Abstract
Hearing loss induces plasticity in excitatory and inhibitory neurotransmitter systems in auditory brain regions. Excitatory-inhibitory balance is also influenced by a range of neuromodulatory regulatory systems, but less is known about the effects of auditory damage on these networks. In this work, we studied the effects of acoustic trauma on neuromodulatory plasticity in the auditory midbrain of CBA/J mice. Quantitative PCR was used to measure the expression of serotonergic and GABAergic receptor genes in the inferior colliculus (IC) of mice that were unmanipulated, sham controls with no hearing loss, and experimental individuals with hearing loss induced by exposure to a 116 dB, 10 kHz pure tone for 3 hours. Acoustic trauma induced substantial hearing loss that was accompanied by selective upregulation of two serotonin receptor genes in the IC. The Htr1B receptor gene was upregulated tenfold following trauma relative to shams, while the Htr1A gene was upregulated threefold. In contrast, no plasticity in serotonin receptor gene expression was found in the hippocampus, a region also innervated by serotonergic projections. Analyses in the IC demonstrated that acoustic trauma also changed the coexpression of genes in relation to each other, leading to an overexpression of Htr1B compared to other genes.. These data suggest that acoustic trauma induces serotonergic plasticity in the auditory system, and that this plasticity may involve comodulation of functionally-linked receptor genes.
Keywords: acoustic trauma, inferior colliculus, serotonin, gene expression, CBA/J mouse
1. Introduction
Damage to the auditory system by trauma, aging, or peripheral deprivation can cause a myriad of molecular and physiological changes (Argence et al., 2006; Dong et al., 2010a,b; Holt et al., 2005; Manzoor et al., 2013; Mossop et al., 2000; Rao et al., 2010; Tadros et al., 2007; Vale and Sanes, 2002). These changes may be viewed as compensatory or even pathological, and could contribute to aberrant perceptual states such as tinnitus (Eggermont and Roberts, 2004; Knipper et al., 2013). One set of well-documented changes important to auditory function is an alteration in the balance between excitatory and inhibitory neurotransmitter pathways (examples reviewed by Caspary et al., 2008; Salvi et al., 2000). However, neuromodulators serve as key points of control for these excitatory-inhibitory interactions in the auditory system of normal-hearing animals (Hurley and Sullivan, 2012), and therefore neuromodulatory plasticity may also play an important role in auditory responses to damage. In this study, we specifically investigated how portions of the neuromodulatory serotonergic system in the inferior colliculus (IC) that actively modulate auditory responses in healthy individuals respond to auditory damage.
Much like excitatory-inhibitory balance in sensory brain regions, neuromodulatory systems respond to peripheral sensory damage. The density of neuromodulatory fibers, the profiles of axonal contacts, and the expression of particular receptor types are altered by damage to sensory organs (Holt et al., 2005; Kang et al., 2013; Qu et al., 2000; Rao et al., 2010; Rhoades et al., 1990; Tadros et al., 2007; Vizuete et al., 1993). One such neuromodulatory pathway is the serotonergic system. Different serotonergic receptors regulate excitatory inhibitory circuits in specific ways (Hurley, 2007; Hurley et al., 2008; Hurley and Sullivan, 2012), and neuromodulatory plasticity in this system could differentially influence processing pathways following damage. However, there is a relatively poor understanding of how neuromodulatory receptors regulating particular features of excitatory and inhibitory neurotransmission respond to sensory damage, and whether such responses support adaptive models of sensory plasticity.
Multiple features of the IC provide an excellent foundation for addressing these issues. Excitatory-inhibitory convergence in the IC shapes important response properties such as frequency tuning and binaural integration in well-understood ways (for example, D’Angelo et al., 2005; Lin and Feng, 2003; Pollak, 2012; Pollak et al. 2002; Yang et al., 1992). Further, specific serotonin receptors regulate distinct components of excitation or inhibition (Hurley, 2006; Hurley and Sullivan, 2012; Wang et al., 2008). For example, the 5-Ht1A receptor decreases excitatory responses and regulates spike timing. The 5-HT1B and the 5-HT2A receptors have both been reported to regulate inhibition; the 1B by disinhibiting neurons (Hurley et al., 2008), the 2A by increasing GABAergic transmission (Wang et al., 2008). Understanding how specific receptors are altered in expression following hearing loss can help develop functional hypotheses on how they contribute to E-I regulation following hearing loss.
Directly related to the goals of this study, both excitatory and inhibitory pathways, and the serotonergic system within the IC also show plasticity following reduction of peripheral input. After cochleotomy or exposure to high-intensity sound, both pre- and post-synaptic components of GABAergic pathways such as the expression of the GABAAα receptor are downregulated, and excitation is upregulated (Argence et al., 2006; Dong et al., 2010a,b; Mossop et al., 2000; Vale et al., 2004; Vale and Sanes, 2000; 2002). Hearing loss with age is accompanied by a change in the serotonergic receptor system of the IC, via an upregulation of the 5-HT2B receptor gene (Tadros et al., 2007). Bilateral cochlear ablation results in a transient downregulation of the 5-HT5B receptor gene (Holt et al., 2005). Monaural acoustic trauma also creates a lower density of serotonergic fibers in the colliculus contralateral to the damaged ear relative to the ipsilateral colliculus (Papesh and Hurley, 2012). Based on this evidence, we hypothesized that acoustic trauma would change the expression of serotonin receptors with identified regulatory effects on excitatory-inhibitory balance in the IC as well.
2. Materials and Methods
2.1 Research subjects and experimental treatments
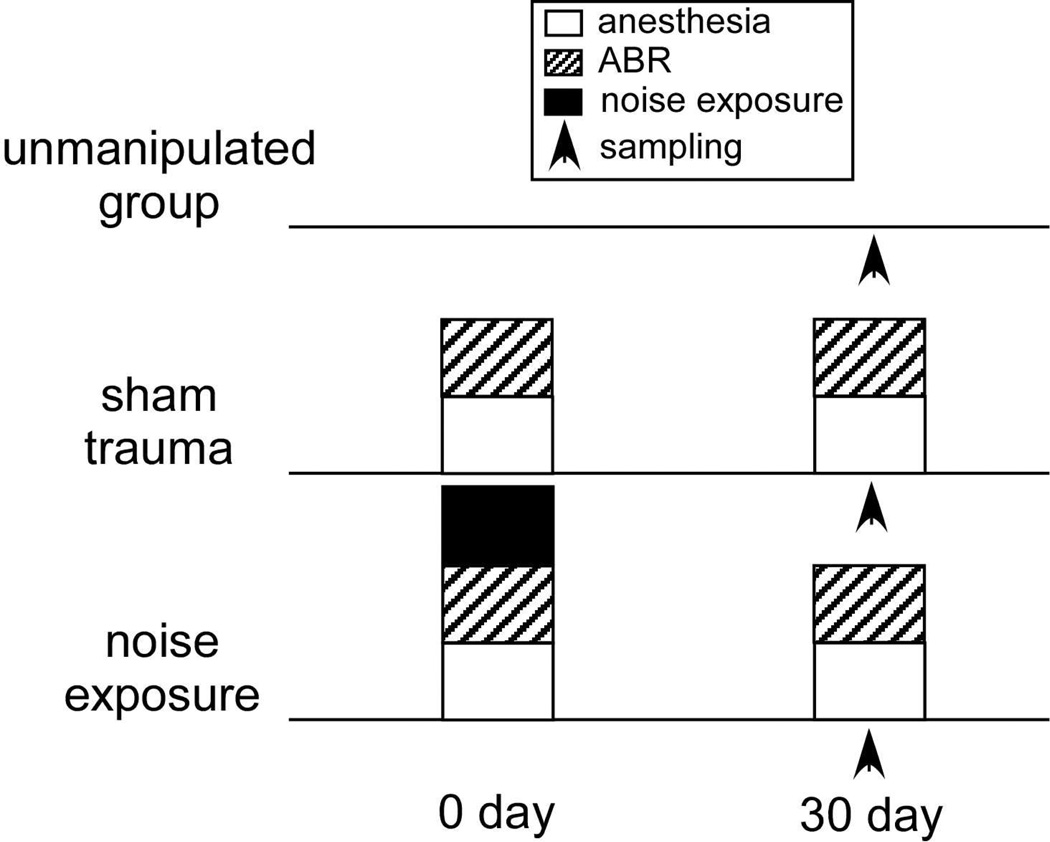
All manipulations were approved by the Bloomington Institutional Animal Care and Use Committee. Male mice of the CBA/J strain at nine weeks of age were purchased from Jackson Laboratories. Upon arrival, the mice were housed individually and held for at least two weeks prior to experiments on a 14:10 hour light/dark cycle. Subjects were divided into three experimental groups: (i) unmanipulated baselines (n=6); (ii) sham procedural controls (n=7) which underwent anesthesia, electrode implantation, and auditory brainstem (ABR) recordings; and (iii) experimental subjects (n=10), which also underwent acoustic trauma (Fig. 1).
Figure 1.
Schematic diagram of experimental groups.
2.2 Auditory Brainstem Responses
To assess acute and sustained hearing loss, ABRS were measured immediately before and after acoustic trauma or sham treatment, and 33–45 days later for most individuals. Two sham control subjects experienced longer recovery times of 53 and 57 days. Subjects were anesthetized with 100 mg/kg ketamine, 5 mg/kg xylazine, and 2 mg/kg of acepromazine. Maintenance doses of anesthesia were provided based on reflexive responses to tail and toe pinches. Three silver wire electrodes (0.010” bare, A-M Systems) were implanted subcutaneously, with the active electrode placed at the vertex of the skull and the reference and ground electrodes placed ventrolateral to the left and right ears, respectively. Electrodes were removed following ABR recording and administration of trauma. Subjects were euthanized and decapitated immediately following measurement of recovery ABRs. Bilateral inferior colliculi and the right hippocampal hemisphere were removed and stored individually in RNAlater for qPCR analyses (Qiagen). Hippocampal samples were not measured for the unmanipulated baseline subjects.
2.3 Neurophysiological and trauma system
Acoustic stimuli were generated and ABRs measured by the custom BATLAB software system (Dr. Donald Gans, Kent State University). ABR and trauma stimuli were presented via a Selenium Super Tweeter ST300-SLF speaker (Selenium) in a free-field binaural arrangement. Speaker output was calibrated using an ACO Pacific ultrasonic microphone system. ABR stimuli consisted of pure tones of 8, 12, 16, and 20 kHz. The trauma stimulus consisted of a 10 kHz pure tone presented at 116dB SPL for 3 hours with an on/off duty cycle of 200/50 ms. The magnitude and duration of this noise exposure produces hearing loss with a relatively low degree of variation among individuals in our hands. Sham subjects remained under anesthesia in the physiological rig for 3 hours without exposure to the trauma tone. Stimuli were passed through a FT-6 antialias filter (Tucker-Davis Technologies) to a PA-5 programmable attenuator (Tucker Davis Technologies), and amplified with a Samson Servo-170 Amplifier (Samson Technologies). Electrode signals were passed through a Grass P-5 series A.C. preamplifier (amplification 100,000×, low-pass 30Hz, high-pass 3 kHz) coupled to a Grass RPS107 power supply (Grass Technologies). ABR thresholds were measured in 5 dB intervals.
2.4 Gene expression
Targeted qPCR measures were performed in lieu of large-scale microarrays to test specific genes of interest. RNA was extracted using a combination of Qiashredder and RNeasy kits (Qiagen). RNA concentration was quantified using a Take-3 spectrophotometer (Biotek), and 0.5 µg of RNA was reverse-transcribed to cDNA using the SuperScript III protocol (Invitrogen Life Technologies). QPCR was performed with either custom Taqman Array plates or Taqman primer/probe assays (Invitrogen Life Technologies) and run on a Stratagene MX3000P qPCR platform (Agilent Technologies), with all samples replicated on independent plates. Raw fluorescence data were processed using the PCR Miner data analysis tool (Zhao and Fernald, 2005).
The following genes were measured using pre-indexed commercial assays (Invitrogen Life Technologies): β-Actin (standard, Mm00607939_s1), Htr1B (Mm00439377_s1), Htr1A (Mm00434106_s1), Htr2A (Mm00555764_m1), Htr3A (Mm00442874_m1), and Gabrg1 (Mm00439047_m1). The expression of 18s rRNA (Mm03928990_g1) and Gabra1 (Mm00439046_m1) were also measured in a subset of individuals. Serotonin receptor genes were selected specifically to focus on receptors that regulate excitatory-inhibitory interactions in the inferior colliculus. The Gabrg1 gene was selected both as a proxy for inhibitory function and for comparison to previous studies using an alternate GABAA subunit on hearing loss in the IC. The effects of acoustic trauma on both Gabrg1 and Gabra1 expression was tested in a subset of individuals to determine if there was a differential effect of trauma on GABAA subunit types. Prior studies have show that the expression of GABAAα mRNA is downregulated with acoustic trauma (Dong et al., 2010a), and protein expression is downregulated with age-related hearing loss (Caspary et al., 1999).
Primer amplification efficiencies and measure replicability were estimated with the PCR miner tool, and gene transcript number relative to β-Actin was calculated using a slightly modified version of the Livak and Schmittgen (2001) method employed by Carleton (2011) to correct for differential efficiencies across primer/probe pairs:
where est is the efficiency of the β-Actin primer, CTst is the critical cycle for the β-Actin standard, eex is the primer efficiency for the experimental target, and CTex is the critical cycle for the target gene. Measures for which the coefficient of variance across replicates exceeded 10%, or detection failed in a single replicate were excluded. A systematic lack of hemispheric asymmetry in gene expression was confirmed with a series of paired-sample T-tests for all three test groups (p > 0.05), so the values from each hemisphere were averaged to obtain a single measure for the IC of each individual while avoiding pseudoreplication. Also, due to this lack of asymmetry values for 18s rRNA and Gabra1 were measured in only one hemisphere from each subject. The failure rates for individual runs were variable across genes, and resulted in the exclusion of the following number of individuals: 0 for Htr1B, 1 for Htr1A, 4 for Htr2A, 11 for Htr3A, and 7 for Gabrg1. Failure rates for genes in the hippocampal samples were much less variable, such that no individuals were excluded for the Htr1B, Htr1A, or Htr2A genes. A total of 4 individuals were excluded from the Htr3A analysis, while 5 were excluded from the Gabrg1 analysis.
2.5 Statistical Analyses
Statistical analyses were chosen to profile (i) changes in mean gene expression of individual genes across experimental groups, (ii) changes in variance within groups, and (iii) the mathematical relationship of expression measures for different changes. Full model tests included the unmanipulated treatment group to assess (i) whether sham procedures altered mean gene expression, and (ii) the native variation in serotonin receptor gene expression and how interindividual variance was modified by experimental treatments. These full models are especially conducive to the latter purpose, and the Brown-Forysthe F-test was used to determine if the variances were statistically different across treatments. The Welch’s ANOVA (which does not strictly assume homoscedasticity) was performed to test across-group mean differences if the F-test was significant. Further pairwise comparisons were made between the sham and trauma groups to specifically test the effect of acoustic trauma on gene expression while controlling for procedural effects of the anesthesia or ABR measurements. Changes to the means of gene expression ratios across groups were tested with ANOVAs. Finally, linear regressions were used to determine the relationship between Htr1B expression and the magnitude of hearing loss at the four frequencies measured. For this analysis, the data from the sham and trauma groups were pooled.
3. Results
3.1 Acoustic trauma induced severe hearing loss
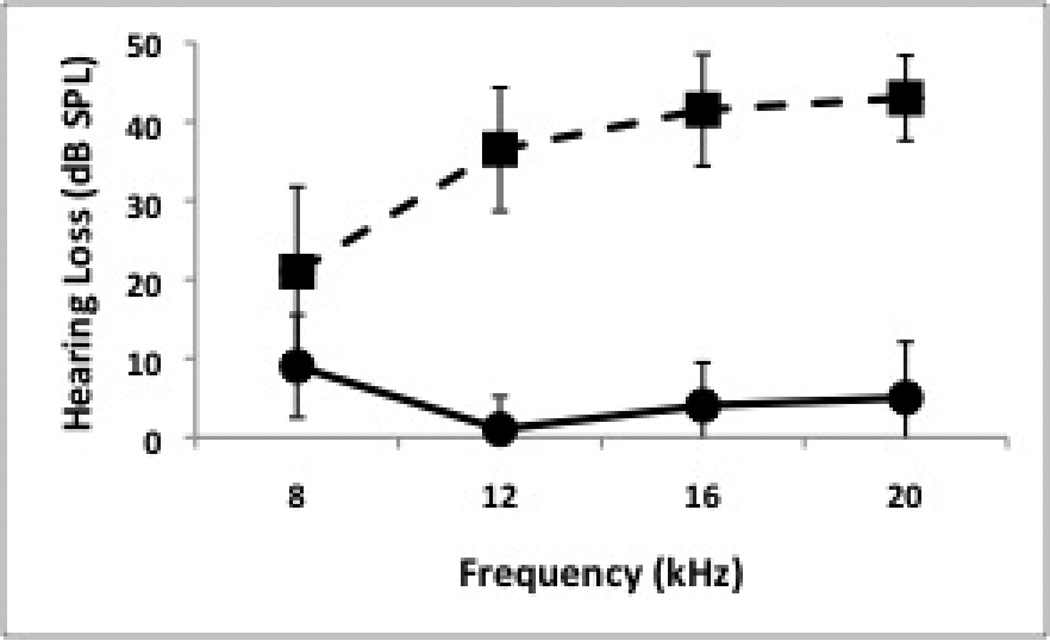
There was minor variation in initial thresholds obtained prior to manipulations, particularly at 16 and 20 kHz, with most individuals having thresholds within 1 step (5 dB) of the mean. Significantly greater hearing loss was observed at all tested frequencies for traumatized subjects compared to sham controls following a minimum of four weeks of recovery, with varying magnitude across frequencies (Fig. 2). In a pattern similar for previous observations with tonal trauma (Dong et al., 2010a,b), hearing loss was relatively minor at 8 kHz (F = 5.16, dF = 1, p = 0.041), and more severe at 12 kHz (F = 87.73, dF = 1, p < 0.0001), 16 kHz (F = 106.44, dF = 1, p = 0.0001) and 20 kHz (F = 136.03, dF = 1, p < 0.0001).
Figure 2.
Hearing loss following recovery for sham controls (n = 5, solid line) and trauma subjects (n = 10, dashed line) as assessed via ABR measures. Error bars are standard deviation.
3.2 Trauma and sham procedures change gene expression
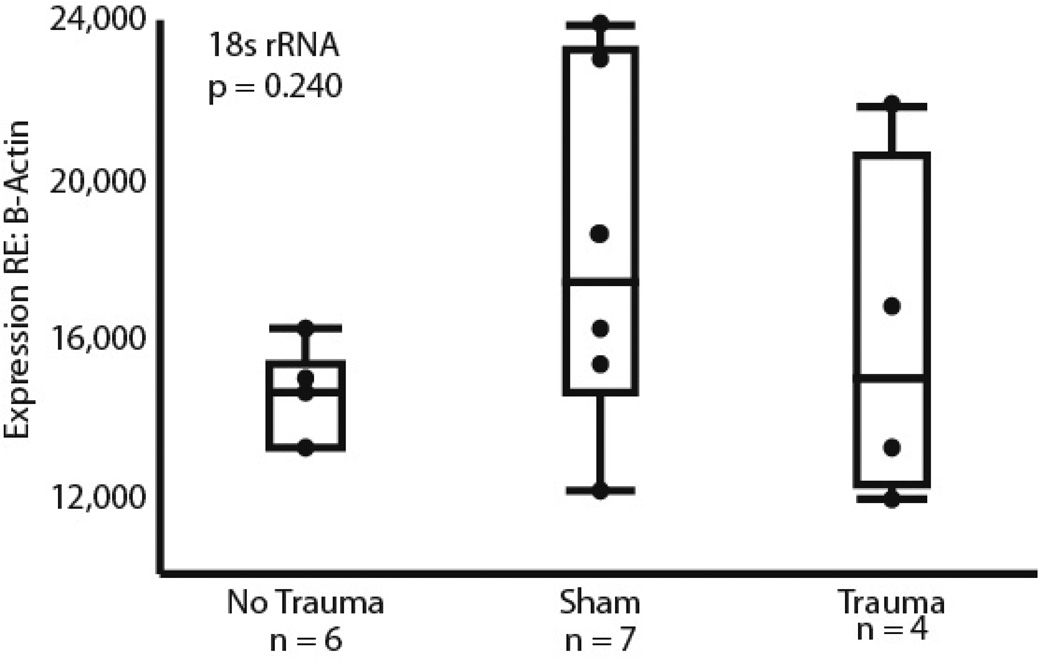
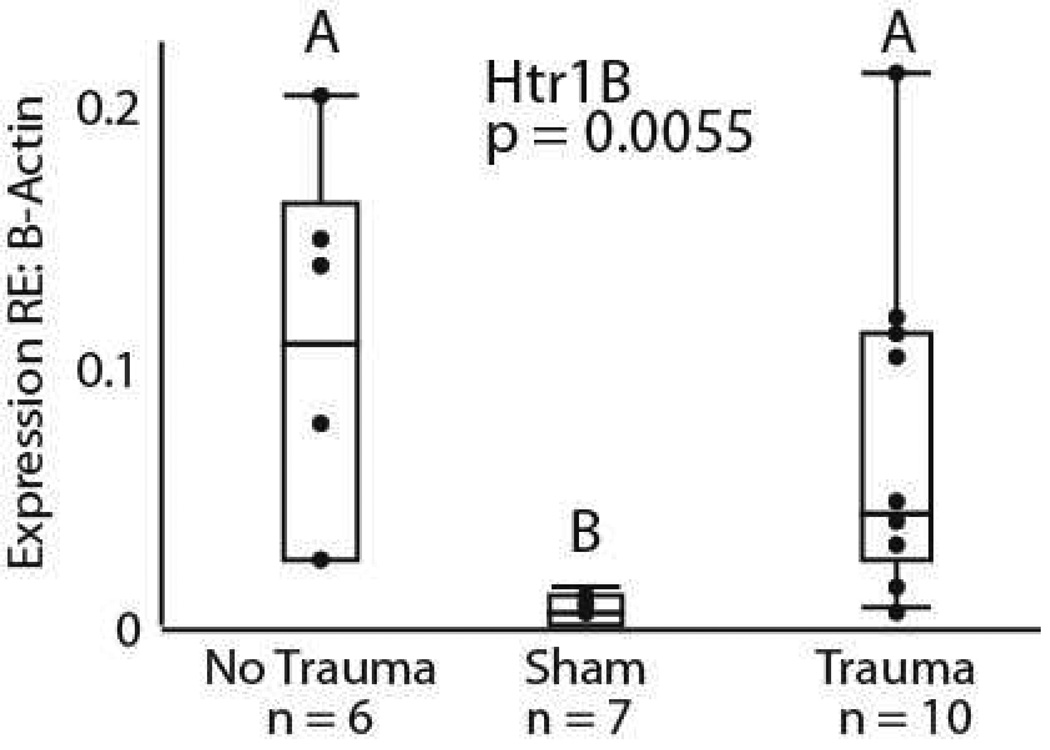
Analyses of the relative expression of β-Actin and 18s rRNA confirmed that there was no change in β-Actin expression relative to a second housekeeper gene due to our experimental treatments (F = 1.591, dF = 2 p = 0.2409, Fig. 3). Because of this, all further comparisons were conducted using β-Actin expression as the sole standard. Having an unmanipulated group, a sham trauma group, and an acoustic trauma group allowed us to assess both whether the sham treatment influenced gene expression relative to the unmanipulated group, and whether acoustic trauma altered gene expression relative to the sham control. In a comparison of all 3 treatment groups with the same statistical model, expression of only the Htr1B gene was significantly influenced by treatment, as shown in figure 4. The sham treatment group differed significantly from both the unmanipulated group (‘no trauma’) and the trauma group, with a lower mean level and lower variance in expression (Welch’s ANOVA, F = 10.06, dF = 2, p = 0.0055; Brown-Forsythe F = 4.16, dF = 2, p = 0.0307). No further significant differences in across-group means were found in the full model for the other genes tested (Htr1A, F = 3.38, dF = 2, p = 0.056; Htr2A, F = 0.695, dF = 2, p = 0.513; Htr3A, F = 1.34, dF = 2, p = 0.309; Gabrg1, F = 0.330, dF = 2, p = 0.725, Gabra1, F = 1.378, dF = 2, p = 0.2842). The differences in Htr1B expression between the sham group and the unmanipulated group suggest that some feature of the procedure associated with acoustic trauma, such as the stress of ABR measurement, or the administration of anesthesia, is capable of influencing gene expression on its own.
Figure 3.
The relationship between the expression of two housekeepr genes: β-actin and 18s rRNA. No significant differences were found across each of the three treatment groups (p = 0.240).
Figure 4.
Htr1B gene expression differences across the three treatment groups. The unmanipulated and trauma groups both have statistically higher means than the sham surgical group (Welch’s ANOVA, p = 0.0055). Statistically unequal variances were also present, with variance being higher in the unmanipulated and trauma groups compared to the sham surgical group (Brown-Forsythe, p = 0.0307).
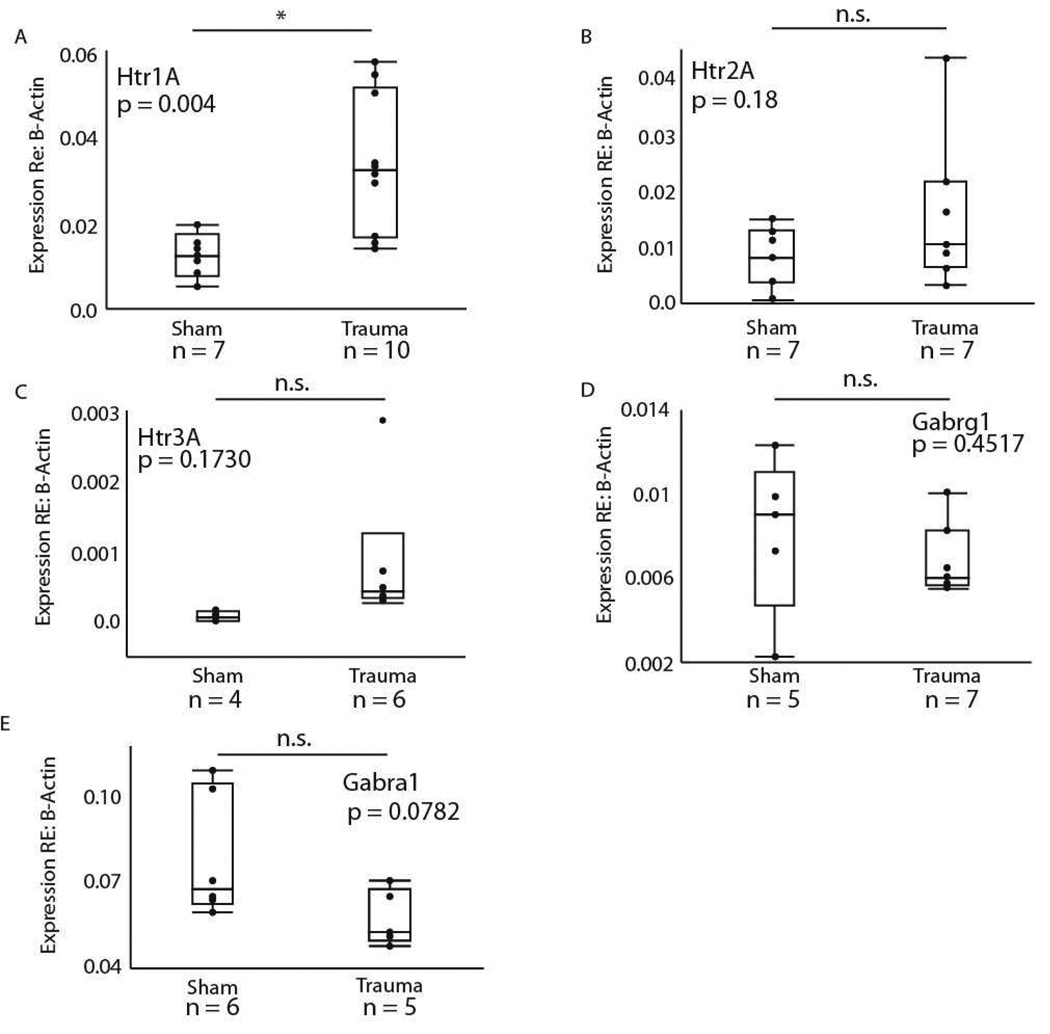
We further compared gene expression in the sham control group and the acoustic trauma group directly. In these pairwise comparisons, gene expression remained significantly higher in the traumatized group for the Htr1B gene (F = 7.4460, dF = 1, p = 0.0155), with the trauma group expressing tenfold higher Htr1B than shams. A significant difference in expression was also found for the Htr1A gene (F = 11.27, dF = 1, p = 0.0043), with the trauma group expressing threefold more Htr1A than shams (Fig. 5A). Using a similar model, no effect was found for the Htr2A (F = 2.0771, dF = 1, p = 0.1751, Fig. 5B), Htr3A (F =2.2386, dF = 1, p = 0.1730, Fig. 5C), Gabrg1 (F = 0.6314, dF = 1, p = 0.4517, Fig. 5D), or Gabra1 genes (F = 3.9487, dF = 1, p = 0.0782, Fig. 5E).
Figure 5.
Pairwise comparisons between the sham surgical and trauma treatments for (A) Htr1A, (B) Htr2A, (C) Htr3A, (D) Gabrg1, and (E) Gabra1 genes in the IC. Gene expression in the trauma group was significantly higher for the Htr1A gene (p = 0.0043). No significant difference was found for the other 4 genes (p > 0.05).
3.3 Ratios of gene expression in the IC
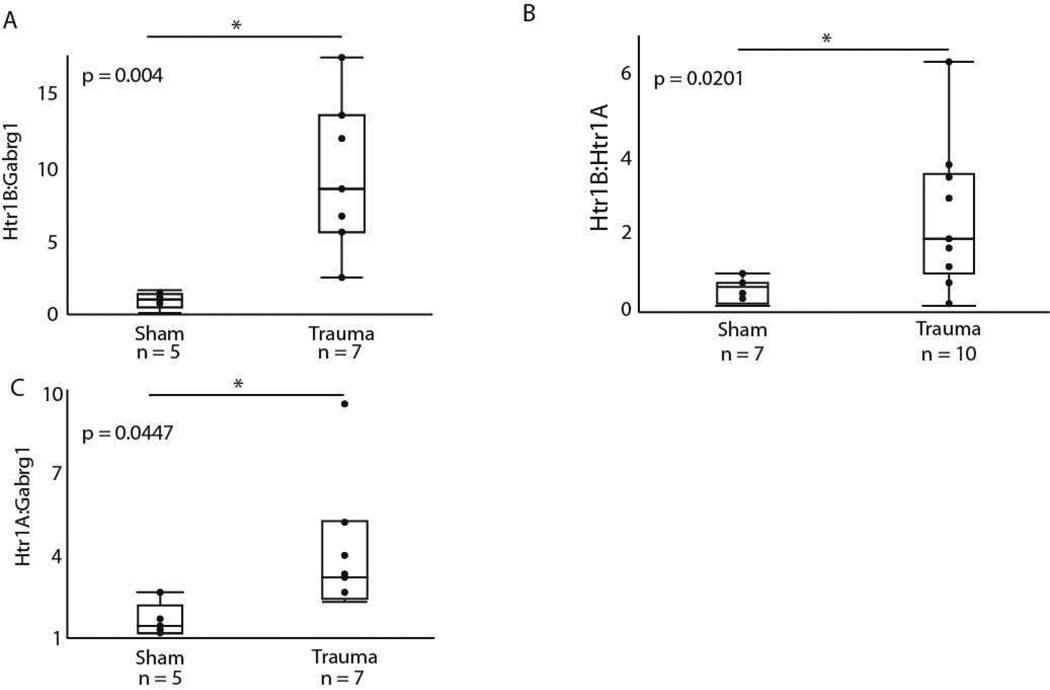
Assessing the co-expression of genes can provide the basis for hypotheses on how a perturbation such as acoustic trauma alters multiple facets of neural circuitry in a coordinated fashion. For this reason, we compared the relationship of three specific gene pairs between the sham and acoustic trauma groups. The primary comparison of interest was the coexpression of the Gabrg1 and Htr1B genes, since these two receptor types likely function in concert to tune auditory responses (Hurley et al., 2008). Trauma significantly increased the ratios of Htr1B:Gabrg1 from 0.992 to 9.439, (F = 13.510, dF = 1, p = 0.0043, Fig. 6A). Two other gene pairs involved in the regulation of excitatory-inhibitory balance were analyzed for comparison. The first of these consisted of the Htr1B and Htr1A genes, with the rationale that baseline changes in the serotonergic system could result in coordinated upregulation of serotonin receptors in general. Trauma significantly increased the ratio of Htr1B:Htr1A from 0.5476 to 2.343 (F = 6.7571, dF = 1, p = 0.0201, Fig. 6B), consistent with the strong upregulation of Htr1B gene expression we observed. We further compared Htr1A with the Gabrg1 gene. The Htr1A:Gabrg1 ratio increased from 1.612 to 4.259 (F = 5.262, dF = 1, p = 0.0447, Fig. 6C) following trauma.
Figure 6.
Ratio of gene expression for (A) Htr1B:Gabrg1, (B) Htr1B:Htr1A, and (C) Htr1A:Gabrg1.
3.4 Htr1B expression in the IC and hearing loss
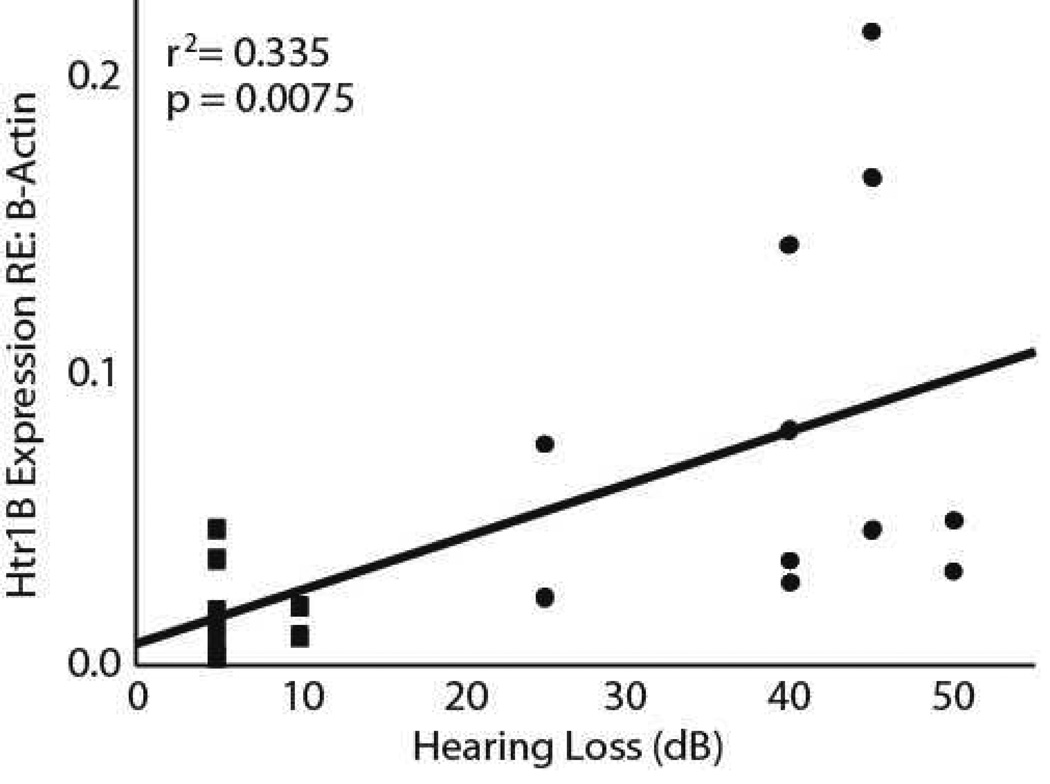
A weak but significant relationship between hearing loss and expression of the Htr1B gene was found for all four frequencies tested (8 kHz: r2 = 0.241, p = 0.0279, 12 kHz: r2 = 0.3401, p = 0.007, 16 kHz: r2 = 0.3350, p = 0.0075, 20 kHz: r2 = 0.329, p = 0.0082). The correlation was consistent across all four frequencies, and is illustrated for 16 kHz in figure 7. However, this relationship is dominated by the difference between individuals with and without hearing loss. For example, no correlation was found between the magnitude of hearing loss and Htr1B expression for any of the frequencies tested within the trauma group (p > 0.05).
Figure 7.
The relationship between hearing loss at 16 kHz and the expression of Htr1B. The squares represent sham trauma subjects and the circles represent trauma subjects.
3.5 Hippocampal serotonin receptor expression following acoustic trauma
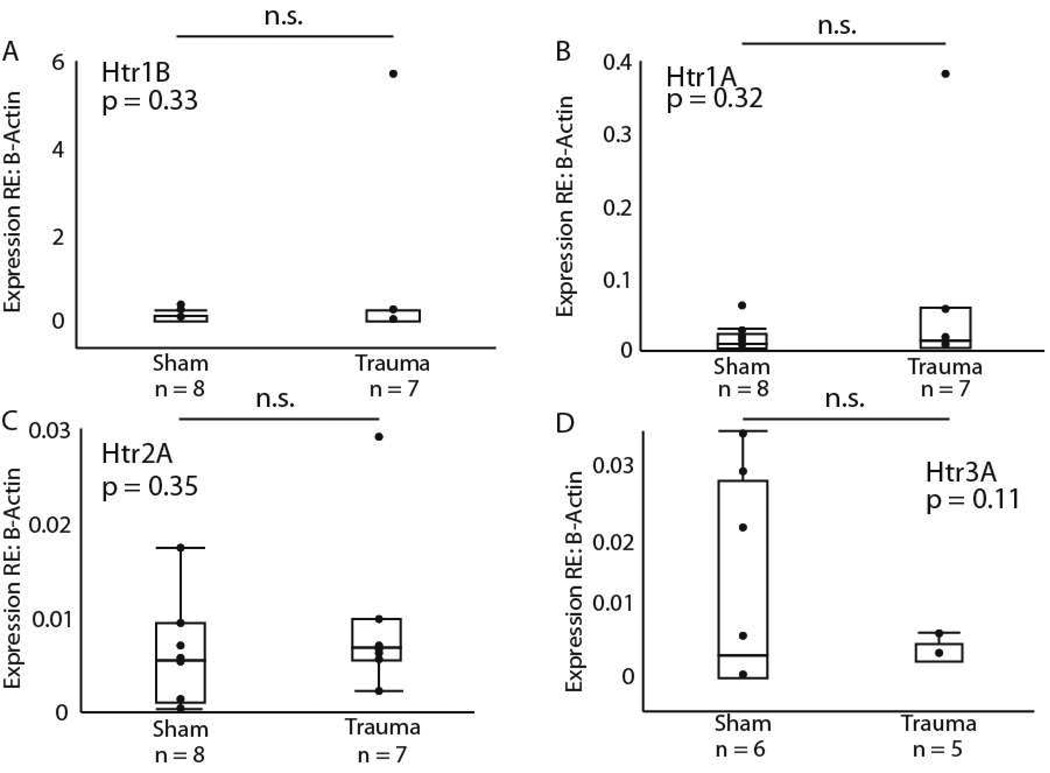
We measured the expression of the serotonergic genes in the hippocampus as well as in the IC. The rationale for this comparison is that the hippocampus is a non-auditory region that receives a dense projection from some of the same serotonergic nuclei as the IC (Oleskevich and Descarries, 1990). We therefore reasoned that measuring receptor expression in the hippocampus would allow us to determine whether any changes in gene expression resulted from a generalized process downstream of the dorsal/median raphe, or represented a more localized process. For the hippocampal measurements, we compared only sham versus acoustic trauma groups. In the hippocampus, no significant differences were found for the expression of the serotonergic Htr1B (F = 1.03, dF = 1, p = 0.33, Fig. 8A), Htr1A (F = 1.05, dF = 1, p = 0.32, Fig. 8B), Htr2A (F = 0.92, dF = 1, p = 0.35, Fig. 8C), or Htr3A (F = 3.06, dF = 1, p = 0.11, Fig. 8D) genes between sham and trauma individuals. In addition, there was no significant effect of acoustic trauma on the expression of Gabrg1 (F = 0.31, dF = 1, p = 0.59).
Figure 8.
Pairwise comparisons between the sham surgical and trauma treatments for the (A) Htr1B, (B) Htr1A, (C) Htr2A, and (D) Htr3A genes in the hippocampus. Gene expression was not significantly different for any of the genes (p > 0.05).
4. Discussion
4.1 Acoustic trauma induces neuromodulatory plasticity in the IC
Our data showed selective upregulation among serotonin receptors in the adult auditory midbrain as a response to sensory damage. This upregulation did not occur in every region innervated by the dorsal raphe nucleus, since it was not observed in the hippocampus. The Htr1B receptor gene displayed particularly remarkable plasticity in the IC, with a tenfold upregulation in trauma subjects compared to sham controls. Also, the expression level of Htr1B in the IC is correlated with hearing loss, but only when sham and trauma subjects are pooled, i.e. there is no relationship between the magnitude of hearing loss and Htr1B expression within the trauma group (Fig. 7). The Htr1A gene was significantly upregulated as well, with expression increasing threefold with trauma. Contrary to prior studies, there was no significant downregulation of the Gabrg1 gene due to high interindividual variance, but the direction and magnitude of a 30% downregulation was similar to that described previously (Dong et al., 2010a). Two previous studies using gene arrays have further identified genes for the 5-HT2B receptor and the 5-HT5B receptor as increasing in the IC with age-induced hearing loss and a transient decrease following bilateral deafening by cochlear ablation, respectively (Holt et al., 2005; Tadros et al., 2007). Although we did not examine the Htr2B or Htr5B genes in this study, these prior findings combined with our data broadly support the contention that peripheral damage causes changes in expression of serotonin receptor genes. It is worth noting that all identified effects on serotonin receptor expression following damage to the auditory system have been upregulations in various regions of the central auditory systems. This may indicate that serotonergic receptor expression is generally globally upregulated following auditory damage, and nonsignificant effects may result from relatively small transcriptional changes or changes with relatively high inter-individual variance. However, the direct comparisons among specific serotonin receptor genes using quantitative PCR in the current study shows that particular receptors show very different magnitudes of upregulation in expression. Because different serotonin receptors have distinct roles in the regulation of excitatory-inhibitory circuitry in the IC (Hurley, 2006; 2007; Hurley et al., 2008; Hurley and Sullivan, 2012), these findings suggest that selective upregulation of receptor expression should be considered part of the suite of specific changes influencing excitatory-inhibitory interactions in the auditory system following hearing loss.
4.2 Surgery, trauma, and their interaction
Our three experimental groups: the unmanipulated, sham, and noise-exposed groups, differed in crucial ways important to the interpretation of our data. The unmanipulated group did not receive anesthesia, did not undergo measurement of ABRs, and was not exposed to noise. This group was thus closest to a ‘native’ state for serotonin and other receptors. The noise-exposed group underwent anesthesia, ABR measurement, and exposure to noise 30 days prior to sacrifice, and anesthesia and ABR measurement immediately before sacrifice (Fig. 1). The ‘sham’ group underwent all of the same treatments as the noise-exposed group, at the same time points and for the same durations, excepting the noise exposure. Therefore, the only difference between the noise-exposed group and the sham group was exposure to noise. We considered an additional control group, a group that would be exposed to noise without anesthesia, which could be compared to the unmanipulated group. We did not include this group because undergoing exposure to noise is a stressful experience, increasing corticosterone levels in mice. Trauma that induces permanent threshold shifts causes more prolonged corticosterone elevations that trauma leading to temporary threshold shifts (Meltser and Canlon, 2011; Tahera et al., 2006a,b), and serotonin levels in the IC increase significantly even at relatively moderate intensities (Hall and Hurley, 2010). The central serotonergic system itself is highly sensitive to stress, which influences the expression and function of serotonin receptors, including the 5-HT1B receptor (for example: Choi et al., 2014; Furay et al., 2011; Hazra et al., 2012; Yohe et al., 2012). For these reasons, stress induced by noise exposure without anesthesia would be a strong confounding factor, making it difficult to attribute gene expression differences between the unmanipulated group and an unanesthetized noise exposed group to noise exposure alone.
Comparing the unmanipulated group to the sham group allowed us to conclude that something about the sham procedures, whether this was administration of anesthesia or recording ABRs, altered expression of one of the serotonin receptor genes, the Htr1B gene, relative to the unmanipulated group. The expression measures for this gene showed large variance in the unmanipulated group, and the sham control treatment reduced expression to low levels in all individuals (Fig. 4). This was true even though other receptor genes did not show these effects. Rao et al. (2010) found similarly dramatic effects of sham surgical procedures in the auditory cortex. In their study, real and sham cochleotomies had similar effects on the ability of serotonin to decrease firing rates, input resistance, and spike rate adaptation. This emphasizes that sham procedures are capable of changing the function of serotonin receptors in auditory brain regions. In the Rao et al. study, cochleotomy had further effects relative to the sham group at a later time point after cochleotomy, preventing serotonin from decreasing excitability. Thus, the sham group in this study allowed a distinction to be made between the effects of procedures associated with the cochleotomy, and of the cochleotomy itself.
In our results, analogous to those of Rao et al., the sham and noise-exposed groups also showed a difference in expression of both the Htr1A gene and the Htr1B gene, with expression of both increasing in the noise-exposed group relative to the sham group. For the Htr1B gene, this had the interesting effect of making expression levels in the unmanipulated and acoustic trauma groups statistically similar, and different from the sham group. Thus, from a descriptive standpoint, the noise exposure interacted with procedures such as anesthetic administration or ABR measurement to cancel each other out. One interpretation of this pattern is that, although sham procedures and noise exposure individually had significant effects on the expression of the Htr1B gene, the unmanipulated and noise-exposed groups do not meaningfully differ.
From a functional perspective, however, the similar mean and variation in Htr1B gene expression in the unmanipulated and noise-exposed groups are likely to lead to different outcomes for activation of this receptor in the IC, assuming that protein levels follow a similar pattern. This is because acoustic trauma is well-documented to change the baseline state of neural circuits in the IC, and because serotonin receptors act by modulating this underlying circuitry. Multiple studies have reported increased spontaneous and evoked activity in the IC following hearing loss, due to changes in both inhibitory and excitatory pathways (Argence et al., 2006; Dong et al., 2010a,b; Mossop et al., 2000; Vale et al., 2004; Vale and Sanes, 2000; 2002). Because the 5-HT1B receptor may suppress GABAergic transmission in the IC (Hurley et al. 2008), one possibility is therefore that equal expression of receptors, in the presence of downregulated inhibitory pathways, could result in a diminution of the effect of the 5-HT1B receptor in noise-exposed versus unmanipulated mice. Noise exposure also changes the baseline state of the serotonergic system itself, altering the density of serotonergic inputs to the IC, with a lower fiber density contralateral to an exposed ear relative to contralateral to a protected ear (Papesh and Hurley 2012). 5-HT1B receptors could therefore also be activated to a different extent in unmanipulated versus noise-exposed mice. Although quantitatively similar, the values for Htr1B gene expression in the unmanipulated and noise-exposed groups are therefore likely to represent different functional outcomes for this receptor. Whether the expression levels for the 5-HT1B receptor in the unmanipulated versus noise exposed groups represent meaningful functional differences, or a truly equivalent functional state because of additive regulation with sham procedures, are two testable competing hypotheses for future studies.
Overall, it is the comparison of the unmanipulated, sham trauma, and noise-exposed groups that reveals an important and biologically relevant feature of this system: that it is sensitive to multiple types of manipulations, including non-auditory ones. This highlights the role of the serotonergic system in auditory regulation; to integrate multiple types of auditory and nonauditory information that are salient to behavior, and to translate this information into changes in auditory processing through serotonin receptors expressed in the IC. Our results suggest that a key regulatory point in this process is at the level of serotonin receptor expression, and in particular the expression of 5-HT1B receptors.
4.3 Correlations of gene expression
Although the Htr1B gene was highly sensitive to our experimental and control manipulations, it showed a consistent pattern with Gabrg1 gene expression; the individuals with the highest expression levels of Htr1B within each group also expressed the most Gabrg1. In sham controls, there was a roughly 1:1 ratio of Htr1B:Gabrg1. Following trauma, this increased to a 10:1 ratio (Fig. 6). Therefore, we propose that changes in the relative expression of these receptor genes may be an important feature of trauma-induced plasticity in the IC. This would also be consistent with current functional models of the role of 5-HT1B receptor activity in regulating GABAergic activity in the IC (Hurley et al., 2008).
4.4 Implications for the regional localization of plasticity
One of the interesting questions surrounding trauma-induced serotonergic plasticity is the degree to which plasticity is manifest in specific brain regions. To address this question, we profiled gene expression in the hippocampus along with the IC. Even though the serotonergic network in the hippocampus shows plasticity in response to factors such as environmental enrichment (Rasmuson et al., 1998), we found no hippocampal plasticity following acoustic trauma. Rather, the observed plasticity was confined to the IC. This highlights the potential for a diffuse neuromodulatory system to regulate sensory processing without direct impacts on other brain functions.
4.5 Potential functional effects of transcriptional changes
The 5-HT1A and 5-Ht1B receptors encoded by the two genes upregulated by acoustic trauma have differing roles in regulating the excitatory-inhibitory circuitry of the IC. Activation of the 5-HT1A receptor reduces responses to auditory stimuli, consistent with the function of this somatodendritic receptor in other brain regions (Hannon and Hoyer, 2008; Hurley, 2007). In contrast, the 5-HT1B receptor enhances responses to auditory stimuli in the IC, and is generally localized to presynaptic axons and terminals of the neurons that express it, where it acts to dampen transmitter release (Sari, 2004). These features suggest a disinhibitory role for the 5-HT1B receptor in the IC (Hurley et al., 2008). Because of these differing roles, the upregulation of these two genes in the IC have interesting functional implications, if one assumes that the changes in expression are reflected in increased levels of functional receptor protein in neuronal membranes. One possible model of activity is that the upregulation of both receptors in concert counteracts the increased excitability of IC neurons that has been observed by multiple authors following induced hearing loss (Dong et al., 2010b; Manzoor et al., 2013; Mulders and Robertson, 2011). In this scenario, increased levels of the 5-HT1A receptor in the trauma group would have a larger suppressive effect than in the control, balancing trauma-evoked hyperexcitability. If the 5-HT1B receptor is expressed by IC neurons projecting to the medial geniculate body, increased levels of this receptor would also gate the output of the IC to a greater extent in mice subject to acoustic trauma than in controls. Together, increased expression of these two receptors could therefore potentially compensate for trauma-induced increases in excitabilty prior to MGB.
A variation on this scenario would occur if the 5-HT1B receptor expression we measured occurred in intrinsic axon collaterals. In this event, increased expression of the 5-HT1B receptor in animals exposed to acoustic trauma could reduce the activity of axon collaterals within the IC. The fact that an agonist of the 5-HT1B receptor disinhibits neurons in the IC, suggests that 5-HT1B receptors would be localized on GABAergic synaptic terminals. Since increased expression of the 5-HT1A and 5-HT1B receptors would result in greater suppression and disinhibition, respectively, this model would result in an antagonistic push-pull interaction between the upregulated serotonin receptor subtypes. Both of these functional hypotheses could be tested using electrophysiological approaches.
Serotonin receptor plasticity following auditory trauma may serve a function other than the explicit auditory processing roles outlined above, in that it facilitates experience-dependent plasticity in some sensory processing regions. In visual cortex, serotonin modulates the reorganization of ocular dominance columns following monocular deprivation (Kojic et al., 1997). In particular, serotonergic activity modifies the activity of inhibitory networks, contributing to reorganization of ocular dominance columns (Baroncelli et al., 2010). This process involves epigenetic effects that modulate gene transcription via histone modification (Vetencourt et al., 2011). Interestingly, these epigenetic effects are directly influenced by the activity of the 5-HT1A receptor type. Given that serotonin facilitates tuning plasticity within the auditory cortex in associative learning paradigms (Suga et al., 2002), it is possible that increased serotonin receptor expression following acoustic trauma could promote input-dependent plasticity.
4.6 Conclusions
The serotonergic system in the inferior colliculus responds to auditory damage with selective plasticity in receptor expression. Plasticity is most pronounced for the Htr1B gene, which plays an important role in regulating the activity of GABAergic inhibition. This suggests that serotonergic plasticity following auditory damage may be an important mechanism regulating shifts in excitatory-inhibitory function.
We studied changes in the inferior colliculus following acoustic trauma.
Serotonin receptor gene expression was upregulated following trauma.
Receptor expression regulation is selective.
Plasticity was observed in important regulatory receptors.
Acknowledgements
This research was funded by grant DC008963 to LMH from NIDCD. Support for MN was provided by the NSF REU program grant DBI-0851607.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial interests.
References
- Argence M, Saez I, Sassu R, Vassias I, Vidal PP, de Waele C. Modulation of inhibitory and excitatory synaptic transmission in rat inferior colliculus after unilateral cochleectomy: an in situ and immunofluorescence study. Neurosci. 2006;141:1193–1207. doi: 10.1016/j.neuroscience.2006.04.058. [DOI] [PubMed] [Google Scholar]
- Baroncelli L, Maffei L, Sale A. New perspectives in amblyopia therapy on adults: a critical role for the excitatory/inhibitory balance. Front. Cellular Neurosci. 2011;5:25. doi: 10.3389/fncel.2011.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroncelli L, Sale A, Viegi A, Vetencourt JFM, De Pasquale R, Baldini S, Maffei L. Experience-dependent reactivation of ocular dominance plasticity in the adult visual cortex. Exp. Neurol. 2010;226:100–109. doi: 10.1016/j.expneurol.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Carleton KL. Quantification of transcript levels with quantitative RT-PCR. In: Orgogozo V, Rockman M, editors. Molecular Methods for Evolutionary Genetics. New York: Springer; 2011. pp. 279–295. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner GF, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory systems. J. Exp. Biol. 2008;211:1781–1791. doi: 10.1242/jeb.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JY, Shin S, Lee M, Jeon TJ, Seo Y, Kim CH, Kim DG, Hoon C, Lee K, Choi TH, Kang JH. Acute physical stress induces the alteration of the serotonin 1A receptor density in the hippocampus. Synapse. 2014 doi: 10.1002/syn.21748. [DOI] [PubMed] [Google Scholar]
- D’Angelo WR, Sterling SJ, Ostapoff EM, Kuwada S. Role of GABAergic inhibition in the coding of interaural time differences of low-frequency sounds in the inferior colliculus. J. Neurophysiol. 2005;93:3390–3400. doi: 10.1152/jn.00956.2004. [DOI] [PubMed] [Google Scholar]
- Dong S, Mulders WHAM, Rodger J, Woo S, Robertson D. Acoustic trauma hyperactivity and changes in gene expression guinea-pig auditory brainstem. Eur. J. Neurosci. 2010;31:1616–1628. doi: 10.1111/j.1460-9568.2010.07183.x. [DOI] [PubMed] [Google Scholar]
- Dong S, Rodger J, Mudlers WHAM, Robsertson D. Tonotopic changes in GABA receptor expression in guinea pig inferior colliculus after partial unilateral hearing loss. Brain Res. 2010;1342:24–32. doi: 10.1016/j.brainres.2010.04.067. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Roberts LE. The neuroscience of tinnitus. TRENDS Neurosci. 2004;27:676–682. doi: 10.1016/j.tins.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Furay AR, McDevitt RA, Miczek KA, Neumaier JF. 5-HT1B mRNA expression after chronic social stress. Behav. Brain Res. 2011;224:350–357. doi: 10.1016/j.bbr.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav. Brain Res. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Hazra R, Guo JD, Dabrowska J, Rainnie DG. Differential distribution of serotonin receptor subtypes in BNSTALG neurons: modulation by unpredictable shock stress. Neurosci. 2012;225:9–21. doi: 10.1016/j.neuroscience.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt AG, Asako M, Lomax CA, MacDonald JW, Tong L, Lomax MI, Altschuler RA. Deafness-related plasticity in the inferior colliculus: gene expression following removal of peripheral activity. J. Neurochem. 2005;93:1069–1086. doi: 10.1111/j.1471-4159.2005.03090.x. [DOI] [PubMed] [Google Scholar]
- Huang X, Mooney RD, Rhoades RW. Effects of serotonin on retinotectal-, corticotectal-, and glutamate-induced activity in the superior colliculus of the hamster. J. Neurophysiol. 1993;70:723–732. doi: 10.1152/jn.1993.70.2.723. [DOI] [PubMed] [Google Scholar]
- Hurley LM. Different serotonin receptor agonists have distinct effects on sound-evoked responses in inferior colliculus. J. Neurophysiol. 2006;96:2177–2188. doi: 10.1152/jn.00046.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM. Activation of the serotonin 1A receptor alters the temporal characteristics of auditory responses in the inferior colliculus. Brain Res. 2007;1181:21–29. doi: 10.1016/j.brainres.2007.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Bohorquez A, Tracy J. The serotonin 1B receptor modulates frequency response curves and spectral integration in the inferior colliculus by reducing GABAergic inhibition. J. Neurophysiol. 2008;100:1656–1667. doi: 10.1152/jn.90536.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Sullivan MR. From behavioral context to receptors: serotonergic modulatory pathways in the IC. Front. Neural. Circ. 2012;6:58. doi: 10.3389/fncir.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HH, Wang CH, Chen HC, Li IH, Cheng CY, Liu RS, Huang WS, Shiue CY, Ma KH. Investigating the effects of noise-induced hearing loss on serotonin transporters in rat brain using 4-[(18)F]-ADAM/small animal PET. Neuroimage. 2013;75:262–269. doi: 10.1016/j.neuroimage.2012.06.049. [DOI] [PubMed] [Google Scholar]
- Knipper M, Van Dijk P, Nunes I, Rüttiger L, Zimmerman U. Advances in the neurobiology of hearing disorders: recent developments regarding the basis of tinnitus and hyperacusis. Prog. Neurobiol. 2013;111:17–33. doi: 10.1016/j.pneurobio.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Kojic L, Gu Q, Douglas RM, Cynader MS. Serotonin facilitates plasticity in kitten visual cortex: an in vitro study. Dev. Brain Res. 1997;101:299–304. doi: 10.1016/s0165-3806(97)00083-7. [DOI] [PubMed] [Google Scholar]
- Kruglikov I, Rudy B. Perisomatic GABA release and thalamocortical integration onto neocortical excitatory cells are regulated by neuromodulators. Neuron. 2008;58:911–924. doi: 10.1016/j.neuron.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WY, Feng AS. GABA is involved in spatial unmasking in the frog auditory midbrain. Neurosci. 2003;23:8143–8151. doi: 10.1523/JNEUROSCI.23-22-08143.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Manzoor NF, Gao Y, Licari F, Kaltenbach JA. Comparison and contrast of noise-induced hyperactivity in the dorsal cochlear nucleus and inferior colliculus. Hear. Res. 2013;295:114–123. doi: 10.1016/j.heares.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltser I, Canlon B. Protecting the auditory system with glucocorticoids. Hear. Res. 2011;281:47–55. doi: 10.1016/j.heares.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Mossop JE, Wilson MJ, Caspary DM, Moore DR. Down-regulation of inhibition following unilateral deafening. Hear. Res. 2000;147:183–187. doi: 10.1016/s0378-5955(00)00054-x. [DOI] [PubMed] [Google Scholar]
- Mulders WHAM, Robertson D. Progressive centralization of midbrain hyperactivity after acoustic trauma. Neurosci. 2011;192:753–760. doi: 10.1016/j.neuroscience.2011.06.046. [DOI] [PubMed] [Google Scholar]
- Oleskevich S, Descarries L. Quantified distribution of the serotonin innervation in adult rat hippocampus. Neurosci. 1990;34:19.s–33.s. doi: 10.1016/0306-4522(90)90301-j. [DOI] [PubMed] [Google Scholar]
- Oliver DL. Neuronal organization in the inferior colliculus. In: Winer JA, Schreiner CE, editors. The Inferior Colliculus. New York: Springer-Verlag; 2005. pp. 69–114. [Google Scholar]
- Oliver DL, Kuwada S, Yin TCT, Haberly LB, Henkel CK. Dendritic and axonal morphology of HRP-injected neurons in the inferior colliculus of the cat. J. Comp. Neurol. 1991;303:75–100. doi: 10.1002/cne.903030108. [DOI] [PubMed] [Google Scholar]
- Papesh MA, Hurley LM. Plasticity of serotonergic innervation of the inferior colliculus in mice following acoustic trauma. Hear. Res. 2012;283:89–97. doi: 10.1016/j.heares.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak GD. Circuits for processing dynamic interaural intensity disparities in the inferior colliculus. Hear. Res. 2012;288:47–57. doi: 10.1016/j.heares.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak GD, Burger RM, Park TJ, Klug A, Bauer EE. Roles of inhibition for transforming binaural properties in the brainstem auditory system. Hear. Res. 2002;168:60–78. doi: 10.1016/s0378-5955(02)00362-3. [DOI] [PubMed] [Google Scholar]
- Qu Y, Eysel UT, Vandesande F, Arckens L. Effect of partial sensory deprivation on monoaminergic neuromodulators in striate cortex of adult cat. Neurosci. 2000;101:863–868. doi: 10.1016/s0306-4522(00)00441-3. [DOI] [PubMed] [Google Scholar]
- Rao D, Basura GJ, Roche J, Daniels S, Mancilla JG, Manis PB. Hearing loss alters serotonergic modulation of intrinsic excitability in auditory cortex. J. Neurophysiol. 2010;104:2693–2703. doi: 10.1152/jn.01092.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmuson S, Olsson T, Henriksson BG, Kelly PAT, Holmes MC, Secki JR, Mohammed AH. Environmental enrichment selectively increases 5-HT1A receptor mRNA expression and binding in the rat hippocampus. Mol. Brain Res. 1998;53:285–290. doi: 10.1016/s0169-328x(97)00317-3. [DOI] [PubMed] [Google Scholar]
- Reitsamer HA, Pflug R, Franz M, Huber S. Dopaminergic modulation of horizontal-cell-axon-terminal receptive field size in the mammalian retina. Vision Res. 2006;46:467–474. doi: 10.1016/j.visres.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Rhoades RW, Mooney RD, Chiaia NL, Bennett-Clarke CA. Development and plasticity of the serotoninergic projection to the hamster’s superior colliculus. J. Comp. Neurol. 1990;299:151–166. doi: 10.1002/cne.902990203. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Wang J, Ding D. Auditory plasticity and hyperactivity following cochlear damage. Hear. Res. 2000;147:261–274. doi: 10.1016/s0378-5955(00)00136-2. [DOI] [PubMed] [Google Scholar]
- Sari Y. Serotonin 1B receptors: from protein to physiological function and behavior. Neurosci. Biobehav. Rev. 2004;28:565–582. doi: 10.1016/j.neubiorev.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Suga N, Xiao Z, Ma X, Ji W. Plasticity and corticofugal modulation for hearing in adult animals. Neuron. 2002;36:9–18. doi: 10.1016/s0896-6273(02)00933-9. [DOI] [PubMed] [Google Scholar]
- Tadros SF, D’Souza M, Zettel ML, Zhu X, Lynch-Erhardt M, Frisina RD. Serotonin 2B receptor: upregulated with age and hearing loss in mouse auditory system. Neurobiol. Aging. 2007;28:1112–1123. doi: 10.1016/j.neurobiolaging.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Tahera Y, Meltser I, Johansson P, Hansson AC, Canlon B. Glucocorticoid receptor and nuclear factor-kB interactions in restraint stress-mediated protection against acoustic trauma. Endocrin. 2006;147:4430–4437. doi: 10.1210/en.2006-0260. [DOI] [PubMed] [Google Scholar]
- Tahera Y, Meltser I, Johansson P, Bian Z, Stierna P, Hansson AC, Canlon B. NF-jB mediated glucocorticoid response in the inner ear after acoustic trauma. J. Neurosci. Res. 2006;83:1066–1076. doi: 10.1002/jnr.20795. [DOI] [PubMed] [Google Scholar]
- Vale C, Juiz JM, Moore DR, Sanes DH. Unilateral cochlear ablation produces greater loss of inhibition in the contralateral inferior colliculus. Eur. J. Neurosci. 2004;20:2133–2140. doi: 10.1111/j.1460-9568.2004.03679.x. [DOI] [PubMed] [Google Scholar]
- Vale C, Sanes DH. Afferent regulation of inhibitory synaptic transmission in the developing auditory midbrain. J. Neurosci. 2000;20:1912–1921. doi: 10.1523/JNEUROSCI.20-05-01912.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale C, Sanes DH. The effect of bilateral deafness on excitatory and inhibitory synaptic strength in the inferior colliculus. Eur. J. Neurosci. 2002;16:2394–2404. doi: 10.1046/j.1460-9568.2002.02302.x. [DOI] [PubMed] [Google Scholar]
- Vetencourt JFM, Tiraboschi E, Spolidoro M, Castren E, Maffei L. Serotonin triggers a transient epigenetic mechanism that reinstates adult visual cortex plasticity in rats. Eur. J. Neurosci. 2011;33:49–57. doi: 10.1111/j.1460-9568.2010.07488.x. [DOI] [PubMed] [Google Scholar]
- Vizuete ML, de la Roza C, Steffen V, Machado A, Cano J. Changes in neurotransmitters in superior colliculus after neonatal enucleation: biochemical and immunocytochemical studies. Neurosci. 1993;56:165–176. doi: 10.1016/0306-4522(93)90571-v. [DOI] [PubMed] [Google Scholar]
- Wang HT, Luo B, Huang YN, Zhou KQ, Chen L. Sodium salicylate suppresses serotonin-induced enhancement of GABAergic spontaneous inhibitory postsynaptic currents in rat inferior colliculus in vitro. Hear. Res. 2008;236:42–51. doi: 10.1016/j.heares.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Yang L, Pollak GD, Resler C. GABAergic circuits sharpen tuning curves and modify response properties in the mustache bat inferior colliculus. J. Neurophysiol. 1992;68:1760–1774. doi: 10.1152/jn.1992.68.5.1760. [DOI] [PubMed] [Google Scholar]
- Yohe LR, Suzuki H, Lucas LR. Aggression is suppressed by acute stress but induced by chronic stress: immobilization effects on aggression, hormones, and cortical 5-HT1B/striatal dopamine D2 receptor density. Cogn. Affect. Behav. Neurosci. 2012;12:446–459. doi: 10.3758/s13415-012-0095-9. [DOI] [PubMed] [Google Scholar]
- Zhao S, Fernald RD. Comprehensive algorithm for quantitative real-time polymerase chain reaction. J. Comput. Biol. 2005;12:1045–1062. doi: 10.1089/cmb.2005.12.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]