteine (NAC) injection for 12 weeks. Total cholesterol is shown in A. White blood cell count is displayed in B. BMCs were stained with anti-CD150 FITC, anti-CD48 PE and LSK antibodies to quantify the percentage of LSK cells (C) and CD150+CD48-LSK cells (D) in BMC. (E) Quantification of GMP frequency in BMCs of mice on HFD with or without NAC treatment. (F) Plaque size in aortic roots of mice on HFD with or without NAC treatment. n=7–11 per group. In the entire NAC injection experiments, 9 of 13 SR-BI+/+ mice were females and 10 of 18 SR-BI−/− mice were females.
HDL negatively regulated HSPC frequency and white blood cell count in PB of human subjects
To investigate if the effect of HDL on mouse HSC and HSPC is also found in human, we studied the correlation between HDL and WBC level in patients (n=37) with coronary heart disease. To avoid the influence of acute infection, individuals with WBC counts of > 10,000 cells/μl or neutrophil frequency above 75% were excluded from the study. The basic characterization of these patients is shown in table 2. Spearman analysis demonstrated a negative correlation between HDL levels and total WBC count in PB (P<0.05, r=−0.3, n=37) (Figure 6A) as well as neutrophils (P<0.05, r=−0.4, n=37) (Figure 6B). As we observed that females had higher HDL-C than males (HDL-C: 1.07 ± 0.050 vs. 0.92 ± 0.040, P<0.05), Multilogistic Regression Analysis was performed to further investigate the association between HDL and WBC count by taking age and sex as co-variates. When taking these variables into account, HDL-C remained negatively correlated with WBC count in these patients (P=0.02). Consistent with previous reports,24, 25 patients received Statins treatment had higher HDL-C. Both Statins and aspirin treatments did not affect WBC count. Next, we measured HSPC (Lin-CD34+CD38-CD45RA−/low) in the blood of patients with low and high levels of HDL. The percentage of HSPC in mononuclear cells in peripheral blood (PBMC) was 0.025% in the patients with low HDL and 0.014% in patients with normal HDL (Figure 6, C and D and Supplementary figure VI, n=6–11, P < 0.05). We could also detect SR-BI expression on human HSPC (n=6, Figure 7E). Hence, as in mouse, in humans HDL levels appear to be correlated with leukocytosis and presence of higher numbers of HSPC, that also express the HDL receptor SR-BI, in the blood.
Discussion
Accumulated studies have described the anti-atherosclerotic properties of HDL. Beside reverse cholesterol transport, HDL promotes endothelium repair to maintain endothelium integrity and inhibits inflammatory cell infiltration to lesion site.26–29 Most of the beneficial regulation of HDL is mediated through HDL receptor Abca1, Abcg1 and SR-BI.26, 30–32 Apart from that, SR-BI also plays a major role in the clearance of lipopolysaccharide.33 Recently, others and we reported that HDL suppresses HSPC proliferation, resulting in the inhibition of leukocytosis and atherosclerosis progression.2, 3, 34 Although we described that SR-BI is expressed on murine HSPC and that HSPC proliferation is inhibited by apoA-I infusion,3 whether and how SR-BI is involved in the HDL-mediated regulation of HSPC is unknown. Here we demonstrate that SR-BI is required for the HDL-mediated regulation of HSPC quiescence, proliferation and differentiation. The signaling pathways underlying the regulation of HDL on HSPC are Akt phosphorylation, p38 MAPK phosphorylation and ROS production downstream of SR-BI.
Hypercholesterolemia stimulates monocyte and HSPC proliferation,2, 3, 35 leading to leukocytosis, monocytosis and atherosclerosis development. Like macrophages, HSPC express Abca1, Abcg1 and apoE,2, 34 all of which regulate intracellular cholesterol homeostasis. Both Abca1/Abcg1 and apoE deficiency impair cholesterol efflux, leading to cholesterol accumulation in lipid rafts in cell membranes. This change of lipid rafts leads to increased localization of receptors such as IL-3/GM-CSF receptor on the surface; and modulates phosphorylation cascades originating from membrane-bound proteins.2, 34 As a result, proliferation and differentiation of HSPC is triggered, resulting in an enhanced white blood cell pool in the PB. In this study, we identified a role for SR-BI in HSPC quiescence vs. proliferation in response to apoA-I. The phenotype of SR-BI−/− mice shared some features in common with Abca1−/−Abcg1−/− and apoE−/− mice in the aspect of HSPC proliferation, leukocytosis and accelerated atherosclerosis induced by hypercholesterolemia. Based on the nature of SR-BI in cholesterol transport and our findings, we assumed that both impaired cholesterol homeostasis and activated phosphorylation cascades may be involved in SR-BI−/− HSPC proliferation. This assumption is supported by several lines of observations: (1) higher HSPC frequency and more HSPC proliferation in SR-BI−/− on HFD than SR-BI+/+ on HFD; (2) apoA-I infusion prohibited Akt phosphorylation in HSPC of DKO mice on HFD but not in SR-BI−/− on HFD, and correspondingly, (3) infusion of human apoA-I reduced HSPC frequency and inhibited HSPC proliferation in DKO mice on HFD, but not in SR-BI−/− mice on HFD; (4) in vitro inhibition of Akt phosphorylation reduced SR-BI−/− LSK expansion more than that of SR-BI+/+ LSK cells when both were isolated from HFD feeding mice. Apart from higher pAkt in HSPC of SR-BI−/− mice on HFD, we also detected more phospho-p38MAPK on HSPC in these mice. Addition of phospho-p38MAPK inhibitor restrained more SR-BI−/− LSK expansion than SR-BI+/+ LSK when they were isolated from mice on HFD. Thus, both Akt phosphorylation and p38MAPK phosphorylation may contribute to HSPC activation and proliferation, which are downstream of SR-BI. However, to fully prove this, in vivo Akt and p38MAPK inhibition studies will be needed, which can technically not be performed. Apart from these findings, we noticed that neither apoA-I infusion nor NAC injection altered cholesterol level in the blood of these mice despite they suppressed HSPC proliferation. These data suggest that intracellular signaling pathways may contribute more substantially than lipid raft to the determination of HSPC cell fate.
As described earlier, HSPC reside in low oxygen BM niche and have low intracellular level of ROS, which help maintain their quiescence and self-renewal capacity.6, 13 In Ataxia telangiectasia (A-T) mutated (Atm−/−) mice, Atm−/− HSPC cycle more active than Atm+/+ HSPC, resulting in HSPC exhaustion and loss of BM reconstitution capacity.11 In Mdm2−/− mice, due to Mdm2 deficiency, p53 expression was up-regulated and ROS production was increased in HSPC, leading to enhanced cell death.36 Prohibition of ROS production by NAC treatment rescued ROS-induced HSPC defects in Atm−/− and Mdm2−/− mice.11, 36 In parallel, the adverse effects of ROS in atherosclerosis development have been extensively studied. All the findings consistently delineated that enhanced ROS production induced endothelial cell death and dysfunction,37, 38 plaque instability by ROS-induced activation of matrix metalloproteinases,39 and inflammation.40, 41 In this study, we investigated the physiological and pathological meaning of ROS in the context of HSPC, HDL, SR-BI and atherosclerosis. We demonstrate that hypercholesterolemia stimulated HSPC proliferation and enhanced ROS production, which was associated with plaque development in SR-BI−/− mice. ApoA-I infusion attenuated HFD-induced ROS production, HSPC expansion and atherosclerosis progression in DKO mice. Similarly, NAC treatment reversed HFD-induced HSPC proliferation and leukocytosis and inhibited atherosclerosis progression in SR-BI−/− mice. Although the contribution of pAkt and phospho-p38MAPK on ROS production in HSPC is yet to be defined, suppression of ROS production in HSPC seemed to have a beneficial role in atherosclerosis. To our knowledge, this is the first study demonstrating a link between hypercholesterolemia, ROS, HSPC activation and atherosclerosis.
By FACS, SR-BI was detected on human and murine HSPC. Furthermore, the number of white blood cells and HSPC in blood was intimately and negatively associated with HDL level in individuals. Taken the mice findings together, we speculated that HDL could regulate human HSPC via SR-BI. Perspective, it would be very interesting to investigate the relationship of HSPC and atherosclerosis in patients with SR-BI mutation and deficiency.
Overall, in line with the previous reports, this study confirmed the link between HSPC proliferation, leukocytosis and atherosclerosis progression. In addition, we demonstrated that the HDL receptor, SR-BI is expressed on HSPC as well as Abca1 and Abcg1. SR-BI could play a critical role in the control of HSPC proliferation and differentiation, therefore, limiting HFD-induced leukocytosis in blood, inflammatory cell infiltration in plaque and plaque progression.
Supplementary Material
Table 1. Basic characteristics of patients enrolled in the correlation and HSPC studies.
Patients diagnosed as coronary heart disease were enrolled in the study. Their disease history and medical measurements are characterized below.
| Parameters | HDL < 1.04 mmol/l | HDL > 1.04 mmol/l | p value |
|---|---|---|---|
| number | 22 | 15 | |
| Age (year) | 64 ± 3.0 | 60 ± 2.7 | 0.33 |
| BMI (kg/m2) | 24.2 ± 1.23 | 23.0 ± 0.79 | 0.44 |
| Systolic pressure (mmHg) | 124.2 ± 4.34 | 125.9 ± 2.22 | 0.75 |
| Diastolic pressure (mmHg) | 78.33 ± 3.66 | 75 ± 3.59 | 0.52 |
| White blood cells (109/l) | 7.6 ± 0.28 | 6.2 ± 0.33 | 0.03* |
| Neutrophils (109/l) | 4.9 ± 0.27 | 3.7 ± 0.28 | 0.004* |
| Red blood cells | 4.2 ± 0.18 | 4.4 ± 0.18 | 0.43 |
| Glucose (mmol/l) | 9.4 ± 1.12 | 9.5 ± 1.01 | 0.97 |
| Platelet | 227 ± 15.4 | 211 ± 15.6 | 0.93 |
| Total cholesterol (mmol/l) | 4.54 ± 0.219 | 5.3 ± 0.315 | 0.046* |
| LDL (mmol/l) | 2.85 ± 0.184 | 3.74 ± 0.323 | 0.014* |
| Total tryglyceride (mmol/l) | 2.12 ± 0.271 | 1.69 ± 0.284 | 0.30 |
compared with patients with low HDL.
Significance.
The athero-protective properties of HDL and SR-BI have been shown in multiple cell types including hepatocytes, macrophages, endothelial cells and endothelial progenitors. Hereby, we demonstrated that HDL suppresses hematopoietic stem/progenitor cell (HSPC) proliferation that is mediated via SR-BI. In addition, we highlighted the link between HDL-mediated inhibition of reactive oxygen species (ROS) content in HSPC, reduced HSPC activation and leukocytosis, and attenuation of atherosclerosis progression, all of which is SR-BI-dependent. Providing that inflammatory cells in atherosclerotic plaque are exclusively derived from HSPC, these findings not only enrich our knowledge of atherosclerosis in the context of HDL, SR-BI and HSPC but also shed lights on therapeutic interventions of atherosclerosis.
Acknowledgments
Hereby, we express our sincere appreciation to Prof. Deneys Van der Westhuyzen for providing SR-BI−/− mice and the littermates and advice for us to generate the colonies, to Dr. Xuhong Wang, Dr. Baoyu Zhang and Dr. Caiguo Yu from the Department of Endocrinology in Lu He hospital for providing patients profiles and blood samples, to Ms. Evelyn De Schryver and Mr. Thomas Vanwelden for their technical assistance, to Prof. Jan Stassen from KU LLeuven to his advice.
Sources of funding
This work was supported by FWO funding (G1508612N) to YMF; Beijing Technology Foundation (Z131100006813018) to DZ; NIH NHLBI R01HL112270, HL64164 and HL112276 to MST; Major National Basic Research Program of the People’s Republic of China (2011CB503900 and 2012CB517505) and National Natural Science Foundation of the People’s Republic of China (30930037 and 81121061) to GL; the Vanwayenberghe fonds, FWO funding (G085111N), and NIH-PO1-CA-65493-06 funding to CMV.
Nonstandard abbreviations
- HDL
high density lipoprotein
- apoA-I
apolipoprotein A-I
- HSPC
hematopoietic stem/progenitor cells
- PB
peripheral blood
- LSK cells
Lin- Sca-1+ cKit+ cells
- SR-BI
scavenger receptor type BI
- LT-HSC
long term hematopoietic stem cells
- ROS
reactive oxygen species
- BM
bone marrow
- WBC
white blood cells
- LDL
low density lipoporotein
- LDLr−/−
low density lipoprotein receptor knockout
- GMP
granulocyte monocyte progenitors
- HFD
high fat diet
- WT
wide type
- DKO
LDLr−/− apoA-I−/−
- NAC
N-acetylcysteine
- pAkt
phospho Akt
- PBMC
mononuclear cells in peripheral blood
- Atm
Ataxia telangiectasia (A-T) mutated
Footnotes
Disclosures
None.
References
- 1.Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, Jacobs DR, Jr, Bangdiwala S, Tyroler HA. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective american studies. Circulation. 1989;79:8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR. Atp-binding cassette transporters and hdl suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng Y, Schouteden S, Geenens R, Van Duppen V, Herijgers P, Holvoet P, Van Veldhoven PP, Verfaillie CM. Hematopoietic stem/progenitor cell proliferation and differentiation is differentially regulated by high-density and low-density lipoproteins in mice. PLoS One. 2012;7:e47286. doi: 10.1371/journal.pone.0047286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miharada K, Karlsson G, Rehn M, Rorby E, Siva K, Cammenga J, Karlsson S. Cripto regulates hematopoietic stem cells as a hypoxic-niche-related factor through cell surface receptor grp78. Cell Stem Cell. 2011;9:330–344. doi: 10.1016/j.stem.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 5.Pollard PJ, Kranc KR. Hypoxia signaling in hematopoietic stem cells: A double-edged sword. Cell Stem Cell. 2010;7:276–278. doi: 10.1016/j.stem.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Juntilla MM, Patil VD, Calamito M, Joshi RP, Birnbaum MJ, Koretzky GA. Akt1 and akt2 maintain hematopoietic stem cell function by regulating reactive oxygen species. Blood. 2010;115:4030–4038. doi: 10.1182/blood-2009-09-241000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maehama T, Dixon JE. The tumor suppressor, pten/mmac1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 8.Geest CR, Buitenhuis M, Groot Koerkamp MJ, Holstege FC, Vellenga E, Coffer PJ. Tight control of mek-erk activation is essential in regulating proliferation, survival, and cytokine production of cd34+-derived neutrophil progenitors. Blood. 2009;114:3402–3412. doi: 10.1182/blood-2008-08-175141. [DOI] [PubMed] [Google Scholar]
- 9.Geest CR, Buitenhuis M, Laarhoven AG, Bierings MB, Bruin MC, Vellenga E, Coffer PJ. P38 map kinase inhibits neutrophil development through phosphorylation of c/ebpalpha on serine 21. Stem Cells. 2009;27:2271–2282. doi: 10.1002/stem.152. [DOI] [PubMed] [Google Scholar]
- 10.Geest CR, Coffer PJ. Mapk signaling pathways in the regulation of hematopoiesis. J Leukoc Biol. 2009;86:237–250. doi: 10.1189/jlb.0209097. [DOI] [PubMed] [Google Scholar]
- 11.Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y, Suda T. Reactive oxygen species act through p38 mapk to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 12.Chen C, Liu Y, Liu R, Ikenoue T, Guan KL, Zheng P. Tsc-mtor maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med. 2008;205:2397–2408. doi: 10.1084/jem.20081297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110:3056–3063. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ueda Y, Royer L, Gong E, Zhang J, Cooper PN, Francone O, Rubin EM. Lower plasma levels and accelerated clearance of high density lipoprotein (hdl) and non-hdl cholesterol in scavenger receptor class b type i transgenic mice. The Journal of biological chemistry. 1999;274:7165–7171. doi: 10.1074/jbc.274.11.7165. [DOI] [PubMed] [Google Scholar]
- 15.Arai T, Wang N, Bezouevski M, Welch C, Tall AR. Decreased atherosclerosis in heterozygous low density lipoprotein receptor-deficient mice expressing the scavenger receptor bi transgene. The Journal of biological chemistry. 1999;274:2366–2371. doi: 10.1074/jbc.274.4.2366. [DOI] [PubMed] [Google Scholar]
- 16.Feng Y, van Eck M, Van Craeyveld E, Jacobs F, Carlier V, Van Linthout S, Erdel M, Tjwa M, De Geest B. Critical role of scavenger receptor-bi-expressing bone marrow-derived endothelial progenitor cells in the attenuation of allograft vasculopathy after human apo a-i transfer. Blood. 2009;113:755–764. doi: 10.1182/blood-2008-06-161794. [DOI] [PubMed] [Google Scholar]
- 17.Covey SD, Krieger M, Wang W, Penman M, Trigatti BL. Scavenger receptor class b type i-mediated protection against atherosclerosis in ldl receptor-negative mice involves its expression in bone marrow-derived cells. Arteriosclerosis, thrombosis, and vascular biology. 2003;23:1589–1594. doi: 10.1161/01.ATV.0000083343.19940.A0. [DOI] [PubMed] [Google Scholar]
- 18.Trigatti B, Rayburn H, Vinals M, Braun A, Miettinen H, Penman M, Hertz M, Schrenzel M, Amigo L, Rigotti A, Krieger M. Influence of the high density lipoprotein receptor sr-bi on reproductive and cardiovascular pathophysiology. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:9322–9327. doi: 10.1073/pnas.96.16.9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vergeer M, Korporaal SJ, Franssen R, Meurs I, Out R, Hovingh GK, Hoekstra M, Sierts JA, Dallinga-Thie GM, Motazacker MM, Holleboom AG, Van Berkel TJ, Kastelein JJ, Van Eck M, Kuivenhoven JA. Genetic variant of the scavenger receptor bi in humans. N Engl J Med. 364:136–145. doi: 10.1056/NEJMoa0907687. [DOI] [PubMed] [Google Scholar]
- 20.West M, Greason E, Kolmakova A, Jahangiri A, Asztalos B, Pollin TI, Rodriguez A. Scavenger receptor class b type i protein as an independent predictor of high-density lipoprotein cholesterol levels in subjects with hyperalphalipoproteinemia. J Clin Endocrinol Metab. 2009;94:1451–1457. doi: 10.1210/jc.2008-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Eck M, Hoekstra M, Hildebrand RB, Yaong Y, Stengel D, Kruijt JK, Sattler W, Tietge UJ, Ninio E, Van Berkel TJ, Pratico D. Increased oxidative stress in scavenger receptor bi knockout mice with dysfunctional hdl. Arterioscler Thromb Vasc Biol. 2007;27:2413–2419. doi: 10.1161/ATVBAHA.107.145474. [DOI] [PubMed] [Google Scholar]
- 22.Wilhelm AJ, Zabalawi M, Owen JS, Shah D, Grayson JM, Major AS, Bhat S, Gibbs DP, Jr, Thomas MJ, Sorci-Thomas MG. Apolipoprotein a-i modulates regulatory t cells in autoimmune ldlr−/−, apoa-i−/− mice. J Biol Chem. 2010;285:36158–36169. doi: 10.1074/jbc.M110.134130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, Reheman A, Gushiken FC, Nolasco L, Fu X, Moake JL, Ni H, Lopez JA. N-acetylcysteine reduces the size and activity of von willebrand factor in human plasma and mice. J Clin Invest. 2011;121:593–603. doi: 10.1172/JCI41062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barter PJ, Brandrup-Wognsen G, Palmer MK, Nicholls SJ. Effect of statins on hdl-c: A complex process unrelated to changes in ldl-c: Analysis of the voyager database. J Lipid Res. 2010;51:1546–1553. doi: 10.1194/jlr.P002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicholls SJ, Tuzcu EM, Sipahi I, Grasso AW, Schoenhagen P, Hu T, Wolski K, Crowe T, Desai MY, Hazen SL, Kapadia SR, Nissen SE. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA. 2007;297:499–508. doi: 10.1001/jama.297.5.499. [DOI] [PubMed] [Google Scholar]
- 26.Seetharam D, Mineo C, Gormley AK, Gibson LL, Vongpatanasin W, Chambliss KL, Hahner LD, Cummings ML, Kitchens RL, Marcel YL, Rader DJ, Shaul PW. High-density lipoprotein promotes endothelial cell migration and reendothelialization via scavenger receptor-b type i. Circ Res. 2006;98:63–72. doi: 10.1161/01.RES.0000199272.59432.5b. [DOI] [PubMed] [Google Scholar]
- 27.Mineo C, Deguchi H, Griffin JH, Shaul PW. Endothelial and antithrombotic actions of hdl. Circ Res. 2006;98:1352–1364. doi: 10.1161/01.RES.0000225982.01988.93. [DOI] [PubMed] [Google Scholar]
- 28.Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of hdl. Circ Res. 2004;95:764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 29.Stannard AK, Khan S, Graham A, Owen JS, Allen SP. Inability of plasma high-density lipoproteins to inhibit cell adhesion molecule expression in human coronary artery endothelial cells. Atherosclerosis. 2001;154:31–38. doi: 10.1016/s0021-9150(00)00444-5. [DOI] [PubMed] [Google Scholar]
- 30.Yvan-Charvet L, Wang N, Tall AR. Role of hdl, abca1, and abcg1 transporters in cholesterol efflux and immune responses. Arterioscler Thromb Vasc Biol. 2010;30:139–143. doi: 10.1161/ATVBAHA.108.179283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yvan-Charvet L, Pagler TA, Seimon TA, Thorp E, Welch CL, Witztum JL, Tabas I, Tall AR. Abca1 and abcg1 protect against oxidative stress-induced macrophage apoptosis during efferocytosis. Circ Res. 2010;106:1861–1869. doi: 10.1161/CIRCRESAHA.110.217281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Da Silva JR, Reilly M, Billheimer JT, Rothblat GH, Rader DJ. Hepatic expression of scavenger receptor class b type i (sr-bi) is a positive regulator of macrophage reverse cholesterol transport in vivo. J Clin Invest. 2005;115:2870–2874. doi: 10.1172/JCI25327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cai L, Ji A, de Beer FC, Tannock LR, van der Westhuyzen DR. Sr-bi protects against endotoxemia in mice through its roles in glucocorticoid production and hepatic clearance. J Clin Invest. 2008;118:364–375. doi: 10.1172/JCI31539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy AJ, Akhtari M, Tolani S, Pagler T, Bijl N, Kuo CL, Wang M, Sanson M, Abramowicz S, Welch C, Bochem AE, Kuivenhoven JA, Yvan-Charvet L, Tall AR. Apoe regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J Clin Invest. 2011;121:4138–4149. doi: 10.1172/JCI57559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swirski FK, Wildgruber M, Ueno T, Figueiredo JL, Panizzi P, Iwamoto Y, Zhang E, Stone JR, Rodriguez E, Chen JW, Pittet MJ, Weissleder R, Nahrendorf M. Myeloperoxidase-rich ly-6c+ myeloid cells infiltrate allografts and contribute to an imaging signature of organ rejection in mice. J Clin Invest. 2010;120:2627–2634. doi: 10.1172/JCI42304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abbas HA, Maccio DR, Coskun S, Jackson JG, Hazen AL, Sills TM, You MJ, Hirschi KK, Lozano G. Mdm2 is required for survival of hematopoietic stem cells/progenitors via dampening of ros-induced p53 activity. Cell Stem Cell. 2010;7:606–617. doi: 10.1016/j.stem.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kojda G, Harrison D. Interactions between no and reactive oxygen species: Pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res. 1999;43:562–571. doi: 10.1016/s0008-6363(99)00169-8. [DOI] [PubMed] [Google Scholar]
- 38.Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res. 2007;100:460–473. doi: 10.1161/01.RES.0000258450.44413.96. [DOI] [PubMed] [Google Scholar]
- 39.Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest. 1996;98:2572–2579. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Basta G, Schmidt AM, De Caterina R. Advanced glycation end products and vascular inflammation: Implications for accelerated atherosclerosis in diabetes. Cardiovasc Res. 2004;63:582–592. doi: 10.1016/j.cardiores.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 41.Aikawa M, Sugiyama S, Hill CC, Voglic SJ, Rabkin E, Fukumoto Y, Schoen FJ, Witztum JL, Libby P. Lipid lowering reduces oxidative stress and endothelial cell activation in rabbit atheroma. Circulation. 2002;106:1390–1396. doi: 10.1161/01.cir.0000028465.52694.9b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.