Abstract
Objectives
Existing treatments for pediatric EoE effectively reduce inflammation. However, impact of treatment on health-related quality of life (HRQoL) over time for pediatric patients with EoE and their families has not been systematically assessed. We hypothesized that individualized multidisciplinary treatment would improve both child and family HRQoL over time, with improvements associated with decreased symptom severity.
Methods
Children with EoE treated in 4 tertiary care centers were enrolled. Baseline assessments occurred at the time of patients’ first evaluation; follow-up assessments occurred at 2 and 6 months after baseline. Presence and severity of 8 EoE symptoms were measured. HRQoL was measured with the PedsQL parent proxy-report (PR), child self-report (CR), and Family Impact Module (FIM). Statistical analyses used mixed-effects modeling to test changes over time for child and family HRQoL.
Results
Ninety-seven children were enrolled (ages 2–18 years; mean age, 7.7 yrs +/− 4.8 y; 78% male; 80% Caucasian). Baseline mean symptom number was 3.5 (SD, 2.3) and symptom severity was 5.5 (SD, 4.5). HRQoL scores were significantly related to symptom scores (P < 0.001). EoE symptom severity decreased during the study (P=0.03). PR PedsQL Total and FIM Total scores improved from baseline to 6 months (respectively, adjusted means 78.4 vs 81.0, P=0.0006; 68.9 vs 70.1, P=0.03). Interactions with baseline symptom severity revealed that subjects with lowest symptom severity showed the most improved HRQoL scores (P=0.0013).
Conclusions
HRQoL improved during the course of evaluation and treatment, with positive changes being strongest for patients with less symptom severity at baseline.
Keywords: pediatric, eosinophilic esophagitis (EoE), health-related quality of life (HRQoL), family impact
Introduction
Eosinophilic esophagitis (EoE) is an increasingly common, chronic disease of the esophagus, occurring in children and adults.1, 2 EoE is marked histologically by evidence of increased eosinophils in the esophagus and clinically by symptoms such as dysphagia, vomiting, and pain. Past studies identified the significant impact of symptoms and complications of EoE on health-related quality of life (HRQoL) of affected children and their families.3, 4 While treatments are effective in inducing clinicopathological remission,5–12 HRQoL over time for pediatric patients with EoE has not been systematically assessed.
Health-related QoL (HRQoL) is considered to be a primary outcome index for children with chronic medical illness since it focuses on patients’ subjective perceptions of their illness and its impact on psychological and social parameters including academics, social interactions, extracurricular activities and emotional functioning.13 Longitudinal studies have used generic HRQoL measures to evaluate treatment outcome for a variety of pediatric conditions, including epilepsy, cystic fibrosis, and type 1 diabetes.14–16 Ingerski and colleagues compared cross-sectional HRQoL data for pediatric patients with Eosinophilic Gastrointestinal Disorders (EGIDs) with other groups of chronically ill pediatric patients and demonstrated that the parents of patients with EGIDs perceived their children to have significantly lower emotional and school HRQoL scores than youth with cystic fibrosis, epilepsy, and type 1 diabetes.17 Using the generic core scales of the PedsQL, Cortina and colleagues found that pediatric patients with Eosinophilic Gastrointestinal Diseases (EGIDs) had significantly poorer HRQoL than healthy controls.18 This negative impact could be explained by patients experiencing persistent symptoms, following daily medical and/or dietary treatments, coping with the burden of elimination diets, and uncertainty about their future given the chronic nature of this disease.
Chronic diseases also impact the HRQoL of families of affected children.19 In fact, parents of chronically ill children with 10 different types of pediatric diseases had significantly lower HRQoL compared with parents of healthy children.20 Families caring for children with chronic diseases were found to have diminished HRQoL in their social life, vitality, sleep, decreased frequency of positive emotions and more depressive feelings. Mothers of children and adolescents with EGIDs had very high levels of stress related specifically to caring for their ill children.21 Reasons for this are unclear but could be explained by a number of factors including the stress involved with the daily monitoring of symptoms and administration of medications and complex diets, frequent participation in numerous medical visits and procedures, dealing with unknown long-term disease outcomes and coping with age-dependent patient behaviors that may develop as a consequence of the disease and treatments.22 Thus, studies suggest that EoE impacts both children and their parents’ HRQoL, but longitudinal measurements of this disease parameter are lacking.
To begin to address this problem, we measured HRQoL for children with EoE and their families at baseline entry into a multi-disciplinary program, and we followed them longitudinally. We hypothesized that treatment would reduce EoE symptoms, leading to an associated improvement in children and families’ HRQoL over time.
Materials and Methods
Participants
We performed a prospective, longitudinal, multi-centered study that enrolled subjects and their families who met the following characteristics: 1) established diagnosis of EoE with diagnosis based on previously identified criteria including: a) the presence of upper intestinal symptoms (e.g., feeding dysfunction, vomiting, heartburn, abdominal pain); b) greater than or equal to 15 eosinophils/high power field in the esophageal mucosa, c) treatment with proton pump inhibitors for 2 months prior to EoE diagnosis, and d) alternative causes for symptoms and eosinophilia, including gastroesophageal reflux disease (GERD), ruled out; 2) between the ages 2–18 years, and 3) new to tertiary care programs at one of four centers: Children’s Hospital Colorado/National Jewish Health, Aurora and Denver, CO; Children’s Hospital of Philadelphia, PA; Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Rady Children’s Hospital, San Diego, CA.
Enrollment, consenting and baseline assessment were completed in person during clinic visits, which allowed instruction for parents and children regarding questionnaire completion and age-appropriate supervision for children ages 5 and older for independent completion of the self-report HRQoL measure. In anticipation of home-based questionnaire completion, research assistants instructed children to respond to self-report questionnaires and they instructed parents to provide necessary help for young children (e.g., with reading) but to allow children to choose their own responses, and to follow this procedure when completing questionnaires at home. Baseline measures were administered at enrollment, with follow-up assessments occurring 2 and 6 months later. Follow-up assessments were web-based, using email and electronic questionnaires.1
All children received standard of care medical evaluations and treatments that included diet modifications and/or topical steroids as directed by each center’s multidisciplinary team. No treatment decisions were based on enrollment in this study. The study was approved by the human subjects review board at each participating site.
Measures
Subjects and families completed web-based questionnaires that included demographic information, medical history, EoE symptoms and their perceived severity, and HRQoL of subjects and their families. Symptoms were assessed using the EoE Clinical Symptom Score scale,23 a brief symptom questionnaire regarding current symptoms and symptom severity. Symptoms queried included 1) heartburn/regurgitation, 2) abdominal pain, 3) nocturnal symptoms, 4) nausea, 5) vomiting, 6) anorexia/early satiety, 7) difficulty swallowing liquids, and 8) difficulty swallowing solids. Symptom severity and frequency was rated as 0 = Not present, 1 = Mild to moderate symptoms on 1–3 days in the previous 2 weeks, 2 = Mild to moderate symptoms on more than 3 days in the previous 2 weeks, or 3 = Severe symptoms interrupting daily activities in the previous 2 weeks. Based on this, two symptom scores were calculated: the number of different symptoms reported present (possible range 0–8) and a severity score based on the sum of severity ratings for symptoms that were present (possible range 0–24).
Children’s HRQoL was assessed with an electronic version of the Pediatric Quality of Life Inventory (PedsQL 4.0), a reliable and validated instrument applicable across pediatric diseases for children ages 2–18 years.24–26 Age-specific PedsQL forms were used for children ages 2 – 4 years, 5 – 7 years, 8 – 12 years, and 13 – 18 years. Parent proxy scores were obtained for subjects ages 2–18 years, and self-report scores were obtained for children ages 5–18 years. This 23-item measure yields a Physical Health, Psychosocial Health and Total score. The Psychosocial Health scale represents the mean of Emotional, Social, and School Functioning subscales. Scores are converted to 100-point scales in which high scores indicate better HRQoL. Internal consistency coefficients for PedsQL 4.0 questionnaires at baseline were: Parent-proxy Total, α = 0.93, Physical Health, α = 0.90, and Psychosocial Health, α = 0.88, and Child self-report Total, α = 0.90, Physical Health, α = 0.83, and Psychosocial Health, α = 0.86. PedsQL PR and CR scores were significantly correlated for all scales and subscales (range of Spearman correlations: r = 0.52 to 0.72; P < 0.0001).
The PedsQL Family Impact Module (FIM)27 is a 36-item reliable and validated measure that was completed by the parent respondent to measure the impact of EoE on the parents and families of the study subjects. The FIM yields a Total score; a Parent HRQoL Summary score that includes subscales for Physical, Emotional, Social, and Cognitive Functioning, Communication, and Worry; and a Family Functioning Summary score that includes Daily Activities and Family Relationships. Scores are converted to 100-point scales, where high scores indicate better HRQoL. In a community sample, families of children with chronic illness had lower FIM scores than families whose children had no chronic illness.28 Internal consistency coefficients for FIM baseline scores in this study were: Total score, α = 0.97, Parent HRQoL Summary scale, α = 0.96, and Family Functioning Summary scale, α = 0.95.
Statistical analysis
Outcome measures were symptom scores, parent and child reports of HRQoL, and FIM scores. Score distributions at baseline were examined for normality, and parametric (t-tests, ANOVA, Tukey post hoc tests) and nonparametric procedures (chi square, Fisher’s Exact Test, Spearman’s rho) were used as appropriate. Spearman correlation coefficients were calculated to test whether quality of life scores at BL, 2 months, and 6 months were correlated with symptom number and severity scores from the same time point. The impact of treatment over time was tested using linear mixed-effects modeling, with symptom severity specific to each time point included as a covariate. This analytic strategy takes into account uneven follow-up times and correlations within subjects. In order to identify relevant covariates for modeling the impact of treatment over time, we examined baseline QoL and symptom scores in relation to demographic, medical history, and treatment variables. The demographic variables that were significant at the P < 0.05 level were then tested in the longitudinal mixed effects models and retained if the P < 0.05 level was maintained along with the time variable. Thus, the age group variable was retained in the longitudinal models associated with the family impact (FIM) scores, but not for the parent-proxy report or child self-report QoL scores. Interaction terms were also tested with the time variable to examine whether differential changes over time by severity score or child age were present. Subjects were required to have a baseline and 6-month follow-up in order to be included in the analysis. Two-month follow-ups were not required because the analysis strategy was able to take this into account without dropping subjects. All statistical tests were evaluated using SAS 9.2 (Copyright (c) 2002–2008 by SAS Institute Inc., Cary, NC, USA), and were 2-sided with an alpha level set at .05. No adjustment for type I error was applied for this observational study.
Results
Subject description
The sample included 97 children from 4 centers: Children’s Hospital Colorado/National Jewish Health (n=60); Children’s Hospital of Philadelphia (n=18); Cincinnati Children’s Hospital Medical Center (n=16), and Rady Children’s Hospital (n=3). Of 126 eligible subjects, 29 were lost to follow-up at the 6-month data point, leaving 97 subjects for analyses. Subjects not followed tended to be older (P = 0.08) and included a significantly smaller proportion of males (P = 0.006), but racial/ethnic background and maternal education level were not different. Number and severity of symptoms at baseline were not different from subjects followed (P = 0.82, P = 0.62, respectively). Baseline parent proxy (PR) and child self-report (CR) PedsQL Total scores were lower for those lost to follow-up (P = 0.03, P = 0.02, respectively), but Family Impact scores were not different (P = 0.60). Demographic characteristics for the sample are shown in Table 1.
Table 1.
Participant characteristics
| Mean (SD) or N (%) | |
|---|---|
| Child Age | 7.7 (4.8) |
| Child Age by Group | |
| Age 2 – 4 Years | 38 (39.2%) |
| Age 5 – 7 Years | 15 (15.5%) |
| Age 8 – 12 Years | 26 (26.8%) |
| Age 13 – 18 Years | 18 (18.6%) |
| Gendera | |
| Male | 73 (77.7%) |
| Female | 21 (22.3%) |
| Racea | |
| Caucasian | 75 (79.8%) |
| Other | 19 (20.2%) |
| Mother’s Educationb | |
| < High School | 6 (7.1%) |
| High School/Some College | 31 (36.5%) |
| College Graduate/Professional Degree | 48 (56.5%) |
| Father’s Educationc | |
| < High School | 6 (8.0%) |
| High School/Some College | 28 (37.3 %) |
| College Graduate/Professional Degree | 41 (54.7%) |
Missing data n=3;
missing data n=12;
missing data n=22
EoE Symptoms and Baseline Treatment
Table 2 shows the baseline frequency of specific EoE symptoms for the entire sample and by treatment group. At study baseline, 91% of subjects were receiving the EoE treatments queried, including swallowed steroids only (SS), dietary restrictions only (DR), or both dietary restrictions and swallowed steroids (Both). Nine percent were not yet receiving either treatment at baseline. Baseline treatment type was not associated with specific EoE symptoms nor with symptom number or severity scores. In general, children with any of the atopic conditions were more likely to be in the DR or Both treatments groups. Among 46 children with documentation of IgE-mediated food allergies, 42 (91%) were in the two groups that included dietary restrictions. There were no differences among treatment groups for child age, gender, race or parent educational level (data not shown).
Table 2.
Treatment at baseline for specific EoE symptom type, symptom burden scores and co-morbid atopic conditions
| Total | Swallowed Steroids (SS) | Dietary Restrictions (DR) | Both SS and DR | Neither SS nor DR | ||
|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | N (%) | P | |
| 97 (100) | 20 (20.6) | 35 (36.1) | 33 (34.0) | 9 (9.3) | ||
| Specific EoE Symptom | ||||||
| Abdominal Pain | 62 (63.9) | 15 (24.2) | 24 (38.7) | 19 (30.7) | 4 (6.5) | 0.329 |
| Heartburn/Regurg | 56 (57.7) | 14 (25.0) | 19 (33.9) | 18 (32.1) | 5 (8.9) | 0.658 |
| Anorexia/early satiety | 53 (54.6) | 12 (22.6) | 18 (34.0) | 21 (39.6) | 2 (3.8) | 0.143 |
| Nausea | 46 (47.4) | 11 (23.9) | 13 (28.3) | 18 (39.1) | 4 (8.7) | 0.446 |
| Nocturnal Symptoms | 38 (39.1) | 7 (18.5) | 19 (50.0) | 11 (29.0) | 1 (2.6) | 0.059 |
| Diff Swallow Solids | 38 (39.1) | 10 (26.3) | 11 (29.0) | 15 (39.5) | 2 (5.3) | 0.331 |
| Vomit | 24 (24.7) | 3 (12.5) | 6 (25.7) | 13 (54.2) | 2 (8.3) | 0.123 |
| Diff Swallow Liquids | 22 (22.6) | 6 (27.4) | 6 (29.3) | 8 (36.4) | 2 (9.1) | 0.736 |
| Co-morbid Atopic Conditions | ||||||
| Food Allergya | 46 (57.5) | 3 (6.5) | 18 (39.3) | 24 (52.2) | 1 (2.2) | <0.0001 |
| Eczema | 32 (33.0) | 2 (6.3) | 14 (43.8) | 14 (43.8) | 2 (6.3) | 0.039 |
| Asthma | 45 (46.4) | 6 (13.3) | 17 (37.8) | 19 (42.2) | 3 (6.7) | 0.204 |
| Allergic Rhinitis | 65 (67.0) | 14 (21.5) | 20 (30.8) | 27 (41.5) | 4 (6.2) | 0.068 |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||
| Symptom Burden | ||||||
| Number Symptoms | 97 | 3.9 (2.0) | 3.3 (2.6) | 3.7 (2.3) | 2.4 (1.5) | 0.38.9 |
| Sx Severity Score | 97 | 6.1 (4.1) | 5.6 (5.2) | 5.7 (4.0) | 3.2 (2.6) | 0.440 |
| No. Atopic Conditionsb | 1.5 (1.0) | 1.1 (0.97) | 1.5 (1.15) | 1.8 (0.81) | 1.0 (0.87) | 0.033c |
Food Allergy, n = 80;
Eczema, Asthma, Allergic Rhinitis;
post hoc by Student’s t: Both > SS & Neither, p < 0.05
Specific EoE symptoms were unrelated to overall symptom burden, measured by number of different EoE symptoms and symptom severity scores, but were weakly related to co-morbid atopic conditions (e-Table 1, http://links.lww.com/MPG/A338). Specific EoE symptoms were associated with varying levels of overall symptom burden. For example, abdominal pain was the most frequently occurring symptom, but children with abdominal pain had fewer other symptoms and low symptom severity scores. With mean ages of 5.9 and 6.1 years, respectively, children with nocturnal symptoms and vomiting were the youngest in the sample. Both indexes of overall symptom burden were negatively correlated with subjects’ age, r = −0.22 (P = 0.034) and r = −0.25 (P = 0.015), respectively.
Determinants of HRQoL scores for subjects and families
We examined baseline levels of HRQoL by subject age and co-morbid conditions. Neither PR nor CR PedsQL™ scores were associated with subject age. However, the Family Impact (FIM) summary scale scores were significantly correlated with child age, indicating that the older the child with EoE, the higher (better) were the scores for Total FIM (r = 0.34, P = 0.001), Parent HRQL (r = 0.28, P = 0.006) and Family Functioning (r = 0.35, P = 0.0004).
Examination of baseline HRQoL for children with co-morbid atopic conditions showed that subjects with asthma had significantly poorer HRQoL for PR and CR PedsQL and Family Impact summary scores in comparison with children without asthma (Table 5). For subjects with allergic rhinitis, significantly greater negative family impact was reported at baseline compared with those without allergies, but QoL scores for the children did not differ by either parent report or child self-report. Subjects with IgE-mediated food allergy and eczema did not have different baseline QoL levels for the children or their families.
Table 5.
Baseline PedsQL and Family Impact scores by co-morbid atopic condition
| Food Allergy | Eczema | Asthma | Allergic Rhinitis | |||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | |
| PedsQ Parent Report | N=46 | N=34 | N=32 | N=65 | N=45 | N=52 | N=65 | N=32 |
| Total Score | 73.8 (17.2) | 76.5 (16.7) | 76.6 (15.1) | 76.0 (18.3) | 70.5 (18.2) | 81.2 (14.7)b | 74.7 (17.0) | 79.4 (17.4) |
| Physical Health | 77.3 (20.9) | 81.0 (18.7) | 80.7 (19.6) | 80.0 (20.6) | 74.6 (21.9) | 85.2 (17.3)b | 79.2 (20.0) | 82.5 (20.8) |
| Psychosocial Functioning | 70.4 (16.4) | 72.0 (17.1) | 72.5 (13.1) | 72.0 (18.6) | 66.3 (17.3) | 77.3 (14.9)c | 70.2 (16.5) | 76.3 (17.3) |
| PedsQL Child Report | N=24 | N=25 | N=14 | N=45 | N=27 | N=32 | N=40 | N=19 |
| Total Score | 75.4 (15.5) | 78.0 (14.2) | 77.5 (11.3) | 78.7 (16.0) | 72.5 (16.3) | 83.4 (11.8)b | 76.9 (15.0) | 81.7 (14.6) |
| Physical Health | 79.3 (18.0) | 81.1 (14.2) | 78.3 (14.9) | 83.1 (16.0) | 75.7 (18.2) | 87.2 (11.1)b | 81.0 (16.3) | 84.0 (14.7) |
| Psychosocial Health | 71.5 (17.1) | 75.0 (16.8) | 76.7 (12.3) | 74.3 (18.5) | 69.3 (18.3) | 79.6 (14.7)a | 72.8 (17.4) | 79.3 (16.1) |
| Family Impact Scale | N=40 | N=34 | N=32 | N=65 | N=45 | N=52 | N=65 | N=32 |
| Total | 65.5 (20.0) | 68.9 (19.2) | 63.0 (19.9) | 68.8 (19.9) | 58.8 (21.1) | 74.0 (16.1)d | 63.3 (20.7) | 74.2 (16.6)a |
| Parent HRQL | 67.9 (20.0) | 70.2 (19.8) | 65.0 (19.5) | 70.8 (20.0) | 60.1 (21.0) | 76.5 (15.6)d | 65.2 (20.7) | 76.3 (16.2)b |
| Family Functioning | 67.3 (22.9) | 73.1 (24.6) | 66.1 (23.9) | 71.1 (24.0) | 59.7 (26.4) | 77.8 (17.9)d | 65.5 (25.6) | 77.4 (18.0)a |
P=0.02;
P< 0.01;
P< 0.001;
P< 0.0001, by t Test
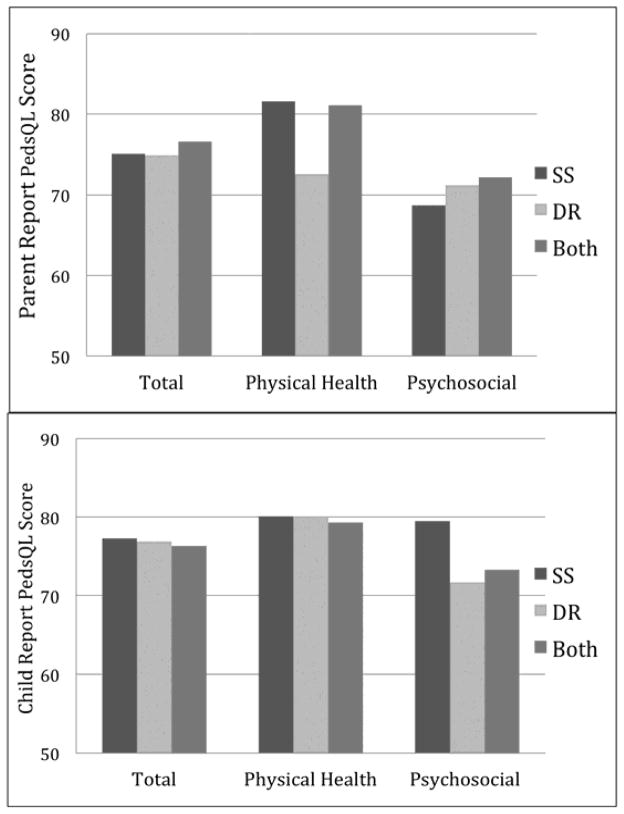
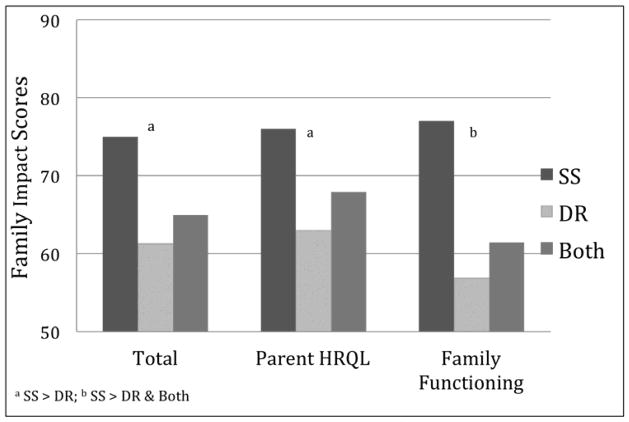
Baseline PedsQL summary and subscale scores did not differ for the SS, DR or Both treatment groups at baseline for either parent proxy report (PR) or child self-report (CR) PedsQL scores (Figure 1). In contrast, Family Impact baseline scores for the Total and Parent HRQL summary scales and the Physical Functioning subscale were higher for the SS group than the DR group, and the Family Functioning scale and Daily Activities subscale were better for the SS treatment group than for DR and Both groups (Figure 2 shows summary scales). See e-Table 2 (http://links.lww.com/MPG/A339) for HRQoL scores for the total sample and by treatment groups, including for subjects receiving no treatment at baseline. Overall, at baseline there was a clear pattern of lower FIM scores and greater impact on families where children were on dietary restrictions, regardless of whether they were also being treated with swallowed steroids.
Figure 1.
Parent Proxy and Child Self-Report PedsQL™ Summary Scales by Treatment Group at Baseline
Figure 2.
Family Impact Summary Scales by Treatment Group at Baseline
Symptoms and HRQoL scores at baseline, 2 months and 6 months
Table 3 shows raw scores for PedsQL PR, CR, and Family Impact scores at baseline, 2 months and 6 months. Table 4 illustrates that number of symptoms and symptom severity were negatively correlated with CR and PR PedsQL scores and with FIM scores. At each time point examined, the higher the symptom number and the more severe the symptoms, the lower the HRQoL score. These findings indicate that the worse a subject’s reported symptoms, the lower was their HRQoL as reported by themselves and their parents, and the greater was the impact on the family.
Table 3.
QoL scores and symptom burden at baseline and at 2- and 6-month follow-up
| Baseline Mean (SD) |
2 months Mean (SD) |
6 months Mean (SD) |
|
|---|---|---|---|
| PedsQL™ Parent-Proxy Report | N=97 | N=84 | N=97 |
| Total Score | 76.2 (17.2) | 79.1 (15.1) | 79.7 (15.8) |
| Physical Health | 80.3 (20.2) | 82.9 (16.9) | 83.9 (16.6) |
| Psychosocial Functioning | 72.2 (16.9) | 75.2 (16.1) | 75.7 (17.6) |
| Emotional Functioning | 65.6 (20.6) | 69.2 (19.0) | 69.7 (21.1) |
| Social Functioning | 82.8 (17.4) | 84.5 (16.8) | 85.7 (16.7) |
| School Functioning | 68.4 (19.8) a | 71.5 (22.3)b | 71.8 (20.2)c |
| PedsQL™ Child Self-Report | N=59 | N=51 | N=58 |
| Total Score | 78.4 (14.9) | 79.7 (16.1) | 81.0 (14.5) |
| Physical Health | 82.0 (15.7) | 83.5 (16.2) | 84.4 (15.7) |
| Psychosocial Functioning | 74.9 (17.1) | 76.0 (18.7) | 77.7 (17.2)) |
| Emotional Functioning | 72.2 (20.5) | 72.0 (21.7) | 77.0 (17.7)) |
| Social Functioning | 82.8 (17.4) | 85.0 (21.4) | 83.1 (21.7) |
| School Functioning | 68.9 (19.6) | 73.7 (22.4) | 75.1 (20.3) |
| Family Impact Module | N=97 | N=81 | N=97 |
| Total | 66.9 (20.0) | 67.6 (20.1) | 69.2 (20.8) |
| Parent HRQL | 68.9 (19.9) | 70.0 (20.1) | 71.1 (21.0) |
| Physical Functioning | 67.7 (23.3) | 68.8 (22.6) | 69.7 (22.5) |
| Emotional Functioning | 61.1 (22.5) | 64.7 (20.9) | 66.5 (21.5) |
| Social Functioning | 76.1 (24.3) | 75.3 (25.3) | 76.2 (25.4) |
| Cognitive Functioning | 72.8 (21.6) | 74.1 (20.6) | 73.9 (23.5) |
| Communication | 69.1 (24.5) | 66.4 (25.5) | 67.9 (24.8) |
| Worry | 45.5 (23.0) | 46.3 (23.8) | 50.2 (24.1) |
| Family Functioning | 69.4 (23.9) | 66.3 (25.2) | 69.7 (26.3) |
| Daily Activities | 65.1 (29.2) | 63.6 (28.6) | 66.5 (30.2) |
| Family Relationships | 74.7 (23.3) | 72.5 (23.1) | 76.3 (20.5) |
| Symptom Burden | |||
| Symptom number | 3.5 (2.3) | 3.2 (2.1) | 3.0 (2.1) |
| Symptom severity | 5.5 (4.5) | 5.1 (4.7) | 4.4 (4.0) |
Note: Due to young children not attending school,
n=88
n= 76;
n= 92
Table 4.
Spearman correlations for Symptom Number and Symptom Severity with QoL scores at baseline, 2 months and 6 months
| Baseline | 2 Months | 6 Months | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Sx No. | Sx Sev | Sx No. | Sx Sev | Sx No. | Sx Sev | |
| PedsQL™ Parent proxy report | N = 97 | N = 84 | N = 97 | |||
| Total score | −0.52 | −0.51 | −0.43 | −0.34b | −0.46 | −0.54 |
| Physical Health | −0.50 | −0.49 | −0.45 | −0.38c | −0.34c | −0.44 |
| Psychosocial Functioning | −0.50 | −0.48 | −0.34b | −0.25a | −0.49 | −0.53 |
| PedsQL™ Child self report | N = 59 | N = 51 | N = 58 | |||
| Total score | −0.59 | −0.57 | −0.46 | −0.41 | −0.51 | −0.64 |
| Physical Health | −0.56 | −0.54 | −0.51c | −0.43 | −0.45c | −0.52 |
| Psychosocial Functioning | −0.51 | −0.49 | −0.41b | −0.37 | −0.51 | −0.61 |
| Family Impact Module | N = 97 | N = 84 | N = 97 | |||
| Total | −0.52 | −0.55 | −0.36c | −0.39c | −0.49 | −0.59 |
| Parent HRQL | −0.50 | −0.51 | −0.41c | −0.39c | −0.40 | −0.52 |
| Family Functioning | −0.43 | −0.46 | −0.31b | −0.32b | −0.45 | −0.51 |
All P-values p < 0.0001, except:
P < 0.05,
P < 0.01,
P < 0.001
Symptom changes over time
To determine whether symptoms changed after entry into a multidisciplinary program, we modeled symptom number and symptom severity scores from baseline through 6 months, controlling for child age. Across all subjects, number of different symptoms did not decrease significantly from BL to 6 months (P = 0.08; adjusted means (SE): BL = 3.4 (0.22); 2m = 3.2 (0.21); 6m = 3.0 (0.25)). However, during this time course, symptom severity scores decreased significantly (P = 0.03; adjusted means (SE): BL = 5.3 (0.44); 2 m = 5.0 (0.41); 6 m = 4.4 (0.49).
HRQoL changes over time
To evaluate the changes in HRQoL over time, we modeled the PedsQL PR, CR and FIM scores from baseline through 6 months follow-up, including baseline symptom severity as a covariate and examining whether starting symptom severity influenced changes of QoL over time by including an interaction term with time. After adjusting for baseline symptom severity, PR PedsQL Total scores and Psychosocial Functioning scale scores increased significantly over time (P = 0.0006; P = 0.0002), although the magnitude of the increases was small (Total: adjusted means at median symptom severity (SE) BL = 78.4 (1.4); 2m = 79.2 (1.3); 6m = 81.0 (1.4); Psychosocial: adjusted means (SE), BL = 74.3 (1.5); 2m = 75.2 (1.4); 6m = 77.1 (1.5). The PR Physical Health score did not increase significantly over time (P = 0.07).
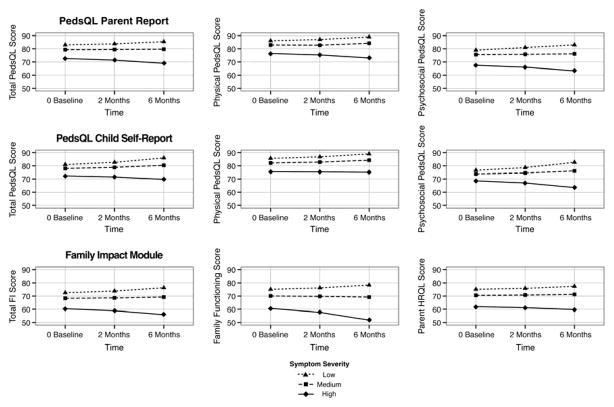
Within the model, symptom severity scores corresponding with follow-up points were strongly and significantly related to PR PedsQL scores over the course of the study (Total: P < 0.0001, Physical: P < 0.0001, Psychosocial: P = 0.0002), suggesting that changes in symptom severity were paralleled by changes in HRQoL scores. For the PR Total and Psychosocial scales, there was a significant interaction for Time*BL symptom severity (PR Total: P = 0.0031; PR Psychosocial: P = 0.0002), indicating that subjects with lower symptom severity scores at baseline had significantly greater improvements in QoL, in comparison with subjects with higher symptom severity scores, for whom QoL improved less or even decreased over time (Figure 3).
Figure 3.
HRQoL outcomes in relation to Low, Medium and High baseline symptom severity
For the CR PedsQL scores, none of the scores changed significantly over time after adjusting for baseline symptom severity. CR PedsQL Total, Physical and Psychosocial scale scores were strongly related only to symptom severity (CR Total: P = 0.0002, Physical: P = 0.002, Psychosocial: P = 0.0002). Figure 3 shows that while Time*BL symptom severity interactions were not statistically significant, the same pattern over time was evident as with the parent reported scores, where subjects with the lowest symptom severity scores at baseline had the greatest improvement in HRQoL.
Finally, we assessed the relationship between symptoms and Family Impact scores for the Total, Parent HRQL and Family Functioning scales over time, adjusting for both symptom severity and subject age. With child age controlled, the mean FIM Total score increased significantly over time (P = 0.03; adjusted means (SE), BL= 68.9 (1.8); 2m = 69.3 (1.6); 6m = 70.1 (1.8)), while there was no significant change with time alone for the Family Functioning or Parent HRQL scores (P = 0.12, P = 0.24). However, Time*symptom severity interactions were significant for FIM Total and Family Functioning scores (P = 0.014; P = 0.01), indicating that the level of subjects’ symptom severity at baseline was significantly associated with changes in family scores over time in a pattern similar to parents’ ratings of children’s HRQoL over time.
Discussion
In this first longitudinal assessment of HRQoL of children and families with EoE, we identified four findings relevant to their care. First, subject age, EoE symptom burden, atopic co-morbidities, and treatment type were associated with baseline ratings of child HRQoL and family impact. Second, EoE symptom severity scores decreased during the study, although number of symptoms did not. Third, symptom burden scores were consistently correlated with HRQoL scores at baseline and follow-up time points. Finally, HRQoL improved during the course of evaluation and treatment, with positive changes being strongest for patients with lower symptom severity at baseline. Together, these results suggest that assessment of and attention to QoL in the care of EoE patients may be beneficial.
In this study, we used standardized measures of HRQoL, the PedsQL Generic Core Scales and Family Impact Module, to assess our subjects and families. At baseline, HRQoL scores reported by both parents and children were generally lower than those of healthy children.29 Although generic measures such as the PedsQL allow comparisons across diseases, the PedsQL generic scales were not designed to assess disease specific physical and psychosocial challenges faced by children with EoE. Nevertheless, in this study all HRQoL scores were strongly related to the number and severity of EoE symptoms at baseline, at 2 months, and at 6 months. This is consistent with other studies of pediatric chronic illness that report a strong association between symptoms and diminished QoL.13, 30
Our results indicated that while PedsQL scores were generally similar to children with other GI disease,17, 29, 31 they were higher than scores reported for children with other EGIDs, such as eosinophilic gastritis, gastroenteritis and colitis.17, 18 Our study focused solely on patients with EoE, a subset of EGIDs that may not carry as much clinical morbidity because of the less extensive involvement of the GI tract. In this regard, patients with other EGIDs may have additional symptoms such as bleeding and diarrhea and may need to be on chronic systemic steroid treatments or require more extensive testing including colonoscopy. Thus, it is not surprising that our patients with EoE have higher HRQoL than those with other EGIDs who have more extensive disease.
In this study of pediatric EoE patients, 79% of the sample had at least one atopic co-morbid condition. The high prevalence of co-morbid atopic disease appeared to contribute HRQoL levels in several ways. Children with asthma had lower child and family QoL at baseline than children without asthma, and those with allergic rhinitis had lower family QoL than those with no allergies. Children with more co-morbid atopic conditions were more likely to be in the group being treated with both swallowed steroids and dietary restrictions. Although having IgE-mediated food allergy was not associated with lower baseline QoL, children with food allergy were more likely to be receiving treatment that included dietary restrictions. In turn, families of children being treated with dietary restrictions at baseline rated greater family impact, with lower QoL scores in multiple domains. Unfortunately, in this study we were not able to distinguish food restrictions directed at IgE-mediated food allergies versus those directed at food sensitivities identified as contributing to EoE inflammation, nor were we able to distinguish the QoL impact of varying levels of dietary restrictions. Although IgE-mediated food allergies and EoE food sensitivities are confounded in everyday life, future studies may be able to separate the influence on HRQoL of food avoidance related to IgE-mediated food allergies from food restrictions as treatment for EoE.
Over the course of the 6 months, our study showed statistically significant decrease in mean symptom severity scores. Despite the mean decrease, treatment impact varied across subjects, and a subset of patients experienced an increase in symptom severity. Similarly, our study showed a statistically significant improvement in HRQoL as measured with the parent reported PedsQL Total and Psychosocial Health scores as well as with the Family Impact Module Total score. Although the mean changes in HRQoL scores did not meet criteria for minimal clinically important improvement,32 the heterogeneity of QoL changes raises questions about factors affecting improved versus decreased HRQoL. Type of treatment, included in the statistical modeling of HRQoL scores, did not have a differential effect on QoL outcomes over time, nor did atopic co-morbidities or specific EoE symptom type. Importantly, baseline data from this observational study suggested that treatment strategies were determined by subjects’ disease status and co-morbidities, consistent with the individualized treatment approaches taken by the participating multidisciplinary teams.
For this follow-up of children with EoE, symptom severity at the time of study entry was the variable most strongly associated with patterns of change in HRQoL, with lower symptom severity at baseline associated with significant increase in QoL over time. The fact that many patients were in treatment at baseline may explain initial correlations between symptoms and HRQoL, but it does not explain why patients with more severe symptoms experienced a decrease in QoL, while those with less severe symptoms reported increased QoL over time. The patients with less symptom severity might have had improved QoL due to the psychological benefits of greater medical attention; the occurrence of placebo responses is well known. Alternatively, patients with high symptom levels may have had previous treatment failure and associated lower QoL. Such patients may have sought further opinions at a tertiary care center and have recalcitrant disease. Another possibility is that treatment was effective in reducing eosinophil levels and other signs of active disease, but those reductions were not associated with perceived symptom reduction. Several studies have documented a lack of agreement between EoE symptom reports and “objective” indexes of disease activity, particularly after treatment has been initiated.23, 33 Because histologic data were not obtained over time in this study, we were unable to examine these relationships. Finally patients with EoE can also experience functional abdominal pain that can be challenging to recognize and treat.
Other studies of pediatric conditions have used the PedsQL Generic Core Scales to measure changes in HRQoL over time. Significant HRQoL improvements have more often been reported with the resolution of acute conditions as compared with chronic illness. For example, PedsQL scores improved significantly from baseline to follow-up for children presenting to an orthopedic clinic for the treatment of fractures.34 In contrast, longitudinal assessments of children with chronic diseases such as cystic fibrosis and epilepsy found little change in overall health status or HRQoL over time, but did identify conditions or treatments with differential effects on outcomes.14, 15 It has become clear that pediatric EoE is a chronic disease, and similar to other chronic diseases, it has a waxing and waning course, a variable time frame for symptom and QoL improvement, a complex relationship between disease activity and symptom experience, and a plethora of patient and family psychosocial factors associated with treatment outcome. Finally, it is possible that the generic HRQoL instrument we used in our study is insufficiently sensitive to EoE-specific concerns for children and for families. The physical and psychosocial items queried with the Generic PedsQL Core Scales and Family Impact Module are not specific to symptoms or to emotional and social challenges experienced by children with EoE, but rather assess general domains of functioning. The recently developed PedsQL EoE Module will allow EoE-specific HRQoL assessment in future studies.35 Nevertheless, while use of the generic HRQoL instruments might be considered a shortcoming in our study, it allowed exploration of the QoL impact of allergic conditions as they co-occur with pediatric EoE, resulting in combined effects on HRQoL.
We demonstrated a number of differences related to age within this sample of children with EoE. Younger children had more symptoms and greater symptom severity. Child age varied with specific EoE symptoms experienced, consistent with previous reports for children with EoE.36–38 Family Impact Total, Parent HRQL, and Family Functioning scores were lowest for the youngest children, with progressively less impact with older children. For parents of young children, there is a high frequency of illness related tasks that are demanding of caregiver time and energy, in addition to intensive parenting demands that occur for healthy children. This is consistent with reports that Pediatric Parenting Stress is greater with younger child age.39
Parents’ responses may be affected not only by more general caregiving tasks for younger children, but also by the number and types of symptoms experienced by children with EoE.40 The youngest children in the sample had nocturnal symptoms, which have a major impact on parents’ HRQoL. Vomiting and anorexia/early satiety, often associated with feeding difficulties, also commonly occurred among the youngest children, consistent with previous reports of EoE symptom presentation in young children.41 Feeding difficulties in other pediatric chronic diseases have been associated with increased parenting stress, as shown for families coping with mealtime behavior problems in their 1–6 year-olds’ type 1 diabetes.42 Abdominal pain was highly prevalent in our sample with 64% of patients reporting this symptom despite being in treatment. Parents of children with chronic pain report significant impact on their own and their families’ functioning.30 Caregiver burden and emotional impact on the family may be decreased not only because of young children’s caregiving needs, but also because of emotional strains experienced by parents of children with nighttime symptoms, food refusal and chronic pain.
While our study is revealing in that it identifies the longitudinal impact of EoE on children and their families, our findings should be interpreted with a number of considerations in mind. We used generic measures of quality of life, which may have limited responsiveness for assessing EoE specific concerns. Patients were self-referred to centers so the population examined might represent a group with more active/difficult to treat EoE, higher levels of anxiety surrounding the condition, or greater co-morbid diseases. These sample characteristics might limit the generalizability of our findings. In some circumstances, a higher level of education about the disease and reassurances from providers in a tertiary care setting might provide an improved HRQoL outside of actual pharmacological/diet based treatments. We could not provide an untreated control group to identify the impact of disease activity on HRQoL. This would only be possible to do by assessing HRQoL prior to treatment and, as we conducted this study in referral centers, this was not possible. We attempted to provide a broad assessment of HRQoL by obtaining both patient and parent input, but responder bias remains a problem. In our sub-analyses examining subject characteristics and HRQoL, we conducted multiple comparisons, and some of these findings may be due to chance. Finally, validated objective measures of EoE disease activity are currently lacking, leaving only child or parent symptom reports as indexes of changes in disease status.
In conclusion, we report that patients and families with EoE who undergo evaluation in a tertiary care EoE center have diminished QoL compared to healthy individuals, diminished QoL is associated with increased symptom severity and, on average, QoL improves over time. Ongoing and new investigations are needed to address specific family factors associated with HRQoL and the impact of specific treatments and their side effects on QoL. The development of EoE specific tools for assessing quality of life and improved measures of EoE disease severity will add immensely to the ability to determine relationships between disease, the manner in which children and parents cope with it, and its impact on HRQoL for both children with EoE and their families.
Supplementary Material
Acknowledgments
Funding Source: This research was conducted with support from the Investigator-Sponsored Study Program of AstraZeneca, LP and was supported by the NIH/NCRR Colorado CTSI Grant Number UL1 RR025780. This work was also supported by K24 DK100303 (GTF).
Footnotes
A REDCap (http://redcapinfo.ucdenver.edu/) electronic version of the PedsQL™ was developed by the Behavioral Medicine Core Laboratory, Clinical & Translational Research Center, Colorado Clinical & Translational Studies Institute (UL1 TR000154), with permission from and in consultation with Dr. James Varni. Appropriate licenses were obtained for use of the PedsQL™. The REDCap electronic version was formatted to be as close as possible to the original paper questionnaires, and development followed procedures previously used by Dr. Varni in creating an electronic version of the PedsQL™. 25
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jpgn.org).
The authors report no conflicts of interest.
Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
References
- 1.Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterol. 2007;133:1342–63. doi: 10.1053/j.gastro.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 2.Liacouras C, Furuta G, Hirano I, et al. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3–20. doi: 10.1016/j.jaci.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 3.Flood EM, Beusterien KM, Amonkar MM, et al. Patient and caregiver perspective on pediatric eosinophilic esophagitis and newly developed symptom questionnaires. Curr Med Res Opin. 2008;24:3369–81. doi: 10.1185/03007990802536900. [DOI] [PubMed] [Google Scholar]
- 4.Franciosi J, Hommel K, DeBrosse C, et al. Quality of life paediatric eosinophilic oesophagities: what is important to patients? Child Care Health Dev. 2011 doi: 10.1111/j.1365-2213.2011.01265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aceves SS, Furuta GT, Spechler SJ. Integrated approach to treatment of children and adults with eosinophilic esophagitis. Gastrointest Endosc Clin N Am. 2008;18(1):195–217. xi. doi: 10.1016/j.giec.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Dellon E, Sheikh A, Speck O, et al. Viscous topical is more effective than nebulized steroid therapy for patients with eosinophilic esophagitis. Gastroenterol. 2012;143:321–4. doi: 10.1053/j.gastro.2012.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonsalves N, Yang G, Doerfler B, et al. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterol. 2012;142:1451–9. doi: 10.1053/j.gastro.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Kagalwalla A, Sentongo T, Ritz S, et al. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2006;4(9):1097–102. doi: 10.1016/j.cgh.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 9.Konikoff MR, Noel RJ, Blanchard C, et al. A randomized, double blind, placebo-controlled trial of fluticasone propionate for pediatric eosinophilic esophagitis. Gastroenterol. 2006;131(5):1381–91. doi: 10.1053/j.gastro.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 10.Spergel J, Brown-Whitehorn T, Cianferoni A, et al. Identification of causative foods in children with eosinophilic esophagitis treated with an elimination diet. J Allergy Clin Immunol. 2012;130:461–7. doi: 10.1016/j.jaci.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 11.Straumann A, Conus S, Degen L, et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterol. 2010;139:1526–37. doi: 10.1053/j.gastro.2010.07.048. [DOI] [PubMed] [Google Scholar]
- 12.Teitelbaum J, Fox V, Twarog F, et al. Eosinophilic esophagitis in children: immunopathological analysis and response to fluticasone propionate. Gastroenterol. 2002;122:1216–25. doi: 10.1053/gast.2002.32998. [DOI] [PubMed] [Google Scholar]
- 13.Maity S, Thomas AG. Quality of life in paediatric gastrointestinal and liver disease: a systematic review. J Pediatr Gastroenterol Nutr. 2007;44:540–54. doi: 10.1097/MPG.0b013e3180332df0. [DOI] [PubMed] [Google Scholar]
- 14.Modi A, Ingerski L, Rausch J, et al. Treatment factors affecting longitudinal quality of life in new onset pediatric epilepsy. J Pediatr Psychol. 2011;36:466–75. doi: 10.1093/jpepsy/jsq114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sawicki G, Rasouliyan L, McMullen A, et al. Longitudinal assessment of health-related quality of life in an observational cohort of patients with cystic fibrosis. Pediatr Pulmonol. 2011;46:36–44. doi: 10.1002/ppul.21325. [DOI] [PubMed] [Google Scholar]
- 16.Hilliard M, Goeke-Morey M, Cogen F, et al. Predictors of diabetes-related quality of life after transitioning to the insulin pump. J Pediatr Psychol. 2009;34:137–46. doi: 10.1093/jpepsy/jsn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ingerski L, Modi A, Hood K, et al. Health-Related Quality of Life across pediatric chronic conditions. J Pediatr. 2010;156:639–44. doi: 10.1016/j.jpeds.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cortina S, McGraw K, deAlarcon A, et al. Psychological functioning of children and adolescents with eosinophil-associated gastrointestinal disorders. Child Health Care. 2010;39:266–78. doi: 10.1080/02739615.2010.515927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stein R, Riessman C. The development of an Impact-on-Family Scale: Preliminary findings. Medical Care. 1980;18:465–72. doi: 10.1097/00005650-198004000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Hatzmann J, Heymans H, Ferrer-i-Carbonell A, et al. Hidden consequences of success in pediatrics: parental health-related quality of life—results from the Care Project. Pediatrics. 2008;122:e1030–8. doi: 10.1542/peds.2008-0582. [DOI] [PubMed] [Google Scholar]
- 21.Taft T, Ballou S, Keefer L. Preliminary evaluation of maternal caregiver stress in pediatric eosinophilic gastrointestinal disorders. J Pediatr Psychol. 2012;37:523–32. doi: 10.1093/jpepsy/jsr118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klinnert MD. Psychological Perspectives on Pediatric Eosinophilic Esophagitis. In: Liacouras CA, Markowitz JE, editors. Eosinophilic Esophagitis. New York: Springer; 2012. pp. 375–394. [Google Scholar]
- 23.Aceves SS, Newbury RO, Dohil MA, et al. A symptom scoring tool for identifying pediatric patients with eosinophilic esophagitis and correlating symptoms with inflammation. Ann Allergy Asthma Immunol. 2009;103:401–6. doi: 10.1016/S1081-1206(10)60359-6. [DOI] [PubMed] [Google Scholar]
- 24.Varni J, Seid M, Kurtin P. The PedsQL 4.0: Reliability and validity of the Pediatric Quality of Life Inventory Version 4. 0 generic core scales in healthy and patient populations. Medical Care. 2001;39:800–12. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Varni J, Limbers C, Burwinkle T, et al. The e-PedsQL™ in Type 1 and Type 2 Diabetes: Feasibility, reliability, and validity of the Pediatric Quality of Life Inventory™ Internet administration. Diabetes Care. 2008;31:672–7. doi: 10.2337/dc07-2021. [DOI] [PubMed] [Google Scholar]
- 26.Young N, Varni J, Snider L, et al. The internet is valid and reliable for child-report: An example using the Activities Scale for Kids (ASK) and the Pediatric Quality of Life Inventory (PedsQL) J Clin Epidemiol. 2009;62:314–20. doi: 10.1016/j.jclinepi.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Varni J, Sherman S, Burwinkle T, et al. The PedsQL Family Impact Module: Preliminary reliability and validity. Health Qual Life Outcomes. 2004;2:55. doi: 10.1186/1477-7525-2-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medrano G, Berlin K, Hobart Davies W. Utility of the PedsQL™ family impact module: assessing the psychometric properties in a community sample. Qual Life Res. 2013 doi: 10.1007/s11136-013-0422-9. [DOI] [PubMed] [Google Scholar]
- 29.Varni JW, Limbers CA, Burwinkle TM. Impaired health-related quality of life in childeren and adolescents with chronic conditions: A comparative analysis of 10 disease clusters and 33 disease categories/severities utilizing the PedsQL 4. 0. Generic Core Scales Health Qual Life Outcomes. 2007;5(43):1–15. doi: 10.1186/1477-7525-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mano K, Khan K, Ladwig R, et al. The impact of pediatric chronic pain on parents’ health-related quality of life and family functioning: Reliability and Validity of the PedsQL 4. 0 Family Impact Module. J Pediatr Psychol. 2011;36:517–27. doi: 10.1093/jpepsy/jsp099. [DOI] [PubMed] [Google Scholar]
- 31.Youssef NN, Langseder A, Verga BJ, et al. Chronic childhood constipation is associated with impaired quality of life: a case-controlled study. J Pediatr Gastroenterol Nutr. 2005;41:56–60. doi: 10.1097/01.mpg.0000167500.34236.6a. [DOI] [PubMed] [Google Scholar]
- 32.Varni J, Burwinkle T, Seid M, et al. The PedsQL™* 4. 0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. 2003;3:329–41. doi: 10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 33.Pentiuk S, Putnam PE, Collins MH, Rothenberg ME. Dissociation between symptoms and histological severity in pediatric eosinophilic esophagitis. J Pediatr Gstroenterol Nutr. 2009;48:152–60. doi: 10.1097/MPG.0b013e31817f0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Varni J, Seid M, Knight T, Uzark K, Szer I. The PedsQLTM 4. 0 Generic Core Scales: Sensitivity, responsiveness, and impact on clinical decision-making. J Behav Medicine. 2002;25:175–93. doi: 10.1023/a:1014836921812. [DOI] [PubMed] [Google Scholar]
- 35.Franciosi J, Hommel K, Bendo C, et al. PedsQL(™) Eosinophilic Esophagitis Module: Feasibility, reliability and validity. J Pediatr Gastroenterol Nutr. 2013;57:57–66. doi: 10.1097/MPG.0b013e31828f1fd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noel R, Putnam P, Rothenberg M. Eosinophilic esophagitis. N Engl J Med. 2004;351:940–1. doi: 10.1056/NEJM200408263510924. [DOI] [PubMed] [Google Scholar]
- 37.Pentiuk SP, Miller CK, Kaul A. Eosinophilic esophagitis in infants and toddlers. Dysphagia. 2007;22:44–8. doi: 10.1007/s00455-006-9040-9. [DOI] [PubMed] [Google Scholar]
- 38.Putnam PE. Eosinophilic esophagitis in children: clinical manifestations. Gastrointest Endosc Clin N Am. 2008;37(2):369–81. doi: 10.1016/j.gtc.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 39.Streisand R, Swift E, Wickmark T, RC, Holmes C. Pediatric parenting stress among parents of children with Type I Diabetes: The role of self-efficacy, responsibility, and fear. J Pediatr Psychol. 2005;30:513–21. doi: 10.1093/jpepsy/jsi076. [DOI] [PubMed] [Google Scholar]
- 40.Klinnert MD, Moore W, Miller J, et al. Impact of eosinophilic esophagitis on quality of life for youth and their families. J Allergy Clin Immunol. 2011;127:S:AB110. [Google Scholar]
- 41.Mukkada V, Haas A, Creskoff Maune N, et al. Feeding dysfunction in children with eosinophilic gastrointestinal diseases. Pediatrics. 2010;126:e1–e7. doi: 10.1542/peds.2009-2227. [DOI] [PubMed] [Google Scholar]
- 42.Powers S, Byars K, Mitchell M, et al. Parent report of mealtime behavior and parenting stress in young children with Type 1 Diabetes and in healthy control subjects. Diabetes Care. 2002;25:313–8. doi: 10.2337/diacare.25.2.313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.