Abstract
NK cells that mediate ADCC play an important role in tumor-specific immunity. We have examined factors limiting specific lysis of tumor cells by CD16.NK-92 cells induced by CNTO 95LF antibodies recognizing αV integrins that are overexpressed on many tumor cells. Although all tested tumor cells were killed by CD16.NK-92 effectors in the presence of the antibodies, the killing of target cells with a low level of ICAM-1 expression revealed a dramatic decrease in their specific lysis at high antibody concentration, revealing a dose limiting effect. A similar effect was also observed with primary human NK cells. The effect was erased after IFN-γ treatment of tumor cells resulting in upregulation of ICAM-1. Furthermore, killing of the same tumor cells induced by Herceptin antibody was significantly impaired in the presence of CNTO 95Ala-Ala antibody variant that blocks αV integrins but is incapable of binding to CD16. These data suggest that αV integrins on tumor cells could compensate for the loss of ICAM-1 molecules, thereby facilitating ADCC by NK cells. Thus, NK cells could exercise cytolytic activity against ICAM-1 deficient tumor cells in the absence of proinflammatory cytokines, emphasizing the importance of NK cells in tumor-specific immunity at early stages of cancer.
Keywords: NK cells, ADCC, tumor cells, adhesion receptors
INTRODUCTION
The development of a strong tumor specific immune response is essential for host defense against cancer. Responses of NK cells that are capable to lyse tumor cells have been shown to play an important role in the first line of tumor-specific host defense [1, 2]. The cytolytic activity of NK cells is regulated by the balance between positive and negative signals induced by various activating and inhibitory receptors [3]. The specificity of NK cell responses is partially mediated by IgG antibodies that recognize cell surface cancer-associated epitopes and induce antibody-dependent cell-mediated cytotoxicity (ADCC) through antibody Fc binding to FcγRIIIa (CD16).
The αV integrins are upregulated on tumor cells and angiogenic endothelial cells, making them attractive therapeutic targets. A number of integrin-specific antibodies have been developed to direct NK cell cytolytic activity against cancer cells [4–6]. One of these antibodies, termed CNTO 95, is currently showing promise in clinical trials [7–11]. This is a fully humanized monoclonal antibody recognizing the αV chain of integrins. CNTO 95 demonstrated low toxicity and is compatible with radiation treatments [12]. However, the ability of this antibody to induce ADCC against tumor cells has not been evaluated in depth.
Here we analyzed the capacity of parental CNTO 95 antibody and their derivatives to induce ADCC against tumor cells by NK92 cells transduced to express CD16 receptor. Because NK-92 cells do not express αV integrins to a detectable level, they provide a unique opportunity to evaluate the potency of CNTO 95 antibody in ADCC. We have found that CNTO 95 binding to αV integrins on ICAM-1 deficient tumor cells diminishes CD16.NK-92-mediated cytotoxicity against the tumor cells in a dose-dependent manner. The killing efficiency was restored in the presence of IFN-γ resulting in upregulation of ICAM-1. These and other data revealed the role of αV integrins on tumor cells in NK cell cytolytic activity and provide evidence that NK cells could successfully attack ICAM-1 deficient tumor cells at the very early stages of cancer in the absence of proinflammatory cytokines.
RESULTS
Factors limiting effectiveness of CNTO 95 antibody in ADCC against tumor cells
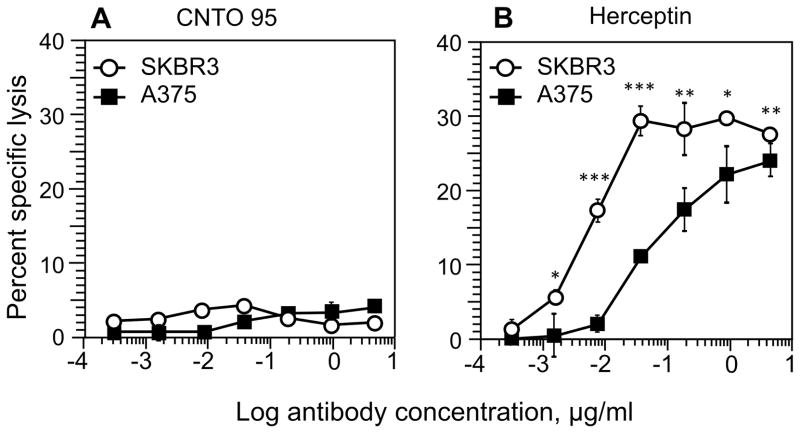
We tested the ability of CNTO 95 to induce ADCC by CD16.NK-92 cells against A375 melanoma cells and SKBR3 breast cancer cells that express αV integrins. The specific lysis of the target cells in the presence of CNTO 95 was almost undetectable (Fig. 1A). In contrast, Herceptin antibody that recognizes Her2/neu receptor on the cell surface of A375 and SKBR3 cells effectively induced robust cytotoxicity against these tumor cells mediated by the CD16.NK-92 cells (Fig. 1B). This was unexpected because the difference in the level of αV integrins on both tumor cells was marginal, and the apparent binding affinities of CNTO 95 and Herceptin to their respective targeting molecules on the cell surface were within the range of the affinity values previously measured for the binding of these antibodies to αV and Her2/neu proteins on the cell surface (Table S1 and Fig. S1A and B, also see refs [10, 13]). In addition, the level of αV expression appeared to be significantly higher than the level of Her2/neu molecules on A375 cells, i.e., 39–138×103 vs. 7–15×103 molecules per cell (Fig. S1B and 2). Nevertheless, A375 cells were effectively killed by CD16.NK-92 in ADCC induced by Herceptin but not CNTO 95 antibodies.
Figure 1.
CD16.NK-92 cytolytic effectors induced ADCC mediated by parental CNTO 95 (A) and Herceptin (B) against melanoma A375 (black squares) and breast cancer SKBR3 (open circles) cells. Increasing concentrations of CNTO 95 or Herceptin antibodies were tested to trigger cytolytic activity by CD16.NK-92 toward the two different cancer cell lines. E:T ratio was 5:1. Data represent mean ± SD from four independent experiments with each condition tested in triplicates in each experiment.
These data prompted us to examine the binding affinity of CNTO 95 and Herceptin to soluble CD16 as well as to CD16 on the surface of live cells. We also analyzed the binding of CNTO 95LF that differs from the parental antibody by a low level of fucosylation of Fc fragment carbohydrates, which affects the structure of the Fc fragment and enhances the binding affinity to CD16 [14–16]. The apparent affinity of CNTO 95 to CD16 (KD=10.8 nM) in a cell free system was 5-times lower than that of Herceptin (KD= 2.1 nM) (Fig. S3A and Table S2). In contrast, the affinity of CNTO 95LF to CD16 (KD=1.0 nM) appeared 2-times stronger than that found for Herceptin (Fig. S3A and Table S2). The apparent affinity of CNTO 95Ala-Ala, which has two mutated residues in the Fc fragment, was 150-times weaker then that of Herceptin (KD=304 nM) (Table S2 and Fig. S4A). Consistent with these data, CNTO 95LF bound stronger than parental CNTO95 to CD16 on live CD16.NK-92 cells (Fig. S3B and Table S2). CNTO 95Ala-Ala did not show any detectable binding to the cell surface CD16 (Fig. S4B). These data suggested that a relatively low affinity of CNTO 95 for CD16 is likely responsible for the inability of these antibodies to induce efficient ADCC against tumor cells.
CNTO 95LF is capable of mediating ADCC but reveals a cell type-dependent bell-shaped dose-response curve
We evaluated the efficiency of CNTO 95LF to induce cytolytic activity by CD16.NK-92 cells against A375 and SKBR3 tumor cells. Up to 70% of A375 cells were specifically lysed by CD16.NK-92 in the presence of CNTO 95LF (Fig. 2A). In contrast, specific lysis of the tumor cells induced by parental CNTO 95 antibodies was barely detectable (Fig. 1A and Fig. 2A). CNTO 95LF also induced specific killing of SKBR3 cells by CD16.NK-92 cells, albeit with less potency (Fig. 2B). The specific lysis of SKBR3 cells induced by parental CNTO 95 was not evident (Fig. 1A and Fig. 2B). Consistent with this, NK-92 expressing the CD16(176F) allele that binds to the antibody Fc fragment with a lower affinity were significantly less potent in killing A375 cells induced by CNTO 95LF (Fig. S5). In control experiments, CNTO 95Ala-Ala antibodies that bind to αV integrin, but do not interact with CD16 were ineffective in inducing ADCC by CD16.NK-92 cells (Fig. 2A and B). Thus, the superiority of CNTO 95LF antibody over parental CNTO 95 antibody in ADCC is explained by a stronger binding to CD16 receptor on cytolytic effectors.
Figure 2.
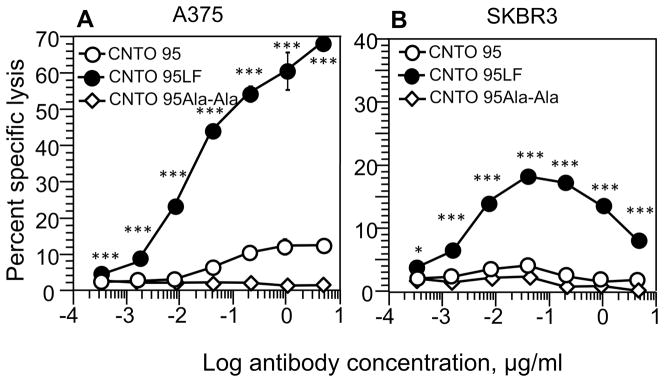
Comparison of the CNTO 95LF antibody mediated cytotoxicity with that induced by parental CNTO 95 antibody. Mutated CNTO 95Ala-Ala antibody was utilized as a negative control. A375 cell line (A) and SKBR3 (B) were used as a target. The 51Cr-labeled target cells were incubated with CD16.NK-92 cells at E:T ratio 5:1 in the presence of indicated concentrations of the antibodies. The specific lysis of the target cells was determined based on the amount of released 51Cr to the culture supernatant. CNTO 95LF, black circles; CNTO 95, open circles; CNTO 95Ala-Ala, open diamonds. Data shown are representative from three to five independent experiments with each condition tested in triplicates in each experiment. Error bars show mean ± SD; *p<0.05, **p<0.001, ***p<0.0001 by two-tailed Student t-test.
There was a significant difference in the dependence of CD16.NK-92-mediated specific lysis of A375 and SKBR3 cells upon the concentration of CNTO95LF antibody. The specific lysis of A375 cells gradually increased with rising antibody concentration, resembling an S-shape killing curve, while the killing curve of SKBR3 cells had a bell shape (Fig. 2A and B). The bell-shape curve of SKBR3 cytolysis did not change in the presence of an excess of human IgG (Fig. S6) providing evidence that the observed high-dose inhibitory effect could not be explained by competition between soluble and target cell-associated antibodies for the binding to CD16 on NK cells. The inhibitory effect was also evident in CNTO 95LF-induced killing of SKBR3 tumor cells by polyclonal primary NK effector cells (Fig. S7). In contrast, Herceptin-induced SKBR3 killing by the NK cells showed a typical S-shaped killing curve (Fig. 1B). These data suggest that blocking of αV integrins at high antibody concentrations preclude the integrins’ interactions with cognate ligands on NK cells to thereby influence the NK cytolytic activity toward SKBR3 target cells.
The αV-containing integrins on tumor cells contribute to NK cell cytolytic activity
To test the role of αV-containing integrins expressed on tumor cells in ADCC exerted by CD16.NK-92 effectors, we compared the killing of A375 and SKBR3 cells induced by Herceptin in the presence or absence of CNTO 95Ala-Ala that specifically binds to the αV chain (data not shown), but did not reveal detectable binding to CD16 on the NK cells (Fig. S4). Fig. 3A and B show that blocking of αV integrins with CNTO 95Ala-Ala antibody significantly impaired effectiveness of Herceptin-mediated cytotoxicity of CD16.NK-92 against both target cells. The concentration of Herceptin required to achieve half maximal specific lysis of A375 (Fig. 3A) and SKBR3 (Fig. 3B) target cells in the presence of CNTO 95Ala-Ala increased by 5- and 20-fold, respectively. The maximal specific lysis of both targets was decreased. The inhibitory effect of CNTO 95Ala-Ala on Herceptin-induced lysis of SKBR3 was concentration-dependent and evident at concentrations above 0.1 μg/ml (0.7 nM)(Fig. 3C). To exclude possible influence of CNTO 95Ala-Ala on the interaction of Herceptin with Her2/neu receptor on target cells, we stained Her2-positive SKBR3 cells with fluorescence-labeled Herceptin in the presence or absence of CNTO 95 and found no difference in the amount of cell surface bound Herceptin (Fig. S8). In another control experiments, the total human IgG did not exert any inhibitory effect, but mouse 17E6 blocking antibody specific for αV integrins inhibited Herceptin-induced target cell lysis (Fig. 3C and Fig. S9A and B). To further reiterate the important role of αV integrins, we exploited SKOV3 ovarian cancer cells, which express both αV and Her2/neu proteins on the cell surface (Fig. S10A and B). We found that CD16.NK-92-mediated killing of these cells induced by Herceptin was also strongly inhibited by CNTO 95Ala-Ala and 17E6 antibodies (Fig. S11).
Figure 3.
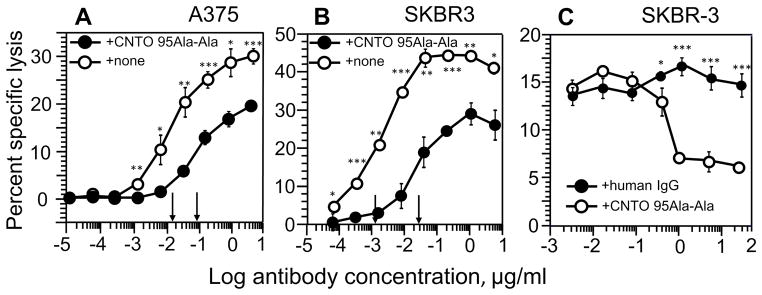
Herceptin-induced ADCC by CD16.NK-92 in the presence or absence of CNTO 95Ala-Ala. Specific lysis of 51Cr-labeled A375 (A) or SKBR-3 (B) cells by CD16.NK-92 (E:T=5:1) was induced by Herceptin antibodies at indicated concentrations in the presence (black circles) or absence (open circles) of 10 μg/ml of CNTO 95Ala-Ala antibody. Arrows indicate the Herceptin concentration required for half-maximal lysis (SD50). (C). CD16.NK-92 and SKBR3 cells were pre-incubated with CNTO 95AlaAla antibodies or normal human IgG at indicated concentrations, and the specific lysis of these target cells by CD16.NK-92 was induced by Herceptin antibodies at 0.04 μg/ml. The data shown are representative from two to five independent experiments with each condition tested in triplicates within every experiment. Data depicted as mean ± SD; *p<0.05, **p<0.001, ***p<0.0001 by two-tailed Student t-test.
These data show that αV-containing integrins on tumor cells facilitate the ADCC activity of human NK cells.
The effect of αV integrins on ADCC depends on the level of ICAM-1 expression on tumor cells
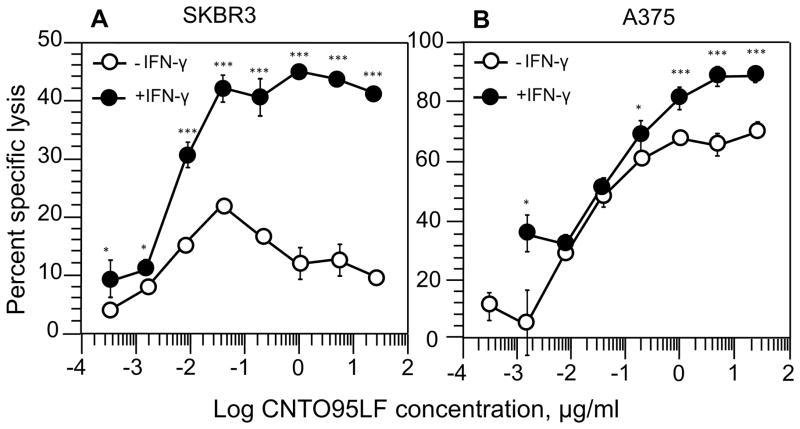
ICAM-1 serves as a ligand for various β2-containing integrins, namely: αLβ2 or LFA-1, αMβ2 or Mac-1, and αXβ2, which are expressed on NK cells [17, 18]. We compared ICAM-1 expression on A375, SKBR3, and SKOV3 cells and found a large difference in the level of ICAM-1 on these tumor cells (Figure S12A, B, and C). The expression of ICAM-1 on A375 cells was significantly higher than that on SKBR3 and SKVO3 cells. To study the role of ICAM-1 in the inhibition of ADCC against SKBR3 and SKOV3 tumor cells (Fig. 2B and 4) at high concentration of CNTO 95LF antibodies, we treated A375 and SKBR3 targets with IFN-γ to stimulate the expression of ICAM-1. IFN-γ treatment resulted in significant upregulation of ICAM-1 expression on SKBR3 cells (Fig. S12B), whereas the ICAM-1 level on A375 cells was practically unchanged (Fig. S12A). Unexpectedly, SKVO3 cells did not respond to IFN-γ treatment by upregulation of ICAM-1 (Fig. 12C) suggesting that the mechanism of upregulation of ICAM-1 is defective in some tumor cells. Comparison of CNTO 95LF-induced killing of IFN-γ treated tumor cells showed that the maximum specific lysis of SKBR3 cells significantly increased (Fig. 5A) as opposed to that of the treated A375 targets (Fig. 5B). The IFN-γ treatment also changed the shape of the killing curve of SKBR3 target cells: the bell-shaped concentration dependence was no longer observed, and the curve assumed an S-shape similar to that for A375 target cells (Fig. 5A and B). A similar effect was observed with polyclonal primary human NK effector cells (Fig. S7). IFN-γ treatment of SKBR3 cells also alleviated the blocking effect of αV-containing integrins on Herceptin-induced ADCC (compare Fig. 3B and Fig. S13). Because IFN-γ treatment did not change the level of αV chain expression on tumor cells (Table S3), the observed increase in ADCC efficiency cannot be attributed to an elevated level of the epitope recognizable by the antibody. However, IFN-γ treatment could also upregulate expression of various ligands for activating and inhibitory receptors on NK cells [19, 20]. To single out the role of ICAM-1 for the observed inhibition of CD16.NK-92-mediated ADCC, we utilized TS1/18 antibodies specific for integrin β2 chain [21]. The presence of TS1/18 antibodies in the extracellular medium significantly decreased the maximum specific lysis of IFN-γ-treated SKBR3 cells (Fig. 6A) and restored the bell-shape of the killing curve. The killing curve of SKBR-3 cells without IFN-γ treatment remained bell-shaped regardless of the presence or absence of TS1/18 antibody (Fig. 6B). Importantly, blocking only αLβ2 integrin (LFA-1) with TS1/22 antibodies [22] did not inhibit the CNTO 95LF-induced destruction of the A375 tumor cells by CD16.NK-92-mediated ADCC (Fig. S14). The inhibitory effect of TS1/18 antibodies on CNTO 95LF-induced killing of A375 cells that express high level of ICAM-1 as opposed to SKBR3 cells was much less profound and did not change a typical shape of the killing curve (Fig. S14). Nevertheless, TS1/18 antibody inhibited the CNTO 95LF-induced killing of A375 cells twice as strongly as it inhibited the killing inhibition of the same cells induced by Herceptin antibodies (data not shown).
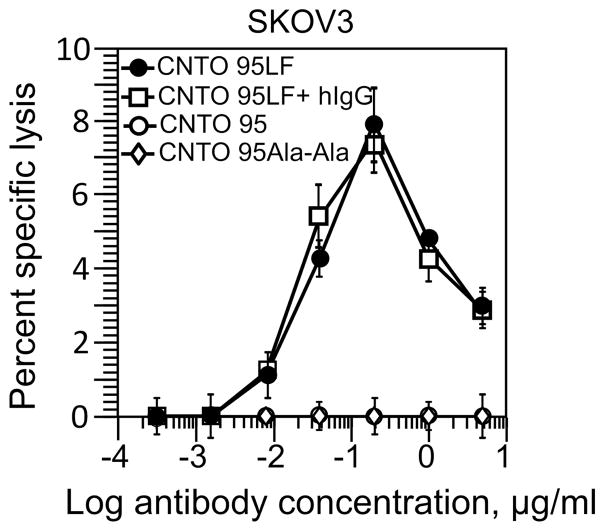
Figure 4.
Specific lysis of SKVO3 tumor cells by CD16.NK-92 induced by CNTO 95LF antibodies. The 51Cr-labeled SKVO3 tumor cells were incubated with CD16.NK-92 cells at E:T ratio 5:1 in the presence of CNTO 95LF (black circles) or CNTO 95 (open circles) or CNTO 95Ala-Ala (open diamonds) antibodies at indicated concentrations. In control experiments, normal human IgG (open squares) were also added (50 μg/ml) along with CNTO 95LF. The data shown are representative from five independent experiments with each antibody concentration tested in triplicates and depicted as mean ± SD. No significant difference by two-tailed Student t-test.
Figure 5.
Effect of IFN-γ treatment on specific lysis of SKBR3 (A) and A375 (B) tumor cells by CD16.NK-92 cells induced by CNTO 95LF antibodies. The target cells were treated with IFN-γ (black circles) for 48h or left untreated (open circle) and the ADCC was evaluated as in Fig. 3. The assay results are representative from three to seven experiments with each condition tested in triplicates in every experiment. Data represent mean ± SD, *p<0.05, **p<0.001, ***p<0.0001 by two-tailed Student t-test.
Figure 6.
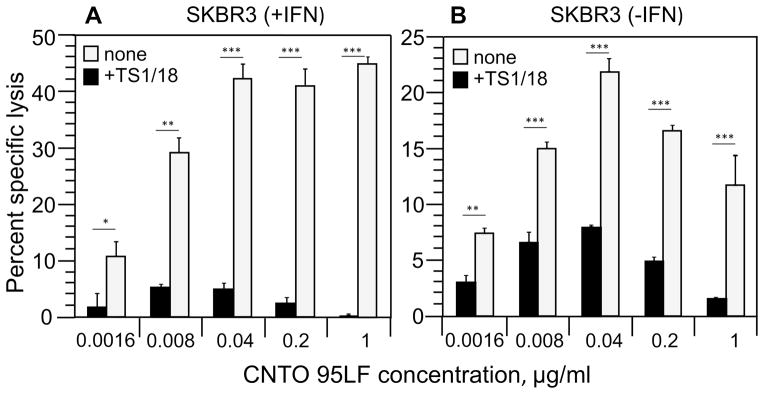
Inhibition of ADCC against tumor cells mediated by CD16.NK-92 cells in the presence of blocking antibodies against β2-integrins. SKBR3 cells were treated with IFN-γ for 48 hours (A) or left untreated (B). The 51Cr-labeled tumor cells were incubated with CD16.NK-92 at E:T ratio 5:1 along with CNTO 95LF antibodies at various concentrations in the presence (black squares) or absence (gray squares) of TS1/18 antibodies (10μg/ml). The percent specific lysis was measured after 4h at 37°C. Results are representative of two independent experiments with each condition tested in triplicates in every experiment and are expressed as mean ± SD, *p<0.05, **p<0.001, ***p<0.0001 by two-tailed Student t-test.
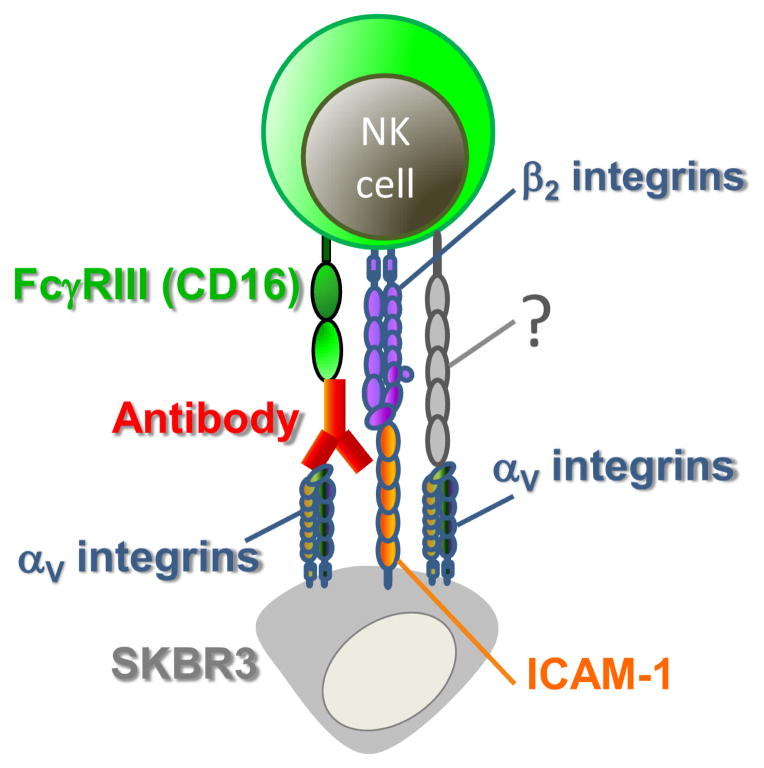
Taken together, the data indicate that engagement of αV integrin on target cells facilitates efficient cytolytic activity of NK cells against tumor cells (Fig. 7) and that the contribution of the αV integrins is modulated by other receptor-ligand interactions that depend on the nature of tumor cells and receptor diversity on NK cells.
Figure 7.
Integrin molecules regulate antibody-dependent cytotoxicity of NK cells against tumor cells (see text for details).
DISCUSSION
Antibodies against tumor-associated antigens are widely used to trigger cytolytic activity of NK cells against tumor cells. However, how the phenotype of various tumor cells could influence ADCC is not well understood. In this study, we thoroughly evaluated killing of two different tumor cells by CD16.NK-92 effectror induced by CNTO 95LF antibodies. We observed a bell-shape killing curve of SKBR3 tumor cells, while specific lysis of A375 melanoma cells was characterized by a typical S-shape curve (Fig. 2B). The difference in the killing was linked to a significantly lower level of ICAM-1 expression on SKBR3 cancer cells as opposed to A375 melanoma cells. The observed dose-limiting effect of the antibodies was not unique for SKBR3 target cells, but was also evident in the killing of SKOV3 tumor cells that express very low level of ICAM-1 (Fig. 4 and Fig. S12C). A similar inhibitory effect resulting in a bell-shape killing curve was observed for Abegrin, a fully humanized anti-integrin αVβ3 antibody [5]. We conclude that low doses of CNTO 95LF antibody can trigger strong ADCC responses through engaging with CD16, but higher doses become inhibitory through antibody-mediated blockade of αV integrins. Thus, our data add to previous evidence that downregulation of adhesion receptors on cancer cells could lead to suppression of NK cell and CTL responses [22–25], and αV integrins can contribute to NK cell-mediated ADCC.
The observed inhibition of ADCC at high concentrations of CNTO 95LF (0.04–5 μg/ml) might be explained by the presence of free CNTO 95LF antibody in assay media. In fact, the free normal IgG that is present in the serum at high concentration (10–12mg/ml or ≈0.1mM) could compete with antibodies for the binding to CD16 and impair ADCC [26]. However, we have found no influence of an excess of normal human IgG on the target cell lysis (Fig. 3C and 4, Fig. S6 and S9B). Most likely, cell-bound CNTO 95LF at the interface of NK-target cell conjugates, whose local (effective) concentration is significantly higher than that of free antibodies, could effectively compete with the free antibody for CD16. Because the inhibition of the specific lysis of tumor cells occurred at high concentrations of CNTO 95LF, we propose that blocking of αV-containing integrins on tumor cells suppresses ADCC. This conclusion is supported by experiments showing that CNTO 95Ala-Ala antibodies or mouse 17E6 antibodies that do not bind to CD16 receptor but specifically interact with the αV chain diminished CD16.NK-92 cytolytic activity (Fig. 3 and Fig. S9A and S11). The contribution of integrin αV chain is decreased but still evident in the presence of high levels of ICAM-1 on tumor cells that can interact with β2 integrins on NK cells (Fig. 3A and S14). Thus, we would like to suggest that additional interactions between αV-containing integrins on tumor cells and their ligands on NK cells facilitate the ability of NK cells to hunt and kill tumor cells.
The nature of ligand on NK-92 cells interacting with αV integrins on tumor cells is not known. Vitronectin and fibronectin are two possible ligands for αV integrins. It has been shown that fibronectin binds to the CD11b (integrin αM, Mac-1) on the surface of mouse NK cells resulting in the recruitment of activated Src [27]. Other ligand candidates may include ADAM family proteins or CD31. The ligand identification will establish the significance of yet another interaction occurring at the interface of NK cell/target cell conjugates and how it influences the cytolytic activity of NK cells.
The observed inhibitory effect of αV integrin blockade on the cytolytic activity of CD16.NK-92 cells demonstrates that changes in the phenotype of the targeted tumors could significantly influence ADCC. In addition, it has been previously shown that engagement of αV integrins on activated NK cells by vitronectin induces a costimulatory signal to complement CD16-mediated signaling [28]. Thus, the αV integrins expressed on either NK cells or on tumor cells can regulate NK cell cytolytic activity. This implies that successful application of tumor-specific antibodies directing the destruction of tumor cells by NK cells requires a personalized approach that includes careful choice of the therapeutic dose of antibody and analysis of the possible contribution of other receptor-ligand interactions occurring between tumor cells and NK cells.
Perhaps not surprisingly, we found that only defucosylated CNTO 95LF antibodies, which bound to CD16(176V) with high affinity, mediated ADCC of tumor cells by CD16.NK-92, whereas parental CNTO 95 antibodies were ineffective. In contrast, weaker ADCC responses were mediated by NK-92 cells expressing the lower affinity CD16(176F) (Fig. S5). These findings may have important implications, because less than 20% of individuals are homozygous (176V/176V) for high affinity CD16 and around 40% of individuals have the homozygous 176F/176F haplotype [29, 30]. This suggests that increased efficiency of natural immunity could be achieved if the affinity of the Fc-CD16 interactions could be modulated during immune response against pathogens or tumors. In support of this, changes in the glycosylation pattern of antibodies during the immune response have been documented [31, 32]. It has also been shown that fucosylation of serum IgG could be modulated during advance stages of cancer [33, 34]. Changes in the IgG glycosylation pattern enhancing affinity of the Fc-CD16 interactions have been reported to appear during antigen-driven maturation of the antibody response [35, 36].
CNTO 95LF antibody induced efficient ADCC even though a significant fraction of cell surface αV chain was blocked (Fig. S15). This is in accord with previous observation that a relatively small number of targeting molecules is sufficient to initiate effective ADCC [37], which could be due to the ability of the antigen-bound antibodies to form clusters at the cell surface. In fact, anti-αV antibodies induce clustering of the cell surface αV integrins [38]. Also, Rituximab induces recruitment of CD20 into caps, accounting for the higher potency of Rituximab in triggering ADCC [39]. In accord with these findings, engineered IgG containing tandem of 3 Fc fragments were exceptionally potent in the induction of NK cell cytolytic activity [40].
In conclusion, our data show that upregulation of αV integrin receptors on tumor cells could compensate for the loss of ICAM-1 molecules and promote ADCC by NK cells. This suggests that NK cells are still capable of targeting ICAM-1-deficient tumor cells in the absence of proinflammatory cytokines. Our results also identify an alternative mechanism by which NK cells can respond to tumors and play an important role in innate the immune response at the very earlier stages of cancer, prior to the development of inflammation.
MATERIALS AND METHODS
Cells, antibodies and proteins
The human NK-92 cell line was transduced by retroviral vector pBMN-NoGFP [41] to express FcγRIIIa receptor (CD16a or 176V allele of CD16 that binds to the antibody Fc fragment with higher affinity [42]). CD16.NK-92 cells [41] were used in all but one (Figure S5) experiments, in which NK-92 cells were transduced with the 176F low affinity allele of CD16. Human tumor cell lines used in the present study were the A375 melanoma cell line, the SKBR3 breast cancer line, and the SKOV3 ovarian cancer cell line.
Herceptin, humanized antibody against human HER2/neu were kindly provided by Dr. Takami Sato (Thomas Jefferson University). Mouse 17E6 blocking antibody (IgG1) recognizing human αV integrin was supplied by Calbiochem. Hybridomas producing mouse TS1/18 blocking antibody (IgG1) specific for human CD18 (β2 integrin chain), mouse TS1/22 blocking antibody (IgG1) specific for human CD11a (LFA-1 αL chain), and mouse HB9580 (R6.5) antibody against human ICAM-1 were purchased from ATCC. W6/32 antibody specific for a common epitope of human MHC class I molecules was purified from culture supernatant of its respective hybridoma. Antibody recognizing His6 tag (Penta-His) was purchased from QIAGEN. Normal human IgG and goat anti-human antibodies conjugated to peroxidase were supplied by Sigma. Goat anti-human IgG labeled with Alexa Fluor 488 were purchased from Life Technology, Invitrogen. Parental CNTO 95, CNTO 95LF and CNTO 95Ala-Ala monoclonal antibodies were provided by Centocor Inc. The CNTO 95Ala-Ala antibodies having 2 substitutions, i.e., L236A and L237A, were produced as previously described [43]. These mutations abrogate binding of the antibodies to CD16, while the recognition of the αV epitope is preserved. The CNTO 95LF were made by producing the antibodies in the YB2/0 cell line that is deficient in fucosyltransferase [44]. Soluble FcγRIIIa protein containing polyhistidine tag at the C-terminal end was supplied by R&D System.
Herceptin and CNTO 95 and their derivatives were labeled with Alexa Fluor 488 (Molecular Probes) according to the manufacturer’s instruction.
Evaluation of the number of epitopes on the target cells
2.0×105 A375 or SKBR3 cells were incubated with serial dilutions of the human receptor-specific antibodies labeled with Alexa Fluor 488 for 30 minutes at 4°C. Alexa Fluor 488-labeled human IgG was used to determine the background binding. The receptor specific antibodies and the human IgG were labeled at approximately 5:1 F/P ratio. The cells were washed free of unreacted material and analyzed by flow cytometry. Alexa Fluor 488 microspheres (Bangs Laboratories, Inc.) were used to quantify the number of fluorescent antibodies bound to the cell surface receptors (see Supplemental Information for more details). The data were analyzed using Bangs Laboratories quantitative software, QuickCal. The following equation was used to analyze the binding of the antibodies to αV-containing integrins on the cell surface:
| (1) |
where N is the experimentally determined number of antibody molecules bound to the receptor at various antibody concentrations [Ab], Nmax is the maximum number of receptor proteins per cell. Kd is the equilibrium dissociation constant. The values of Nmax and Kd were determined with Ordinary Least Squares (OLS) regression analysis using Excel software (Microsoft Inc.) with Solver add-on.
Binding of the αV-specific antibodies to CD16 receptor
To determine the strength of the antibody binding to soluble His6-tagged CD16 (R&D System) we exploited a previously described a sandwich ELISA assay [45, 46] with some modifications. 96-well plates were coated with 2 μg/ml of Penta-His antibody and were then blocked with 1% BSA in PBS. Soluble His6-tagged CD16 was added to the wells at 2 μg/ml and plates were further incubated for 2 hours at room temperature. The wells were then washed and Herceptin or CNTO 95 or CNTO 95LF or CNTO 95Ala-Ala antibodies were added at concentrations ranging from 0.01 to 100 μg/ml, and the wells were blocked with 1% BSA. Wells in which mouse IgG was added instead of the human antibody of interest were used as a negative control. After overnight incubation at 4°C anti-human IgG antibody conjugated with peroxidase were added at 1:500 dilutions and the plates were incubated for about 12 hours at 4° C. The assay was developed with o-phenylendiamine, and the absorbance was measured at 490 nm. Apparent Kd values for interactions of the antibodies with plate-bound soluble CD16 receptor were determined from the best fit of the experimental points to Equation 1.
CD16.NK-92 cells were washed and resuspended in 1% BSA/PBS containing various dilutions of CNTO 95 or CNTO 95LF or CNTO 95Ala-Ala for 30 minutes at 4° C. The cells were washed free of unreacted material and incubated with goat anti-human IgG antibody conjugated with AlexaFluor 488. The cells were then quickly washed and the fluorescent intensity of the cell surface bound antibodies was measured by Beckman-Coulter flow cytometer. Cells stained with the secondary antibody only were utilized as negative control. Apparent equilibrium dissociation constants (Kd) were derived from the best fit of measured MFI values to the curve described by Equation 1.
51Cr-release ADCC Assay
Target cells were harvested using DPBS containing 3 mM EDTA, washed and then labeled with 51Cr. 5.0×103 51Cr-labeled target cells and 2.5×104 CD16.NK-92 effector cells were combined with antibodies at various concentrations in a total volume of 200 μl in 96-well round bottom plate. The plates were incubated in a CO2 incubator for 4h at 37°C and radioactivity of the supernatants was analyzed on a Wizard automatic gamma counter (PerkinElmer). The percentage of specific lysis was calculated as previously described [47].
Supplementary Material
Acknowledgments
This work was supported by NIH grants to Y.S. (AI52812; CA131973) and K.S.C. (CA083859). Some financial support for this work was provided by the Centocor Research & Development Sponsored Program. We thank all members of Sykulev lab for valuable discussions of the manuscript and providing other support.
Abbreviations
- ADCC
antibody-dependent cell-mediated cytotoxicity
- αLβ2 integrin or LFA-1
lymphocyte function-associated antigen 1
Footnotes
Potential Conflict of Interest
CD16.NK-92 cells are protected by patents or pending patents by K.S.C. and licensed by Conkwest, Inc. (www.conkwest.com).
References
- 1.Purdy AK, Campbell KS. Natural killer cells and cancer: regulation by the killer cell Ig-like receptors (KIR) Cancer Biol Ther. 2009;8:2211–2220. doi: 10.4161/cbt.8.23.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol. 12:239–252. doi: 10.1038/nri3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nemeth JA, Nakada MT, Trikha M, Lang Z, Gordon MS, Jayson GC, Corringham R, Prabhakar U, Davis HM, Beckman RA. Alpha-v integrins as therapeutic targets in oncology. Cancer Invest. 2007;25:632–646. doi: 10.1080/07357900701522638. [DOI] [PubMed] [Google Scholar]
- 5.Mulgrew K, Kinneer K, Yao XT, Ward BK, Damschroder MM, Walsh B, Mao SY, Gao C, Kiener PA, Coats S, Kinch MS, Tice DA. Direct targeting of alphavbeta3 integrin on tumor cells with a monoclonal antibody, Abegrin. Mol Cancer Ther. 2006;5:3122–3129. doi: 10.1158/1535-7163.MCT-06-0356. [DOI] [PubMed] [Google Scholar]
- 6.Gutheil JC, Campbell TN, Pierce PR, Watkins JD, Huse WD, Bodkin DJ, Cheresh DA. Targeted antiangiogenic therapy for cancer using Vitaxin: a humanized monoclonal antibody to the integrin alphavbeta3. Clin Cancer Res. 2000;6:3056–3061. [PubMed] [Google Scholar]
- 7.Mullamitha SA, Ton NC, Parker GJ, Jackson A, Julyan PJ, Roberts C, Buonaccorsi GA, Watson Y, Davies K, Cheung S, Hope L, Valle JW, Radford JA, Lawrance J, Saunders MP, Munteanu MC, Nakada MT, Nemeth JA, Davis HM, Jiao Q, Prabhakar U, Lang Z, Corringham RE, Beckman RA, Jayson GC. Phase I evaluation of a fully human anti-alphav integrin monoclonal antibody (CNTO 95) in patients with advanced solid tumors. Clin Cancer Res. 2007;13:2128–2135. doi: 10.1158/1078-0432.CCR-06-2779. [DOI] [PubMed] [Google Scholar]
- 8.O’Day S, Pavlick A, Loquai C, Lawson D, Gutzmer R, Richards J, Schadendorf D, Thompson JA, Gonzalez R, Trefzer U, Mohr P, Ottensmeier C, Chao D, Zhong B, de Boer CJ, Uhlar C, Marshall D, Gore ME, Lang Z, Hait W, Ho P. A randomised, phase II study of intetumumab, an anti-alphav-integrin mAb, alone and with dacarbazine in stage IV melanoma. Br J Cancer. 2011;105:346–352. doi: 10.1038/bjc.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Q, Manning CD, Millar H, McCabe FL, Ferrante C, Sharp C, Shahied-Arruda L, Doshi P, Nakada MT, Anderson GM. CNTO 95, a fully human anti alphav integrin antibody, inhibits cell signaling, migration, invasion, and spontaneous metastasis of human breast cancer cells. Clin Exp Metastasis. 2008;25:139–148. doi: 10.1007/s10585-007-9132-4. [DOI] [PubMed] [Google Scholar]
- 10.Trikha M, Zhou Z, Nemeth JA, Chen Q, Sharp C, Emmell E, Giles-Komar J, Nakada MT. CNTO 95, a fully human monoclonal antibody that inhibits alphav integrins, has antitumor and antiangiogenic activity in vivo. Int J Cancer. 2004;110:326–335. doi: 10.1002/ijc.20116. [DOI] [PubMed] [Google Scholar]
- 11.Hersh EM, O’Day SJ, Powderly J, Khan KD, Pavlick AC, Cranmer LD, Samlowski WE, Nichol GM, Yellin MJ, Weber JS. A phase II multicenter study of ipilimumab with or without dacarbazine in chemotherapy-naive patients with advanced melanoma. Invest New Drugs. 2011;29:489–498. doi: 10.1007/s10637-009-9376-8. [DOI] [PubMed] [Google Scholar]
- 12.Ning S, Nemeth JA, Hanson RL, Forsythe K, Knox SJ. Anti-integrin monoclonal antibody CNTO 95 enhances the therapeutic efficacy of fractionated radiation therapy in vivo. Mol Cancer Ther. 2008;7:1569–1578. doi: 10.1158/1535-7163.MCT-08-0288. [DOI] [PubMed] [Google Scholar]
- 13.Nagy P, Friedlander E, Tanner M, Kapanen AI, Carraway KL, Isola J, Jovin TM. Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a herceptin-resistant, MUC4-expressing breast cancer cell line. Cancer Res. 2005;65:473–482. [PubMed] [Google Scholar]
- 14.Stewart R, Thom G, Levens M, Guler-Gane G, Holgate R, Rudd PM, Webster C, Jermutus L, Lund J. A variant human IgG1-Fc mediates improved ADCC. Protein Eng Des Sel. 2011;24:671–678. doi: 10.1093/protein/gzr015. [DOI] [PubMed] [Google Scholar]
- 15.Ferrara C, Grau S, Jager C, Sondermann P, Brunker P, Waldhauer I, Hennig M, Ruf A, Rufer AC, Stihle M, Umana P, Benz J. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc Natl Acad Sci U S A. 2011;108:12669–12674. doi: 10.1073/pnas.1108455108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrara C, Stuart F, Sondermann P, Brunker P, Umana P. The carbohydrate at FcgammaRIIIa Asn-162. An element required for high affinity binding to non-fucosylated IgG glycoforms. J Biol Chem. 2006;281:5032–5036. doi: 10.1074/jbc.M510171200. [DOI] [PubMed] [Google Scholar]
- 17.Timonen T, Gahmberg CG, Patarroyo M. Participation of CD11a-c/CD18, CD2 and RGD-binding receptors in endogenous and interleukin-2-stimulated NK activity of CD3-negative large granular lymphocytes. Int J Cancer. 1990;46:1035–1040. doi: 10.1002/ijc.2910460615. [DOI] [PubMed] [Google Scholar]
- 18.Sadhu C, Harris EA, Staunton DE. Enhancement of Natural Killer cell cytotoxicity by a CD18 integrin-activating antibody. Biochem Biophys Res Commun. 2007;358:938–941. doi: 10.1016/j.bbrc.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 19.Bui JD, Carayannopoulos LN, Lanier LL, Yokoyama WM, Schreiber RD. IFN-dependent down-regulation of the NKG2D ligand H60 on tumors. J Immunol. 2006;176:905–913. doi: 10.4049/jimmunol.176.2.905. [DOI] [PubMed] [Google Scholar]
- 20.Gustafson KS, Ginder GD. Interferon-gamma induction of the human leukocyte antigen-E gene is mediated through binding of a complex containing STAT1alpha to a distinct interferon-gamma-responsive element. J Biol Chem. 1996;271:20035–20046. doi: 10.1074/jbc.271.33.20035. [DOI] [PubMed] [Google Scholar]
- 21.Huang C, Zang Q, Takagi J, Springer TA. Structural and functional studies with antibodies to the integrin beta 2 subunit. A model for the I-like domain. J Biol Chem. 2000;275:21514–21524. doi: 10.1074/jbc.M002286200. [DOI] [PubMed] [Google Scholar]
- 22.Anikeeva N, Somersalo K, Sims TN, Thomas VK, Dustin ML, Sykulev Y. Distinct role of lymphocyte function-associated antigen-1 in mediating effective cytolytic activity by cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 2005;102:6437–6442. doi: 10.1073/pnas.0502467102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Somersalo K, Anikeeva N, Sims TN, Thomas VK, Strong RK, Spies T, Lebedeva T, Sykulev Y, Dustin ML. Cytotoxic T lymphocytes form an antigen-independent ring junction. J Clin Invest. 2004;113:49–57. doi: 10.1172/JCI200419337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beal AM, Anikeeva N, Varma R, Cameron TO, Norris PJ, Dustin ML, Sykulev Y. Protein kinase C theta regulates stability of the peripheral adhesion ring junction and contributes to the sensitivity of target cell lysis by CTL. J Immunol. 2008;181:4815–4824. doi: 10.4049/jimmunol.181.7.4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu D, Bryceson YT, Meckel T, Vasiliver-Shamis G, Dustin ML, Long EO. Integrin-dependent organization and bidirectional vesicular traffic at cytotoxic immune synapses. Immunity. 2009;31:99–109. doi: 10.1016/j.immuni.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Preithner S, Elm S, Lippold S, Locher M, Wolf A, da Silva AJ, Baeuerle PA, Prang NS. High concentrations of therapeutic IgG1 antibodies are needed to compensate for inhibition of antibody-dependent cellular cytotoxicity by excess endogenous immunoglobulin G. Mol Immunol. 2006;43:1183–1193. doi: 10.1016/j.molimm.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Zhang T, Liu S, Yang P, Han C, Wang J, Liu J, Han Y, Yu Y, Cao X. Fibronectin maintains survival of mouse natural killer (NK) cells via CD11b/Src/beta-catenin pathway. Blood. 2009;114:4081–4088. doi: 10.1182/blood-2009-05-219881. [DOI] [PubMed] [Google Scholar]
- 28.Rabinowich H, Lin WC, Amoscato A, Herberman RB, Whiteside TL. Expression of vitronectin receptor on human NK cells and its role in protein phosphorylation, cytokine production, and cell proliferation. J Immunol. 1995;154:1124–1135. [PubMed] [Google Scholar]
- 29.Lehrnbecher T, Foster CB, Zhu S, Leitman SF, Goldin LR, Huppi K, Chanock SJ. Variant genotypes of the low-affinity Fcgamma receptors in two control populations and a review of low-affinity Fcgamma receptor polymorphisms in control and disease populations. Blood. 1999;94:4220–4232. [PubMed] [Google Scholar]
- 30.Lazar GA, Dang W, Karki S, Vafa O, Peng JS, Hyun L, Chan C, Chung HS, Eivazi A, Yoder SC, Vielmetter J, Carmichael DF, Hayes RJ, Dahiyat BI. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci U S A. 2006;103:4005–4010. doi: 10.1073/pnas.0508123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wuhrer M, Porcelijn L, Kapur R, Koeleman CA, Deelder A, de Haas M, Vidarsson G. Regulated glycosylation patterns of IgG during alloimmune responses against human platelet antigens. J Proteome Res. 2009;8:450–456. doi: 10.1021/pr800651j. [DOI] [PubMed] [Google Scholar]
- 32.Selman MH, de Jong SE, Soonawala D, Kroon FP, Adegnika AA, Deelder AM, Hokke CH, Yazdanbakhsh M, Wuhrer M. Changes in antigen-specific IgG1 Fc N-glycosylation upon influenza and tetanus vaccination. Mol Cell Proteomics. 2012;11:M111 014563. doi: 10.1074/mcp.M111.014563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alley WR, Jr, Vasseur JA, Goetz JA, Svoboda M, Mann BF, Matei DE, Menning N, Hussein A, Mechref Y, Novotny MV. N-linked glycan structures and their expressions change in the blood sera of ovarian cancer patients. J Proteome Res. 2012;11:2282–2300. doi: 10.1021/pr201070k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kodar K, Stadlmann J, Klaamas K, Sergeyev B, Kurtenkov O. Immunoglobulin G Fc N-glycan profiling in patients with gastric cancer by LC-ESI-MS: relation to tumor progression and survival. Glycoconj J. 2012;29:57–66. doi: 10.1007/s10719-011-9364-z. [DOI] [PubMed] [Google Scholar]
- 35.Eisen HN, Siskind GW. Variations in Affinities of Antibodies during the Immune Response. Biochemistry. 1964;3:996–1008. doi: 10.1021/bi00895a027. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt AG, Xu H, Khan AR, O’Donnell T, Khurana S, King LR, Manischewitz J, Golding H, Suphaphiphat P, Carfi A, Settembre EC, Dormitzer PR, Kepler TB, Zhang R, Moody MA, Haynes BF, Liao HX, Shaw DE, Harrison SC. Preconfiguration of the antigen-binding site during affinity maturation of a broadly neutralizing influenza virus antibody. Proc Natl Acad Sci U S A. 2013;110:264–269. doi: 10.1073/pnas.1218256109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prang N, Preithner S, Brischwein K, Goster P, Woppel A, Muller J, Steiger C, Peters M, Baeuerle PA, da Silva AJ. Cellular and complement-dependent cytotoxicity of Ep-CAM-specific monoclonal antibody MT201 against breast cancer cell lines. Br J Cancer. 2005;92:342–349. doi: 10.1038/sj.bjc.6602310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castel S, Pagan R, Mitjans F, Piulats J, Goodman S, Jonczyk A, Huber F, Vilaro S, Reina M. RGD peptides and monoclonal antibodies, antagonists of alpha(v)-integrin, enter the cells by independent endocytic pathways. Lab Invest. 2001;81:1615–1626. doi: 10.1038/labinvest.3780375. [DOI] [PubMed] [Google Scholar]
- 39.Rudnicka D, Oszmiana A, Finch DK, Strickland I, Schofield DJ, Lowe DC, Sleeman MA, Davis DM. Rituximab causes a polarization of B cells that augments its therapeutic function in NK-cell-mediated antibody-dependent cellular cytotoxicity. Blood. 2013;121:4694–4702. doi: 10.1182/blood-2013-02-482570. [DOI] [PubMed] [Google Scholar]
- 40.Nagashima H, Tezuka T, Tsuchida W, Maeda H, Kohroki J, Masuho Y. Tandemly repeated Fc domain augments binding avidities of antibodies for Fcgamma receptors, resulting in enhanced antibody-dependent cellular cytotoxicity. Mol Immunol. 2008;45:2752–2763. doi: 10.1016/j.molimm.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 41.Miah SM, Campbell KS. Expression of cDNAs in human Natural Killer cell lines by retroviral transduction. Methods Mol Biol. 2010;612:199–208. doi: 10.1007/978-1-60761-362-6_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koene HR, Kleijer M, Algra J, Roos D, von dem Borne AE, de Haas M. Fc gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H phenotype. Blood. 1997;90:1109–1114. [PubMed] [Google Scholar]
- 43.Xu D, Alegre ML, Varga SS, Rothermel AL, Collins AM, Pulito VL, Hanna LS, Dolan KP, Parren PW, Bluestone JA, Jolliffe LK, Zivin RA. In vitro characterization of five humanized OKT3 effector function variant antibodies. Cell Immunol. 2000;200:16–26. doi: 10.1006/cimm.2000.1617. [DOI] [PubMed] [Google Scholar]
- 44.Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, Uchida K, Anazawa H, Satoh M, Yamasaki M, Hanai N, Shitara K. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 2003;278:3466–3473. doi: 10.1074/jbc.M210665200. [DOI] [PubMed] [Google Scholar]
- 45.Anikeeva N, Lebedeva T, Sumaroka M, Kalams SA, Sykulev Y. Soluble HIV-specific T-cell receptor: expression, purification and analysis of the specificity. J Immunol Meth. 2003b;277:75–86. doi: 10.1016/s0022-1759(03)00179-0. [DOI] [PubMed] [Google Scholar]
- 46.Martinez-Hackert E, Anikeeva N, Kalams SA, Walker BD, Hendrickson WA, Sykulev Y. Structural basis for degenerate recognition of natural HIV peptide variants by cytotoxic lymphocytes. J Biol Chem. 2006;281:20205–20212. doi: 10.1074/jbc.M601934200. [DOI] [PubMed] [Google Scholar]
- 47.Beal AM, Anikeeva N, Varma R, Cameron TO, Norris PJ, Dustin ML, Sykulev Y. Protein kinase C theta regulates stability of the peripheral adhesion ring junction and contributes to the sensitivity of target cell lysis by CTL. J Immunol. 2008;181:4815–4824. doi: 10.4049/jimmunol.181.7.4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.