Abstract
A growing body of evidence suggests that in utero and early-life exposure to arsenic may have detrimental effects on children, even at the low to moderate levels common in the United States and elsewhere. In a sample of 170 mother–infant pairs from New Hampshire, we determined infant exposure to in utero arsenic by evaluating infant toenails as a biomarker using inductively coupled plasma mass spectrometry. Infant toenail arsenic concentration correlated with maternal postpartum toenail concentrations (Spearman’s correlation coefficient 0.34). In adjusted linear models, a doubling of maternal toenail arsenic concentration was associated with a 53.8% increase in infant toenail arsenic concentration as compared with 20.4% for a doubling of maternal urine arsenic concentration. In a structural equation model, a doubling of the latent variable integrating maternal toenail and urine arsenic concentrations was associated with a 67.5% increase in infant toenail arsenic concentration. A similar correlation between infant and maternal postpartum toenail concentrations was observed in a validation cohort of 130 mother–infant pairs from Rhode Island. In utero exposure to arsenic occurs through maternal water and dietary sources, and infant toenails appear to be a reliable biomarker for estimating arsenic exposure during the critical window of gestation.
Keywords: biological markers, biomarkers, arsenic, prenatal exposure
INTRODUCTION
Arsenic is a ubiquitous metalloid that is found in both inorganic (iAs) and organic forms and can readily cross the placental barrier and appear in fetal tissue.1,2 Early-life exposure to arsenic has been associated with an increase in cancer, cardiovascular, and all-cause mortality3 and other health changes later in life including neurobehavioral effects.4-9 As gestation is a period of rapid cellular differentiation and proliferation, the fetus may be particularly vulnerable to the effects of environmental toxicants.10,11 In utero arsenic exposure has been associated with adverse health events such as low birth weight, increased risk of infection and diarrheal disease, and higher infant mortality.12-18
Inorganic arsenic species, including arsenate (AsV+) and arsenite (AsIII+), accumulate in keratin-rich tissues of the integumentary system, and thus toenails can serve as a biomarker of internal dose19 for up to 6–12 months in adults.20,21 Beginning at ~10 weeks of gestation, human nails develop in utero, and toenails at birth range from 3.2 to 5.7 mm in length.22 In general, the growth rate of nails is accelerated in early life and only begins to slow around 10 years of age.23 Therefore, infant toenails collected at birth likely estimate iAs exposure during the entire window of gestation as opposed to short-term measures of arsenic exposure, such as blood and urine, that reflect hours or days since exposure.24 From a practical perspective, toenails have the added advantage of being minimally invasive to collect and inexpensive to store.
Prior studies that used infant toenails as a biomarker of in utero exposure were conducted in highly exposed populations.17,25,26 Therefore, in a sample of US mother-infant pairs exposed to relatively low levels of arsenic, we examined the reliability of infant toenails as a biomarker of in utero exposure and evaluated whether maternal exposure to water and food (particularly rice and rice products)27,28 influenced infant toenail concentration.
MATERIALS AND METHODS
The study protocols for the New Hampshire Birth Cohort Study (NHBCS) and the Rhode Island Child Health Study (RICHS) were approved by the Committee for the Protection of Human Subjects at Dartmouth College and by the Institutional Review Boards for Women and Infants’ Hospital and Brown University respectively. All study participants from both cohorts provided written informed consent.
Sample Collection
The NHBCS is an ongoing prospective study that began in 2009 and includes over 1000 women from New Hampshire between the ages of 18 and 45 years, with a singleton pregnancy, and who report having a private well as their primary home water source. During enrollment at a study clinic (typically at 24–28 weeks of gestation), study participants provided a spot urine sample and completed a prenatal questionnaire that collects information about their pregnancy, including the estimated amount of home tap water consumed daily and a 3-day dietary recall questionnaire that specifically asks for the number of eight-ounce cups of cooked rice and rice cereals consumed daily. Participants were also provided with a kit to collect a home drinking water sample using a commercially washed, high-density polyethylene bottle that meets the Environmental Protection Agency’s standards for water collection. Urine and water samples were frozen at −20 °C until analysis. At 2 weeks postpartum, an information packet was mailed to study participants requesting maternal and infant toenail clipping samples within 8 weeks of birth; toenails were stored at room temperature until analysis.
To validate our primary association of interest (infant and maternal toenail arsenic concentration), we also examined the association between infant and maternal toenail arsenic concentration in 130 mother-infant pairs from the RICHS, which utilized similar toenail collection procedures as the NHBCS.29 More than 90% of participants in the RICHS use public water sources (as a selection criteria, all NHBCS participants use private water sources) and therefore exposure to arsenic was presumably lower in the RICHS than the NHBCS. Study participants in the RICHS were older (73.1% older than 30 years in RICHS compared with 52.4% in the NHBCS) and more likely to be obese (23.8% in RICHS compared with 17.1% in the NHBCS). By design, RICHS oversampled both low and high birth weight babies, and thus had a higher proportion of infants who were low birth weight (6.9% were <2500 g in RICHS compared with 2.3% in the NHBCS).
Trace Element Analysis
Infant toenail samples were collected from NHBCS participants in prelabeled collection vials. Upon analysis, samples were weighed and digested in Optima nitric acid (Fisher Scientific, St. Louis, MO, USA) by low-pressure microwave digestion at the Trace Element Analysis (TEA) Core Laboratory (Dartmouth College, Hanover, NH, USA).30 After digestion, the final sample weight was recorded and samples were then analyzed for total arsenic, measured in μg total arsenic per g toenail (μg/g), using inductively coupled plasma mass spectrometry (ICPMS) on an Agilent 7700× (Agilent Technologies Headquarters, Santa Clara, CA, USA). Arsenic was detected in all but one infant toenail sample. Among samples with detectable levels, infant toenail arsenic concentration ranged from 0.001 to 1.21 μg/g.
Maternal toenail samples were collected in paper envelopes, and analysis entailed an additional washing procedure that included the manual removal of any visible dirt and five washes in an ultrasonic bath using Triton X-100 (LabChem, Pittsburgh, PA, USA) and acetone followed by deionized water. Toenails were then dried before low-pressure microwave digestion and ICPMS analysis. Arsenic was detected in all maternal toenails and ranged from 0.001 to 0.41 μg/g.
Maternal urine samples were analyzed at the University of Arizona within 1 month of collection. Samples were analyzed for individual species of arsenic using a combination of high-performance liquid chromatography (HPLC) and ICPMS that is able to detect five arsenic species, including arsenate, arsenite, monomethylarsonic acid (MMA), dimethylarsinic acid (DMA), and arsenobetaine.31 As unmetabolized arsenobetaine is believed to be non-toxic, we estimated total arsenic by summing arsenate, arsenite, MMA, and DMA.27,32 Detection limits for the four individual arsenic species ranged from 0.10 to 0.15 μg/l and 158, 59, 29, and 0 of the 170 samples for arsenate, arsenite, MMA, and DMA, respectively, were below the detection limit. For samples below the detection limit, we assigned a value equal to the detection limit divided by 2. The Cayman’s Creatinine Assay Kit (Cayman Chemical Company, Ann Arbor, MI, USA) was used to determine urinary creatinine.
Tap water samples were analyzed at Dartmouth’s TEA core for total arsenic concentration also by ICPMS on an Agilent 7700× (Agilent Technologies Headquarters).27 The detection limit for arsenic in tap water samples ranged between 0.009 and 0.074 μg/l, and 96% of samples were above the detection limit.
Statistical Analysis
We used several different approaches to evaluate the reliability of infant toenail arsenic concentrations as a biomarker of in utero exposure. First, we used Spearman’s correlation coefficients to explore relationships between maternal exposure variables and infant toenail arsenic concentration. Next, we examined univariate relationships between characteristics collected by the NHBCS and infant toenail arsenic concentration using analysis of variance (ANOVA) on geometric means to identify candidate covariates for our final models; we then used linear models to adjust for confounding factors.
To improve model fit and normalize residuals, we log10-transformed arsenic biomarker variables. Exponentiated coefficients from our models therefore represent the percent change in infant toenail arsenic concentration based on an increase in the respective maternal exposure variable.33 More specifically, in models where both dependent and independent variables were log10-transformed, 2β represents the percent change in the dependent variable based on a relative doubling of the independent variable. Finally, we examined potential non-linear relationships between our dependent and independent variables using LoWeSS (locally weighted scatterplot smoothing). Study participants with missing covariate data were excluded in our final regression models. Our final regression model with maternal toenail concentration as an independent variable included both infant and maternal season of toenail collection, and our final model with maternal urine concentration as an independent variable included creatinine concentration to account of urinary dilution. Estimates of maternal arsenic intake (both from water and rice) were included in a single model.
Measurements of infant toenail arsenic concentration considered laboratory imprecision. Because infant toenails were digested in the collection vials and sample weight was determined by subtracting the pre- and post-digestion vial weights, some samples had a negative sample weight because of imprecision in measurements. To account for this, we subtracted the largest negative sample weight from all samples and examined vial batch as a fixed effect in our initial linear models. As we found no evidence of confounding by vial batch, we excluded this effect from our final models.
Metabolism of iAs differs among individuals and the relative concentration of urinary arsenic species can be used to estimate arsenic methylation capacity.34 Therefore, we examined maternal arsenic metabolism (as measured by relative urinary arsenic species) as a potential effect modifier in the relationship between infant and maternal toenail arsenic concentration. To do so, we identified mothers above the median for iAs/total arsenic, MMA/total arsenic, and DMA/total arsenic proportions, and for MMA to iAs ratio (primary methylation index) and DMA to MMA ratio (secondary methylation index). Interaction terms were tested in linear models between these and maternal toenail arsenic concentration.
We also evaluated maternal water and rice consumption as potential contributors to arsenic exposure. For water, we calculated μg of arsenic consumed per day by multiplying the mothers’ home tap water arsenic concentration (μg/l) by the daily water consumption reported in the prenatal questionnaire. Self-report of water consumption in a questionnaire has been shown to be a valid assessment of water consumption in other populations—for instance, Spearman’s correlation coefficient between water consumption reported in a food frequency questionnaire compared with dietary recalls is 0.52 among women.35 As previous studies have done, we calculated rice consumption from the most recent 2 days by converting cups of cooked rice or rice cereal to g of dry rice consumed daily.27
Finally, as we had multiple correlated measures of maternal arsenic exposure (i.e., toenails, urine, and arsenic from water consumption),26 we combined the maternal arsenic biomarker variables into a latent variable using structural equation modeling (SEM). We then explored including estimated arsenic from water and rice consumption in the SEM model and evaluated the relationship between the latent variable for maternal arsenic biomarkers and infant toenail arsenic concentration.
All analyses were performed using Stata version 12.1 statistical software (StataCorp, College Station, TX, USA).
RESULTS
Infant toenail arsenic concentration in the NHBCS ranged from 0.000 to 1.21 μg/g with a median of 0.06 μg/g (interquartile range (IQR): 0.03–0.11) and maternal toenail arsenic concentration ranged from 0.001 to 0.41 μg/g with a median of 0.06 μg/g (IQR: 0.04–0.09). Maternal urinary total arsenic (excluding arsenobetaine) ranged from 0.45 to 58.3 μg/l with a median of 3.88 μg/l (IQR: 1.77–7.03).
Infant and maternal toenail arsenic concentrations in the NHBCS differed little across select characteristics (Table 1). However, for both infant and maternal toenails, specimens varied by season of collection (ANOVA P-value <0.01 to 0.02); consequently, our models included season of toenail collection as covariates.
Table 1.
Geometric mean of infant and maternal toenail arsenic concentration according to characteristics collected by the New Hampshire Birth Cohort Study.
|
Geometric mean (SD) toenail arsenic concentration, mg/g
|
|||
|---|---|---|---|
| No. (%) | Infant | Maternal | |
| Infant characteristics | |||
| Sex | |||
| Male | 72 (42.4) | 0.06 (2.49) | — |
| Female | 87 (51.2) | 0.07 (2.64) | — |
| Unknown | 11 (6.4) | 0.09 (2.18) | — |
| Breast feeding status | |||
| Yes | 131 (77.0) | 0.06 (2.63) | — |
| No | 30 (17.6) | 0.08 (2.20) | — |
| Unknown | 9 (5.3) | 0.08 (2.17) | — |
| Age at toenail collection | |||
| < 10 Weeks | 112 (65.9) | 0.07 (2.62) | — |
| ≥ 10 Weeks | 58 (34.1) | 0.07 (2.41) | — |
| Birth weight | |||
| Low weight, < 2500 g | 4 (2.3) | 0.06 (1.75) | — |
| Normal weight, ≥2500g | 155 (91.2) | 0.06 (2.58) | — |
| Unknown | 11 (6.5) | 0.09 (2.18) | — |
| Season of toenail collection | ** | ||
| Spring | 48 (28.2) | 0.08 (2.06) | — |
| Summer | 34 (20.0) | 0.10 (2.92) | — |
| Fall | 35 (20.6) | 0.06 (2.10) | — |
| Winter | 53 (31.2) | 0.05 (2.77) | — |
| Maternal characteristics | |||
| Age at conception | |||
| <30 Years | 65 (38.2) | 0.06 (2.51) | 0.06 (2.05) |
| ≥30 Years | 89 (52.4) | 0.07 (2.49) | 0.06 (2.11) |
| Unknown | 16 (9.4) | 0.06 (3.03) | 0.06 (2.26) |
| Prepregnancy BMI, kg/m2 | |||
| Normal weight, <25.0 | 94 (55.3) | 0.07 (2.56) | 0.06 (2.18) |
| Overweight, ≥25.0 to<30.0 | 39 (22.9) | 0.06 (2.64) | 0.06 (2.17) |
| Obese, Z30.0 | 29 (17.1) | 0.05 (2.32) | 0.05 (1.79) |
| Unknown | 8 (4.7) | 0.05 (2.47) | 0.08 (1.49) |
| Smoking status | |||
| Smoker | 7 (4.1) | 0.08 (2.73) | 0.10 (2.07) |
| Non-smoker | 148 (87.1) | 0.06 (2.55) | 0.06 (1.96) |
| Unknown | 15 (8.8) | 0.08 (2.48) | 0.05 (3.53) |
| Parity | |||
| First live birth | 61 (35.9) | 0.06 (2.47) | 0.05 (1.97) |
| 1 Or more live births | 105 (61.8) | 0.07 (2.56) | 0.07 (2.15) |
| Unknown | 4 (2.4) | 0.06 (2.99) | 0.07 (2.15) |
| Gestational diabetes | |||
| Yes | 7 (4.1) | 0.04 (2.57) | 0.05 (2.00) |
| No | 163 (95.9) | 0.07 (2.53) | 0.06 (2.10) |
| Season of toenail collection | * | ||
| Spring | 50 (29.4) | — | 0.05 (2.47) |
| Summer | 25 (14.7) | — | 0.06 (1.64) |
| Fall | 51 (30.0) | — | 0.07 (1.80) |
| Winter | 44 (25.9) | — | 0.07 (2.09) |
| Mean daily energy intake | |||
| <2000 kcal | 86 (50.6) | 0.08 (2.48) | 0.07 (2.07) |
| ≥ 2000 kcal | 70 (41.2) | 0.05 (2.42) | 0.05 (2.15) |
| Unknown | 14 (8.2) | 0.08 (3.22) | 0.07 (1.74) |
| Rice consumption | * | ||
| Yes | 36 (21.2) | 0.09 (2.41) | 0.06 (2.35) |
| No | 134 (78.8) | 0.06 (2.53) | 0.06 (2.03) |
| Arsenic from water | ** | ** | |
| < 10 μg/day | 126 (74.1) | 0.06 (2.33) | 0.05 (1.93) |
| ≥ 10 μg/day | 29 (17.1) | 0.11 (2.72) | 0.13 (1.93) |
| Unknown | 15 (8.8) | 0.08 (3.10) | 0.07 (1.71) |
Abbreviation: BMI, body mass index.
P<0.05;
P<0.01. Analysis of variance (ANOVA) was used to compare geometric means across categories.
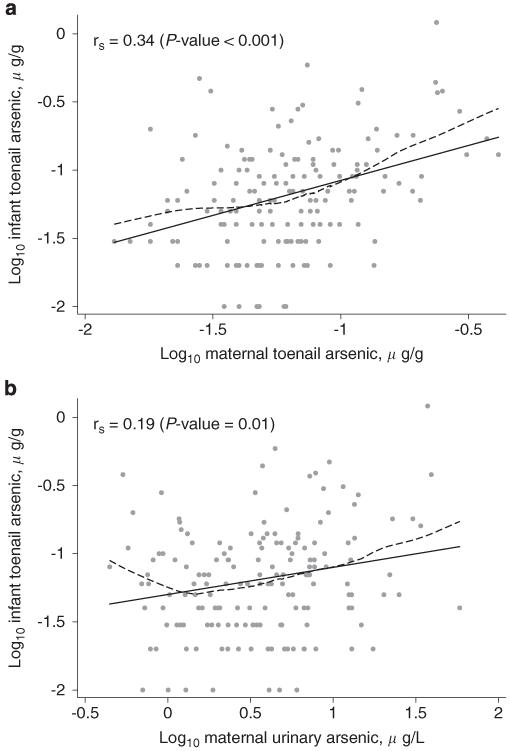
Infant toenail arsenic concentration was correlated with both maternal toenail arsenic concentration and maternal urinary arsenic, and the correlation was stronger with maternal toenail arsenic concentration (Figure 1). A similar correlation between infant and maternal toenail arsenic concentration was observed for 130 mother–infant pairs in the RICHS (rs = 0.29, P-value <0.001).
Figure 1.
The relationship between log10-transformed infant toenail arsenic concentration and maternal biomarkers including log10-transformed maternal toenail arsenic concentration (a) and log10-transformed maternal urinary arsenic concentration (b). In both panels, the rs represents Spearman’s correlation coefficient, the black line represents least-squares regression, and the dashed line represents LoWeSS (locally weighted scatterplot smoothing) moving average fitted curve.
In the NHBCS, the relationship between infant and maternal toenail arsenic concentration differed somewhat by the relative concentration of urinary arsenic species. Among mothers above the median ratio for urinary DMA to MMA, Spearman’s correlation coefficient was 0.29 (P-value <0.001) compared with 0.36 (P-value <0.01) for those below the median.
In a linear model adjusted for infant and maternal season of toenail collection, a doubling of maternal toenail arsenic concentration was associated with a 53.8% (95% confidence interval (CI): 33.9–76.7) increase in infant toenail arsenic concentration (Table 2). Similarly, in models adjusted for urinary creatinine concentration,36 a doubling of total urinary arsenic concentration was associated with a 20.4% (95% CI: 2.7–41.1) increase.
Table 2.
Change in infant toenail arsenic concentration in relation to maternal arsenic exposure variables.
| Exposure | No. | Percent change in infant toenail arsenic concentration (95% CI) |
|---|---|---|
| Maternal toenail arsenic | 162 | |
| Toenail arsenic, μg/ga | 53.8% (33.9, 76.7) | |
| Season of maternal toenail collection | ||
| Spring | 0.0% (reference) | |
| Summer | − 12.6% (− 46.9, 43.9) | |
| Fall | − 20.6% (− 54.5, 38.8) | |
| Winter | − 17.1% (− 48.9, 34.5) | |
| Season of infant toenail collection | ||
| Spring | 0.0% (reference) | |
| Summer | 36.7% (− 12.1, 112.6) | |
| Fall | − 14.5% (− 51.7, 51.1) | |
| Winter | − 37.9% (− 62.2, 1.9) | |
| Maternal urinary arsenic | 143 | |
| Total urinary arsenic, μg/la,b | 20.4% (2.7, 41.1) | |
| Urinary creatinine, mg/dl | − 0.2% ( − 0.7, 0.3) | |
| Maternal arsenic intake variables | 149 | |
| Arsenic from water, 10 μg/dayc | 14.5% (5.7, 23.9) | |
| Rice consumption, 0.25 cooked cup/day | 16.9% (1.7, 34.4) |
Abbreviation: CI, confidence interval.
All coefficients have been exponentiated and represent the change in infant toenail arsenic concentration.
Increase based on a doubling of the exposure.
Total urinary arsenic excludes arsenobetaine.
Estimated by total arsenic concentration of tap water and self-reported amount of daily consumption.
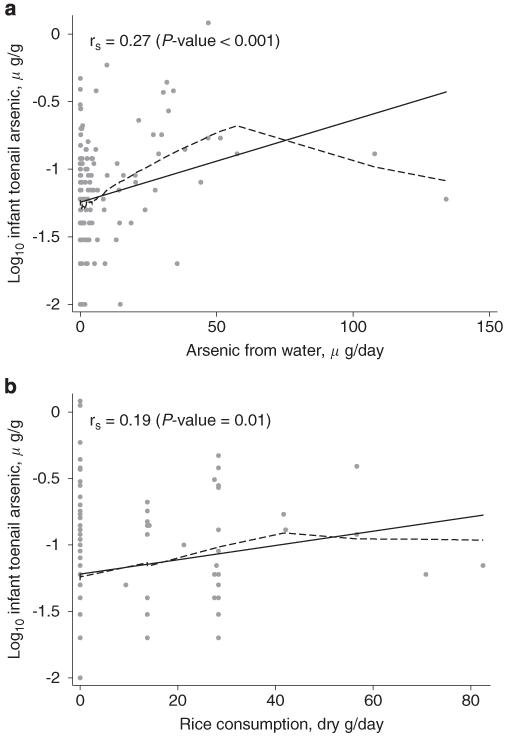
Both arsenic from water and rice intake were related to infant toenail arsenic concentration (Table 2 and Figure 2). Adjusted for rice consumption, a 10 μg/day increase in arsenic from water consumption was associated with a 14.5% (95% CI: 5.7–23.9) increase in infant toenail arsenic concentration. However, adjusted for arsenic intake from water, an increase of 0.25 cooked cup/day of rice consumption was associated with a 16.9% (95% CI: 1.7–34.4) increase in infant toenail arsenic concentration.
Figure 2.
The relationship between log10-transformed infant toenail arsenic concentration and maternal arsenic intake variables including arsenic from water (a) and rice consumption (b). In both panels, the rs represents Spearman’s correlation coefficient, the black line represents least-squares regression, and the dashed line represents LoWeSS (locally weighted scatterplot smoothing) moving average fitted curve.
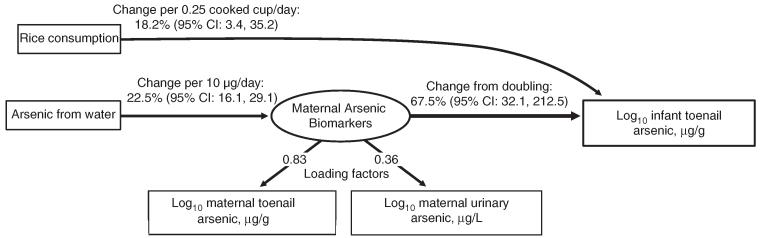
Our SEM including maternal arsenic biomarkers (toenail and urine concentrations as a latent variable) and arsenic intake variables (arsenic from water and rice consumption) exhibited an excellent fit with the data (likelihood ratio P-value comparing our model with a fully saturated model = 0.48; Figure 3). A doubling of the latent maternal arsenic biomarker variable was associated with a 67.5% (95% CI: 32.1–212.5) increase in infant toenail arsenic concentration. Rice consumption was related through a direct pathway from maternal ingestion. In our model, 0.25 cups/day of cooked rice was associated with an 18.2% (95% CI: 3.4–35.2) increase in infant toenail arsenic concentration.
Figure 3.
Structural equation model based on observed maternal biomarkers and arsenic intake variables. The latent maternal arsenic biomarkers variable includes both log10-transformed maternal toenail arsenic concentration and log10-transformed maternal urinary arsenic concentration as loading factors. Effect estimates represent the change based on the respective unit increase in the corresponding independent variable. The P-value of the likelihood ratio comparing the above model with a fully saturated model is 0.48, the root mean squared error approximation is 0.00, the comparative fit index is 1.00, and Tucker–Lewis index is 1.01.
DISCUSSION
In this US population exposed to relatively low levels of arsenic, infant toenail arsenic concentrations were correlated with two maternal biomarkers—toenails and urine—but more strongly with toenail concentrations. The stronger correlation is likely because maternal arsenic toenail concentration represents a more integrated, longer-term biomarker. These data suggest that infant toenails can be used as a reliable biomarker to estimate in utero exposure. Moreover, maternal arsenic intake from water and diet was directly related to infant exposure, as measured by infant toenail arsenic concentration. This finding has important implications in understanding the sources of arsenic exposure.
Although prior studies have used a wide variety of biomarkers to estimate in utero exposure to arsenic,8,17,19,25,26,37-41 few have evaluated the use of infant toenails. These are confined to highly exposed regions of the world such as Bangladesh and West Bengal.17,25,26 The findings from our study, conducted in a larger US study population, and confirmed in an independent cohort, are consistent with those from more highly exposed populations. Indeed, they suggest that the association between maternal exposure and infant toenail arsenic may be even stronger. For example, the sole prior study, to our knowledge, comparing infant and maternal toenail arsenic concentration reported Pearson’s correlation coefficient of 0.24 (n = 52, P-value 0.12).17 Potentially because of seasonal dietary patterns, we also found = that season of toenail collection is a factor that should be used for adjustment in statistical models, consistent with previous studies of adult populations exposed to low levels of arsenic.42 In addition to being a reliable biomarker of in utero exposure in populations exposed to low levels of arsenic via drinking water, our findings indicate that infant toenails are sensitive enough to detect very low levels from sources such as diet, as most of the RICHS validation cohort consume public regulated drinking water with levels presumably <10 μg/l arsenic. Furthermore, the similar correlations between infant and maternal toenail arsenic concentration between the NHBCS and RICHS suggest the association is reliable in populations of different sociodemographic characteristics.
We found some evidence that the relationship between infant and toenail arsenic concentration may be modified by maternal arsenic metabolism, and this warrants further investigation. Arsenic metabolism differs by age and sex and has been attributed to both genetic (e.g., via arsenic methyltransferase) and nutritional factors.43-45 There is evidence as well that arsenic metabolism is accelerated during pregnancy (manifested by higher relative concentrations of DMA in urine and a higher DMA to MMA ratio or secondary methylation index) that could, in theory, protect the developing fetus from arsenic toxicity particularly early in gestation.34 Although we can only speculate, the weaker correlation between infant and maternal toenail arsenic concentration we observed among women with a higher DMA to MMA ratio suggests that infants of mothers with a more efficient arsenic metabolism may have reduced fetal exposure.
In our study, infant toenail arsenic concentrations were related to all measures of maternal arsenic exposure including both biomarkers and estimates of arsenic intake. Similar to prior work,27,28 our results suggest that dietary sources of arsenic exist in US populations. The overall association between maternal rice consumption and infant toenail arsenic concentration is similar to that of urinary arsenic concentrations recently reported in US children (an increase of 0.25 cooked cups/day was associated with a 16.9% increase in infant toenail arsenic concentration in this study compared with a 14.2% increase in urinary arsenic concentration28). This is an important finding for future health policies aimed at public protection from environmental hazards and further evidence that maternal factors directly influence fetal exposure. Maternal rice intake was directly associated with infant toenail arsenic concentrations, suggesting that infant toenails may be more sensitive to estimating in utero exposure than maternal toenail concentration. This may be because of the fact that infant toenails develop within the in utero environment. Because toenails in the NHBCS were collected near the time of birth, we assumed that infant toenails would be less prone to external contamination, and therefore our laboratory analysis did not entail a washing step for infant toenails. Future studies should examine potential external contamination of infant nails and whether timing of collection following birth affects arsenic exposure estimates.
Overall, our study indicates that fetal exposure to arsenic may occur directly from maternal water and dietary sources and that infant toenails are an integrated biomarker of in utero exposure. Toenails have the advantage of being non-invasive to collect and convenient to store; however, because of their small size, infant toenails can be more challenging to handle in the laboratory than adult nail specimens. In our study, the small size of infant toenail specimens may have introduced additional measurement error (because of a loss in precision of the pre- and post-weight calculation method) and is a limitation of infant toenails as a biomarker that must be acknowledged. Nevertheless, our findings suggest that infant toenails can be used as a reliable biomarker to estimate in utero arsenic exposure even at relatively low levels of maternal arsenic exposure, and thus can be considered in the design of future environmental studies that seek to analyze the impacts of common levels of arsenic exposure on children’s health.
ACKNOWLEDGEMENTS
This work was supported by grants P20 ES018175, P01 ES022832, and R01 ES022223 from the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH); grant R25CA134286 from the National Cancer Institute (NCI), NIH; and RD-83459901 and RD-83544201 from the Environmental Protection Agency (EPA), and grant R01 MH094609 from the National Institute of Mental Health (NIMH). MA Davis was supported by Award Number K01AT006162 from the NIH. The NIEHS, NIMH, NIH, NCI, and EPA were not involved in the design and conduct of the study or collection, management, analysis, and interpretation of the data. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIMH, NIH, NCI, and EPA. In addition, the EPA does not endorse the purchase of any commercial products or services mentioned in the publication.
ABBREVIATIONS
- AsIII+
arsenite
- AsV+
arsenate
- BMI
body mass index
- DMA
dimethylarsinic acid
- HPLC
high performance liquid chromatography
- iAs
inorganic arsenic
- ICPMS
inductively coupled plasma mass spectrometry
- LoWeSS
locally weighted scatterplot smoothing
- MMA
monomethylarsonic acid
- NHBCS
New Hampshire Birth Cohort Study
- RICHS
Rhode Island Child Health Study
- SEM
structural equation modeling
- TEA
Trace Element Analysis
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Hall M, Gamble M, Slavkovich V, Liu X, Levy D, Cheng Z, et al. Determinants of arsenic metabolism: blood arsenic metabolites, plasma folate, cobalamin, and homocysteine concentrations in maternal-newborn pairs. Environ Health Perspect. 2007;115:1503–1509. doi: 10.1289/ehp.9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Concha G, Vogler G, Lezcano D, Nermell B, Vahter M. Exposure to inorganic arsenic metabolites during early human development. Toxicol Sci. 1998;44:185–190. doi: 10.1006/toxs.1998.2486. [DOI] [PubMed] [Google Scholar]
- 3.Rahman M, Sohel N, Yunus M, Chowdhury ME, Hore SK, Zaman K, et al. Increased childhood mortality and arsenic in drinking water in matlab, Bangladesh: a population-based cohort study. PLoS One. 2013;8:e55014. doi: 10.1371/journal.pone.0055014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamadani JD, Tofail F, Nermell B, Gardner R, Shiraji S, Bottai M, et al. Critical windows of exposure for arsenic-associated impairment of cognitive function in pre-school girls and boys: a population-based cohort study. Int J Epidemiol. 2011;40:1593–1604. doi: 10.1093/ije/dyr176. [DOI] [PubMed] [Google Scholar]
- 5.Wasserman GA, Liu X, Parvez F, Ahsan H, Factor-Litvak P, Kline J, et al. Water arsenic exposure and intellectual function in 6-year-old children in Araihazar, Bangladesh. Environ Health Perspect. 2007;115:285–289. doi: 10.1289/ehp.9501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wasserman GA, Liu X, Parvez F, Ahsan H, Factor-Litvak P, van Geen A, et al. Water arsenic exposure and children’s intellectual function in Araihazar, Bangladesh. Environ Health Perspect. 2004;112:1329–1333. doi: 10.1289/ehp.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Ehrenstein OS, Poddar S, Yuan Y, Mazumder DG, Eskenazi B, Basu A, et al. Children’s intellectual function in relation to arsenic exposure. Epidemiology. 2007;18:44–51. doi: 10.1097/01.ede.0000248900.65613.a9. [DOI] [PubMed] [Google Scholar]
- 8.Parvez F, Wasserman GA, Factor-Litvak P, Liu X, Slavkovich V, Siddique AB, et al. Arsenic exposure and motor function among children in Bangladesh. Environ Health Perspect. 2011;119:1665–1670. doi: 10.1289/ehp.1103548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farzan SF, Karagas MR, Chen Y. In utero and early life arsenic exposure in relation to long-term health and disease. Toxicol Appl Pharmacol. 2013;272:384–390. doi: 10.1016/j.taap.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vahter M. Health effects of early life exposure to arsenic. Basic Clin Pharmacol Toxicol. 2008;102:204–211. doi: 10.1111/j.1742-7843.2007.00168.x. [DOI] [PubMed] [Google Scholar]
- 11.Vahter M, Nermell B, Hamadam J, Raqib R. Arsenic metabolism and toxicity in early life-interactions with nutrition. Epidemiology. 2009;20:S247–S247. [Google Scholar]
- 12.Rahman A, Persson LA, Nermell B, El Arifeen S, Ekstrom EC, Smith AH, et al. Arsenic exposure and risk of spontaneous abortion, stillbirth, and infant mortality. Epidemiology. 2010;21:797–804. doi: 10.1097/EDE.0b013e3181f56a0d. [DOI] [PubMed] [Google Scholar]
- 13.Rahman A, Vahter M, Ekstrom EC, Persson LA. Arsenic exposure in pregnancy increases the risk of lower respiratory tract infection and diarrhea during infancy in Bangladesh. Environ Health Perspect. 2011;119:719–724. doi: 10.1289/ehp.1002265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahman A, Vahter M, Ekstrom EC, Rahman M, Golam Mustafa AH, Wahed MA, et al. Association of arsenic exposure during pregnancy with fetal loss and infant death: a cohort study in Bangladesh. Am J Epidemiol. 2007;165:1389–1396. doi: 10.1093/aje/kwm025. [DOI] [PubMed] [Google Scholar]
- 15.Rahman A, Vahter M, Smith AH, Nermell B, Yunus M, El Arifeen S, et al. Arsenic exposure during pregnancy and size at birth: a prospective cohort study in Bangladesh. Am J Epidemiol. 2009;169:304–312. doi: 10.1093/aje/kwn332. [DOI] [PubMed] [Google Scholar]
- 16.Saha KK, Engstrom A, Hamadani JD, Tofail F, Rasmussen KM, Vahter M. Pre- and postnatal arsenic exposure and body size to 2 years of age: a cohort study in rural bangladesh. Environ Health Perspect. 2012;120:1208–1214. doi: 10.1289/ehp.1003378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huyck KL, Kile ML, Mahiuddin G, Quamruzzaman Q, Rahman M, Breton CV, et al. Maternal arsenic exposure associated with low birth weight in Bangladesh. J Occup Environ Med. 2007;49:1097–1104. doi: 10.1097/JOM.0b013e3181566ba0. [DOI] [PubMed] [Google Scholar]
- 18.Kippler M, Wagatsuma Y, Rahman A, Nermell B, Persson LA, Raqib R, et al. Environmental exposure to arsenic and cadmium during pregnancy and fetal size: a longitudinal study in rural Bangladesh. Reprod Toxicol. 2012;34:504–511. doi: 10.1016/j.reprotox.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Kile ML, Houseman EA, Rodrigues E, Smith TJ, Quamruzzaman Q, Rahman M, et al. Toenail arsenic concentrations, GSTT1 gene polymorphisms, and arsenic exposure from drinking water. Cancer Epidemiol Biomarkers Prev. 2005;14:2419–2426. doi: 10.1158/1055-9965.EPI-05-0306. [DOI] [PubMed] [Google Scholar]
- 20.Garland M, Morris JS, Rosner BA, Stampfer MJ, Spate VL, Baskett CJ, et al. Toenail trace element levels as biomarkers: reproducibility over a 6-year period. Cancer Epidemiol Biomarkers Prev. 1993;2:493–497. [PubMed] [Google Scholar]
- 21.Karagas MR, Le CX, Morris S, Blum J, Lu X, Spate V, et al. Markers of low level arsenic exposure for evaluating human cancer risks in a US population. Int J Occup Med Environ Health. 2001;14:171–175. [PubMed] [Google Scholar]
- 22.Seaborg B, Bodurtha J. Nail size in normal infants. Establishing standards for healthy term infants. Clin Pediatr (Phila) 1989;28:142–145. doi: 10.1177/000992288902800309. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton JB, Terada H, Mestler GE. Studies of growth throughout the lifespan in Japanese: growth and size of nails and their relationship to age, sex, heredity, and other factors. J Gerontol. 1955;10:401–415. doi: 10.1093/geronj/10.4.401. [DOI] [PubMed] [Google Scholar]
- 24.Orloff K, Mistry K, Metcalf S. Biomonitoring for environmental exposures to arsenic. J Toxicol Environ Health B. 2009;12:509–524. doi: 10.1080/10937400903358934. [DOI] [PubMed] [Google Scholar]
- 25.Intarasunanont P, Navasumrit P, Waraprasit S, Chaisatra K, Suk WA, Mahidol C, et al. Effects of arsenic exposure on DNA methylation in cord bloodsamples from newborn babies and in a human lymphoblast cell line. Environ Health. 2012;11:31. doi: 10.1186/1476-069X-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samanta G, Das D, Mandal BK, Chowdhury TR, Chakraborti D, Pal A, et al. Arsenic in the breast milk of lactating women in arsenic-affected areas of West Bengal, India and its effect on infants. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2007;42:1815–1825. doi: 10.1080/10934520701566785. [DOI] [PubMed] [Google Scholar]
- 27.Gilbert-Diamond D, Cottingham KL, Gruber JF, Punshon T, Sayarath V, Gandolfi AJ, et al. Rice consumption contributes to arsenic exposure in US women. Proc Natl Acad Sci USA. 2011;108:20656–20660. doi: 10.1073/pnas.1109127108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis MA, Mackenzie TA, Cottingham KL, Gilbert-Diamond D, Punshon T, Karagas MR. Rice consumption and urinary arsenic concentrations in U.S. children. Environ Health Perspect. 2012;120:1418–1424. doi: 10.1289/ehp.1205014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marsit CJ, Maccani MA, Padbury JF, Lester BM. Placental 11-beta hydroxysteroid dehydrogenase methylation is associated with newborn growth and a measure of neurobehavioral outcome. PLoS One. 2012;7:e33794. doi: 10.1371/journal.pone.0033794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amaral AF, Porta M, Silverman DT, Milne RL, Kogevinas M, Rothman N, et al. Pancreatic cancer risk and levels of trace elements. Gut. 2011;61:1583–1588. doi: 10.1136/gutjnl-2011-301086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le XC, Lu X, Ma M, Cullen WR, Aposhian HV, Zheng B. Speciation of key arsenic metabolic intermediates in human urine. Anal Chem. 2000;72:5172–5177. doi: 10.1021/ac000527u. [DOI] [PubMed] [Google Scholar]
- 32.Navas-Acien A, Francesconi KA, Silbergeld EK, Guallar E. Seafood intake and urine concentrations of total arsenic, dimethylarsinate and arsenobetaine in the US population. Environ Res. 2011;111:110–118. doi: 10.1016/j.envres.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. Linear, Logistic, Survival, and Repeated Measures Models. Springer; New York, NY: 2012. [Google Scholar]
- 34.Gardner RM, Nermell B, Kippler M, Grander M, Li L, Ekstrom EC, et al. Arsenic methylation efficiency increases during the first trimester of pregnancy independent of folate status. Reprod Toxicol. 2011;31:210–218. doi: 10.1016/j.reprotox.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Haftenberger M, Heuer T, Heidemann C, Kube F, Krems C, Mensink GB. Relative validation of a food frequency questionnaire for national health and nutrition monitoring. Nutr J. 2010;9:36. doi: 10.1186/1475-2891-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113:192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahamed S, Kumar Sengupta M, Mukherjee A, Amir Hossain M, Das B, Nayak B, et al. Arsenic groundwater contamination and its health effects in the state of Uttar Pradesh (UP) in upper and middle Ganga plain, India: a severe danger. Sci Total Environ. 2006;370:310–322. doi: 10.1016/j.scitotenv.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 38.Mazumder DN, Majumdar KK, Santra SC, Kol H, Vicheth C. Occurrence of arsenicosis in a rural village of Cambodia. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2009;44:480–487. doi: 10.1080/10934520902719886. [DOI] [PubMed] [Google Scholar]
- 39.Pearce DC, Dowling K, Gerson AR, Sim MR, Sutton SR, Newville M, et al. Arsenic microdistribution and speciation in toenail clippings of children living in a historic gold mining area. Sci Total Environ. 2010;408:2590–2599. doi: 10.1016/j.scitotenv.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 40.Wickre JB, Folt CL, Sturup S, Karagas MR. Environmental exposure and fingernail analysis of arsenic and mercury in children and adults in a Nicaraguan gold mining community. Arch Environ Health. 2004;59:400–409. doi: 10.3200/AEOH.59.8.400-409. [DOI] [PubMed] [Google Scholar]
- 41.Tsuji JS, Van Kerkhove MD, Kaetzel RS, Scrafford CG, Mink PJ, Barraj LM, et al. Evaluation of exposure to arsenic in residential soil. Environ Health Perspect. 2005;113:1735–1740. doi: 10.1289/ehp.8178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karagas MR, Tosteson TD, Blum J, Klaue B, Weiss JE, Stannard V, et al. Measurement of low levels of arsenic exposure: a comparison of water and toenail concentrations. Am J Epidemiol. 2000;152:84–90. doi: 10.1093/aje/152.1.84. [DOI] [PubMed] [Google Scholar]
- 43.Schlawicke Engstrom K, Broberg K, Concha G, Nermell B, Warholm M, Vahter M. Genetic polymorphisms influencing arsenic metabolism: evidence from Argentina. Environ Health Perspect. 2007;115:599–605. doi: 10.1289/ehp.9734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gamble MV, Liu X, Slavkovich V, Pilsner JR, Ilievski V, Factor-Litvak P, et al. Folic acid supplementation lowers blood arsenic. Am J Clin Nutr. 2007;86:1202–1209. doi: 10.1093/ajcn/86.4.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hall MN, Liu X, Slavkovich V, Ilievski V, Pilsner JR, Alam S, et al. Folate, cobalamin, cysteine, homocysteine, and arsenic metabolism among children in Bangladesh. Environ Health Perspect. 2009;117:825–831. doi: 10.1289/ehp.0800164. [DOI] [PMC free article] [PubMed] [Google Scholar]