Abstract
Receptor tyrosine kinases (RTKs) are involved in regulation of key process in roles in endothelial biology including proliferation, migration and angiogenesis. It is now generally accepted that RTK signaling occurs intracellularly as well as on the plasma membrane although many important details remain to be worked out. Endocytosis and subsequent intracellular trafficking spatiotemporally regulate RTK signaling while “signaling endosomes” provide a platform for the compartmentalization of signaling events. This review summarizes recent advances in our understanding of endothelial RTK endocytosis and signaling using vascular endothelial growth factor receptor-2 as a paradigm.
Keywords: endocytosis, endosomes, signaling, VEGFR2, NRP1, synectin, Myosin-VI, FGFR1, EGFR
Introduction
Vascular networks reach every organ system in the body. In addition to supplying oxygen and nutrients to tissue as well as a variety of hormones, the vasculature is able to directly affect function of different organs via direct paracrine secretion of various molecules. Endothelial cells are the primary source of these factors and play a central role in most, if not all, kinds of biological activities, physiological or pathological, that take place in blood vessels. In addition, the endothelium plays a central role in the maintenance of normal vasculature, including regulation of its permeability, stability and proliferation.1–3
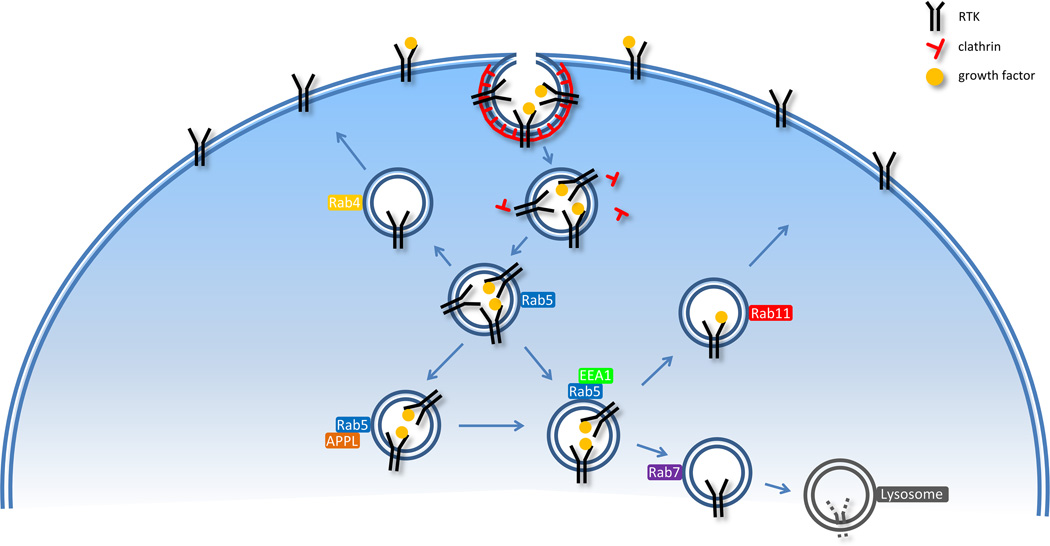
Endothelial cells respond to a variety of biochemical and mechanical inputs via a number of different receptor systems. Among these, receptor tyrosine kinases (RTKs) such as vascular endothelial growth factor receptor-2 (VEGFR2) and fibroblast growth factor receptor-1 (FGFR1) are essential regulators of such essential endothelial cell activities as proliferation, migration, angiogenesis and vascular permeability. Whilst traditionally RTKs are thought to signal from the plasma membrane, recent studies have demonstrated that a number of critical signaling events occur in the cytoplasm, following RTK endocytosis, which bring them into close proximity with either downstream signaling targets or phosphatases.4 Interestingly, the extent to which a specific RTK’s signaling is affected by endocytosis is different for different receptors. This could be due to different endocytosis pathways or intracellular trafficking routes that these receptors take (Fig. 1).
Figure 1.
General RTK endocytosis and recycling pathways. Ligand binding induces RTK activation and internalization (only clathrin-mediated pathway is shown). Internalized RTK undergoes trafficking to Rab5-positive endosomes, where they are sorted for either degradation or recycling. The degradation pathway is mediated by Rab7, whereas the recycling pathway is dependent upon Rab4 (short-loop) or Rab11 (long-loop). Specific details may vary for each particular RTK.
To date, EGFR (epidermal growth factor receptor) has been one of the best-studied receptors in regard to the connection between its trafficking and signaling.5 In addition to plasma membrane signaling, much of EGFR signaling output occurs from various endocytic compartments as its uptake and intracellular trafficking brings EGFR into association with a series of adaptor proteins involved in various signaling cascade.6 In particular, EGFR activates ERK1/2 (extracellular signal-regulated kinases 1 and 2)7 and AKT8 signaling from EEA1-positive early endosomes.
Endocytosis of EGFR as well as of other receptors is regulated by the Rab family proteins.9 The Rabs are GTP-binding proteins that switch between active GTP-bound and inactive GDP-bound forms. The switch between the two states is mediated by guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs). The Rab proteins regulate various stages of cellular transport, including vesicle budding, endosome trafficking, and tethering of vesicles with target membranes.10 Different Rab proteins share structural similarity, but have unique subcellular localization and effector proteins, which enable them to be involved in different stages of cellular transport.9
Here we review the recent progress in the understanding of endocytosis and signaling of the two endothelial RTKs- VEGFR2 and FGFR1 and the effect this process has on their signaling and biological activities.
Endocytosis pathways of VEGFR2 and FGFR1
VEGFR2 is the primary signaling receptor for VEGF-A, the endothelial growth factor central to much of the blood vessel biology, as well as a related ligand VEGF-C. Following binding of its ligands, VEGFR2 undergoes dimerization and activation characterized by phosphorylation of key tyrosine residues in its cytoplasmic domain.11 This is followed by endocytosis via the clathrin/dynamin-mediated pathway.12 In addition to this ligand-dependent endocytosis, two other forms of VEGFR2 endocytosis have also been described: a slow constitutive recycling and shear stress-induced VEGFR2 endocytosis.
In unstimulated endothelial cells, VEGFR2 is located both on the plasma membrane and in EEA1-positive early endosomes where its presence is thought to be due to constitutive endocytosis.13 The endosomal population of VEGFR2 undergoes short-loop Rab4-dependent recycling to the plasma membrane, rather than long-loop Rab11-dependent recycling.14 The function of the constitutive VEGFR2 endocytosis and recycling remains unclear. Interestingly, ligand-independent VEGFR2 activation has been reported to occur in the Golgi of endothelial cells in response to hyperglycemia.15 Further investigation of the trafficking pathway involved could help our understanding of ligand-independent VEGFR2 functions.
Shear stress induces VEGFR2 endocytosis via phosphorylation of CD31 (PECAM), a transmembrane protein thought to be involved in shear stress sensing.16 In this process, blood flow induces formation of a VE-cadherin–β-catenin–VEGFR2 complex17 and aforementioned PECAM phosphorylation. Once phosphorylated, PECAM facilitates VEGFR2 transactivation by a Src family kinase18 leading to activation of downstream signaling events such as ERK1/2 activation19 and eNOS phosphorylation.20 The shear stress-induced VEGFR2 endocytosis is caveolae-dependent21 and the trafficking pathway involved is largely unknown.
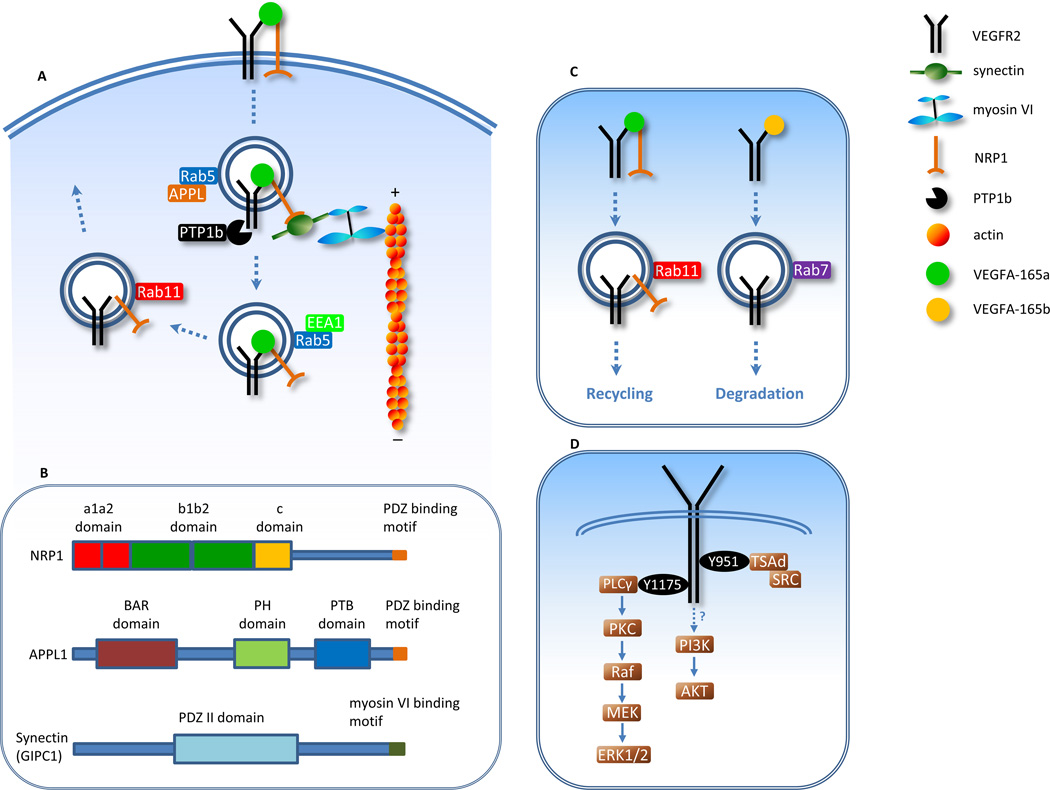
Ligand (VEGF)-activated VEGFR2 endocytosis and subsequent intracellular trafficking is a tightly controlled process. The ligand-induced auto-phosphorylation of the receptor’s kinase domain increases the rate of its internalization22 that proceeds via clathrin-coated pits. AP2 and other adaptor proteins are involved in the recruitment of clathrin to plasma membrane, where clathrin undergoes polymerization, forming lattice-like coat.23 Dynamin mediates the membrane fission and budding of clathrin-coated vesicles.24 After internalization, the clathrin lattice is disassembled, leaving the uncoated vesicle to traffic towards endosomes.23 VEGFR2 undergoes Rab5-dependent trafficking to early endosomes.14, 25 In an unusual departure from conventional endosomal trafficking, VEGFR2 trafficking is in part dependent on the synectin-myosin-VI complex (Fig. 2A), as deletion of either molecule causes delayed VEGFR2 appearance in EEA1-positive early endosomes.26
Figure 2.
VEGFR2 endocytosis and recycling pathways. (A) VEGFR2 trafficking requires the synectin-myosin-VI complex, which is recruited by NRP1 after receptor internalization. (B) The PDZ binding motif on NRP1 and APPL1 mediates binding to the PDZ domain of synectin. Synectin forms a complex with myosin-VI via its myosin-VI binding motif. (C) The internalized VEGFR2-NRP1 receptor complex undergoes Rab11-dependent recycling. In the absence of NRP1, VEGFR2 is degraded via Rab7-positive late endosome. (D) VEGFR2: Src activation is downstream of phosphorylation of tyrosine 951 and ERK1/2 activation is downstream of phosphorylation of tyrosine 1175. The mechanism of VEGF-induced AKT activation is unclear.
Synectin (GIPC1) is a single domain PDZ protein that can bind to a number of transmembrane proteins and receptors including another VEGF-A receptor neuropilin-1 (NRP1), nerve growth factor receptor TrkA13, non-tyrosine kinase fibroblast growth factors receptor Syndecan-427 and α5 and α6 integrins28 among others. In addition to the PDZ domain, synectin also has the ability to bind intracellular motor protein myosin-VI (Fig. 2B). Myosin-VI is a unique member of the myosin family due to its ability to move towards the minus end of actin filaments. This allows it to move cargo from the plasma membrane into the cell along actin filaments. Myosin-VI plays a role in different stages of endocytosis, such as receptor internalization and sorting, through interaction with different binding partners.29 Synectin, which associates with peripheral endocytic vesicles, binds myosin-VI and facilitates trafficking towards early endosomes. 30 The association between synectin and myosin-VI has been demonstrated in neurons31 and epithelial cells32–34, besides endothelial cells.26, 35
The recruitment of the synectin-myosin-VI complex to Rab5-positive endosomes usually involves adaptor proteins APPL1 and 2.36, 37 However, in the case of VEGFR2, the role of APPL appears to be played by NRP125 that is able to bind VEGF-A165 via its extracellular VEGF binding site and myosin-VI via its cytoplasmic domain (Fig. 2B). In this model, VEGF-A is thought to form a complex with VEGFR2 and NRP1 that is then endocytosed and trafficked together. 38
Once in early endosomes, VEGFR2 can undergo either lysosomal degradation or recycling back to plasma membrane. Approximately 40% of VEGFR2 is degraded within 30 minutes of VEGF stimulation39 with the receptor sorted for lysosomal degradation via Rab739. Proteasome- dependent degradation of VEGFR2 has also been reported that is, in part, regulated by VEGFR2-mediated activation of p38-MAPK that lessens the receptor’s degradation by the 26S proteasome system.40 The remainder of endocytosed receptors undergoes recycling. While constitutively endocytosed VEGFR2 may recycle in a Rab-4 dependent manner, VEGF stimulation switches VEGFR2 recycling to a Rab11-dependent pathway.14
NRP1 plays a critical role, not only in the recruitment of synectin in VEGFR2 endocytosis, but also in VEGFR2 recycling to the membrane. Stimulation of HUVECs with VEGF-A165a that binds both VEGFR2 and NRP1 leads VEGFR2 to Rab11-dependent recycling, whereas stimulation with VEGF-A165b which does not bind NRP1 causes Rab7-dependent degradation of VEGFR2 (Fig. 2C)14. In this manner NRP1 promotes both VEGFR2 endocytosis and recycling to the membrane and therefore relatively increases the proportion of VEGFR2 available for VEGF binding.
Similarly to VEGFR2, signaling by FGFR1, the principal FGF receptor in the endothelium, is also regulated by its endocytosis.41, 42 The receptor’s endocytosis can proceed via a number of different pathways. Depletion of E-Syt2, an endocytic adaptor protein that interacts with both activated FGFR1 and the clathrin adaptor complex AP-2, inhibits FGFR1 endocytosis and signaling in Xenopus embryo, suggesting clathrin-mediated pathway.41 FGFR1 was also reported to interact with caveolin-1 and the knockdown of caveolin-1 decreases FGF2-induced activation of AKT and ERK1/2 in ovine fetoplacental artery endothelial (oFPAE) cells.43 Finally, in endothelial cells FGFR1 endocytosis can proceed via macropinocytosis.3 Thus, it is likely that for FGFR1 the predominant mode of uptake is cell-type specific.
Once internalized, FGFR1 is found in Rab5/EEA1-positive early endosomes 3, 44 from where the majority of the activated receptors are sorted for degradation via Lamp1-positive late endosomes while a relatively small proportion is recycled back to the plasma membrane for further stimulation 45.
Endocytosis-dependent regulation of VEGFR2 signaling
One unique aspect of VEGFR2 endocytosis is its regulation by a number of transmembrane and cytosolic interacting proteins, which play a role in either VEGFR2 internalization or degradation. A group of such proteins involved in VEGFR2 internalization are Dab2, ephrin-B2 and PAR-3. Dab246, a clathrin-associated sorting protein, and the cell polarity regulator PAR-3 interact with the transmembrane protein ephrin-B2 and VEGFR2. Disruption of this interaction by silencing of Dab2 or PAR-3 causes reduced VEGFR2 internalization and impaired VEGF-induced angiogenesis. After RTKs are internalized into early endosomes, a proportion of the receptors are modified by ubiquitin and then sorted for lysosomal degradation. CCM347 and myoferlin48 respectively associate with VEGFR2 in endothelial cells and serve to enhance VEGFR2 stability by preventing receptor degradation.
Apart from internalization and degradation, VEGFR2 signaling is regulated by protein tyrosine phosphatases (PTPs) such as VE-PTP and PTP1b. VE-PTP is a transmembrane phosphatase that associates via its extracellular domain with VE-Cadherin at endothelial cell-cell junctions.49 VEGF stimulation causes the activation and translocation of VEGFR2 to the junctions where the receptor is silenced by VE-PTP. VE-PTP also abolishes tyrosine phosphorylation of VE-Cadherin mediated by VEGFR2 and thus enhances EC junction integrity.50 Silencing of VE-PTP boosts VEGFR2 signaling activity while also increasing permeability.51
Unlike VE-PTP, PTP1b affects VEGFR2 signaling further away from the plasma membrane. PTP1b is an ER-membrane bound phosphatase with its catalytic domain facing the cytosol.52 The extensive ER network that reaches cell periphery enables PTP1b to come in close proximity with internalized VEGFR2 as it undergoes intracellular trafficking. Delayed trafficking of VEGFR2 to EEA1-positive endosomes observed in synectin or myosin-VI null endothelial cells or in endothelial cells expressing a truncated NRP1 with its cytoplasmic domain deleted results in reduced VEGF-dependent activation of ERK1/2 pathway. 25, 26 This can be corrected by suppression of PTP1b expression or activity, thus suggesting that the delay in VEGFR2 trafficking exposes the receptor to prolonged dephosphorylation by PTP1b.53
It is unclear in which subcellular compartment VEGFR2 is dephosphorylated by PTP1b. Studies of EGFR-PTP1b interactions showed that endosomes and ER form close membrane contacts which is the possible site of contact between the two molecules.54 On the other hand, a study of insulin receptor trafficking found the interaction between IR and PTP1b in a peri-nuclear endosomal compartment.55 Thus it is likely that PTP1b interacts with different RTKs in different subcellular compartment or compartment interfaces. Since RTK signaling is regulated both spatially and temporally, when and where they are deactivated by protein tyrosine phosphatases has a critical impact on the downstream signaling events. The driving force behind the formation of these compartment interfaces and the recruitment of PTP1b to the contact sites remains to be elucidated.
Another interesting aspect of PTP1b function is its potential to regulate RTK endocytosis. The ESCRT-0 complex, which is composed of the ubiquitin-binding proteins STAM 1 and 2 and their binding partner Hrs, binds to ubiquitin moieties on RTKs and facilitate the formation of multivesicular bodies which then fuse with lysosomes56. STAM2 is a PTP1b substrate and the latter’s knockdown increases STAM2 phosphorylation57 which in turn influences its function and localization. Therefore by affecting STAM2 phosphorylation, PTP1b potentially affects the degradation of activated RTKs. It is unclear whether PTP1b specifically affects VEGFR2 lysosomal degradation. VEGF stimulation resulted in increased phosphorylation of Hrs and the co-localization of VEGFR2 with the HRS/STAM complex13, suggesting the involvement of this protein complex in VEGFR2 trafficking.
Biology of RTK endocytosis in endothelium
Increasing evidence from a number of studies showed the essential role of endocytosis in the optimal activation of RTKs and subsequent signaling events. 58, 59 Endocytosis brings the receptors into close proximity of downstream targets or keeps them away from the dephosphorylation of protein tyrosine phosphatases. Generally RTKs have more than one downstream signaling pathway, yet the pathways are not necessarily activated at exactly the same time point, or for the same duration, or at the same subcellular localization. The relationship between RTK endocytosis and signaling requires better knowledge of the localization of their downstream targets. It is likely that each downstream signaling pathway has a unique “signaling compartment” along the RTK trafficking route. Such “signaling compartments” enable tight regulation of activation and inactivation of the signaling pathways. Knowledge on this aspect of receptor biology is still largely insufficient. Current understanding of signaling compartments regulating VEGFR2 signaling pathways is discussed below.
ERK1/2 activation and angiogenesis
VEGFR2 mediates endothelial cell proliferation, angiogenesis, arteriogenesis and controls vascular lumen size via the activation of ERK1/2 26, 60, 61. VEGF activates ERK1/2 through the classical Raf-MEK-ERK signaling cascade (Fig. 2D) 62. FGF2 also induces ERK1/2 activation 63. And FGF2-dependent ERK1/2 signaling plays a key role in the maintenance of VEGFR2 expression 1. In both cases, ERK1/2 activation depends on receptor internalization, as inhibition of internalization abrogates VEGF- and FGF2-induced ERK1/2 signaling64.
Knockdowns of Rab7 or Rab11 increase VEGF-induced ERK1/2 activation26. As these interventions, respectively, cause reduced degradation or recycling of VEGFR2, this suggests that ERK1/2 signaling occurs in a subcellular compartment preceding these compartments. ERK1/2 signaling has been associated with clathrin-coated vesicles (CCVs), early endosomes and late endosomes. Evidence for the role of CCVs and early endosomes comes from studies of neurotrophin receptor TrkA in neurons. A number of signaling components of the Ras-MAPK pathway, such as Ras, C-Raf, Mek and ERK1/2, were found to be present in CCVs in unstimulated cells and phospho-ERK1/2 was significantly enriched in CCVs after NGF treatment 65. On the contrary, AKT and phospho-AKT were not detectable in CCVs before or after NGF stimulation65. Phospho-ERK1/2 presence has also been demonstrated in Rab5-positive early endosomes using confocal microscopy and increased phospho-ERK1/2 in Rab5-positive endosomes was observed after NGF treatment66. Furthermore, ERK1/2 activation was demonstrated to occur in EEA1-positive endosomes in endothelial cells, as delayed trafficking of activated VEGFR2 to this endosomal compartment caused impaired ERK1/2 activation.25 PI3K and phospho-AKT were also present in early endosomes67. The detection of ERK1/2 signaling in CCVs is notable, considering the transient phase of these vesicles and rapid assembly and disassembly of clathrin coats in the vesicles, making CCVs a seemingly unsuitable compartment for signaling events to take place.
Late endosome involvement in ERK1/2 signaling was reported in studies of EGFR signaling. The MEK1 partner protein MP1 and the late endosomal adaptor protein p14 form a scaffold complex that recruits MAPK to late endosomes68. Upon EGF stimulation, activated ERK1/2 was found to co-localize with p14 and MP1 on late endosomes. RNAi-mediated silencing of MP1 or p14 caused reduced EGF-induced ERK1/2 activation69. Taken together, ERK1/2 signaling is found to span a variety of subcellular compartments, from CCVs to late endosomes.
AKT activation and endothelial cell survival
VEGF promotes endothelial cell survival or anti-apoptotic signaling pathways via the anti-apoptotic kinase AKT/PKB (Fig. 2D). Several studies have suggested that AKT activation requires VEGFR2 internalization and endocytosis to a similar extent as ERK1/2 activation.26, 70, 71 As discussed above, AKT activation was not detected in clathrin-coated vesicles in studies of neurotrophin receptor TrkA in neurons.65 Interestingly, in the context of EGFR signaling, AKT, by immunostaining, has been shown to be closely associated with two Rab5 effectors APPL1 and APPL272, which are located on a subset of Rab5-positive early endosomes.36 A knockdown of APPL1 in zebrafish led to a strongly diminished IGF-induced AKT phosphorylation, whereas IGF-induced ERK1/2 phosphorylation remained largely unchanged.72 This is in agreement with the findings that ERK1/2 signals in endosomal compartments other than just early endosomes. Therefore APPL1 depletion has a minimal impact on IGF-induced ERK1/2 activation. On the contrary, IGF-induced AKT signaling is confined to Rab5/APPL-positive endosomes. APPL1 is also involved in regulating signaling of EGFR 36, TrkA 13, and adiponectin receptors 73. It is unclear whether APPL1 is also regulates VEGFR2 endocytosis and signaling, since NRP1 is the molecule in endothelial cells that mediates the recruitment of the synectin-myosin-VI complex to activated VEGFR2. It remains to be elucidated whether VEGFR2-induced AKT activation depends on APPL1 and 2, and whether the signaling endosome for AKT is receptor-specific.
Src activation, endothelial cell migration and vascular permeability
Activated VEGFR2 recruits the signaling adaptor TSAd (T-cell-specific adaptor) which promotes Src activation (Fig. 2D). Src mediates Src-FAK (focal adhesion kinase) signaling activity which is involved in endothelial cell migration and vascular permeability 74. The process of cell migration includes cell polarization, lamellipodia formation, and the assembly and disassembly of focal adhesions while vascular permeability is associated with the integrity of EC junctions. Both types of activities involve signaling events in specialized compartments of endothelial cells and the endosomal trafficking and signaling events in these contexts are different from what is discussed above. Inactive Src resides in the perinuclear region75 which, according to other studies,76 is likely to be Rab7-positive late endosomes. In contrast, active Src is not observed in the perinuclear region suggesting rapid translocation upon activation.76 Once activated, Src moves to focal adhesions or cell-cell contact sites39 through the Rab11-dependent recycling pathway75 or the ESCRT (endosomal-sorting complexes required for transport) pathway71 according to different studies. Src becomes more active as it progresses from the perinuclear region to the plasma membrane, demonstrating an “activation gradient” along the trafficking route.75 This finding suggests that Src activation only begins after it exits late endosomes and enters the recycling endosome. Compared to ERK1/2 and AKT which are activated in early endosomes upon VEGF stimulation, Src is activated in a later signaling compartment, probably on the VEGFR2 recycling route.
Conclusions
There has been tremendous progress in recent years in studies of spatial and temporal aspects of RTK signaling. It is now clear that RTK endocytosis and signaling are closely related events. Endocytosis regulates signaling spatiotemporally as cytoplasmic uptake and trafficking bring activated RTKs into close proximity to downstream signaling molecules and signaling regulators such as protein tyrosine phosphatases. As each RTK has several downstream signaling targets, each signaling pathway has a unique “signaling endosome” or “signaling compartment”. It is further likely that these differ for different RTKs. Such setting allows for spatial or temporal separation of individual signaling pathway and may to a considerable extent determine specificity of each RTK signaling.
In endothelial cells, current evidence suggests that VEGFR2 undergoes VEGF-induced endocytosis via clathrin-mediated pathway 77, whereas FGF2-induced FGFR1 endocytosis is clathrin-independent 3, 43. Although ERK1/2 activation has been reported to occur in multiple endosomal compartments, the bulk of signaling activity is likely to originate from Rab5/EEA1-positive early endosomes in endothelial cells 25, 26. It is likely that VEGFR2, FGFR1 and other receptors have different downstream signaling endosomes. Further studies are needed to fully unravel signaling mechanisms of endothelial receptor tyrosine kinases.
Significance.
This review focuses on the endocytosis of specific RTKs in endothelial cells and summarizes in particular the current knowledge regarding VEGFR2 endocytosis and signaling. The rapidly changing understanding of how this key endothelial RTK operates provides new insights into biology of key endothelium-dependent processes such as angiogenesis and arteriogenesis and offer the possibility of new therapeutic approaches to currently some of the most intractable cardiovascular illnesses.
Acknowledgments
Sources of Funding: Supported in part by NIH grants R01 HL084691 and P01 HL107205
Abbreviations
- EGFR
epidermal growth factor receptor
- VEGFR2
vascular endothelial growth factor receptor-2
- NRP1
Neuropilin 1
- FGFR1
fibroblast growth factor receptor-1
- ERK1/2
extracellular signal-regulated kinases 1 and 2
Footnotes
Disclosures
None
Reference
- 1.Murakami M, Nguyen LT, Hatanaka K, Schachterle W, Chen PY, Zhuang ZW, Black BL, Simons M. Fgf-dependent regulation of vegf receptor 2 expression in mice. The Journal of clinical investigation. 2011;121:2668–2678. doi: 10.1172/JCI44762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murakami M, Nguyen LT, Zhuang ZW, Moodie KL, Carmeliet P, Stan RV, Simons M. The fgf system has a key role in regulating vascular integrity. The Journal of clinical investigation. 2008;118:3355–3366. doi: 10.1172/JCI35298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elfenbein A, Lanahan A, Zhou TX, Yamasaki A, Tkachenko E, Matsuda M, Simons M. Syndecan 4 regulates fgfr1 signaling in endothelial cells by directing macropinocytosis. Science signaling. 2012;5:ra36. doi: 10.1126/scisignal.2002495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorkin A, von Zastrow M. Endocytosis and signalling: Intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10:609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hupalowska A, Miaczynska M. The new faces of endocytosis in signaling. Traffic. 2012;13:9–18. doi: 10.1111/j.1600-0854.2011.01249.x. [DOI] [PubMed] [Google Scholar]
- 6.Sorkin A, Goh LK. Endocytosis and intracellular trafficking of erbbs. Experimental cell research. 2009;315:683–696. doi: 10.1016/j.yexcr.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 7.Kranenburg O, Verlaan I, Moolenaar WH. Dynamin is required for the activation of mitogen-activated protein (map) kinase by map kinase kinase. The Journal of biological chemistry. 1999;274:35301–35304. doi: 10.1074/jbc.274.50.35301. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Pennock S, Chen X, Wang Z. Endosomal signaling of epidermal growth factor receptor stimulates signal transduction pathways leading to cell survival. Molecular and cellular biology. 2002;22:7279–7290. doi: 10.1128/MCB.22.20.7279-7290.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ceresa BP. Regulation of egfr endocytic trafficking by rab proteins. Histology and histopathology. 2006;21:987–993. doi: 10.14670/HH-21.987. [DOI] [PubMed] [Google Scholar]
- 10.Zerial M, McBride H. Rab proteins as membrane organizers. Nature reviews. Molecular cell biology. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 11.Koch S, Tugues S, Li X, Gualandi L, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem J. 2011;437:169–183. doi: 10.1042/BJ20110301. [DOI] [PubMed] [Google Scholar]
- 12.Pasula S, Cai X, Dong Y, Messa M, McManus J, Chang B, Liu X, Zhu H, Mansat RS, Yoon SJ, Hahn S, Keeling J, Saunders D, Ko G, Knight J, Newton G, Luscinskas F, Sun X, Towner R, Lupu F, Xia L, Cremona O, De Camilli P, Min W, Chen H. Endothelial epsin deficiency decreases tumor growth by enhancing vegf signaling. The Journal of clinical investigation. 2012;122:4424–4438. doi: 10.1172/JCI64537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ewan LC, Jopling HM, Jia H, Mittar S, Bagherzadeh A, Howell GJ, Walker JH, Zachary IC, Ponnambalam S. Intrinsic tyrosine kinase activity is required for vascular endothelial growth factor receptor 2 ubiquitination, sorting and degradation in endothelial cells. Traffic. 2006;7:1270–1282. doi: 10.1111/j.1600-0854.2006.00462.x. [DOI] [PubMed] [Google Scholar]
- 14.Ballmer-Hofer K, Andersson AE, Ratcliffe LE, Berger P. Neuropilin-1 promotes vegfr-2 trafficking through rab11 vesicles thereby specifying signal output. Blood. 2011;118:816–826. doi: 10.1182/blood-2011-01-328773. [DOI] [PubMed] [Google Scholar]
- 15.Warren CM, Ziyad S, Briot A, Der A, Iruela-Arispe ML. A ligand-independent vegfr2 signaling pathway limits angiogenic responses in diabetes. Science signaling. 2014;7:ra1. doi: 10.1126/scisignal.2004235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 17.Shay-Salit A, Shushy M, Wolfovitz E, Yahav H, Breviario F, Dejana E, Resnick N. Vegf receptor 2 and the adherens junction as a mechanical transducer in vascular endothelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:9462–9467. doi: 10.1073/pnas.142224299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiu YJ, McBeath E, Fujiwara K. Mechanotransduction in an extracted cell model: Fyn drives stretch- and flow-elicited pecam-1 phosphorylation. The Journal of cell biology. 2008;182:753–763. doi: 10.1083/jcb.200801062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tai LK, Zheng Q, Pan S, Jin ZG, Berk BC. Flow activates erk1/2 and endothelial nitric oxide synthase via a pathway involving pecam1, shp2, and tie2. The Journal of biological chemistry. 2005;280:29620–29624. doi: 10.1074/jbc.M501243200. [DOI] [PubMed] [Google Scholar]
- 20.Jin ZG, Wong C, Wu J, Berk BC. Flow shear stress stimulates gab1 tyrosine phosphorylation to mediate protein kinase b and endothelial nitric-oxide synthase activation in endothelial cells. The Journal of biological chemistry. 2005;280:12305–12309. doi: 10.1074/jbc.M500294200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu J, Bergaya S, Murata T, Alp IF, Bauer MP, Lin MI, Drab M, Kurzchalia TV, Stan RV, Sessa WC. Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. The Journal of clinical investigation. 2006;116:1284–1291. doi: 10.1172/JCI27100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dougher M, Terman BI. Autophosphorylation of kdr in the kinase domain is required for maximal vegf-stimulated kinase activity and receptor internalization. Oncogene. 1999;18:1619–1627. doi: 10.1038/sj.onc.1202478. [DOI] [PubMed] [Google Scholar]
- 23.McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nature reviews. Molecular cell biology. 2011;12:517–533. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- 24.Schmid SL, Frolov VA. Dynamin: Functional design of a membrane fission catalyst. Annual review of cell and developmental biology. 2011;27:79–105. doi: 10.1146/annurev-cellbio-100109-104016. [DOI] [PubMed] [Google Scholar]
- 25.Lanahan A, Zhang X, Fantin A, Zhuang Z, Rivera-Molina F, Speichinger K, Prahst C, Zhang J, Wang Y, Davis G, Toomre D, Ruhrberg C, Simons M. The neuropilin 1 cytoplasmic domain is required for vegf-a-dependent arteriogenesis. Developmental cell. 2013;25:156–168. doi: 10.1016/j.devcel.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lanahan AA, Hermans K, Claes F, Kerley-Hamilton JS, Zhuang ZW, Giordano FJ, Carmeliet P, Simons M. Vegf receptor 2 endocytic trafficking regulates arterial morphogenesis. Developmental cell. 2010;18:713–724. doi: 10.1016/j.devcel.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao Y, Li M, Chen W, Simons M. Synectin, syndecan-4 cytoplasmic domain binding pdz protein, inhibits cell migration. J Cell Physiol. 2000;184:373–379. doi: 10.1002/1097-4652(200009)184:3<373::AID-JCP12>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 28.Tani TT, Mercurio AM. Pdz interaction sites in integrin alpha subunits. T14853, tip/gipc binds to a type i recognition sequence in alpha 6a/alpha 5 and a novel sequence in alpha 6b. The Journal of biological chemistry. 2001;276:36535–36542. doi: 10.1074/jbc.M105785200. [DOI] [PubMed] [Google Scholar]
- 29.Tumbarello DA, Kendrick-Jones J, Buss F. Myosin vi and its cargo adaptors - linking endocytosis and autophagy. Journal of cell science. 2013;126:2561–2570. doi: 10.1242/jcs.095554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buss F, Kendrick-Jones J. How are the cellular functions of myosin vi regulated within the cell? Biochem Biophys Res Commun. 2008;369:165–175. doi: 10.1016/j.bbrc.2007.11.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yano H, Ninan I, Zhang H, Milner TA, Arancio O, Chao MV. Bdnf-mediated neurotransmission relies upon a myosin vi motor complex. Nature neuroscience. 2006;9:1009–1018. doi: 10.1038/nn1730. [DOI] [PubMed] [Google Scholar]
- 32.Aschenbrenner L, Lee T, Hasson T. Myo6 facilitates the translocation of endocytic vesicles from cell peripheries. Mol Biol Cell. 2003;14:2728–2743. doi: 10.1091/mbc.E02-11-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naccache SN, Hasson T, Horowitz A. Binding of internalized receptors to the pdz domain of gipc/synectin recruits myosin vi to endocytic vesicles. Proc Natl Acad Sci U S A. 2006;103:12735–12740. doi: 10.1073/pnas.0605317103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reed BC, Cefalu C, Bellaire BH, Cardelli JA, Louis T, Salamon J, Bloecher MA, Bunn RC. Glut1cbp(tip2/gipc1) interactions with glut1 and myosin vi: Evidence supporting an adapter function for glut1cbp. Molecular biology of the cell. 2005;16:4183–4201. doi: 10.1091/mbc.E04-11-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valdembri D, Caswell PT, Anderson KI, Schwarz JP, Konig I, Astanina E, Caccavari F, Norman JC, Humphries MJ, Bussolino F, Serini G. Neuropilin-1/gipc1 signaling regulates alpha5beta1 integrin traffic and function in endothelial cells. PLoS Biol. 2009;7:e25. doi: 10.1371/journal.pbio.1000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miaczynska M, Christoforidis S, Giner A, Shevchenko A, Uttenweiler-Joseph S, Habermann B, Wilm M, Parton RG, Zerial M. Appl proteins link rab5 to nuclear signal transduction via an endosomal compartment. Cell. 2004;116:445–456. doi: 10.1016/s0092-8674(04)00117-5. [DOI] [PubMed] [Google Scholar]
- 37.Varsano T, Dong MQ, Niesman I, Gacula H, Lou X, Ma T, Testa JR, Yates JR, 3rd, Farquhar MG. Gipc is recruited by appl to peripheral trka endosomes and regulates trka trafficking and signaling. Mol Cell Biol. 2006;26:8942–8952. doi: 10.1128/MCB.00305-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eichmann A, Simons M. Vegf signaling inside vascular endothelial cells and beyond. Current opinion in cell biology. 2012;24:188–193. doi: 10.1016/j.ceb.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gampel A, Moss L, Jones MC, Brunton V, Norman JC, Mellor H. Vegf regulates the mobilization of vegfr2/kdr from an intracellular endothelial storage compartment. Blood. 2006;108:2624–2631. doi: 10.1182/blood-2005-12-007484. [DOI] [PubMed] [Google Scholar]
- 40.Meyer RD, Srinivasan S, Singh AJ, Mahoney JE, Gharahassanlou KR, Rahimi N. Pest motif serine and tyrosine phosphorylation controls vascular endothelial growth factor receptor 2 stability and downregulation. Molecular and cellular biology. 2011;31:2010–2025. doi: 10.1128/MCB.01006-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jean S, Mikryukov A, Tremblay MG, Baril J, Guillou F, Bellenfant S, Moss T. Extended-synaptotagmin-2 mediates fgf receptor endocytosis and erk activation in vivo. Developmental cell. 2010;19:426–439. doi: 10.1016/j.devcel.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 42.Sandilands E, Akbarzadeh S, Vecchione A, McEwan DG, Frame MC, Heath JK. Src kinase modulates the activation, transport and signalling dynamics of fibroblast growth factor receptors. EMBO reports. 2007;8:1162–1169. doi: 10.1038/sj.embor.7401097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng L, Liao WX, Luo Q, Zhang HH, Wang W, Zheng J, Chen DB. Caveolin-1 orchestrates fibroblast growth factor 2 signaling control of angiogenesis in placental artery endothelial cell caveolae. Journal of cellular physiology. 2012;227:2480–2491. doi: 10.1002/jcp.22984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bryant DM, Wylie FG, Stow JL. Regulation of endocytosis, nuclear translocation, and signaling of fibroblast growth factor receptor 1 by e-cadherin. Molecular biology of the cell. 2005;16:14–23. doi: 10.1091/mbc.E04-09-0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Irschick R, Trost T, Karp G, Hausott B, Auer M, Claus P, Klimaschewski L. Sorting of the fgf receptor 1 in a human glioma cell line. Histochemistry and cell biology. 2013;139:135–148. doi: 10.1007/s00418-012-1009-1. [DOI] [PubMed] [Google Scholar]
- 46.Nakayama M, Nakayama A, van Lessen M, Yamamoto H, Hoffmann S, Drexler HC, Itoh N, Hirose T, Breier G, Vestweber D, Cooper JA, Ohno S, Kaibuchi K, Adams RH. Spatial regulation of vegf receptor endocytosis in angiogenesis. Nat Cell Biol. 2013;15:249–260. doi: 10.1038/ncb2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He Y, Zhang H, Yu L, Gunel M, Boggon TJ, Chen H, Min W. Stabilization of vegfr2 signaling by cerebral cavernous malformation 3 is critical for vascular development. Science signaling. 2010;3:ra26. doi: 10.1126/scisignal.2000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bernatchez PN, Acevedo L, Fernandez-Hernando C, Murata T, Chalouni C, Kim J, Erdjument-Bromage H, Shah V, Gratton JP, McNally EM, Tempst P, Sessa WC. Myoferlin regulates vascular endothelial growth factor receptor-2 stability and function. The Journal of biological chemistry. 2007;282:30745–30753. doi: 10.1074/jbc.M704798200. [DOI] [PubMed] [Google Scholar]
- 49.Nawroth R, Poell G, Ranft A, Kloep S, Samulowitz U, Fachinger G, Golding M, Shima DT, Deutsch U, Vestweber D. Ve-ptp and ve-cadherin ectodomains interact to facilitate regulation of phosphorylation and cell contacts. The EMBO journal. 2002;21:4885–4895. doi: 10.1093/emboj/cdf497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hayashi M, Majumdar A, Li X, Adler J, Sun Z, Vertuani S, Hellberg C, Mellberg S, Koch S, Dimberg A, Koh GY, Dejana E, Belting HG, Affolter M, Thurston G, Holmgren L, Vestweber D, Claesson-Welsh L. Ve-ptp regulates vegfr2 activity in stalk cells to establish endothelial cell polarity and lumen formation. Nature communications. 2013;4:1672. doi: 10.1038/ncomms2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mellberg S, Dimberg A, Bahram F, Hayashi M, Rennel E, Ameur A, Westholm JO, Larsson E, Lindahl P, Cross MJ, Claesson-Welsh L. Transcriptional profiling reveals a critical role for tyrosine phosphatase ve-ptp in regulation of vegfr2 activity and endothelial cell morphogenesis. FASEB J. 2009;23:1490–1502. doi: 10.1096/fj.08-123810. [DOI] [PubMed] [Google Scholar]
- 52.Hernandez MV, Sala MG, Balsamo J, Lilien J, Arregui CO. Er-bound ptp1b is targeted to newly forming cell-matrix adhesions. Journal of cell science. 2006;119:1233–1243. doi: 10.1242/jcs.02846. [DOI] [PubMed] [Google Scholar]
- 53.Zhang X, Lanahan AA, Simons M. Vegfr2 trafficking: Speed doesn't kill. Cell Cycle. 2013;12:2163–2164. doi: 10.4161/cc.25536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eden ER, White IJ, Tsapara A, Futter CE. Membrane contacts between endosomes and er provide sites for ptp1b-epidermal growth factor receptor interaction. Nature cell biology. 2010;12:267–272. doi: 10.1038/ncb2026. [DOI] [PubMed] [Google Scholar]
- 55.Romsicki Y, Reece M, Gauthier JY, Asante-Appiah E, Kennedy BP. Protein tyrosine phosphatase-1b dephosphorylation of the insulin receptor occurs in a perinuclear endosome compartment in human embryonic kidney 293 cells. The Journal of biological chemistry. 2004;279:12868–12875. doi: 10.1074/jbc.M309600200. [DOI] [PubMed] [Google Scholar]
- 56.Abella JV, Park M. Breakdown of endocytosis in the oncogenic activation of receptor tyrosine kinases. American journal of physiology. Endocrinology and metabolism. 2009;296:E973–E984. doi: 10.1152/ajpendo.90857.2008. [DOI] [PubMed] [Google Scholar]
- 57.Stuible M, Abella JV, Feldhammer M, Nossov M, Sangwan V, Blagoev B, Park M, Tremblay ML. Ptp1b targets the endosomal sorting machinery: Dephosphorylation of regulatory sites on the endosomal sorting complex required for transport component stam2. The Journal of biological chemistry. 2010;285:23899–23907. doi: 10.1074/jbc.M110.115295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Polo S, Di Fiore PP. Endocytosis conducts the cell signaling orchestra. Cell. 2006;124:897–900. doi: 10.1016/j.cell.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 59.von Zastrow M, Sorkin A. Signaling on the endocytic pathway. Current opinion in cell biology. 2007;19:436–445. doi: 10.1016/j.ceb.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mavria G, Vercoulen Y, Yeo M, Paterson H, Karasarides M, Marais R, Bird D, Marshall CJ. Erk-mapk signaling opposes rho-kinase to promote endothelial cell survival and sprouting during angiogenesis. Cancer cell. 2006;9:33–44. doi: 10.1016/j.ccr.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 61.Wheeler-Jones C, Abu-Ghazaleh R, Cospedal R, Houliston RA, Martin J, Zachary I. Vascular endothelial growth factor stimulates prostacyclin production and activation of cytosolic phospholipase a2 in endothelial cells via p42/p44 mitogen-activated protein kinase. FEBS letters. 1997;420:28–32. doi: 10.1016/s0014-5793(97)01481-6. [DOI] [PubMed] [Google Scholar]
- 62.Takahashi T, Ueno H, Shibuya M. Vegf activates protein kinase c-dependent, but ras-independent raf-mek-map kinase pathway for DNA synthesis in primary endothelial cells. Oncogene. 1999;18:2221–2230. doi: 10.1038/sj.onc.1202527. [DOI] [PubMed] [Google Scholar]
- 63.Ichise T, Yoshida N, Ichise H. Fgf2-induced ras-mapk signalling maintains lymphatic endothelial cell identity by upregulating endothelial-cell-specific gene expression and suppressing tgfbeta signalling through smad2. Journal of cell science. 2014;127:845–857. doi: 10.1242/jcs.137836. [DOI] [PubMed] [Google Scholar]
- 64.Gourlaouen M, Welti JC, Vasudev NS, Reynolds AR. Essential role for endocytosis in the growth factor-stimulated activation of erk1/2 in endothelial cells. The Journal of biological chemistry. 2013;288:7467–7480. doi: 10.1074/jbc.M112.446401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Howe CL, Valletta JS, Rusnak AS, Mobley WC. Ngf signaling from clathrin-coated vesicles: Evidence that signaling endosomes serve as a platform for the ras-mapk pathway. Neuron. 2001;32:801–814. doi: 10.1016/s0896-6273(01)00526-8. [DOI] [PubMed] [Google Scholar]
- 66.Delcroix JD, Valletta JS, Wu C, Hunt SJ, Kowal AS, Mobley WC. Ngf signaling in sensory neurons: Evidence that early endosomes carry ngf retrograde signals. Neuron. 2003;39:69–84. doi: 10.1016/s0896-6273(03)00397-0. [DOI] [PubMed] [Google Scholar]
- 67.Kuruvilla R, Ye H, Ginty DD. Spatially and functionally distinct roles of the pi3-k effector pathway during ngf signaling in sympathetic neurons. Neuron. 2000;27:499–512. doi: 10.1016/s0896-6273(00)00061-1. [DOI] [PubMed] [Google Scholar]
- 68.Teis D, Taub N, Kurzbauer R, Hilber D, de Araujo ME, Erlacher M, Offterdinger M, Villunger A, Geley S, Bohn G, Klein C, Hess MW, Huber LA. P14-mp1-mek1 signaling regulates endosomal traffic and cellular proliferation during tissue homeostasis. J Cell Biol. 2006;175:861–868. doi: 10.1083/jcb.200607025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Teis D, Wunderlich W, Huber LA. Localization of the mp1-mapk scaffold complex to endosomes is mediated by p14 and required for signal transduction. Developmental cell. 2002;3:803–814. doi: 10.1016/s1534-5807(02)00364-7. [DOI] [PubMed] [Google Scholar]
- 70.Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Luthi U, Barberis A, Benjamin LE, Makinen T, Nobes CD, Adams RH. Ephrin-b2 controls vegf-induced angiogenesis and lymphangiogenesis. Nature. 2010;465:483–486. doi: 10.1038/nature09002. [DOI] [PubMed] [Google Scholar]
- 71.Sawamiphak S, Seidel S, Essmann CL, Wilkinson GA, Pitulescu ME, Acker T, Acker-Palmer A. Ephrin-b2 regulates vegfr2 function in developmental and tumour angiogenesis. Nature. 2010;465:487–491. doi: 10.1038/nature08995. [DOI] [PubMed] [Google Scholar]
- 72.Schenck A, Goto-Silva L, Collinet C, Rhinn M, Giner A, Habermann B, Brand M, Zerial M. The endosomal protein appl1 mediates akt substrate specificity and cell survival in vertebrate development. Cell. 2008;133:486–497. doi: 10.1016/j.cell.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 73.Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, Fang Q, Christ-Roberts CY, Hong JY, Kim RY, Liu F, Dong LQ. Appl1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nature cell biology. 2006;8:516–523. doi: 10.1038/ncb1404. [DOI] [PubMed] [Google Scholar]
- 74.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. Vegf receptor signalling - in control of vascular function. Nature reviews. Molecular cell biology. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 75.Sandilands E, Cans C, Fincham VJ, Brunton VG, Mellor H, Prendergast GC, Norman JC, Superti-Furga G, Frame MC. Rhob and actin polymerization coordinate src activation with endosome-mediated delivery to the membrane. Developmental cell. 2004;7:855–869. doi: 10.1016/j.devcel.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 76.Kasahara K, Nakayama Y, Kihara A, Matsuda D, Ikeda K, Kuga T, Fukumoto Y, Igarashi Y, Yamaguchi N. Rapid trafficking of c-src, a non-palmitoylated src-family kinase, between the plasma membrane and late endosomes/lysosomes. Experimental cell research. 2007;313:2651–2666. doi: 10.1016/j.yexcr.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 77.Horowitz A, Seerapu HR. Regulation of vegf signaling by membrane traffic. Cellular signalling. 2012;24:1810–1820. doi: 10.1016/j.cellsig.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]