Abstract
Background
Endothelial microparticles (EMP) are membrane vesicles shed from endothelial cell in response to injury, activation or apoptosis. Kidney transplantation (KTx) is the treatment of choice for patients with end stage kidney disease (ESKD). The aim of this study was to analyze changes in EMP and serum creatinine (SCr) in patients following KTx.
Methods
Blood was periodically collected from patients before (pre-KTx) and after KTx for two months. EMP were identified as CD31+/CD42b− microparticles and quantified by fluorescence-activated cell scanning.
Results
This study included 213 KTx, 14 kidney/pancreas (KPTx) recipients and 60 healthy donors prior to donation. The recipients were divided into 5 groups based on the cause of ESKD. No differences in the quantity of circulating EMP were seen in the pre-KPTx or KTx recipient sera and healthy donor sera. Patients with ESKD secondary to diabetes mellitus, obstructive/inherited kidney disease and autoimmune disease had a decrease in both circulating EMP and SCr by day 60 after KTx.
Conclusion
Reduction in both circulating EMP and SCr was seen after kidney KTx in patients with selective ESKD.
Keywords: kidney transplantation, circulating microparticles, allograft rejection
Introduction
Annually, more than 27,000 solid organ transplants are performed in the USA, which includes more than 18,000 kidney transplants, more than 6,000 liver transplants and more than 2,000 heart transplants (1). Over the past few years, graft survival rates have improved due to more efficient immunosuppressive therapies and transplantation techniques. Thus, the population of patients with a functioning solid allograft has significantly increased. However, allograft rejection remains one of the main causes for allograft failure. Usually, monitoring of renal allograft function is evaluated by serum creatinine (SCr) levels in patients. Unfortunately, SCr level elevation is a nonspecific marker of renal allograft dysfunction, as it may occur in many different conditions. Elevated SCr is seen in acute kidney injury secondary to extrarenal etiologies (such as disturbances in systemic circulation and renal blood flow, urinary outlet obstruction) or non-rejection related causes (such as infection) (2). The “gold standard” test for the assessment of allograft rejection is renal allograft biopsy, which is an invasive, expensive and relatively risky procedure (3). Therefore, the need for a reliable and clinically significant marker of renal allograft rejection is emerging, as early detection of graft rejection is important for efficient patient care and management.
Microparticles are submicron (0.1–1 µm) membrane vesicles released from the plasma membrane during their activation, injury and (or) apoptosis (4–6). They express cell surface proteins and cytoplasmic components of their parent cell (7). Formation of microparticles is a tightly regulated process and the levels of circulating microparticles is increased in patients with vascular diseases, diabetes, infection, metabolic diseases and cancer (4, 8–10).
The pool of circulating microparticles is contributed to by several different cell types, including platelets, leukocytes and endothelial cells, where endothelium-derived microparticles (EMP) represent about 10–15% of the total microparticle population (4–6). It has been previously demonstrated that levels of circulating EMP may be used as a surrogate marker of endothelial cell dysfunction (6, 8, 11).
Kidney allograft rejection occurs via cellular, humoral or combined mechanisms. In many cases, the endothelium is the main target of the recipient immune system. We had previously demonstrated that circulating EMP levels decrease in patients following liver allograft implantation (12). There is evidence that in patients with kidney allografts, EMP also change after transplantation, but these data were obtained from a limited population of patients (13).
The aim of the current study was to investigate changes in circulating EMP and SCr levels in a large population of patients after kidney transplantation and determine whether these changes are different in patients with various underlying causes of end stage kidney disease (ESKD).
Material and Methods
Subjects
The study population consisted of consecutive patients admitted to The Ohio State University Wexner Medical Center for kidney transplantation between October 2011 and May 2013. In addition, blood samples were collected from consecutive living donors before nephrectomy and used as healthy control. The study was approved by the Institutional Review Board and all participants were considered eligible after their written informed consent. The patient population is described in the Results.
Preparation of microparticles from Plasma
Blood samples were collected from the patients into EDTA-containing tubes and processed for microparticle isolation as described earlier (9, 12). Briefly, platelet-free plasma (PFP) was obtained after an initial centrifugation at 1500g for 10 minutes followed by a second centrifugation at 1500g for 15 minutes. Samples were aliquoted and frozen at −80°C until further use.
Antibodies
The following antibodies were used: fluorescein isothiocyanate-Annexin V (FITC-Annexin) (Invitrogen, Carlsbad, CA), phycoerytherin-conjugated anti-CD31 (PE-CD31), anthocyanin-conjugated anti-CD45b (APC-CD45b) (BD Biosciences, San Jose, CA), 1µm polystyrene beads (Sigma, St. Louis, MO).
Immunolabeling and Flow Cytometry of Microparticles
Endothelial-derived microparticles were labeled in 100µl of PFP using PE-CD31 and APC-CD45b for 45 minutes at room temperature. In addition, these samples were labeled with FITC-Annexin according to the manufacturer’s protocol (18) and analyzed on a Becton-Dickinson FACScan flow cytometer (Becton-Dickinson, Franklin Lakes, NJ). Gating parameters were defined using 1µm standard polystyrene beads. Microparticles were defined using forward-scatter analysis. The time necessary for counting 10000 events was determined and microparticle concentration was calculated using the formula MP= (1000 × Num × 60)/(V × t), where MP is concentration of microparticles (mL/1); Num is number of particles passed through flow cytometer; V is volume speed (60 µL/min); and t is time (seconds), as we described earlier (11, 12, 14).
Statistics
Descriptive statistics was used to characterize patient’s demographic data. Data are presented as mean ± standard deviation, unless specified otherwise. Mixed models were applied to the data using the EMP percent change from baseline as the outcome variable and the following as potential predictor variables: baseline EMP, day, ESKD group and SCr percent change from baseline.
Results
Demographics of patients and immunosuppression
During the study period, 257 recipients of kidney or simultaneous kidney/pancreas allograft were recruited, which represents 86% of the patients who received renal allografts at The Ohio State University Wexner Medical Center (OSUWMC) within the same period of time. In addition, blood samples were obtained from 60 consecutive living donors before nephrectomy. For the final analysis, only recipients of the first renal allograft were included to avoid confounding the EMP changes that may be associated with sensitization, development of donor specific antibodies or previous immunosuppression therapy. The demographic characteristics of patients included into the final study cohort (227 patients) are provided in Table 1.
Table 1.
Demographics of patients included into the study
| # of patients |
Age, years |
Gender | Race | Allograft | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD |
Male | Female | Caucasian | African- American |
Others | CAD | LD | ||
| Group1 | 14 | 38.9±8.5 | 10 | 4 | 7 | 6 | 1 | 14 | n/a |
| Group2 | 62 | 55.8±10.2 | 35 | 27 | 38 | 20 | 4 | 30 | 32 |
| Group3 | 43 | 47.9±12.4 | 25 | 18 | 40 | 3 | 0 | 13 | 30 |
| Group4 | 18 | 42.6±14.4 | 9 | 9 | 14 | 2 | 2 | 9 | 9 |
| Group5 | 90 | 47.8±12.9 | 51 | 39 | 66 | 19 | 5 | 46 | 44 |
| Total | 227 | 49.0±12.8 | 130 | 97 | 165 | 50 | 12 | 112 | 115 |
| Healthy donors | 60 | 43.1±11.0 | 11 | 49 | 51 | 7 | 2 | n/a | n/a |
Group 1 – patients with end stage kidney disease secondary to diabetic nephropathy because of diabetes mellitus type I, who received kidney/pancreas simultaneous allografts;
Group 2 - patients with end stage kidney disease secondary to diabetic nephropathy because of diabetes mellitus type I or type II, who received kidney allograft only;
Group 3 – patients with end stage kidney disease secondary to congenital kidney disease or acquired obstructive nephropathy;
Group 4 – patients with end stage kidney disease secondary to immune-complex mediated glomerulonephritides (IgA nephropathy, membranous glomerulonephritis, lupus nephritis);
Group 5 – patients with unknown/unclassified end stage kidney disease.
CAD – cadaveric donor renal allograft; LD – living donor renal allograft.
Data are present as number of patients or mean ±SD, when applicable.
The 227 patients were divided into groups based on the cause of ESKD as following: Group 1 – patients with ESKD secondary to diabetic nephropathy because of diabetes mellitus type I, who received a simultaneous kidney/pancreas transplant; Group 2 - patients with ESKD secondary to diabetic nephropathy (diabetes mellitus type I or type II), who received kidney allograft only; Group 3 – patients with ESKD secondary to congenital causes or acquired obstructive nephropathy; Group 4 – patients with ESKD secondary to immune-complex mediated glomerulonephritides (IgA nephropathy, membranous glomerulonephritis, lupus nephritis); Group 5 – patients with unknown/unclassified ESKD.
Baseline immune suppression consisted of rabbit antithymocyte globulin (ATG) induction (1.25 mg/kg/day) with a short, 5 day course of steroid treatment. Maintenance immune suppression consisted of rapamune (Sirolimus) started on post-transplant day 0 and delayed cyclosporine (Neoral) begun on day 2 or 3 following recovery of renal function. Rapamune was dosed to achieve a target serum level of 10 ng/ml and cyclosporine was dosed to achieve a C2 (concentration 2 hours after the last dose) of 1000 ng/ml.
Rejection episodes occurred within the first year after the transplantation were included. Initial treatment of acute cellular rejection episodes consisted of steroids. ATG was administered for steroid resistant episodes. Antibody-mediated rejection or combined cellular and antibody-mediated rejection episodes were treated with combinations of ATG, steroids, IVIG, and apheresis.
Endothelial microparticles and serum creatinine levels before and after kidney transplantation
EMPs and SCr levels were analyzed in blood plasma before (baseline) and periodically at days 7, 14 and 21 after transplantation and monthly thereafter. Unfortunately, the number of follow ups beyond 2 months post-transplant was low; therefore we report herein only changes in EMP and SCr up to 2 months post-transplant. Blood samples from living donors collected before nephrectomy were used as healthy controls.
There were no significant differences between baseline EMP levels in healthy controls and patients with ESKD, even when the patients were stratified by ESKD. SCr levels were significantly higher in all ESKD patients as compared to healthy controls, regardless of the ESKD etiology.
We analyzed EMP and SCr changes from baseline in each individual patient two months post-transplant. EMP levels did not change significantly 2 month post-transplant when they were analyzed for all patients. However, when patients were stratified by ESKD etiology, there was a decrease in circulating EMP levels in patients with diabetes mellitus who received kidney allograft only (group 2), patients with obstructive/inherited isolated kidney disease (group 3) and patients with immune-complex mediated glomerulonephritides (group 4).
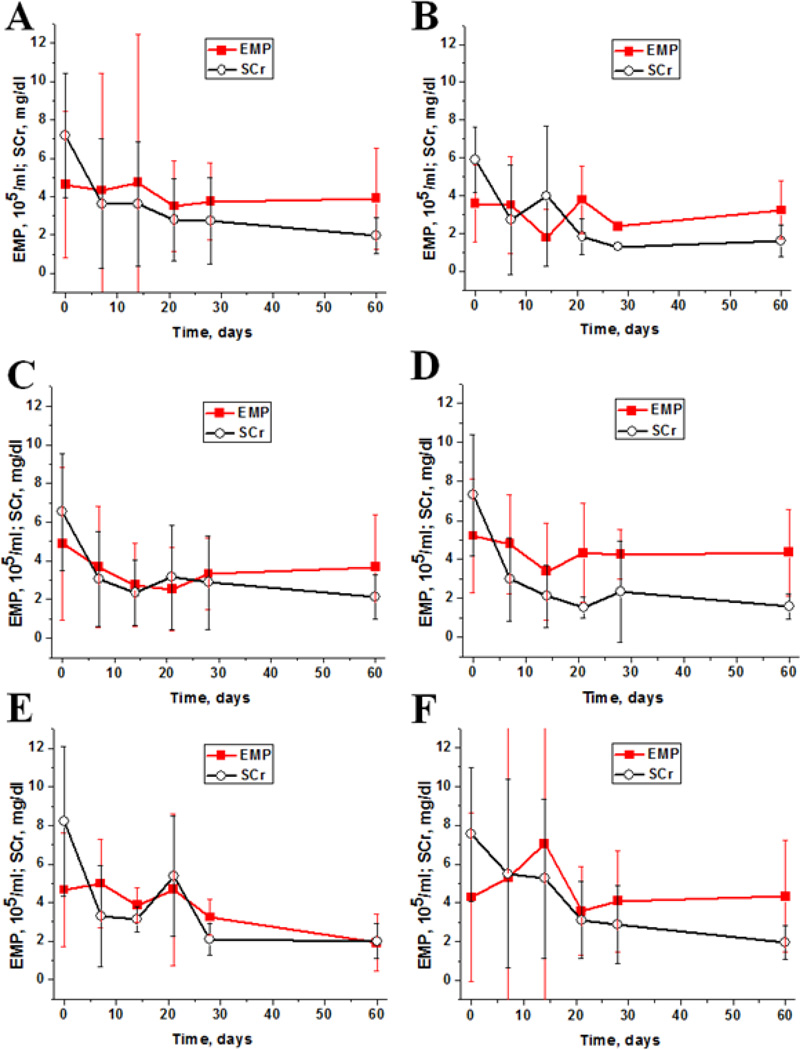
Changes in circulating EMP and SCr levels stratified by ESKD are shown in Figure 1. In all patients SCr significantly decreased after transplantation, typically by week 1. There was a decrease in circulating EMP levels in patients with ESKD secondary to diabetes mellitus (Figure 1, B and C) and obstructive/inherited isolated kidney disease (Figure 1, D) two weeks post-transplant. EMP levels steadily decreased in patients with immune-complex mediated glomerulonephritides (Figure 1, E), though these changes did not reach statistical significance due to low patient numbers. No trends in EMP posttransplant changes were seen in patients with unclassified ESKD (Figure 1, F).
Figure 1. Early posttransplant changes in endothelial microparticles and serum creatinine levels.
A - early posttransplant changes in endothelial microparticles (EMP) and serum creatinine (SCr) levels in all patients. Time 0 depicts baseline (before transplantation).
B - early posttransplant changes in EMP and SCr in patients with kidney/pancreas allografts.
C - early posttransplant changes in EMP and SCr in patients with diabetes received kidney allograft only.
D - early posttransplant changes in EMP and SCr in patients with congenital/obstructive kidney disease.
E - early posttransplant changes in EMP and SCr in patients with immune complex mediated glomerulonephritides.
F - early posttransplant changes in EMP and SCr in patients with unclassified native kidney disease.
Statistical modeling of endothelial microparticles and serum creatinine changes in patients with kidney transplantation
Mixed models were applied to the data using the EMP percent change from baseline as the outcome variable and the following as potential predictor variables: baseline EMP, day post-transplant, ESKD group and SCr percent change from baseline. Two models were run in total: 1. First 60 days not including SCr percent change from baseline as a predictor. 2. First 60 days including SCr percent change from baseline as a predictor (Table 2).
Table 2.
Mixed models analysis using the EMP percent change from baseline as the outcome variable.
| Effect | First 60 days | Including SC as predictor | ||||||
|---|---|---|---|---|---|---|---|---|
| Num DF |
Den DF |
F Value |
Pr > F | Num DF |
Den DF |
F Value |
Pr > F | |
| EMP BL | 1 | 123 | 8.56 | 0.0041 | 1 | 121 | 9.63 | 0.0024 |
| ESKD group | 4 | 123 | 4.19 | 0.0033 | 4 | 121 | 4.45 | 0.0022 |
| Day posttransplant | 4 | 123 | 0.51 | 0.7279 | 4 | 121 | 0.39 | 0.8138 |
| EMP BL*ESKD | 4 | 123 | 4.43 | 0.0022 | 4 | 121 | 4.71 | 0.0015 |
| SCr percent change | n/a | n/a | n/a | n/a | 1 | 121 | 2.72 | 0.1020 |
Num DF - the degrees of freedom of the numerator
Den DF - the degrees of freedom of the denominator
F Value - folded F-statistic, F' = max(s12,s22)/min(s12,s22)
Pr > F - the two-tailed significance probability
EMP – endothelial microparticles
BL – baseline (before the transplantation) levels
ESKD – end stage kidney disease
SCr – serum creatinine
The results of this modeling point out that neither day post-transplant nor SCr percent change from baseline are significant in either model, indicating that the time and SCr percent change are not significant predictors of EMP percent change. However, baseline EMP and ESKD group were significant predictors in all models. The interaction between the baseline EMP levels and ESKD group was found to be significant, indicating that the relationship between EMP baseline levels and the EMP percent change differ by ESKD group.
Endothelial microparticle changes associated with acute rejection
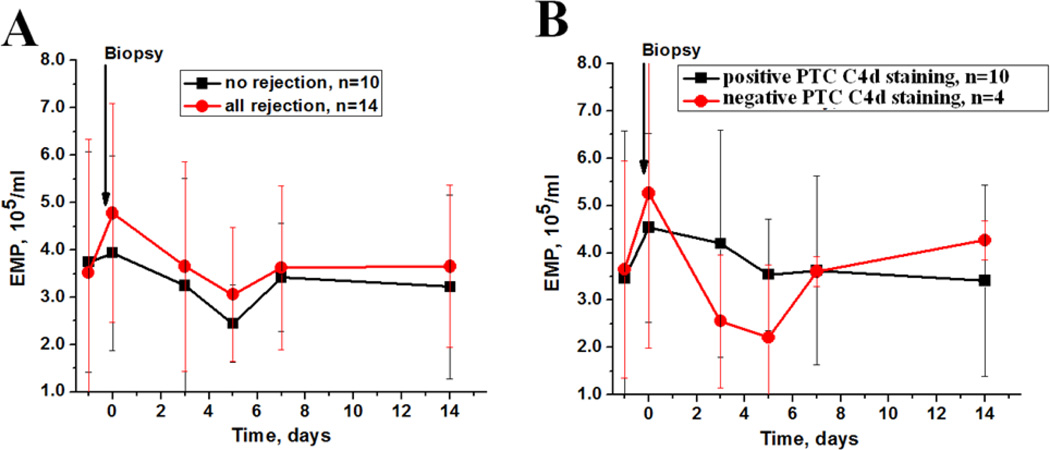
During the study period, 24 (11%) patients had indication kidney allograft biopsies. Fourteen (58%) kidney allograft biopsies showed morphologic features of acute cellular rejection (4 with negative peritubular capillary (PTC) C4d staining and 10 with positive PTC C4d staining) and ten biopsies had other morphologic findings not associated with acute rejection, mainly acute tubular necrosis or suggestive of acute pyelonephritis.
Analysis of EMP changes associated with the allograft biopsy revealed that there is an increase in circulating EMP levels associated with rejection, whereas in patients with no rejection the levels of circulating EMP did not change (Figure 2, A). When we stratified patients by the PTC C4d staining, we found that after treatment for rejection, circulating EMP were rapidly decreased in patients with negative PTC C4d, but circulating EMP levels decrease was slower in patients with positive PTC C4d (Figure 2, B).
Figure 2. Changes in endothelial microparticles in patients with allograft dysfunction who underwent a kidney allograft biopsy.
A – changes in endothelial microparticles (EMP) in patients with allograft dysfunction who underwent kidney allograft biopsy with (solid circle) and without (solid square) histological features of acute rejection. Biopsy time is show by an arrow.
B - changes in EMP in patients with acute rejection stratified by the peritubular capillaries (PTC) C4d staining. Biopsy time is show by an arrow.
Discussion
This is the first study analyzing early post-transplant changes in circulating EMP levels after kidney transplantation in a large population of patients. Long-term changes in circulating microparticles levels in patients with kidney transplantation were previously reported in a smaller patient population (15, 16).
Recent evidence indicates increased levels of circulating EMP in patients with different cardiovascular diseases, including atherosclerosis, coronary artery diseases, systemic and pulmonary hypertension (17–20). The levels of endothelium-derived microparticles were altered in patients after a cardiovascular surgery, including cardiopulmonary bypass (21) and heart transplantation (22). In kidney transplant recipients, levels of circulating EMP were significantly decreased one year after transplantation, as compared to pretransplant values in the same patients. Interestingly, patients on cyclosporine and azathioprine immunosuppressive regimen had lower EMP levels post-transplant, as compared to patients who were on tacrolimus and mycophenolate immunosuppressive treatment (16).
In our study, we describe not only early post-transplant changes in circulating EMP, but changes in patients with different causes of ESKD. ESKD has varying etiologies and pathogenesis, and it may be inappropriate to study all diseases in one group. Indeed, when we analyzed EMP changes in general or in patients with unknown ESKD, there were no observed changes in circulating EMP levels. However, when we stratified patients by the cause of ESKD, we found that in certain ESKD cohorts there is a trend toward a decrease in posttransplant circulating EMP. Indeed, using mixed model analyses, the ESKD group was a significant determinant of EMP changes. Unfortunately, follow up data were available for a limited number of patients, but even these limited data shows differences between patients with different ESKD.
Of note, in our study there was no difference in circulating EMP levels between healthy controls and patients with ESKD. It is an unexpected finding, which is difficult to explain. However, a possible contributing factor may be that the recipients of kidney allograft were on dialysis, which may affect circulating microparticles (23).
We found that there are different EMP changes in patients with kidney allograft dysfunction. Thus, in patients with kidney allograft dysfunction not related to acute rejection, circulating EMP levels did not change, but in patients with biopsy proven acute rejection the number of circulating EMP associated with rejection was elevated (Figure 2, A). When we stratified patients by PTC C4d staining, we found that in patients with negative PTC C4d circulating EMP levels decreased faster than in patients with positive PTC C4d (Figure 2, B). This finding indicates endothelial cell injury or activation, which is reflected by circulating EMP changes.
Conclusions
Our data indicate that there is a decrease in circulating EMP levels after kidney transplantation in patients with selective ESKD. Circulating EMP change differently in patients with positive and negative PTC C4d staining on kidney allograft biopsy, suggesting that circulating EMP levels reflect endothelial cell injury.
Highlights.
-
-
We analyzed changes in endothelial microparticles (EMP) after kidney transplantation
-
-
EMP change after kidney transplantation in selected patient population
-
-
EMP show different dynamics in peritubular capillary C4d positive or negative biopsies
Acknowledgment
This study was supported by the NIDDK R21 grant DK090587 to SVB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Annual Data Report of the US Organ Procurement and Transplantation Network (OPTN) and the Scientific Registry of Transplant Recipients (SRTR) Introduction. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13(Suppl 1):8. doi: 10.1111/ajt.12018. [DOI] [PubMed] [Google Scholar]
- 2.de Fijter JW. Rejection and function and chronic allograft dysfunction. Kidney international Supplement. 2010;(119):S38. doi: 10.1038/ki.2010.421. [DOI] [PubMed] [Google Scholar]
- 3.Williams WW, Taheri D, Tolkoff-Rubin N, Colvin RB. Clinical role of the renal transplant biopsy. Nature reviews Nephrology. 2012;8(2):110. doi: 10.1038/nrneph.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burger D, Schock S, Thompson CS, Montezano AC, Hakim AM, Touyz RM. Microparticles: biomarkers and beyond. Clinical science. 2013;124(7):423. doi: 10.1042/CS20120309. [DOI] [PubMed] [Google Scholar]
- 5.Burger D, Touyz RM. Cellular biomarkers of endothelial health: microparticles, endothelial progenitor cells, and circulating endothelial cells. Journal of the American Society of Hypertension : JASH. 2012;6(2):85. doi: 10.1016/j.jash.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Brodsky SV, Satoskar A, Nadasdy T. Endothelial microparticles in transplant patients - great potential but a long way to go. Frontiers in bioscience. 2012;4:876. doi: 10.2741/E426. [DOI] [PubMed] [Google Scholar]
- 7.Tesse A, Martinez MC, Meziani F, Hugel B, Panaro MA, Mitolo V, et al. Origin and biological significance of shed-membrane microparticles. Endocrine, metabolic & immune disorders drug targets. 2006;6(3):287. doi: 10.2174/187153006778249976. [DOI] [PubMed] [Google Scholar]
- 8.Brodsky SV, Gealekman O, Chen J, Zhang F, Togashi N, Crabtree M, et al. Prevention and reversal of premature endothelial cell senescence and vasculopathy in obesity-induced diabetes by ebselen. Circulation research. 2004;94(3):377. doi: 10.1161/01.RES.0000111802.09964.EF. [DOI] [PubMed] [Google Scholar]
- 9.Augustine D, Ayers LV, Lima E, Newton L, Lewandowski AJ, Davis EF, et al. Dynamic Release and Clearance of Circulating Microparticles During Cardiac Stress. Circulation research. 2013 doi: 10.1161/CIRCRESAHA.114.301904. [DOI] [PubMed] [Google Scholar]
- 10.Lovren F, Verma S. Evolving role of microparticles in the pathophysiology of endothelial dysfunction. Clinical chemistry. 2013;59(8):1166. doi: 10.1373/clinchem.2012.199711. [DOI] [PubMed] [Google Scholar]
- 11.Brodsky SV, Zhang F, Nasjletti A, Goligorsky MS. Endothelium-derived microparticles impair endothelial function in vitro. American journal of physiology Heart and circulatory physiology. 2004;286(5):H1910. doi: 10.1152/ajpheart.01172.2003. [DOI] [PubMed] [Google Scholar]
- 12.Brodsky SV, Facciuto ME, Heydt D, Chen J, Islam HK, Kajstura M, et al. Dynamics of circulating microparticles in liver transplant patients. Journal of gastrointestinal and liver diseases : JGLD. 2008;17(3):261. [PubMed] [Google Scholar]
- 13.Bakouboula B, Morel O, Faller AL, Freyssinet JM, Toti F. Significance of membrane microparticles in solid graft and cellular transplantation. Front Biosci (Landmark Ed) 2011;16:2499. doi: 10.2741/3868. [DOI] [PubMed] [Google Scholar]
- 14.Brodsky SV, Malinowski K, Golightly M, Jesty J, Goligorsky MS. Plasminogen activator inhibitor-1 promotes formation of endothelial microparticles with procoagulant potential. Circulation. 2002;106(18):2372. doi: 10.1161/01.cir.0000033972.90653.af. [DOI] [PubMed] [Google Scholar]
- 15.Al-Massarani G, Vacher-Coponat H, Paul P, Arnaud L, Loundou A, Robert S, et al. Kidney transplantation decreases the level and procoagulant activity of circulating microparticles. American journal of transplantation. 2009;9(3):550. doi: 10.1111/j.1600-6143.2008.02532.x. [DOI] [PubMed] [Google Scholar]
- 16.Al-Massarani G, Vacher-Coponat H, Paul P, Widemann A, Arnaud L, Loundou A, et al. Impact of immunosuppressive treatment on endothelial biomarkers after kidney transplantation. American journal of transplantation. 2008;8(11):2360. doi: 10.1111/j.1600-6143.2008.02399.x. [DOI] [PubMed] [Google Scholar]
- 17.Boulanger CM, Amabile N, Tedgui A. Circulating microparticles: a potential prognostic marker for atherosclerotic vascular disease. Hypertension. 2006;48(2):180. doi: 10.1161/01.HYP.0000231507.00962.b5. [DOI] [PubMed] [Google Scholar]
- 18.Mallat Z, Hugel B, Ohan J, Leseche G, Freyssinet JM, Tedgui A. Shed membrane microparticles with procoagulant potential in human atherosclerotic plaques: a role for apoptosis in plaque thrombogenicity. Circulation. 1999;99(3):348. doi: 10.1161/01.cir.99.3.348. [DOI] [PubMed] [Google Scholar]
- 19.Bernal-Mizrachi L, Jy W, Fierro C, Macdonough R, Velazques HA, Purow J, et al. Endothelial microparticles correlate with high-risk angiographic lesions in acute coronary syndromes. International journal of cardiology. 2004;97(3):439. doi: 10.1016/j.ijcard.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 20.Bernal-Mizrachi L, Jy W, Jimenez JJ, Pastor J, Mauro LM, Horstman LL, et al. High levels of circulating endothelial microparticles in patients with acute coronary syndromes. American heart journal. 2003;145(6):962. doi: 10.1016/S0002-8703(03)00103-0. [DOI] [PubMed] [Google Scholar]
- 21.Nieuwland R, Berckmans RJ, Rotteveel-Eijkman RC, Maquelin KN, Roozendaal KJ, Jansen PG, et al. Cell-derived microparticles generated in patients during cardiopulmonary bypass are highly procoagulant. Circulation. 1997;96(10):3534. doi: 10.1161/01.cir.96.10.3534. [DOI] [PubMed] [Google Scholar]
- 22.Garcia S, Chirinos J, Jimenez J, Del Carpio Munoz F, Canoniero M, Jy W, et al. Phenotypic assessment of endothelial microparticles in patients with heart failure and after heart transplantation: switch from cell activation to apoptosis. The Journal of heart and lung transplantation. 2005;24(12):2184. doi: 10.1016/j.healun.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Daniel L, Fakhouri F, Joly D, Mouthon L, Nusbaum P, Grunfeld JP, et al. Increase of circulating neutrophil and platelet microparticles during acute vasculitis and hemodialysis. Kidney international. 2006;69(8):1416. doi: 10.1038/sj.ki.5000306. [DOI] [PubMed] [Google Scholar]