Abstract
The aim of this study was to determine the effects of cochlear implant (CI) use on behavioral frequency discrimination ability in partially deafened cats. We hypothesized that the additional information provided by the CI would allow subjects to perform better on a frequency discrimination task. Four cats with a high frequency hearing loss induced by ototoxic drugs were first trained on a go/no-go, positive reinforcement, frequency discrimination task and reached asymptotic performance (measured by d’ - detection theory). Reference frequencies (1, 4, and 7 kHz) were systematically rotated (Block design) every 9 to 11 days to cover the hearing range of the cats while avoiding bias arising from the order of testing. Animals were then implanted with an intracochlear electrode array connected to a CI and speech processor. They then underwent 6 months of continuous performance measurement with the CI turned on, except for one month when the stimulator was turned off. Overall, subjects performed the frequency discrimination task significantly better with their CI turned on than in the CI-off condition (3-way ANOVA, p<0.001). The analysis showed no dependence on subject (3-way ANOVA, subject x on-off condition, p>0.5); however, the CI only significantly improved performance for two (1 and 7 kHz) of the three reference frequencies. In this study we were able to show, for the first time, that cats can utilize information provided by a CI in performing a behavioral frequency discrimination task.
Keywords: Partial hearing loss, cochlear implant, behavioral frequency discrimination
1. Introduction
Profound sensorineural hearing loss is successfully treated by intracochlear electrical stimulation (ICES) of the auditory nerve via a cochlear implant (CI). Improvement in the speech perception ability of cochlear implantees over the post-implantation period has been shown in various clinical studies (Blamey et al., 2012, Blamey et al., 1996a, e.g., Wilson and Dorman, 2008). Initial perceptual quality and rate of improvement over time largely depend on the amount of pre-implantation hearing experience and auditory training. As selection criteria for CI recipients have eased, more patients with preserved low frequency hearing have received CIs (for review see Turner et al., 2008). Several clinical studies report improvement of overall hearing performance of subjects with residual hearing in one ear (possibly with a hearing aid) in addition to ICES in the other, sometimes referred to as bimodal hearing (Von Ilberg et al., 2011, Mok et al., 2006, Firszt et al., 2008, Ching et al., 2006). For example, Dorman et al. (2007) showed an increase of 20% in performance of patients with CI in one ear and a hearing aid in another on word and sentence recognition tasks when electric stimulation was added (EAS). Other studies report very little or no bimodal benefit (Mok et al., 2006, Tyler et al., 2002). The latter findings might be at least partially explained by the finding that pitch percepts evoked by ICES can correspond to tones up to 3 octaves lower than those predicted from the place of stimulation (Blamey et al., 1996b). The mechanisms that underlie auditory perception in response to combined electric and acoustic stimulation (EAS) remain unclear. Although the residual hearing of partially deaf subjects is usually in the low frequency region, whereas the CI usually stimulates the high-frequency region, the degree of overlap between the regions is variable and significant overlap in this region could cause electric and acoustic perception interference.
Frequency discrimination ability is a factor in determining how well human subjects can recognize pitch, and separate auditory streams, which in turn affects speech recognition ability (Rose and Moore, 2005). To study the effects of interactions between electric and acoustic stimulation on frequency discrimination, we developed a novel behavioral task (Benovitski et al., 2014) to test frequency discrimination in the partially deaf cat model. We have previously shown that using this task, cats can learn a frequency discrimination task and demonstrate stable and repeatable performance (Benovitski et al., 2014). The use of a partial hearing, chronically stimulated animal model allows us to determine performance changes on a behavioral frequency discrimination task by adding and removing ICES in the same animal. While other studies have used conditioning to provide animals with behaviorally relevant auditory experience (Kral et al., 2006, Klinke et al., 1999), these studies did not allow performance to be measured. Others have used avoidance conditioning to train cats to detect stimulation thresholds (Vollmer et al., 2001, Beitel et al., 2000, Vollmer and Beitel, 2011) and discriminate changes in modulation frequency (Vollmer et al., 2001). In those experiments individual electrodes were tested one at a time and subjects received only electric but not acoustic stimulation.
The aim of this experiment was to determine whether CI use affects the ability of partially deaf animals to perform a frequency discrimination task. We hypothesized that additional information provided by the CI would allow partially deafened animals to perform better on a frequency discrimination task. To our knowledge, this is the first study to test frequency discrimination in partially hearing animals implanted with a CI.
2. Method
2.1 Subjects
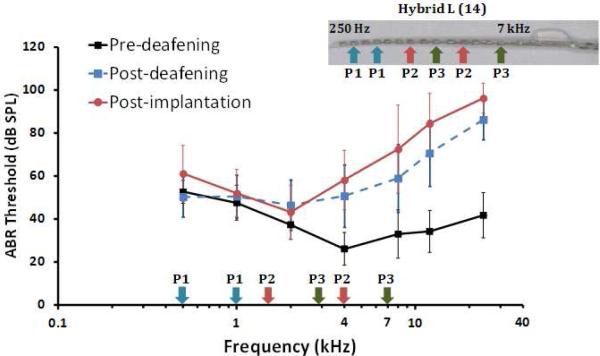
Four healthy cats with otoscopically normal tympanic membranes were used in the present study. All procedures were in accordance with Australian Code of Practice for the Care and Use of Animals for Scientific Purposes and with the guidelines laid down by the National Institutes of Health in the US regarding the care and use of animals for experimental procedures, and were approved by the Royal Victorian Eye and Ear Hospital Animal Research and Ethics Committee. Subjects were partially deafened between 7.4 to 8.3 months of age by daily subcutaneous injections of Kanamycin (200 mg/kg; kanamycin monosulphate, Sigma, USA). After 17 days, hearing condition was checked via tone-specific auditory brain response (ABR) recordings (Coco et al., 2007) and injections continued until a partial high frequency hearing loss was achieved to model an EAS CI recipient (Irving et al., in preperationpress). High frequency hearing loss (normal hearing up to 2 kHz, figure 1) was confirmed using a standard ABR. The difference in thresholds between two ears was not significant (4-way ANOVA, ear side x cat x pre/post CI x frequency, p> 0.9).
Figure 1.
ABR audiograms of cat C5 before and after bilateral deafening. Values are means (n = 8) of thresholds for monaural stimulation of the left and right ears, and error bars represent standard deviation. The inset represents unilateral electrode array placement relative to the characteristic frequencies of the electrode locations in the cochlea. P1, P2, and P3 represent the different pairs’ frequencies and electrodes activated during the psychophysical experiment.
Subjects were implanted unilaterally (left side) with a Hybrid L 14-electrode intra-cochlear array between 13.3 to 14.4 months of age. The tip of electrode array was approximately 10.5 mm from the round window, resulting in the most apical electrode being located at approximately the 4-kHz place as represented in figure 1 (for details about the Hybrid L array and implantation procedure see Shepherd et al., 2011).
Each subject was chronically stimulated using a clinical stimulator and speech processor (Cochlear Limited) carried in a harness worn by the animals which did not limit the animal's ability to move (Fallon et al., 2009). A standard stimulation strategy (SPEAK; McDermott, 1989) with a clinical electrode-frequency allocation map was used. The 14 electrodes from apex to base were allocated to the following frequencies 187, 312, 562, 812, 1062, 1312, 1562, 1937, 2312, 2812, 3437, 4187, 5187, 6312, and 7937 Hz. The stimulation rate was 500 pulses per second per electrode. During the stimulator-on phases of the behavioral task, the following electrode pairs were preferentially activated by the acoustic stimulus: electrodes 13 and 10 (pair 1), 8 and 4 (pair 2), 5 and 1 (pair 3). The frequency-place map was not optimized to match the characteristic frequencies of the electrode locations in the cochlea, thus overlap of electric and acoustic cochlear stimulation can be expected, as shown in figure 1. After the unilateral CI implantation, acoustic signals were not blocked from the implanted nor the contralateral ear meaning that cats were acoustically stimulated in both ears and electrically stimulated in one ear only resulting in a combination of hybrid-bimodal stimulation.
2.2 Behavioral procedure
After deafening, cats were trained on a go/no-go, positive reinforcement, frequency discrimination task developed in our laboratory (For details regarding stability, repeatability and other aspects of the behavioural method, please see Benovitski et al., 2014). Testing was carried out in the animals’ regular housing facility where they were required to respond to pairs of tones by moving toward and away from the device depending on whether the members of a tone pair were different or the same in frequency. A hit (animal approached during a different frequency stimulus pair) was followed by food reward. A correct rejection (CR) was recorded if same-frequency tone pairs were presented without an approach from the animal, after which a new trial could be activated with no delay. A false alarm (FA) (approaching the food source after presentation of a same-frequency tone pair) was followed by a 20 second penalty timeout, during which a green light was presented and a new trial could not be initiated. A miss (failing to approach after presentation of a different-frequency pair) was followed by 0 to 6 seconds of penalty time out without any light indication. Tone pips of 195 ms with 4.3 ms rising edge and 49 ms falling edge were used. The interval between the two tones within a pair was 750 ms, and that between two pairs was 2350 ms.
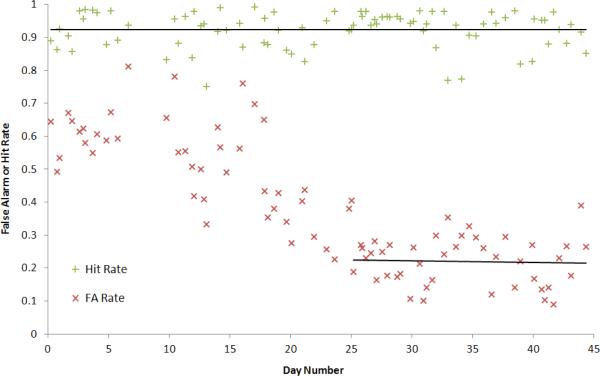
To minimize any response biases, after the end of the first week of procedural training on a different frequency to the test stimuli, presentation of different (target) and same (non-target) frequency tone pairs was randomized and the proportion set to 50% target and 50% non-target. The ratio between the frequency of the tones (▲/f) was fixed at 0.6 during the entire experiment where the three pairs of tones were (1, 0.4 kHz), (4, 1.6 kHz), and (7, 2.8 kHz). Sound pressure level (SPL) was set at 80 dB and the intensity of the tones was roved by ±5 dB to minimize loudness cues. All cats reached asymptotic performance within the first 1.5 (±0.5) months (see figure 2 for example). The training periods of 2 cats were before implantation and the training period of the other 2 cats was interrupted by the implantation.
Figure 2.
Behavioral performance represented in hit and false alarm (FA) rates (plus and cross signs) for cat C4 before starting reference frequency rotations. The frequencies presented during training were 2 and 0.5 kHz. Each point was calculated over a window of 100 trials. The two lines are linear approximations for hit and FA rates over the last 45 and 20 days respectively. Hit rate is stable (slope=2E-06; R2= 3E-07) at 0.92 throughout 45 days and FA stabilized (slope= -4E-04; R2= 1E-03) at 0.23 over the final 20 days indicating good frequency discrimination ability.
Experimental data was collected throughout a 4.3 to 5.5 month period of continuous performance measurement after the conclusion of the training period. The CI stimulator was turned off for a period of one month during this period (Table 1). Reference frequencies (1, 4, and 7 kHz) were systematically rotated (Block design) every 9 to 11 days to cover the hearing range of the cats while balancing the order of testing across both cats and reference frequencies.
Table 1.
Animal details
| Cat | Age at deafening | Age at implantation | Age at EAS start | Age at stimulator turn off | Age of stimulator turn on | Age at EAS end |
|---|---|---|---|---|---|---|
| C2 | 7.4 | 14.3 | 15.7 | 17.8 | 18.8 | 20 |
| C3 | 7.4 | 14.4 | 15.7 | 17.8 | 18.8 | 20.3 |
| C4 | 8.3 | 13.3 | 15.77 | 19 | 20 | 21.3 |
| C5 | 8.3 | 13.3 | 15.77 | 19 | 20 | 21 |
All ages are in months and EAS stands for electric and acoustic stimulation.
2.3 Data analysis
Hit and FA rates (see figures 2 and 3A for example) were analyzed in 100-trial bins. Performance was measured using detection theory by calculating d’ (d-prime); d' = z(hit rate) - z(FA rate), where z is the inverse cumulative normal distribution with a mean of 0 and standard deviation of 1 (Heeger, 1997). To help eliminate trials during which animals were possibly not fully engaged in the discrimination task, a test session was defined as more than 5 consecutive trials that were less than 5 minutes apart (Benovitski et al., 2014). Trials outside of sessions did not contribute to performance calculation. A repeated measures 3-way analysis of variance with the variables stimulator condition (on and off), subjects (n=4), and reference frequencies (1, 4, and 7 kHz), was conducted to determine the effect of an EAS on frequency discrimination ability.
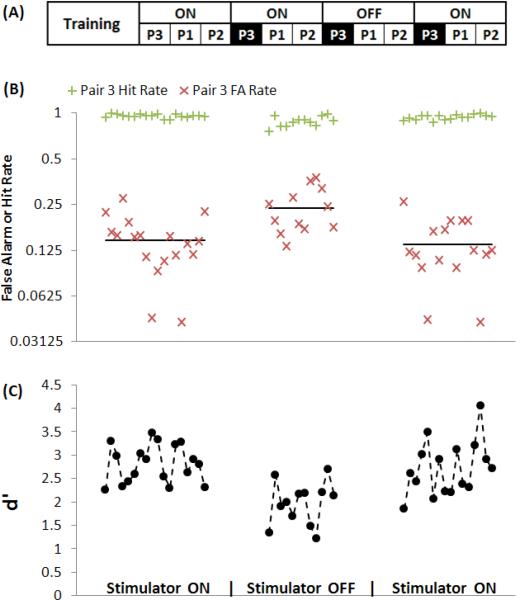
Figure 3.
Example of stimulator turn-off effect on performance of cat C4. A: Experimental time-line. Each block (P1, P2, and P3) represent 9 to 11 days of different pairs of tones (1, 4, and 7 kHz reference) presented throughout the experiment. B: Hit and FA rates for 3 different periods of testing at 7 kHz reference frequency (pair 3, 2.8 & 7 kHz) which was presented at times marked in A. Each point is cumulative calculation of 100-trial window. Solid lines represent FA average of each period. C: Performance represented by d’, calculated from hit and FA rates in B. Increase in FA causes reduction of d’ which means reduction in frequency discrimination ability.
3. Results
Overall cats’ performance on the frequency discrimination task was stable over long periods of time (20 days, Figure 2) and repeatable after 2 months (FA rate, figure 3). This is in accordance with data collected during the method validation (Benovitski et al., 2014). Subjects had a drop in performance, resulting from an increase in the FA rate, after turning off the stimulator, which did not recover over time; while performance returned to baseline after the stimulation was turned back on (e.g. solid lines in figure 3B).
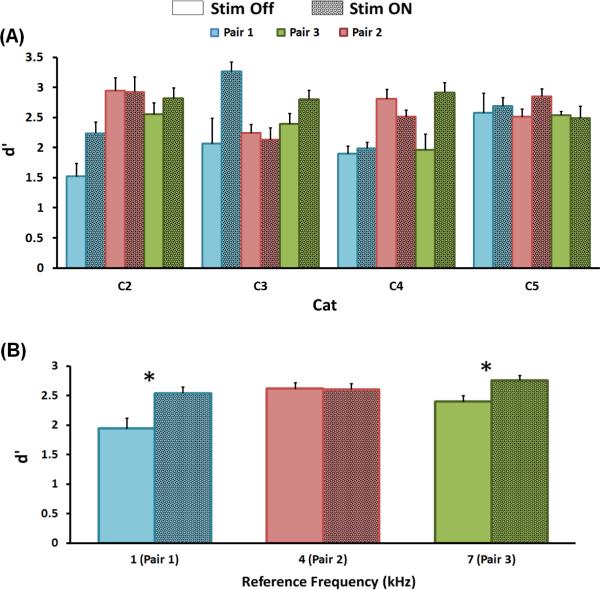
Figure 4A shows behavioral performance for each cat, reference frequency (pair number) and stimulation condition (On/Off). As can be seen, performance was variable among individual animals. C2's performance on Pair 1 with the stimulator off, was the worst, but showed a 0.6 increase in d’ with the stimulator on. A similar increase in performance was seen for C3 for Pair 1 and C4 Pair 3. In contrast, C5 exhibited generally high performance, with no clear effect of stimulation, possibly due to performance hitting a ceiling level (see Discussion). Figure 4B shows the behavioral performance at each of the three reference frequencies, averaged across cats, when the CI was turned on and off. Subjects performed the frequency discrimination task significantly better (d’ = 2.64 with standard error of means of 0.34, averaged across 3 reference frequencies) with the CI on compared to the off condition (d’ = 2.32 with standard error of means of 0.11; 3-way ANOVA, p<0.001). This result was not significantly different among subjects (3-way ANOVA, subject x on-off condition, p>0.5). However, there was a significant dependence on the reference frequency used (3-way ANOVA, reference frequency x on-off condition, p<0.03). For reference frequencies 1 and 7 kHz, d’ increased by 0.6 and 0.36 respectively while reduced by 0.01 for 4 kHz. Post hoc t-tests considering on-off condition for the 1, 4, and 7 kHz reference frequencies were p=0.0015, p=0.4725, and p=0.0041 respectively.
Figure 4.
A: Behavioral performance with cochlear stimulator on and off for 3 different frequency pairs. B: Averaged performance across 4 subjects. Performance is measured in d’ calculated from the last 5, 100-trial sessions (e.g. last 5 data points of each period in figure 3). Error bars represent standard error of the means. Color is matched to the arrows in figure 1. Asterisks mark reference frequencies for which performance is significantly better (Bonferroni correction) when the stimulator is turned on (Post hoc t-tests considering on off condition for the 1, 4, and 7 kHz reference frequencies were p=0.0015, p=0.4725, and p=0.0041 respectively).
4. Discussion
Partial hearing animals were used to model a clinical condition of CI use in subjects with residual hearing. This study shows, for the first time, that partially deafened cats can use the information provided by a CI to improve performance on a frequency discrimination task compared to using their residual hearing alone. The increase in frequency discrimination performance is despite the fact that the use of a clinical CI system meant that frequencies represented by the CI were also represented by the residual hearing at different place along the cochlea (i.e. significant disruption to the normal tonotopic representation). This dual representation might have caused the ICES to interfere with the acoustic stimulation resulting in a performance drop after adding ICES. Additionally, previous studies have reported a marked suppression of the acoustic evoked auditory nerve compound action potential by ICES in guinea pigs (Von Ilberg et al., 1999), which again may have been expected to result in decreased performance.
Multiple clinical studies have assessed speech recognition performance of partially hearing subjects who received bimodal EAS (e.g. a CI and a hearing aid in the contralateral ear). The general trend is that patients with bimodal EAS perform better on speech recognition tasks (for review see Von Ilberg et al., 2011, Firszt et al., 2008, Ching et al., 2006); however, subject variability is large and some individuals do not benefit from bimodal EAS. For example, a study by Mok et al. (2006) showed that less than half of their 14 patients had a significant bimodal benefit. Another study by Tyler et al. (2002) showed a bimodal EAS advantage for only one of the three patients for words and none for sentences. In fact, in some rare cases hearing performance can even deteriorate (Hamzavi et al., 2004, Armstrong et al., 1997). These studies conclude that perceptual ability can be heavily influenced by the amount and quality of residual hearing while bimodal interaction might be a secondary factor. The animal model used in this experiment is somewhat different from the usual bimodal clinical case. The residual hearing is similar in the two ears, and there is no amplification in the non-implanted ear. In the clinical studies, patients have a hearing aid in one ear, usually that with the better hearing thus direct comparison in performance is impracticable. Nevertheless this animal model allows direct, within animal, assessment of CI use under conditions where deafening method and time, as well as previous acoustic experience and environment, are well controlled.
To our knowledge there have been no previous studies of behavioral frequency discrimination performance of partial hearing animals receiving ICES. There have been multiple studies which assessed ICES in animal behavioral models (Vollmer et al., 2001, Vollmer and Beitel, 2011, Kral et al., 2006, Klinke et al., 1999, Beitel et al., 2000). However, results from these studies are not comparable with results from the present experiment due to: 1) effects of electrical stimulation differ significantly between partially and profoundly deaf cochlea (Von Ilberg et al., 2011); 2) none of the above studies used a partial hearing, ICES behavioral model; and 3) conditioning and detection tasks are different from discrimination tasks in terms of their objectives.
Behavioral performance during the current experiment was measured while presenting EAS or acoustic stimulation only. As performance on electrode discrimination alone was not available, we can only speculate about the performance on the same task with only electric stimulation being provided to the animals. Worse performance could be expected with electric stimulation alone, especially if delivered through a clinical speech processor with multiple electrodes activated at the same time. One study on which we base this assumption compared performance of normal hearing, hearing impaired, and CI subjects on a spectral shape discrimination task and concluded that even hearing impaired subjects are doing better than CI users (Henry et al., 2005). In another study, three EAS patients with residual hearing, implanted with the Iowa/Nucleus Hybrid 10-mm short-electrode array performed significantly better compared to standard CI (electric stimulation only) (Turner et al., 2004).
One limitation of the present study is the possibility of a behavioral measurement ceiling effect which could partially explain the lack of EAS benefit at 4 kHz (Pair 2). This is evident from figure 4A where it is clear that the performance of cats C2, C3, and C5 was already high (d'>2.5) for Pair 2 before turning the CI on, although it must be noted that this does not hold for C3. The hit rate was saturated for all the subjects so the d’ differences were primarily due to variations in FA rate. The maximum d’ for 100 trials (ideal observer: 49 hits, 1 miss, 49 CRs, and 1 FA) is 4.1; however, in practice a d’ of 4 is rarely achieved (see figure 3). In an attempt to stay within the usable range of d’, the difficulty level (▲f/f) was set to 0.6 as in pilot experiments it generated a d’ level of 2. Nevertheless, it is possible that elevation in d’ at 4 kHz could not be observed due to the superior performance in the stimulator off condition resulting in a performance ceiling effect.
Another limitation is that the SPL roving used to minimize loudness cues was probably not sufficient, especially with the 7 kHz reference frequency. The difference between hearing thresholds for 7 and 2.8 kHz was around 50dB post implantation. The 80 dB SPL tones may have been almost inaudible to cat C5 at 7kHz but 40 dB above threshold at 2.8kHz; therefore it is possible that some animals may have been able to perform some part of the auditory only task using just a loudness cue. However, that CI use improved performance was also seen with the lowest frequency reference, where the auditory threshold was the most similar, for which the loudness roving should have been sufficient. Finally, it is worth noting that the ICES levels were set relatively to the electrically evoked ABR thresholds, and therefore should not have contained any residual loudness cues.
Several studies of partial deafness suggest that frequencies closer to the lesion edge are over-represented in the cortex (Robertson and Irvine, 1989, Harrison et al., 1991, for review see Pienkowski and Eggermont, 2011). This over-representation has been proposed to underlie the better frequency discrimination performance in that frequency region seen in some patients (as shown by McDermott et al., 1998). However, it is clear from figure 4B that performance at 7 kHz was not greater than for the other 2 reference frequencies. Close examination of cortical activity will be necessary in future experiments to examine possibility that having a larger area of cortex representing given frequencies does not necessarily mean better frequency discrimination.
Most human bimodal clinical studies suggest that patients with residual hearing who receive bimodal auditory input (e.g. CI in one ear and a hearing aid in the other) perform better compared to patients receiving either acoustic or electric input alone. While the low-frequency hearing of the animals in the current study is better than the majority of patients in those studies, there is a growing trend towards implanting patients with increasing amounts of low-frequency hearing. Using this animal model also allows testing of hearing loss treatments such as neurotrophins (Pettingill et al., 2007) and novel electric stimulation strategies (e.g. Focused Multipolar Stimulation (van den Honert and Kelsall, 2007)) in ways not possible in human subjects.
Highlights.
Cats were trained on a positive reinforcement frequency discrimination task.
Regardless of high frequency hearing loss, cats could be trained within a month.
Task performance was tested with a cochlear implant turned on and off.
Animals performed the task better with the cochlear implant turned on.
Intracochlear electric stimulation did not impede acoustic perception alone.
Acknowledgments
This work was funded by the National Institutes of Health (HHS-N-263-2007-00053-C), the National Health and Medical Research Council of Australia and The Department of Electronic Engineering, La-Trobe University. The Bionics Institute acknowledges the support it receives from the Victorian Government through its Operational Infrastructure Support Program. The authors are grateful to Andrew Wise for implant surgeries; Alison Neil, Nicole Critch and Amy Morley for technical assistance; Sam Irvine for advice; Sue Pierce for veterinary advice; Sue Mckay for animal maintenance; and Dexter Irvine for comments on the earlier versions of the manuscript.
Abbreviations
- ICES
intracochlear electrical stimulation
- CI
cochlear implant
- EAS
electric and acoustic stimulation
- FA
false alarm
- CR
correct rejection
- ▲f/f
ratio between frequency difference and reference frequency
- SPL
sound pressure level
- d’
d-prime
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ARMSTRONG M, PEGG P, JAMES C, BLARNEY P. Speech perception in noise with implant and hearing aid. Otology & Neurotology. 1997;18:S140–S141. [PubMed] [Google Scholar]
- BEITEL RE, VOLLMER M, SNYDER RL, SCHREINER CE, LEAKE PA. Behavioral and neurophysiological thresholds for electrical cochlear stimulation in the deaf cat. Audiology and Neurotology. 2000;5:31–38. doi: 10.1159/000013863. [DOI] [PubMed] [Google Scholar]
- BENOVITSKI YB, BLAMEY PJ, RATHBONE GD, FALLON JB. An automated psychoacoustic testing apparatus for use in cats. Hearing research. 2014;309:1–7. doi: 10.1016/j.heares.2013.11.002. [DOI] [PubMed] [Google Scholar]
- BLAMEY P, ARNDT P, BERGERON F, BREDBERG G, BRIMACOMBE J, FACER G, LARKY J, LINDSTRÖM B, NEDZELSKI J, PETERSON A. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants. Audiology and Neurotology. 1996a;1:293–306. doi: 10.1159/000259212. [DOI] [PubMed] [Google Scholar]
- BLAMEY P, ARTIERES F, BAŞKENT D, BERGERON F, BEYNON A, BURKE E, DILLIER N, DOWELL R, FRAYSSE B, GALLÉGO S. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants: an update with 2251 patients. Audiology and Neurotology. 2012;18:36–47. doi: 10.1159/000343189. [DOI] [PubMed] [Google Scholar]
- BLAMEY PJ, DOOLEY GJ, PARISI ES, CLARK GM. Pitch comparisons of acoustically and electrically evoked auditory sensations. Hearing research. 1996b;99:139–150. doi: 10.1016/s0378-5955(96)00095-0. [DOI] [PubMed] [Google Scholar]
- CHING TY, INCERTI P, HILL M, VAN WANROOY E. An overview of binaural advantages for children and adults who use binaural/bimodal hearing devices. Audiology and Neurotology. 2006;11:6–11. doi: 10.1159/000095607. [DOI] [PubMed] [Google Scholar]
- COCO A, EPP SB, FALLON JB, XU J, MILLARD RE, SHEPHERD RK. Does cochlear implantation and electrical stimulation affect residual hair cells and spiral ganglion neurons? Hearing research. 2007;225:60–70. doi: 10.1016/j.heares.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DORMAN MF, GIFFORD RH, SPAHR AJ, MCKARNS SA. The benefits of combining acoustic and electric stimulation for the recognition of speech, voice and melodies. Audiology and Neurotology. 2007;13:105–112. doi: 10.1159/000111782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FALLON JB, SHEPHERD RK, BROWN M, IRVINE DR. Effects of neonatal partial deafness and chronic intracochlear electrical stimulation on auditory and electrical response characteristics in primary auditory cortex. Hearing Research. 2009;257:93–105. doi: 10.1016/j.heares.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIRSZT JB, REEDER RM, SKINNER MW. Restoring hearing symmetry with two cochlear implants or one cochlear implant and a contralateral hearing aid. J Rehabil Res Dev. 2008;45:749–67. doi: 10.1682/jrrd.2007.08.0120. [DOI] [PubMed] [Google Scholar]
- HAMZAVI J, MARCEL POK S, GSTOETTNER W, BAUMGARTNER W-D. Speech perception with a cochlear implant used in conjunction with a hearing aid in the opposite ear. International Journal of Audiology. 2004;43:61–65. doi: 10.1080/14992020400050010. [DOI] [PubMed] [Google Scholar]
- HARRISON R, NAGASAWA A, SMITH D, STANTON S, MOUNT R. Reorganization of auditory cortex after neonatal high frequency cochlear hearing loss. Hearing research. 1991;54:11–19. doi: 10.1016/0378-5955(91)90131-r. [DOI] [PubMed] [Google Scholar]
- HENRY BA, TURNER CW, BEHRENS A. Spectral peak resolution and speech recognition in quiet: normal hearing, hearing impaired, and cochlear implant listeners. The Journal of the Acoustical Society of America. 2005;118:1111. doi: 10.1121/1.1944567. [DOI] [PubMed] [Google Scholar]
- IRVING S, WISE AK, MILLARD RE, SHEPHERD RK, FALLON JB. A partial hearing animal model for chronic electro-acoustic stimulation. Journal of Neural Engineering. doi: 10.1088/1741-2560/11/4/046008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLINKE R, KRAL A, HEID S, TILLEIN J, HARTMANN R. Recruitment of the auditory cortex in congenitally deaf cats by long-term cochlear electrostimulation. science. 1999;285:1729–1733. doi: 10.1126/science.285.5434.1729. [DOI] [PubMed] [Google Scholar]
- KRAL A, TILLEIN J, HEID S, KLINKE R, HARTMANN R. Cochlear implants: cortical plasticity in congenital deprivation. Progress in brain research. 2006;157:283–402. doi: 10.1016/s0079-6123(06)57018-9. [DOI] [PubMed] [Google Scholar]
- MCDERMOTT H. An advanced multiple channel cochlear implant. Biomedical Engineering, IEEE Transactions on. 1989;36:789–797. doi: 10.1109/10.32112. [DOI] [PubMed] [Google Scholar]
- MCDERMOTT HJ, LECH M, KORNBLUM MS, IRVINE DR. Loudness perception and frequency discrimination in subjects with steeply sloping hearing loss: possible correlates of neural plasticity. The Journal of the Acoustical Society of America. 1998;104:2314. doi: 10.1121/1.423744. [DOI] [PubMed] [Google Scholar]
- MOK M, GRAYDEN D, DOWELL RC, LAWRENCE D. Speech perception for adults who use hearing aids in conjunction with cochlear implants in opposite ears. Journal of Speech, Language and Hearing Research. 2006;49:338. doi: 10.1044/1092-4388(2006/027). [DOI] [PubMed] [Google Scholar]
- PETTINGILL LN, RICHARDSON RT, WISE AK, O'LEARY SJ, SHEPHERD RK. Neurotrophic factors and neural prostheses: potential clinical applications based upon findings in the auditory system. Biomedical Engineering, IEEE Transactions on. 2007;54:1138–1148. doi: 10.1109/TBME.2007.895375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIENKOWSKI M, EGGERMONT JJ. Cortical tonotopic map plasticity and behavior. Neuroscience & Biobehavioral Reviews. 2011;35:2117–2128. doi: 10.1016/j.neubiorev.2011.02.002. [DOI] [PubMed] [Google Scholar]
- ROBERTSON D, IRVINE DR. Plasticity of frequency organization in auditory cortex of guinea pigs with partial unilateral deafness. Journal of Comparative Neurology. 1989;282:456–471. doi: 10.1002/cne.902820311. [DOI] [PubMed] [Google Scholar]
- ROSE MM, MOORE BC. The relationship between stream segregation and frequency discrimination in normally hearing and hearing-impaired subjects. Hearing research. 2005;204:16–28. doi: 10.1016/j.heares.2004.12.004. [DOI] [PubMed] [Google Scholar]
- SHEPHERD R, VERHOEVEN K, XU J, RISI F, FALLON J, WISE A. An improved cochlear implant electrode array for use in experimental studies. Hearing Research. 2011;277:20–27. doi: 10.1016/j.heares.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TURNER CW, GANTZ BJ, VIDAL C, BEHRENS A, HENRY BA. Speech recognition in noise for cochlear implant listeners: benefits of residual acoustic hearing. The Journal of the Acoustical Society of America. 2004;115:1729. doi: 10.1121/1.1687425. [DOI] [PubMed] [Google Scholar]
- TURNER CW, REISS LA, GANTZ BJ. Combined acoustic and electric hearing: preserving residual acoustic hearing. Hearing research. 2008;242:164–171. doi: 10.1016/j.heares.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TYLER RS, PARKINSON AJ, WILSON BS, WITT S, PREECE JP, NOBLE W. Patients utilizing a hearing aid and a cochlear implant: speech perception and localization. Ear and hearing. 2002;23:98–105. doi: 10.1097/00003446-200204000-00003. [DOI] [PubMed] [Google Scholar]
- VAN DEN HONERT C, KELSALL DC. Focused intracochlear electric stimulation with phased array channels. The Journal of the Acoustical Society of America. 2007;121:3703. doi: 10.1121/1.2722047. [DOI] [PubMed] [Google Scholar]
- VOLLMER M, BEITEL RE. Behavioral training restores temporal processing in auditory cortex of long-deaf cats. Journal of neurophysiology. 2011;106:2423–2436. doi: 10.1152/jn.00565.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOLLMER M, BEITEL RE, SNYDER RL. Auditory detection and discrimination in deaf cats: psychophysical and neural thresholds for intracochlear electrical signals. Journal of Neurophysiology. 2001;86:2330. doi: 10.1152/jn.2001.86.5.2330. [DOI] [PubMed] [Google Scholar]
- VON ILBERG CA, BAUMANN U, KIEFER J, TILLEIN J, ADUNKA OF. Electric-acoustic stimulation of the auditory system: a review of the first decade. Audiology and Neurotology. 2011;16:1–30. doi: 10.1159/000327765. [DOI] [PubMed] [Google Scholar]
- VON ILBERG CA, KIEFER J, TILLEIN J, PFENNINGDORFF T, HARTMANN R, STÜRZEBECHER E, KLINKE R. Electric-acoustic stimulation of the auditory system. New technology for severe hearing loss. ORL; Journal for oto-rhino-laryngology and its related specialties. 1999;61:334. doi: 10.1159/000027695. [DOI] [PubMed] [Google Scholar]
- WILSON BS, DORMAN MF. Cochlear implants: A remarkable past and a brilliant future. Hearing Research. 2008;242:3–21. doi: 10.1016/j.heares.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]