Abstract
The potential human health effects of bisphenol A (BPA) exposure are a public health concern. In order to design adequately powered epidemiological studies to address potential health effects, data on the reproducibility of BPA concentration in serial urine specimens taken during pregnancy are needed. To provide additional data on the reproducibility of maternal urine specimens, 80 women in the Generation R Study (Rotterdam, NL) contributed a spot urine specimen at < 18, 18–25, and > 25 weeks of pregnancy. Reproducibility, estimated by the intraclass correlation coefficient, was 0.32 (95% confidence interval: 0.18, 0.46), and, on a creatinine basis, 0.31 (95% confidence interval: 0.16, 0.47). While the ICC observed in the Generation R Study is slightly higher than previous reproducibility studies of BPA, it nevertheless indicates a high degree of within person variability, which presents challenges for designing well-powered epidemiologic studies.
Keywords: Endocrine disruptor, epidemiology, environmental exposure, intraclass correlation coefficient, misclassification
Introduction
Bisphenol A (BPA), an essential component of polycarbonate plastics and epoxy resins, is a monomeric compound used in a variety of consumer, industrial, and medical applications. Examples include reusable water bottles and food containers, thermal receipts, metallic food and beverage containers, polyvinyl chloride stretch films, dental sealants, and medical tubing (1). Despite being detectable in the urine of nearly all people living in industrialized countries (2), exposure to BPA is episodic, varying in dose and duration. In addition, the vast majority of BPA appears to be rapidly excreted, with a half-life in urine—the preferred biomarker—of < 6 hours (3). Thus an estimate of exposure based on a spot urine specimen is likely to misclassify individuals with regard to their average long-term exposure, for example, over 9 months of pregnancy. This misclassification has implications for epidemiologic studies where BPA is the exposure of interest, affecting exposure assessment, study design, statistical power, and attenuating exposure-disease associations (4). To date, some epidemiologic studies have shown large effect sizes for child health outcomes in relation to maternal urinary BPA concentrations (5), suggesting that BPA may be an environmental contaminant of great public health interest. However, results are mixed and sometimes contradictory (6, 7). With this in mind, the present study was designed to determine the reproducibility of BPA using three urine specimens collected from a subset of 80 pregnant women participating in the Generation R Study.
Material and Methods
Participants for the present study were selected from the Generation R Study, an ongoing prospective cohort study in Rotterdam, the Netherlands (8). In total, 9 778 women who resided in the study area and had a delivery date between April 2002 and January 2006 were enrolled (61% of those approached to participate in the study). From February 2004 to January 2006, spot urine specimens were collected when women presented for ultrasound examinations during the early (< 18 weeks of gestational age), middle (18–25 weeks of gestational age), and latter (> 25 weeks of gestational age) periods of pregnancy (9). All samples were collected between 8 a.m. and 8 p.m. in 100mL polypropylene urine collection containers that were kept at room temperature for a maximum of 3 hours and transported to a dedicated laboratory facility of the regional laboratory in Rotterdam, the Netherlands (STAR-MDC), and frozen in 20mL portions in 25mL polypropylene vials at −20°C (10). In total, 2 083 women provided a complete set of three urine specimens, 1 161 women provided two specimens, and one urine specimen was available for 754 women. From the 2 083 women with a complete set of three urine specimens, we excluded women with missing data on maternal age, ethnicity, education level, pre-pregnancy body mass index (BMI), perinatal smoking habits, gravidity, parity, and gestational age at child’s birth. We also excluded women for whom child’s birthweight and Child Behavior Checklist scores at age 3 years were missing. These exclusions were applied in order to maximize the number of participants available for pilot studies of reproductive and neurobehavioral outcomes in relation to prenatal BPA exposures, and also to select a reproducibility sample that would be most generalizable to future studies in the Generation R cohort. Once these exclusions were applied, 80 women for the present reproducibility study were selected at random from the 1 024 women with three urine specimens and complete data (as described above). This was based on an expected intraclass correlation coefficient between 0.10–0.65 for three repeated measurements, with a desired precision of 0.30–0.40 (i.e. 95% confidence interval width) (11). This study protocol underwent human subjects review at Erasmus Medical Center, Rotterdam, the Netherlands (IRB Registration #: IRB00001482) and the National Institutes of Health, Bethesda, Maryland (Protocol #: MEC217.595/2002/202).
Total BPA concentration was determined at the Institute for Preventive and Occupational Medicine in Bochum, Germany using a high performance liquid chromatography – mass spectrometry method (on line SPE HPLC – MS/MS) described previously (12). The limit of detection (LOD) was 0.05µg/L, and the limit of quantification was 0.1µg/L. The lower LOD in the present study is in part due to the fact that our method was solely focused on BPA, which allowed us to maximize the precision of its measurement. The between-batch coefficient of variation (CV) was 6.1%, calculated from a pooled urine sample. Laboratory BPA contamination was minimized by limiting specimen handling, using minimal solvent for extraction, the lack of derivatization, and the use of an automated on-line extraction. The concentration of unconjugated (free) BPA was also determined for 26 samples based on having a high total BPA concentration relative to the entire pool of 240 samples. In all but one sample, which had 9.1% unconjugated BPA, all samples had unconjugated BPA that comprised less than 1.4% of the total BPA concentration measured. Urinary creatinine concentrations were also determined (LOD 10 mg/dL) (13), and the between-batch CV using the pooled urine sample was 5.5%. Fifteen women for whom at least one creatinine concentration was below 10 mg/dL (the limit of detection) were excluded from all creatinine- based analyses.
Spearman correlation coefficients between pairs of urinary BPA concentrations across pregnancy were estimated. To estimate reproducibility across all three points in time during pregnancy, we estimated the intraclass correlation coefficient (ICC) and 95% confidence intervals for (natural log) BPA as [between person variance / (between person + within person variance)] using the IRR package in R (14). Values can range from 0 to 1, where ICC < 0.4 indicates poor reproducibility, 0.4 to < 0.75 indicates fair to good reproducibility, and ≥0.75 indicates excellent reproducibility (15). We also conducted bivariate analyses to examine potential predictors of maternal BPA concentration. Natural log maternal BPA concentrations were modeled as a function of each predictor using the MIXED procedure in SAS (Version 9.3; SAS Institute Inc., Cary, NC, USA), and geometric mean BPA concentrations for each category of the predictor are presented.
Results
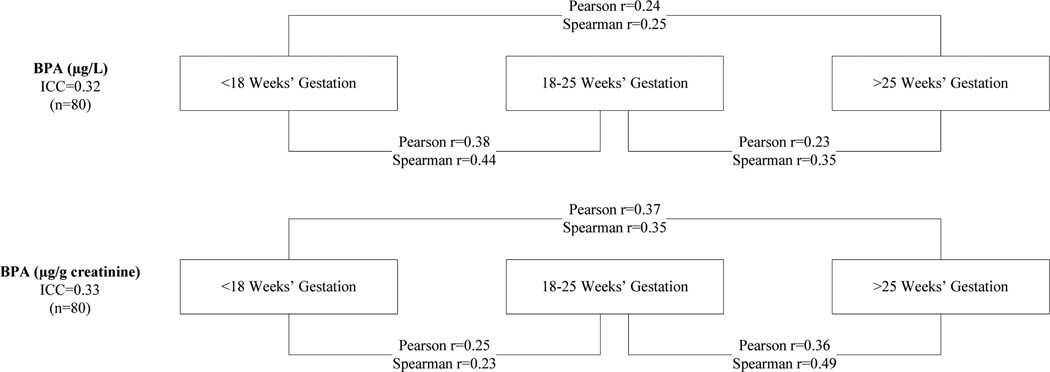
The 80 women selected for the present study were a random sample of the 1 024 women with 3 urine specimens and complete data. The resulting group of women in our study tended to be older, more likely to be Dutch or of other European ethnicity, and were generally more educated compared to those women enrolled in the Generation R Study (n=9 778) (Table 1). Total maternal BPA concentrations were detectible in all specimens. On average (±standard deviation), maternal urine specimens were collected at 13(±1.7), 20(±0.7), and 30(±0.7) weeks of gestation, and median BPA concentrations were 1.1, 1.5, and 1.6 µg/L across these three sampling periods (Table 2). Median BPA concentrations expressed on a creatinine-basis were 3.0, 3.0, and 2.6 µg/g creatinine across the same three sampling periods (Table 2). Geometric mean creatinine concentrations were 53.0, 57,0, and 51.2 mg/dL across the same sampling periods. BPA concentrations in the present study were higher than those reported in a previous analysis of a subset of pregnant women in the Generation R Study who had only 1 urine specimen collected after 20 weeks of pregnancy (9), likely owing to the greater proportion of older, Dutch, and better-educated women participating in the present study, factors which have been associated with higher BPA concentrations in the Generation R Study (9). Pairwise measures of BPA concentrations were moderately correlated (0.13≤rspearman≤ 0.49), regardless of whether they were expressed on a volumetric or creatinine basis (Figure 1). BPA measurements (ug/L) closer in time were more strongly correlated, although this was not true for the creatinine basis measures. The ICCs for three measures of urinary BPA were 0.32 (95% confidence interval: 0.18, 0.46), and on a creatinine basis, 0.31 (95% confidence interval: 0.16, 0.47), indicating low reproducibility. When we excluded the sample with 9.1% unconjugated BPA, the volumetric and creatinine basis ICCs were both 0.31.
Table 1.
Characteristics of participants in the BPA reliability subset, those with a complete set of pregnancy urine specimens, and the overall Generation R cohort.
| Characteristic | BPA subset (n=80) |
Three urine specimens and complete data (n=1 024) |
Three urine specimens (n=2 083) |
Generation R Cohort (n=9 778) |
P value1 |
|---|---|---|---|---|---|
| Number of pregnancies in study (%)2 | <0.0001 | ||||
| 1 | 82.5 | 85.4 | 86.4 | 83.8 | |
| 2 | 17.5 | 14.5 | 13.4 | 5.1 | |
| 3 | 0 | 0.2 | 0.1 | 0.1 | |
| Missing | 0 | 0 | 0.2 | 11.1 | |
| Age at enrollment (years)3 | 31.9±4.4 | 31.1±4.3 | 29.30±5.0 | 29.9±5.4 | <0.0001 |
| Parity (%) | 0.17 | ||||
| 0 | 52.5 | 62.5 | 57.5 | 53.0 | |
| 1 | 36.3 | 27.7 | 29.3 | 29.0 | |
| ≥2 | 11.2 | 9.8 | 12.3 | 14.2 | |
| Missing | 0 | 0 | 0.9 | 3.8 | |
| Ethnicity (%) | <0.0001 | ||||
| Dutch, other European | 73.8 | 70.2 | 48.4 | 45.4 | |
| Surinamese | 7.5 | 5.9 | 8.2 | 7.8 | |
| Moroccan | 2.5 | 3.2 | 6.6 | 5.7 | |
| Turkish | 7.5 | 7.7 | 9.0 | 7.8 | |
| Dutch Antilles | 1.3 | 2.1 | 2.5 | 3.0 | |
| Cape Verdian | 1.3 | 1.7 | 3.4 | 3.6 | |
| Others | 6.3 | 9.3 | 17.9 | 16.2 | |
| Missing | 0 | 0 | 4.0 | 10.5 | |
| Highest completed education (%) | <0.0001 | ||||
| Primary school | 7.5 | 6.3 | 9.4 | 9.8 | |
| Intermediate | 25.0 | 35.0 | 41.0 | 40.3 | |
| Higher | 67.5 | 58.7 | 43.9 | 37.4 | |
| Missing | 0 | 0 | 5.7 | 12.5 |
P values compare the BPA subset (n=80) to the Generation R Cohort (n=9778). P values are calculated by Fisher’s exact test for number of pregnancies in the study, by chi-square for parity, ethnicity, and education, and by t-test for age.
Percents may not sum to 100 because of rounding.
Mean ± standard deviation
Table 2.
Summary statistics for maternal urinary BPA concentrations in the Generation R cohort according to time of measurement.
| BPA measure | GM1 | Min | 25th Percentile | Median | 75th Percentile | Max |
|---|---|---|---|---|---|---|
| BPA (µg/L) (n=80) | ||||||
| < 18 weeks | 1.3 | 0.1 | 0.6 | 1.1 | 3.3 | 14.1 |
| 18–25 weeks | 1.5 | 0.2 | 0.6 | 1.5 | 3.2 | 64.8 |
| > 25 weeks | 1.6 | 0.2 | 0.8 | 1.6 | 2.6 | 47.5 |
| BPA (µg/g creatinine) (n=65) | ||||||
| < 18 weeks | 3.1 | 0.9 | 1.6 | 3.0 | 5.5 | 23.2 |
| 18–25 weeks | 3.3 | 0.8 | 2.0 | 3.0 | 4.1 | 40.4 |
| > 25 weeks | 3.2 | 0.6 | 1.8 | 2.6 | 4.2 | 43.3 |
Geometric mean
Figure 1.
Pairwise Spearman and (loge) Pearson correlation coefficients and ICC measures for BPA on a wet-weight and creatinine basis.
Overall, there was not strong evidence that any of the characteristics examined in Table 3 were related to maternal BPA concentration, though there was some suggestion that older maternal age at enrollment was associated with higher BPA concentrations on a volumetric and creatinine basis. Since our study was not powered to examine predictors of exposure, inferences concerning determinants of exposure should be interpreted with caution.
Table 3.
Mean maternal urinary BPA concentrations as a function of maternal characteristics.
| BPA µg/L (n=80) |
BPA µg/g creatinine (n=65) |
|||
|---|---|---|---|---|
| Maternal characteristic | N1 (obs) | GM (95% CI)2 | N (obs) | GM (95% CI)2 |
| Age at enrollment | ||||
| 20–29 | 21 (63) | 1.2 (0.8, 1.8) | 18 (54) | 2.5 (1.9, 3.3) |
| 30–34 | 44 (132) | 1.3 (1.0, 1.8) | 34 (102) | 3.1 (2.6, 3.8) |
| ≥35 | 15 (45) | 2.1 (1.3, 3.4) | 13 (39) | 3.9 (2.8, 5.5) |
| p-value for trend3 | 0.51 | 0.24 | ||
| Parity | ||||
| 0 | 42 (126) | 1.4 (1.1, 1.9) | 33 (99) | 3.3 (2.7, 4.1) |
| 1 | 29 (87) | 1.4 (1.0, 2.0) | 24 (72) | 2.8 (2.2, 3.6) |
| ≥2 | 9 (27) | 1.5 (0.8, 2.9) | 8 (24) | 3.1 (2.0, 4.9) |
| p-value for trend | 0.68 | 0.15 | ||
| Completed education | ||||
| Primary School | 6 (18) | 0.7 (0.3, 1.5) | 4 (12) | 2.2 (1.3, 3.8) |
| Intermediate | 20 (60) | 1.7 (1.2, 2.6) | 18 (54) | 2.9 (2.2, 4.0) |
| Higher | 54 (164) | 1.4 (1.1, 1.8) | 43 (129) | 3.3 (2.7, 3.9) |
| p-value for trend | 0.46 | 0.21 | ||
| Pre-pregnancy BMI | ||||
| <20 | 12 (36) | 2.0 (1.2, 3.4) | 9 (27) | 4.3 (3.0, 6.3) |
| 20–24 | 51 (153) | 1.2 (0.9, 1.5) | 42 (126) | 2.7 (2.3, 3.3) |
| ≥25 | 17 (51) | 2.0 (1.3, 3.2) | 14 (42) | 3.6 (2.7, 5.0) |
| p-value for trend | 0.52 | 0.52 | ||
| Smoking during pregnancy | ||||
| Never | 68 (204) | 1.5 (1.2, 1.8) | 55 (165) | 3.2 (2.7, 3.7) |
| Until pregnancy known | 4 (12) | 1.5 (0.6, 3.8) | 5 (15) | 3.7 (1.9, 7.3) |
| Continued after known | 8 (24) | 1.2 (0.6, 2.3) | 2 (6) | 2.3 (1.5, 3.8) |
| p-value for trend | 0.58 | 0.29 | ||
N refers to the number of unique women, while (obs) refers to the number of observations modeled as a function of each predictor
Geometric Mean and 95% confidence interval
P value based on a quantitative (continuous) term for each variable
Discussion
To date, two other studies of pregnant women have estimated ICCs using three urinary BPA measures, and have observed slightly lower ICCs, ranging from 0.10 to 0.25 (16, 17). Overall however, median BPA concentrations in those studies—2.0, 1.8, and 1.3 µg/L in the HOME Study (16), and 1.5, 1.3, and 1.2 µg/L in the EARTH Study (17)—were similar to the present study. Although there are countless dietary and behavioral factors that may explain differences in the reproducibility of urinary BPA measures across populations, differences in reported ICCs may be due to factors relating to the design of the reproducibility studies. For example, in the present study, the urine collection interval between adjacent collections was, on average, 7 and 10 weeks. In both the EARTH and HOME studies, urine specimens were collected at greater intervals: 13 and 15 weeks in the EARTH study, and 10 and 13 weeks in the HOME study. The shorter interval between collection times in Generation R may explain our slightly higher ICC, assuming samples taken over longer time intervals are less correlated.
Maternal creatinine concentrations in the present study were approximately one-half of the geometric mean concentrations reported in the HOME study (16) and lower than those among pregnant women in a Spanish cohort (18). The women in our study were better hydrated at the time of specimen collection as a consequence of urine being collected at the time of a clinic visit for a prenatal ultrasound. This resulted in creatinine-basis BPA concentrations that were up to 3-fold greater than urinary BPA concentrations expressed on a volumetric basis. Nevertheless, the ICCs were similar for BPA expressed on a volumetric and creatinine basis.
The renal system undergoes dynamic changes over the course of pregnancy, and kidney function has been associated with urinary BPA concentrations (19). These normal physiological changes during pregnancy likely result in changing BPA and creatinine concentrations over trimesters, unrelated to exposure. In other words, exposure may be identical at two points in pregnancy, but because of changes to the renal system, measurements in urine may differ, and would contribute to the low reproducibility of BPA concentrations across pregnancy.
Finally, recent studies have noted that external contamination by unconjugated BPA may occur during the collection, handling, and processing of urine specimens (20, 21). To address this in the present study, we quantified unconjugated BPA concentrations in 26 samples having high total BPA, and found that in all but one sample, unconjugated BPA comprised less than 1.4% of the total BPA concentration. When the sample with higher concentrations of unconjugated BPA (9.1% of total BPA concentration) was removed from our analysis, the ICCs were essentially unchanged. This strongly suggests that external contamination by unconjugated BPA was minimal in this study.
Taken as a whole, the present study and the extant literature indicate that there is considerable variability within women in their measured urinary BPA concentrations during pregnancy.
Acknowledgements
We thank Joe Braun for providing additional results from the EARTH study, and Walter Rogan and Jane Hoppin who provided comments on an earlier draft of the manuscript. We also thank Kelly Thevenet-Morrison who provided programming assistance.
This research received support from the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences, the Erasmus Medical Center and Erasmus University Rotterdam, the Netherlands Organization for Health Research and Development (ZonMw), and from NIH grant K12-ES019852.
Footnotes
The authors declare they have no potential competing financial interests.
References
- 1.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007 Aug-Sep;24(2):139–177. doi: 10.1016/j.reprotox.2007.07.010. PubMed PMID: 17825522. eng. [DOI] [PubMed] [Google Scholar]
- 2.Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environmental health perspectives. 2010 Aug;118(8):1055–1070. doi: 10.1289/ehp.0901716. PubMed PMID: 20338858. Pubmed Central PMCID: 2920080. Epub 2010/03/27. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volkel W, Colnot T, Csanady GA, Filser JG, Dekant W. Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chemical research in toxicology. 2002 Oct;15(10):1281–1287. doi: 10.1021/tx025548t. PubMed PMID: 12387626. Epub 2002/10/22. eng. [DOI] [PubMed] [Google Scholar]
- 4.White E, Armstrong BK, Saracci R. Principles of exposure measurement in epidemiology : collecting, evaluating, and improving measures of disease risk factors. 2nd ed. xi. Oxford; New York: Oxford University Press; 2008. p. 428. [Google Scholar]
- 5.Braun JM, Hauser R. Bisphenol A and children's health. Curr Opin Pediatr. 2011 Apr;23(2):233–239. doi: 10.1097/MOP.0b013e3283445675. PubMed PMID: 21293273. Epub 2011/02/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braun JM, Kalkbrenner AE, Calafat AM, Yolton K, Ye X, Dietrich KN, et al. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics. 2011 Nov;128(5):873–882. doi: 10.1542/peds.2011-1335. PubMed PMID: 22025598. Pubmed Central PMCID: 3208956. Epub 2011/10/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perera F, Vishnevetsky J, Herbstman JB, Calafat AM, Xiong W, Rauh V, et al. Prenatal bisphenol a exposure and child behavior in an inner-city cohort. Environmental health perspectives. 2012 Aug;120(8):1190–1194. doi: 10.1289/ehp.1104492. PubMed PMID: 22543054. Pubmed Central PMCID: 3440080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaddoe VW, Mackenbach JP, Moll HA, Steegers EA, Tiemeier H, Verhulst FC, et al. The Generation R Study: Design and cohort profile. European journal of epidemiology. 2006;21(6):475–484. doi: 10.1007/s10654-006-9022-0. PubMed PMID: 16826450. eng. [DOI] [PubMed] [Google Scholar]
- 9.Ye X, Pierik FH, Hauser R, Duty S, Angerer J, Park MM, et al. Urinary metabolite concentrations of organophosphorous pesticides, bisphenol A, and phthalates among pregnant women in Rotterdam, the Netherlands: the Generation R study. Environ Res. 2008 Oct;108(2):260–267. doi: 10.1016/j.envres.2008.07.014. PubMed PMID: 18774129. Pubmed Central PMCID: 2628162. Epub 2008/09/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaddoe VW, Bakker R, van Duijn CM, van der Heijden AJ, Lindemans J, Mackenbach JP, et al. The Generation R Study Biobank: a resource for epidemiological studies in children and their parents. European journal of epidemiology. 2007;22(12):917–923. doi: 10.1007/s10654-007-9209-z. PubMed PMID: 18095172. Pubmed Central PMCID: 2190786. Epub 2007/12/21. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonett DG. Sample size requirements for estimating intraclass correlations with desired precision. Stat Med. 2002 May 15;21(9):1331–1335. doi: 10.1002/sim.1108. PubMed PMID: 12111881. [DOI] [PubMed] [Google Scholar]
- 12.Koch HM, Kolossa-Gehring M, Schroter-Kermani C, Angerer J, Bruning T. Bisphenol A in 24 h urine and plasma samples of the German Environmental Specimen Bank from 1995 to 2009: a retrospective exposure evaluation. Journal of exposure science & environmental epidemiology. 2012 Nov;22(6):610–616. doi: 10.1038/jes.2012.39. PubMed PMID: 22617719. [DOI] [PubMed] [Google Scholar]
- 13.Larsen K. Creatinine assay in the presence of protein with LKB 8600 Reaction Rate Analyser. Clinica chimica acta; international journal of clinical chemistry. 1972 May;38(2):475–476. doi: 10.1016/0009-8981(72)90146-5. PubMed PMID: 5026368. Epub 1972/05/01. eng. [DOI] [PubMed] [Google Scholar]
- 14.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing V, Austria. ISBN 3-900051-07-0. 2012 URL http://wwwR-projectorg/,.
- 15.Fleiss JL. The design and analysis of clinical experiments. xiv. New York: Wiley; 1986. p. 432. [Google Scholar]
- 16.Braun JM, Kalkbrenner AE, Calafat AM, Bernert JT, Ye X, Silva MJ, et al. Variability and predictors of urinary bisphenol A concentrations during pregnancy. Environmental health perspectives. 2011 Jan;119(1):131–137. doi: 10.1289/ehp.1002366. PubMed PMID: 21205581. Pubmed Central PMCID: 3018492. Epub 2011/01/06. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braun JM, Smith KW, Williams PL, Calafat AM, Berry K, Ehrlich S, et al. Variability of urinary phthalate metabolite and bisphenol A concentrations before and during pregnancy. Environmental health perspectives. 2012 May;120(5):739–745. doi: 10.1289/ehp.1104139. PubMed PMID: 22262702. Pubmed Central PMCID: 3346778. Epub 2012/01/21. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casas M, Valvi D, Luque N, Ballesteros-Gomez A, Carsin AE, Fernandez MF, et al. Dietary and sociodemographic determinants of bisphenol A urine concentrations in pregnant women and children. Environ Int. 2013 Jun;56:10–18. doi: 10.1016/j.envint.2013.02.014. PubMed PMID: 23542682. [DOI] [PubMed] [Google Scholar]
- 19.You L, Zhu X, Shrubsole MJ, Fan H, Chen J, Dong J, et al. Renal function, bisphenol A, and alkylphenols: results from the National Health and Nutrition Examination Survey (NHANES 2003–2006) Environmental health perspectives. 2011 Apr;119(4):527–533. doi: 10.1289/ehp.1002572. PubMed PMID: 21147601. Pubmed Central PMCID: 3080936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Longnecker MP, Harbak K, Kissling GE, Hoppin JA, Eggesbo M, Jusko TA, et al. The concentration of bisphenol A in urine is affected by specimen collection, a preservative, and handling. Environ Res. 2013 Oct;126:211–214. doi: 10.1016/j.envres.2013.07.002. PubMed PMID: 23899777. Pubmed Central PMCID: 3805685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye X, Zhou X, Hennings R, Kramer J, Calafat AM. Potential external contamination with bisphenol A and other ubiquitous organic environmental chemicals during biomonitoring analysis: an elusive laboratory challenge. Environmental health perspectives. 2013 Mar;121(3):283–286. doi: 10.1289/ehp.1206093. PubMed PMID: 23458838. Pubmed Central PMCID: 3621191. [DOI] [PMC free article] [PubMed] [Google Scholar]