Abstract
HIV-associated neurocognitive disorders (HAND) afflict about half of HIV-infected patients. HIV-infected cells shed viral proteins, such as the transactivator of transcription (Tat), which can cause neurotoxicity by over activation of NMDA receptors (NMDARs). Here, we show that Tat causes a time-dependent, biphasic change in NMDA-evoked increases in intracellular Ca2+ concentration ([Ca2+]i). NMDA-evoked responses were potentiated following 2 h exposure to Tat (50 ng/mL). Tat-induced potentiation of NMDA-evoked increases in [Ca2+]i peaked by 8 h and then adapted by gradually reversing to baseline by 24 h and eventually dropping below control by 48 h. Tat-induced potentiation of NMDA-evoked responses was blocked by inhibition of lipoprotein receptors (LRP) or Src tyrosine kinase. Potentiation was unaffected by inhibition of nitric oxide synthase (NOS). However, NOS activity was required for adaptation. Adaptation was also prevented by inhibition of soluble guanylate cyclase (sGC) and cGMP-dependent protein kinase (PKG). Together, these findings indicate that Tat potentiates NMDA-evoked increases in [Ca2+]i via LRP-dependent activation of Src and that this potentiation adapts via activation of the NOS/sGC/PKG pathway. Adaptation may protect neurons from excessive Ca2+ influx and could reveal targets for the treatment of HAND.
Introduction
Cognitive function is impaired in 30-55% of patients infected with human immunodeficiency virus (HIV) (Cysique et al. 2004, Tozzi et al. 2005, Heaton et al. 2011). HIV-associated neurocognitive disorders (HAND) range in severity from a subtle reduction in information processing speed to significant functional impairment (Antinori et al. 2007, Heaton et al. 2004). Despite effectively managing viral load with combined anti-retroviral therapy (cART), the prevalence of HAND remains persistently high (Heaton et al. 2010) and may be increasing due to prolonged patient lifespans. Currently, the efficacy of drugs to treat HAND is insufficient and the options are few.
In the brain, HIV infects macrophages and microglia, but not neurons (Watkins et al. 1990). Thus, HIV-induced neurotoxicity is indirect and results from the release of neurotoxic agents such as inflammatory cytokines, nitric oxide, glutamate, and viral proteins (Genis et al. 1992, Eugenin et al. 2007, Jiang et al. 2001, Nath 2002, Kaul et al. 2001). The transactivator of transcription (Tat) is a protein shed from HIV-infected cells and detected in the sera and CNS of HIV-infected patients (Chang et al. 1997, Hudson et al. 2000). The level of anti-Tat antibodies in the CSF of HIV-infected patients without cognitive dysfunction is higher than in patients with HAND, suggesting antibody responses against Tat may be neuroprotective (Bachani et al. 2013). Despite significant improvement in the efficacy of cART for treating HIV infection, current regimens remain unable to halt the production of Tat (Li et al. 2009).
Cognitive decline in patients with HAND correlates with synaptodendritic damage (Ellis et al. 2007). Expression of Tat in transgenic rodent models causes loss of excitatory synapses resulting in learning and memory impairment (Carey et al. 2012, Fitting et al. 2012). In vitro, Tat-induced synapse loss (Kim et al. 2008) and neuronal death (Eugenin et al. 2007) are initiated by NMDAR-mediated Ca2+ influx. Tat potentiates NMDA-evoked increases in intracellular Ca2+ concentration ([Ca2+]i) in hippocampal neurons (Haughey et al. 2001). Most studies of Tat- induced changes in NMDAR function are acute (min to h) while the neurotoxic effects of Tat occur over a prolonged time scale (h to days). Treating primary hippocampal neurons with Tat for 24 h causes loss of excitatory synapses (Kim et al. 2008) and simultaneous gain of inhibitory synapses (Hargus & Thayer 2013) indicating that Tat evokes adaptive changes in the synaptic composition of neurons. Such neuroadaptations may be a mechanism to cope with excess excitatory input. Notably, these adaptive changes are prevented by pharmacologic inhibition of the NMDAR (Shin et al. 2012) indicating that the NMDAR is essential for synaptic neuroadaptation. How NMDA-evoked [Ca2+]i responses are affected by prolonged exposure to Tat, when adaptive changes in synaptic composition occur, is the focus of this study.
Here, we examined changes in NMDA-evoked increases in [Ca2+]i during 48 h exposure to Tat. We found that Tat evoked a biphasic change in NMDA-evoked [Ca2+]i responses. Tat initially potentiated the NMDA-evoked increase in [Ca2+]i via the low-density lipoprotein receptor-related protein (LRP) and activation of Src kinase. Tat-induced potentiation subsequently adapted by gradually returning to baseline levels. Adaptation resulted from activation of the nitric oxide synthase (NOS) / soluble guanylate cyclase (sGC) / protein kinase G (PKG) signaling pathway. This study suggests a changing role for the NMDAR during the course of HIV neurotoxicity with implications for treatment of HAND.
Experimental Methods
Drugs and Reagents
Materials were obtained from the following sources: HIV-1 Tat (Clade B, full length recombinant) was from the NIH AIDS Research and Reference Reagent Program (HIV-1 Tat protein (full length, Clade B) from John Brady and DAIDS, NIAID and from Prospec Tany TechnoGene Ltd. (Rehovot, Israel); recombinant rat low-density lipoprotein receptor-related protein associated protein 1 (RAP) was from Fitzgerald Industries International (Concord, MA); Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum, horse serum, fura-2-acetomethyl ester (Fura-2-AM), and glycine were from Invitrogen (Carlsbad, CA); NMDA, NGNitro-L-Arginine Methyl Ester (L-NAME), and 1H-(1,2,4)oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) were from Sigma-Aldrich (St. Louis, MO); 4-amino-5-(4-chloro- phenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2) and 4-amino-7-phenylpyrazol[3,4-d]pyrimidine (PP3), 8R,9S,11S)-(-)-9-methoxy-carbamyl-8-methyl-2,3,9,10-tetrahydro-8,11-epoxy-1H,8H,11H-2,7b,11a-trizadibenzo-(a,g)-cycloocta-(c,d,e)-trinden-1-one (KT5823), and (1R*,2S*)-erythro-2-(4-Benzylpiperidino)-1-(4-hydroxyphenyl)-1-propanol hemitartrate (ifenprodil) were from Tocris (Bristol, United Kingdom); 2-(2-amino-3-methyoxyphenyl)-4 H-1-benzopyran-4-one (PD98059) was from Cell Signaling Technology (Danvers, MA); recombinant rat interleukin-1β was from R&D Systems (Minneapolis, MN).
DNA Constructs
Dominant-negative (DN)-Fyn with the K299M mutation and DN-Src with the K295M mutation in pRK5 were kindly provided by Dr. Filippo Giancotti (Memorial Sloan Kettering Cancer Center, New York, NY); pTagRFP-N was from Evrogen (Moscow, Russia). The catalytically inactive mutant of full-length PKG1α containing the amino terminal regulatory region fused to GFP (G1αR-GFP) in pEGFP-N1 (Clontech) was kindly provided by Darren Browning (Georgia Regents University). The G1αR fragment was removed via digestion with EcoR1 and BamH1 (New England Biolabs), and then inserted into the multiple cloning site of DsRed2-N1 (Clontech). Mammalian expression vectors containing GluN1, GluN2A, and GluN2B were kindly provided by Stephen Traynelis (Emory University).
Cell Culture
In accordance with the University of Minnesota's Institutional Animal Care and Use Committee and the NIH guide for the care and use of laboratory animals, maternal rats were euthanized by CO2 inhalation and fetuses were removed on embryonic day 17. Rat hippocampal neurons were grown in primary culture as described previously (Li et al. 2012). Hippocampi were dissected and placed in Ca2+- and Mg2+-free HEPES-buffered Hanks’ salt solution (HHSS), pH 7.45. HHSS contained the following (in mM): HEPES 20, NaCl 137, CaCl2 1.3, MgSO4 0.4, MgCl2 0.5, KCl 5.0, KH2PO4 0.4, Na2HPO4 0.6, NaHCO3 3.0, and glucose 5.6. Cells were dissociated by triturating through a 5 mL pipette and a flame-narrowed Pasteur pipette and then re-suspended in DMEM without glutamine, supplemented with 10% fetal bovine serum and penicillin/streptomycin (100 U/mL and 100 mg/mL, respectively). Dissociated cells were then plated at a density of 60,000 to 80,000 cells/dish onto a 25-mm-round cover glass (#1) pre-coated with matrigel (150 μL, 0.2 mg/mL). Neurons were grown in a humidified atmosphere of 10% CO2 at 37°C and fed on days 1 and 7 by exchange of 75% of the media with DMEM supplemented with 10% horse serum and penicillin/streptomycin. Cells used in these experiments were cultured without mitotic inhibitors resulting in a mixed glial-neuronal culture consisting of 18 ± 2% neurons, 70 ± 3% astrocytes, and 9 ± 3% microglia as indicated by immunocytochemistry (Kim et al. 2011). The cultures used for experimentation were grown for 12 to 15 days in vitro (DIV).
[Ca2+]i imaging
Intracellular Ca2+ concentration ([Ca2+]i) was recorded as previously described (Li et al. 2013) with minor modifications. Cells were loaded by incubation with 5 μM fura-2 AM in 0.04% pluronic acid in HHSS for 30 min at 37°C followed by washing in the absence of indicator for 10 min. HIV-1 Tat and respective drugs were present during fura-2-AM loading, but were absent during the wash. Coverslips containing fura-2 loaded cells were transferred to a recording chamber, placed on the stage of an Olympus IX71 microscope (Melville, NY), and viewed through a 20X objective. Excitation wavelength was selected with a galvanometer-driven monochromator (8-nm slit width) coupled to a 75-W xenon arc lamp (Optoscan; Cairn Research). [Ca2+]i was monitored using sequential excitation of fura-2 at 340 and 380 nm; image pairs were collected every 1 s. For experimental recordings, cells were superfused at a rate of 1-2 mL/min with HHSS for 1 min followed by 30 s perfusion of Mg2+-free HHSS that contained 200 μM glycine and either 10 or 100 μM NMDA. Fluorescence images (510/40 nm) were projected onto a cooled charge-coupled device camera (Cascade 512B; Roper Scientific) controlled by MetaFluor software (Molecular Devices). After background subtraction, the 340-and 380-nm image pairs were converted to [Ca2+] using the formula [Ca2+]i = Kdβ(R – Rmin)/(Rmax – R) (Grynkiewicz et al. 1985). The dissociation constant (Kd) for fura-2 was 145 nM. β is the ratio of fluorescence intensity acquired with 380 nm excitation measured in Ca2+-free buffer (1 mM EGTA) and buffer containing saturating Ca2+ (5 mM). R is 340 nm / 380 nm fluorescence intensity ratio. Rmin, Rmax, and β were determined in a series of calibration experiments on intact cells. Rmin and Rmax values were generated by applying 10 μM ionomycin in Ca2+-free buffer (1 mM EGTA) and saturating Ca2+ (5 mM), respectively. Values for Rmin, Rmax, and β were 0.37, 9.38, and 6.46, respectively. These calibration constants were applied to all experimental recordings. To generate pseudocolor images, a binary mask was generated by applying an intensity threshold to the 380 nm image and applied to [Ca2+]i images with colors assigned as indicated by the calibration bars in the figures. The neuronal cell body was selected as the region of interest for all recordings. All neurons within the imaging field were included in the analysis and no exclusions were made. For time course experiments, coverslips from the same cell culture plating were treated in parallel and each coverslip imaged only once.
Transfection
Rat hippocampal neurons were transfected between 11-12 days in vitro using a modification of a calcium phosphate protocol described previously (Li et al. 2012). Briefly, hippocampal cultures were incubated for 30 min in DMEM supplemented with 1 mM kynurenic acid, 10 mM MgCl2, and 5 mM HEPES. A DNA/calcium phosphate precipitate containing 1 μg plasmid DNA per well was prepared, allowed to form for 30 min at 21°C then added to the culture. After 90 min of incubation, cells were washed once with DMEM supplemented with MgCl2 and HEPES and then returned to conditioned media.
Biotinylation and immunoblotting
For biotinylation experiments, 7.5 × 106 hippocampal cells were plated in 100 × 20 mm petri dishes. Cells were treated with Tat alone or with an inhibitor of protein kinase G (KT5823) 1 h prior to the addition of Tat. Forty-eight hours later the media was removed and cells were washed three times with ice-cold PBS (pH 8.0) then incubated with 2 mM NHS-PEG4 Biotin (Thermo Fisher Scientific Inc.) in ice-cold PBS with slow shaking at 4 °C for 30 min protected from light. Cells processed in parallel but not treated with biotin served as negative controls. Biotin was gently removed and cells were washed four times with ice-cold PBS. Cells were collected in PBS and centrifuged at 10,000 × g for 1 min. Following centrifugation, supernatants were aspirated and pellets were re-suspended in 250 μL lysis buffer consisting of PBS, 10% Triton, 20% SDS, and a mixture of protease inhibitors (10 μL PMSF, 100 μL HALT). Samples were sonicated with 3 × 1 s pulses and incubated with rocking for 30 min at 4 °C. Debris was pelleted in a microcentrifuge at 14,000 × g at 4 °C for 15 min and supernatants retained. 30 μL of sample (“Total protein”) was removed, and the rest of the sample was mixed by rotation for 30 min at 4 °C with 60 μL of a 50% slurry of NeutrAvidin beads (Thermo Fisher Scientific Inc.). Beads were washed 3 times with lysis buffer and bound proteins eluted in 75 μL of 2X SDS sample buffer plus 25 μL 1 M DTT by heating at 75°C for 30 min. For immunoblotting, 3 μL 1M DTT was added to 15 μL of sample, and the mixture was incubated at 75°C for 10 min. Samples were then loaded onto 8% Bis-Tris gels and run in a Tris–glycine buffer under reducing conditions. Samples were transferred using the iBlot transfer system (Invitrogen) onto nitrocellulose membranes and probed with a rabbit anti-GluN2B polyclonal antibody (1:200 dilution, Millipore). An IRDye 800CW donkey anti-rabbit secondary antibody (LiCor Biosciences; Lincoln, NE, USA) was used at a dilution of 1:1000. Visualization and quantification of band intensity was performed using the Odyssey Imaging System (LiCor Biosciences). Integrated intensity values were recorded for all lanes and normalized to the intensity of the untreated control.
Statistical analysis
For [Ca2+]i imaging studies, an individual experiment (n=1) was defined as the response from a single neuron on a single coverslip. Changes in NMDA-evoked increases in [Ca2+]i are presented as mean ± SEM. Each experiment was replicated using at least 4 separate coverslips from at least 2 separate cultures. For biotinylation experiments, an individual experiment (n=1) was defined as the change in surface-to-total GluN2B from a single 100 × 20 mm petri dish. Changes in GluN2B surface expression are presented mean ± SEM. Each experiment was replicated using at least 10 separate petri dishes from 10 separate cultures. Significance was determined by one-way ANOVA with Tukey's post hoc test for multiple comparisons (OriginPro v8.5).
Results
Tat potentiates NMDA-evoked [Ca2+]iresponses via LRP and a Src family kinase
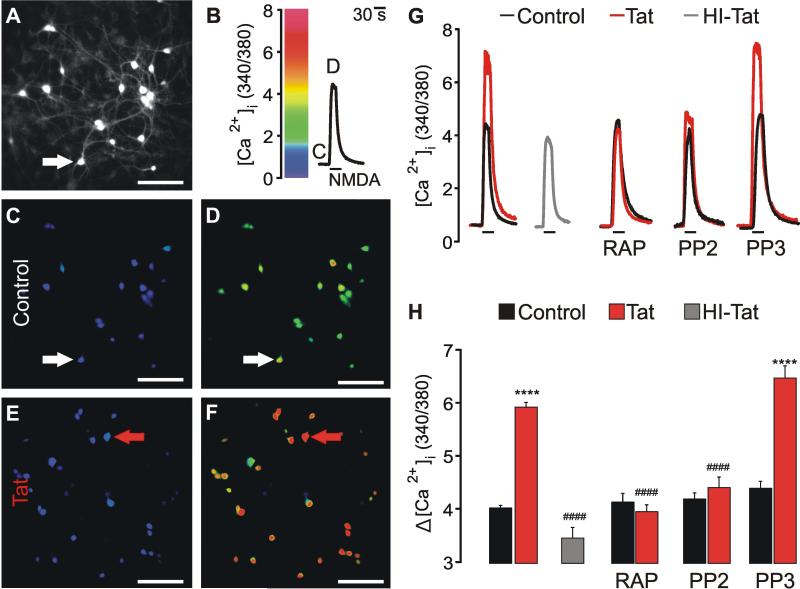
Treating primary hippocampal neurons with Tat potentiated NMDA-evoked increases in [Ca2+]i (Haughey et al. 2001). We replicated this finding using fura-2-based digital imaging of rat hippocampal neurons in vitro. As shown in Figure 1, increases in [Ca2+]i evoked by 100 μM NMDA were potentiated following 40 min treatment with 100 nM Tat, but not by 100 nM heat-inactivated (HI, 85°C × 30 min) Tat.
Fig. 1.
HIV-1 Tat potentiates NMDA-evoked [Ca2+] responses via LRP and a SFK. [Ca2+]i was recorded using fura-2-based digital imaging (Methods). A, representative image shows fura-2 loaded neurons (scale bar = 100 μM). B, trace shows change in [Ca2+]i expressed as 340 nm / 380 nm intensity ratio for the cell identified by the arrow in A. Cells were superfused with 100 μM NMDA (30 s) at the time indicated by the horizontal bar. C-F, pseudocolor images show [Ca2+]i before (C, E) and during (D, F) 30 s application of 100 μM NMDA for control (C, D) and Tat treated (100 nM × 40 min; E, F) cells. Images were scaled as shown in B. Images were collected at times indicated by the letters annotating the traces in B. G, representative traces show NMDA-evoked [Ca2+]i increases for control  cells, Tat-treated cells
cells, Tat-treated cells  , and cells treated with heat-inactivated (HI) Tat
, and cells treated with heat-inactivated (HI) Tat  . Control
. Control  and Tat-treated
and Tat-treated  traces were from the cells indicated by the arrows in C-D and E-F, respectively. Cells were pretreated for 1 h prior to Tat application with 50nM RAP, 10 μM PP2, or 10 μM PP3 as indicated. H, Bar graph shows net [Ca2+]i increase evoked by 100 μM NMDA in control
traces were from the cells indicated by the arrows in C-D and E-F, respectively. Cells were pretreated for 1 h prior to Tat application with 50nM RAP, 10 μM PP2, or 10 μM PP3 as indicated. H, Bar graph shows net [Ca2+]i increase evoked by 100 μM NMDA in control  cells, cells treated with 100 nM Tat
cells, cells treated with 100 nM Tat  , or 100 nM heat-inactivated (HI) Tat
, or 100 nM heat-inactivated (HI) Tat  for 40 min. Cells were pretreated with 50nM RAP, 10 μM PP2, or 10 μM PP3 as indicated. ****p<0.0001 relative to respective control; ####p<0.0001 relative to Tat alone as determined by one-way ANOVA with 9 levels followed by Tukey's post-test for multiple comparisons.
for 40 min. Cells were pretreated with 50nM RAP, 10 μM PP2, or 10 μM PP3 as indicated. ****p<0.0001 relative to respective control; ####p<0.0001 relative to Tat alone as determined by one-way ANOVA with 9 levels followed by Tukey's post-test for multiple comparisons.
Tat is internalized via the LRP (Liu et al. 2000) and Tat-induced neurotoxicity requires binding to the LRP with subsequent NMDA receptor (NMDAR)-mediated Ca2+ influx (Eugenin et al. 2007, Kim et al. 2008). We sought to determine whether the LRP was required for Tat-induced NMDAR potentiation by using the LRP antagonist, receptor-associated protein (RAP). RAP binds to the LRP and prevents neuronal uptake of Tat (Bu 2001). Application of RAP (50 nM) 1 h prior to Tat application completely blocked Tat-induced potentiation (Fig. 1 G, H), suggesting that Tat potentiates NMDAR function via the LRP.
Tyrosine phosphorylation can potentiate NMDAR currents (Wang & Salter 1994). Tat-induced NMDAR potentiation occurs via tyrosine kinase-dependent phosphorylation of GluN2A and GluN2B (Haughey et al, 2001), although the specific tyrosine kinase involved in NMDAR potentiation remains unknown. Src family kinases (SFKs) enhance NMDAR function (Kohr & Seeburg 1996), therefore we used the SFK inhibitor, PP2, to determine the role of SFKs in Tat-induced potentiation of NMDARs. Pretreatment for 1 h with PP2 (10 μM), but not its inactive analog PP3 (10 μM), completely blocked the Tat-induced potentiation of NMDA–evoked responses (Fig. 1 G, H). Thus, Tat-induced potentiation of NMDA-evoked increases in [Ca2+]i requires the activation of a SFK.
Tat induces a biphasic change in NMDA-evoked increases in [Ca2+]i
Up to 40 ng/mL of Tat has been detected in the sera of HIV-infected patients (Xiao et al. 2000). Previously, we have observed Tat-induced changes in synapse number following treatment with 50 ng/mL (3.6 nM) Tat for 24 h (Kim et al. 2008, Hargus & Thayer 2013). Thus, we hypothesized that 3.6 nM Tat would potentiate the NMDA-evoked increase in [Ca2+]i, but that these changes might require longer exposure to develop compared with 100 nM Tat. To test this hypothesis, we recorded NMDA-evoked [Ca2+]i responses following treatment with 50 ng/mL Tat within a 48 h window (Fig. 2). Control and Tat-treated cultures were imaged in parallel. Exposure to Tat for 2 h potentiated NMDA-evoked increases in [Ca2+]i. Tat-induced potentiation of the NMDA-evoked response peaked by 8 h then adapted, returning to baseline by 24 h and dropping below control by 48 h.
Fig. 2.
HIV-1 Tat-induced a biphasic change in NMDA-evoked [Ca2+]i responses. A, representative traces show NMDA-evoked [Ca2+]i (100 μM x 30 s) increases from control  neurons or neurons treated with 50 ng/mL Tat
neurons or neurons treated with 50 ng/mL Tat  for the times indicated above the traces. B, plot summarizes changes in NMDA-evoked [Ca2+]i responses under control
for the times indicated above the traces. B, plot summarizes changes in NMDA-evoked [Ca2+]i responses under control  conditions or after treatment with Tat
conditions or after treatment with Tat  for 0 to 48 h. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 relative to control as determined by one-way ANOVA with 24 levels followed by Tukey's post-test for multiple comparisons.
for 0 to 48 h. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 relative to control as determined by one-way ANOVA with 24 levels followed by Tukey's post-test for multiple comparisons.
Tat is susceptible to degradation by the Ca2+-activated protease, calpain-1 (Passiatore et al. 2009). To examine the possibility that adaptation of NMDAR potentiation was due to the degradation of Tat, cultures received either one (0 h) or two (0 h, 12 h) applications of Tat (50 ng/mL). Following 16 h exposure to Tat, NMDAR function was potentiated in neurons treated with Tat once or twice at 39 ± 2% and 22 ± 2% larger than control, respectively. By 24 h, amplitudes of NMDA-evoked Ca2+ influx from single and double Tat-treated groups returned to baseline levels and were comparable to control suggesting that adaptation of the NMDA-evoked response is not due to the degradation of Tat. Taken together, these data indicate that Tat evokes a biphasic change in NMDAR function. In an attempt to determine whether enhanced Ca2+ flux during the Tat-induced potentiation phase was required for subsequent adaptation, we blocked NMDAR-mediated Ca2+ influx with the readily reversible NMDAR antagonist AP5 during Tat exposure. However, chronic NMDAR antagonism in the absence of Tat enhanced NMDA-evoked responses, consistent with a previous report showing that chronic NMDAR blockade produced a compensatory increase in the surface expression of NMDARs (Crump et al. 2001). Thus, we were unable to determine whether potentiation was necessary for adaptation.
Application of 100 μM NMDA evoked [Ca2+]i increases in Tat-treated cells that peaked near saturation of the fura-2 Ca2+ indicator. A concentration-response experiment was conducted to determine if Tat-induced potentiation was observed when lower NMDA concentrations were used to probe NMDAR function. Tat-induced potentiation of NMDA-evoked responses was independent of NMDA concentration and was 35 ± 2%, 14 ± 3% and 32 ± 1% larger than control for 10, 30, and 100 μM NMDA, respectively. Thus, to enable more accurate measurement of the [Ca2+]i responses, we conducted the remaining experiments using 10 μM NMDA.
Tat-induced potentiation of the NMDA-evoked increase in [Ca2+]i is reversible
Tat-induced potentiation was prevented by inhibition of LRP or SFKs (Fig. 1). We next determined whether Tat-induced potentiation of NMDARs could be reversed by inhibition of LRP or SFKs after the potentiation was already established. Changes in NMDA-evoked increases in [Ca2+]i were studied after 16, 24, 32, or 48 h exposure to Tat. Either RAP (50 nM) or PP2 (10 μM) was applied 1 h prior to Ca2+ imaging in the continued presence of Tat (50 ng/mL). Exposure to Tat for 16 h potentiated NMDA-evoked increases in [Ca2+]i (Fig. 3 A, E). Potentiation was reversed by application of RAP or PP2 1 h prior to evoking the test response with NMDA (Fig. 3 A, E). Potentiation of NMDA-evoked responses adapted by 24 h (Fig. 3 B, F) and then dropped below control by 32 h (Fig. 3 C, G) and 48 h (Fig. 3 D, H). RAP and PP2 did not affect control cultures, but further reduced NMDA-evoked [Ca2+]i responses in cultures treated with Tat for 24 to 48 h (Fig. 3 B-D, F-H). These data indicate that Tat-induced potentiation of NMDA-evoked increase in [Ca2+]i requires sustained activation of LRP and SFK. Furthermore, this potentiation pathway remains activated during the adaptation process as indicated by the reduced amplitude of NMDA-evoked [Ca2+]i responses in fully-adapted cultures (24-48 h treated with Tat).
Fig. 3.
HIV-1 Tat-induced potentiation of NMDA-evoked increases in [Ca2+]i is reversible. A-D, representative traces show NMDA-evoked [Ca2+]i increases from control  neurons or neurons treated with 50 ng/mLTat
neurons or neurons treated with 50 ng/mLTat  for the times indicated above the traces. NMDA (10 μM × 30 s) was applied by superfusion at the times indicated by the horizontal bars. Cells were pretreated with 50 nM RAP or 10 μM PP2 1 h prior to superfusion with 10 μM NMDA as indicated. E-H, Bar graph shows net [Ca2+]i increase evoked by 10 μM NMDA in control
for the times indicated above the traces. NMDA (10 μM × 30 s) was applied by superfusion at the times indicated by the horizontal bars. Cells were pretreated with 50 nM RAP or 10 μM PP2 1 h prior to superfusion with 10 μM NMDA as indicated. E-H, Bar graph shows net [Ca2+]i increase evoked by 10 μM NMDA in control  cells or cells treated with Tat
cells or cells treated with Tat  . Cells were pretreated for 1 h with 50nM RAP or 10 μM PP2 as indicated. **p<0.01, ****p<0.0001 relative to respective control; #p<0.05, ####p<0.0001 relative to Tat alone as determined by separate, one-way ANOVAs with 6 levels for each treatment time followed by Tukey's post-test for multiple comparisons.
. Cells were pretreated for 1 h with 50nM RAP or 10 μM PP2 as indicated. **p<0.01, ****p<0.0001 relative to respective control; #p<0.05, ####p<0.0001 relative to Tat alone as determined by separate, one-way ANOVAs with 6 levels for each treatment time followed by Tukey's post-test for multiple comparisons.
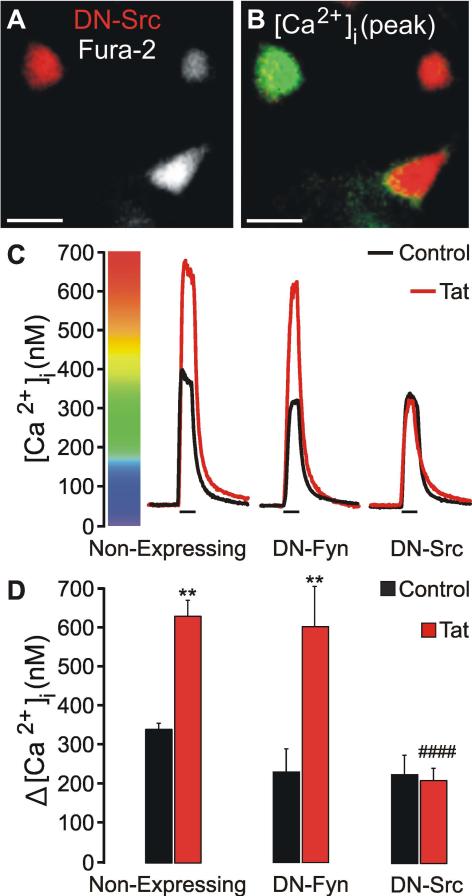
Src kinase mediates Tat-induced potentiation of NMDA-evoked increases in [Ca2+]i
The SFKs, Src and Fyn, have been shown to enhance NMDAR function (Kohr & Seeburg 1996). Following exposure to Tat, Src but not Fyn, associates with NMDARs (King et al. 2010). Due to the potential off-target effects of PP2 (Bain et al. 2007) and the lack of drugs that are selective for Src versus Fyn, we next used a genetic approach to determine which SFK is responsible for potentiating the NMDA-evoked increase in [Ca2+]i. Primary hippocampal cultures were co-transfected with plasmids encoding a red-fluorescent protein (pTag-RFP-N) and dominant-negative (DN)-Fyn or DN-Src (Fig. 4 A-B). Twenty-four hours after transfection, cells were treated with Tat (50 ng/mL) for 16 h, loaded with fura-2, and then imaged. Non-expressing cells in the same imaging field served as controls. Tat potentiated NMDA-evoked [Ca2+]i responses in non-expressing controls and cells expressing DN-Fyn (Fig. 4 C, D). However, expression of DN-Src completely blocked Tat-induced potentiation (Fig. 4 C, D). These data suggest that Tat-induced activation of Src kinase potentiates NMDA-evoked [Ca2+]i responses.
Fig. 4.
Src kinase mediates Tat-induced NMDAR potentiation. Cells were treated with Tat (50 ng/mL) for 16 h as indicated. A, representative image shows neuron expressing DN-Src and DsRed2 in a field of fura-2 loaded neurons (Scale bar = 25 μM). B, pseudocolor image shows peak [Ca2+]i response to 10 μM NMDA (30 s) after 16 h Tat treatment. Image is scaled to bar in C. C, representative traces show NMDA-evoked [Ca2+]i increases for control  and Tat-
and Tat- treated neurons. Recordings from non-expressing cells were from the same imaging field as cells expressing DN-Fyn or DN-Src. D, Bar graph shows net [Ca2+]i increase evoked by 10 μM NMDA (30 s) in control neurons
treated neurons. Recordings from non-expressing cells were from the same imaging field as cells expressing DN-Fyn or DN-Src. D, Bar graph shows net [Ca2+]i increase evoked by 10 μM NMDA (30 s) in control neurons  or neurons treated with 50 ng/mL Tat
or neurons treated with 50 ng/mL Tat  for 16 h. **p<0.01 relative to respective control; ####p<0.0001 relative to non-expressing, Tat-treated cells as determined by one-way ANOVA with 6 levels followed by Tukey's post test for multiple comparisons.
for 16 h. **p<0.01 relative to respective control; ####p<0.0001 relative to non-expressing, Tat-treated cells as determined by one-way ANOVA with 6 levels followed by Tukey's post test for multiple comparisons.
Tat-induced potentiation of NMDA-evoked increases in [Ca2+]iadapts via the NOS/sGC/PKG pathway
We next wanted to better understand the mechanism of adaptation following Tat-induced NMDAR potentiation. Because adaptation of Tat-induced NMDAR potentiation occurred in the presence of sustained Src activation, we hypothesized that adaptation resulted from a sustained increase in NMDAR-mediated Ca2+ influx. NMDAR-mediated Ca2+ influx following exposure to Tat leads to sustained production of nitric oxide (NO) (Eugenin et al. 2007). To determine whether NO production was mediating the Tat-induced adaptation in NMDAR function, we used a pharmacological approach. Cultures were pretreated with L-NAME (100 μM), an inhibitor of nitric oxide synthase (NOS), 1 h prior to and during exposure to Tat for 16, 24, 32, and 48 h. L-NAME did not affect Tat-induced NMDAR potentiation (Fig. 5 A, E) but did prevent adaptation of the NMDA-evoked increase in [Ca2+]i for 48 h (Fig. 5 B-D, F-H).
Fig. 5.
Tat-induced potentiation of NMDA-evoked increases in [Ca2+]i adapt via a NOS/sGC/PKG pathway. A-D, representative traces show NMDA-evoked [Ca2+]i increases from control neurons  or neurons treated with 50 ng/mL Tat
or neurons treated with 50 ng/mL Tat  for the times indicated above the traces. NMDA (10 μM × 30 s) was applied at the times indicated by the horizontal bars. Cells were untreated or pretreated 1 h prior to addition of Tat with 100 μM L-NAME, 1 μM ODQ, or 10 μM KT5823 as indicated. E-H, Bar graphs show net [Ca2+]i increase evoked by 10 μM NMDA (30 s) in control neurons
for the times indicated above the traces. NMDA (10 μM × 30 s) was applied at the times indicated by the horizontal bars. Cells were untreated or pretreated 1 h prior to addition of Tat with 100 μM L-NAME, 1 μM ODQ, or 10 μM KT5823 as indicated. E-H, Bar graphs show net [Ca2+]i increase evoked by 10 μM NMDA (30 s) in control neurons  or neurons treated with 50 ng/mL Tat
or neurons treated with 50 ng/mL Tat  for 16, 24, 32, or 48 h.*p<0.05; ****p<0.0001 relative to respective control; ####p<0.0001 relative to Tat alone as determined by separate, one-way ANOVAs with 8 levels for each treatment time followed by Tukey's post-test for multiple comparisons.
for 16, 24, 32, or 48 h.*p<0.05; ****p<0.0001 relative to respective control; ####p<0.0001 relative to Tat alone as determined by separate, one-way ANOVAs with 8 levels for each treatment time followed by Tukey's post-test for multiple comparisons.
NO can activate soluble guanylate cyclase (sGC) resulting in the synthesis of cyclic guanosine monophosphate (cGMP) (Arnold et al. 1977). To determine whether Tat-induced NO production was mediating the adaptation of NMDA-evoked responses by elevating cGMP, we treated cultures with ODQ (1 μM), an inhibitor of sGC, 1 h prior to and during exposure to Tat for 16, 24, 32, and 48 h. ODQ did not affect Tat-induced NMDAR potentiation (Fig. 5 A, E) but did prevent adaption of NMDA-evoked [Ca2+]i responses for 48 h (Fig. 5 B-D, F-H).
Elevated cGMP activates cGMP-dependent protein kinase (PKG) (Kuo & Greengard 1972). To determine whether Tat-induced NO production was mediating the adaptation of NMDA-evoked [Ca2+]i responses by activating PKG, we treated cultures with the PKG inhibitor, KT5823 (10 μM), 1 h prior to and during exposure to Tat for 16, 24, 32, and 48 h. KT5823 did not affect Tat-induced NMDAR potentiation (Fig 5 A, E), but did prevent adaptation of NMDA-evoked increases in [Ca2+]i for 48 h (Fig. 5 B-D, F-H).
To confirm that adaptation was mediated by PKG we complimented these pharmacological studies using a genetic approach. The catalytically inactive mutant of PKG1α, G1αR, acts in a dominant-negative manner to inhibit PKG activity (Browning et al. 2001). This dominant-negative PKG construct (DN-PKG) was expressed in neurons; cells were then treated with Tat for 16 or 24 h, loaded with Fura-2, and then imaged. Exposure to Tat for 16 h potentiated the NMDA-evoked increase in [Ca2+]i in non-expressing controls and neurons expressing DN-PKG. By 24 h, NMDA-evoked responses adapted in non-expressing cells, but expression of DN-PKG prevented adaptation (Fig. 6). Taken together, these data suggest that adaptation of NMDA-evoked responses following Tat-induced potentiation occurs via a NOS/cGMP/PKG pathway.
Fig. 6.
PKG mediates adaptation of NMDA-evoked responses following Tat-induced potentiation. A, representative images show neuronal expression of DN-PKG-DsRed2 in a field of neurons treated with Tat for 16 h or 24 h and loaded with fura-2 (scale bar = 50 μM). B, pseudocolor images show peak [Ca2+]i response to 10 μM NMDA (30 s) following 16 h or 24 h Tat treatment. Images are scaled to bar in C and E. C and E, representative traces show NMDA-evoked [Ca2+]i increases for control  and Tat-treated
and Tat-treated  cells following 16 h (C) or 24 h (E) exposure to Tat. Recordings from non-expressing cells were from the same imaging field as cells expressing DN-PKG. D and F, Bar graphs show net [Ca2+]i increase evoked by 10 μM NMDA (30 s) in control neurons
cells following 16 h (C) or 24 h (E) exposure to Tat. Recordings from non-expressing cells were from the same imaging field as cells expressing DN-PKG. D and F, Bar graphs show net [Ca2+]i increase evoked by 10 μM NMDA (30 s) in control neurons  or neurons treated with 50 ng/mL Tat
or neurons treated with 50 ng/mL Tat  for 16 h (D) or 24 h (F). *p<0.01; ***p<0.001 relative to respective control; ####p<0.0001 relative to non-expressing, Tat-treated cells as determined by separate, one-way ANOVA with 4 levels for each treatment time followed by Tukey's post test for multiple comparisons.
for 16 h (D) or 24 h (F). *p<0.01; ***p<0.001 relative to respective control; ####p<0.0001 relative to non-expressing, Tat-treated cells as determined by separate, one-way ANOVA with 4 levels for each treatment time followed by Tukey's post test for multiple comparisons.
PKG activates extracellular signal-regulated kinase (ERK) leading to the expression of neuroplasticity-associated proteins such as c-Fos, Egr-1, Arc, and BDNF (Gallo & Iadecola 2011). Therefore, we tested the hypothesis that Tat-induced activation of PKG stimulates ERK signaling to affect NMDA-evoked [Ca2+]i responses. The MAP kinase kinase 1 (MEK1) inhibitor PD98059 (50 μM) was applied 1 h prior to and during treatment with Tat for 16 or 24 h. PD98059 inhibits ERK1/2 phosphorylation and prevents expression of the aforementioned neuroplasticity-associated proteins (Gallo & Iadecola). Tat-induced NMDAR potentiation was comparable in the absence and presence of PD98059 at 49 ± 3% and 62 ± 8% larger than control, respectively. By 24 h, amplitudes of NMDA-evoked increases in [Ca2+]i from both treatment groups returned to baseline and were comparable to control, suggesting that activation of ERK signaling is not required for Tat-induced potentiation or adaptation of NMDARs.
To determine if adaptation following NMDAR potentiation is unique to Tat, we conducted a time course experiment using interleukin-1β (IL-1β). IL-1β is a pro-inflammatory cytokine that potentiates NMDAR-mediated currents (Liu et al. 2013) and NMDA-evoked [Ca2+]i increases via activation of a SFK (Viviani et al. 2003). We replicated this result and found that 5 min exposure to IL-1β (50 pg/mL) potentiated NMDA-evoked [Ca2+]i responses. IL-1β-induced NMDAR potentiation persisted for longer than 24 h then NMDA-evoked [Ca2+]i responses adapted, gradually reversing towards baseline by 48 h (Fig S1). Pretreatment for 1 h with the soluble guanylate cyclase inhibitor ODQ had no effect on IL-1β-induced NMDAR potentiation and failed to prevent adaptation. Thus, while adaptation of potentiated NMDA-evoked Ca2+ responses may be common, it appears that the mechanism of adaptation is stimulus specific.
Tat does not affect surface expression of GluN2B
We hypothesized that the reduction in the amplitude of NMDA-evoked [Ca2+]i increases following 48 h exposure to Tat was due to NMDAR internalization. We first determined the GluN2 subtype mediating the NMDA-evoked response using a pharmacological approach (Fig. S2). We recorded an initial NMDA evoked control response (response #1), allowed the cell to recover for 5 min in presence of ifenprodil (10 μM), a selective GluN2B antagonist (Williams 1993), and then evoked a second response in the continued presence of ifenprodil (response #2). The majority (74 ± 1%) of the NMDA-evoked increase in [Ca2+]i was mediated by ifenprodil-sensitive GluN2B-containing NMDARs, consistent with previous reports indicating that GluN2B preferentially localizes to extrasynaptic sites (Tovar & Westbrook 1999), including the neuronal cell body (She et al. 2012). Additionally, the response amplitude in the presence of ifenprodil was not different in untreated control cells compared to cells treated with Tat for 48 h (Fig. S2), suggesting that the ifenprodil-insensitive component was not participating in the Tat-induced adaptive changes. Thus, even though both GluN2A- and GluN2B-containing NMDARs are susceptible to internalization (Lavezzari et al. 2004), we focused on the surface expression of GluN2B subunits because the NMDA-evoked increase in [Ca2+]i was predominantly mediated by GluN2B-containing NMDARs, the surface expression of GluN2B is more dynamic than GluN2A (Groc et al. 2006), and our region of interest focused on the neuronal cell body.
Next, we determined whether 48 h exposure to Tat affected GluN2B surface expression in primary hippocampal cultures using a biotinylation approach. To confirm the selectivity of our antibody for GluN2B, we first transfected HEK293 cells with plasmids encoding GluN1 with GluN2A or GluN2B and quantified protein expression via Western blot. The antibody selectively labeled GluN2B, but not GluN2A (data not shown). As shown in Fig. 7, GluN2B surface expression was unaffected by 48 h exposure to Tat (50 ng/mL). Addition of KT5823 (10 μM) 1 h prior to and during treatment with Tat for 48 h also had no effect on GluN2B surface expression. We conducted a time course experiment in which cultures were treated with Tat (50 ng/mL) for 0, 8, 24, and 48 h. GluN2B surface expression was unaffected by Tat exposure at all time points (data not shown). Furthermore, treating cultures with 100 nM Tat for 1 h did not affect surface expression of GluN2B-containing NMDARs (data not shown). To confirm that the assay was sufficiently sensitive to detect changes in NMDAR surface expression, we induced NMDAR internalization by a 5 min pretreatment with glycine followed by 5 min treatment with glycine + NMDA (Nong et al. 2003). Treatment with glycine + NMDA evoked a 62 ± 33% decrease in GluN2B surface expression while 48 h treatment with Tat (50 ng/mL) tested in parallel had no effect (data not shown). These data indicate that adaptation following Tat-induced NMDAR potentiation is not due to internalization of GluN2B.
Fig. 7.
Tat does not affect surface expression of GluN2B-containing NMDARs. A, representative Western blot showing total and surface GluN2B. B, Bar graph shows GluN2B surface expression, expressed as the ratio of surface-to-total GluN2B. Cultures were left untreated ( , n=10), treated with 50 ng/mL Tat (
, n=10), treated with 50 ng/mL Tat ( , n=10) for 48 h, or treated with 10 μM KT5823 (
, n=10) for 48 h, or treated with 10 μM KT5823 ( , n=11) 1 h prior to the addition of Tat for 48 h. Data are expressed as mean +/- SEM. One-way ANOVA with 3 conditions was performed followed by Tukey's post-test for multiple comparisons.
, n=11) 1 h prior to the addition of Tat for 48 h. Data are expressed as mean +/- SEM. One-way ANOVA with 3 conditions was performed followed by Tukey's post-test for multiple comparisons.
Discussion
NMDARs are implicated in many neurodegenerative disorders including HAND (Young et al. 1988, Snyder et al. 2005, Spalloni et al. 2013, Rossi et al. 2013, Kaul et al. 2001, Potter et al. 2013). The HIV-1 protein Tat is a neurotoxin involved in the neuropathogenesis of HIV (Nath 2002, King et al. 2006). Here, we used fura-2-based Ca2+ imaging to investigate the effects of Tat on NMDA-evoked [Ca2+]i responses in rat hippocampal neurons in vitro. Tat potentiated NMDA-evoked increases in [Ca2+]i by LRP-dependent activation of Src kinase. Intriguingly, NMDA-evoked responses adapted following Tat-induced potentiation by activation of the NOS/sGC/PKG pathway. Adaptation of NMDA-evoked responses may be a novel neuroprotective mechanism to prevent excessive Ca2+ influx through NMDARs and could identify new targets for the treatment of HAND. The signaling pathways responsible for the biphasic modulation of NMDARs during exposure to Tat are summarized in Figure 8.
Fig. 8.
Proposed mechanism of Tat-induced NMDAR potentiation and adaptation. Tat interacts with LRP leading to the activation Src-kinase. Src kinase potentiates NMDA-evoked [Ca2+]i responses. Increased Ca2+ flux activates NOS to produce NO. NO binds to sGC stimulating the synthesis of cGMP. cGMP then activates PKG leading to the reduction of NMDA-evoked [Ca2+]I responses. Solid and dashed arrows represent direct and potentially indirect connections, respectively.
Tat has been detected in the brains of patients with HIV (Del Valle et al. 2000, Hudson et al. 2000, Wiley et al. 1996). Tat is shed by HIV-infected cells (Steinaa et al. 1994, Chang et al. 1997) and can affect NMDAR function directly and indirectly. Previous work showed that Tat directly activates the NMDAR (Song et al. 2003) resulting in neurotoxicity (Li et al. 2008). Other reports indicate that Tat indirectly enhances NMDAR function via binding to and relieving the inhibitory effect of Zn2+ (Chandra et al. 2005) or via tyrosine kinase-mediated phosphorylation of NMDARs (Haughey et al. 2001). We found that Tat indirectly potentiated NMDA-evoked increases in [Ca2+]i as indicated by prevention of potentiation by the LRP antagonist, RAP. Tat also induces synapse loss (Kim et al. 2008, Shin et al. 2012) and cell death (Eugenin et al. 2007) via an LRP-dependent mechanism (Liu et al. 2000). LRP1 recognizes over 30 ligands with high affinity (Herz & Strickland 2001). Some of these agents alter NMDAR function. For example, activated α2-macroglobulin (Qiu et al. 2002) and amyloid-β (Snyder et al. 2005) inhibit NMDA-evoked currents and ApoE4 potentiates NMDAR function (Qiu et al. 2003). We used a combined pharmacological and genetic approach to show that the previously described potentiation of NMDA-evoked [Ca2+]i responses by Tat (Haughey et al. 2001) was mediated by LRP-dependent activation of Src. The mechanism by which Tat activates Src following binding to LRP is unclear. Ligand binding to LRP activates SFKs (Bock & Herz 2003; Shi et al. 2009). Neurons from animals with an inactivating knock-in mutation in the Lrp1 gene exhibit reduced phosphorylation of GluN2B tyrosine-1472, although this particular mutation increased both LRP1 and GluN2B surface expression (Maier et al. 2013). Thus, it's possible that Tat binding to the LRP activates a signaling cascade resulting in phosphorylation of NMDARs. Alternatively, following internalization, Tat causes fundamental changes in endolysosomal structure and function (Hui et al. 2012); thus, it's possible that Tat disrupts endolysosomal membrane integrity and escapes the endosome to activate Src. NMDAR function could be directly affected by Src-mediated phosphorylation (Salter & Kalia 2004) or indirectly via activation of other Src substrates.
Tat-induced potentiation of the NMDA-evoked increase in [Ca2+]i followed a biphasic time course. The Tat-induced NMDAR potentiation peaked at 8 h and then NMDA-evoked responses returned to baseline by 24 h eventually dropping below control by 48 h. Down-regulation of NMDAR function over the course of hours to days has been described previously following ethanol exposure (Wu et al. 2011) and for excitatory conditions such as benzodiazepine withdrawal (Shen & Tietz 2011). Adaptation following Tat-induced NMDAR potentiation has not been previously described, although it does correlate with adaptive changes in synapse number induced by Tat. Twenty-four hour exposure to 50 ng/ml Tat produced a 50 ± 7% loss of glutamatergic synapses and a simultaneous 38 ± 3% increase in GABAergic synapses (Kim et al. 2008, Hargus & Thayer 2013). We suggest that Tat-induced synaptic changes and attenuation of NMDA-evoked [Ca2+]i responses are part of a neuroprotective response orchestrated by the cell to reduce excess excitatory input.
Adaptation of NMDA-evoked [Ca2+]i responses resulted from the activation of a NOS/sGC/PKG signaling pathway. A series of pharmacological and genetically-expressed inhibitors of this pathway prevented adaptation without affecting potentiation of NMDARs or NMDA-evoked responses in cells that were not exposed to Tat. PKG-mediated phosphorylation of the vasodilator-stimulated phosphoprotein (VASP), a protein expressed in neurons and used to assess PKG activity (Butt et al. 1994, Wang & Robinson 1997), increased following 24 h exposure to Tat (Shin & Thayer 2014) providing further support for Tat-induced activation of an NO-PKG pathway. Thus, this pathway was only activated after exposure to Tat, possibly resulting from potentiation. Sustained Tat-induced NO production results following the formation of a macromolecular complex composed of LRP, PSD95, NMDAR, and nNOS (Eugenin et al. 2007). NO inhibits NMDA receptor function (Manzoni et al. 1992), although this feedback inhibition is generally thought to result from the direct nitrosylation of NMDARs (Lei et al. 1992, Lipton & Stamler 1994) rather than activation of PKG. Activation of PKG leads to suppression of NMDAR-mediated currents (Furukawa & Mattson 1998), however the mechanism by which PKG attenuates NMDAR function is unclear.
Adaptation of potentiated NMDA-evoked [Ca2+]i increases is not unique to Tat. IL-1β potentiated NMDA-evoked responses which subsequently adapted following prolonged exposure, although it appears that the mechanism of adaptation is stimulus specific. Adaptation of Tat-induced NMDAR potentiation does not appear to result from attenuation of Src activity. The SFK inhibitor, PP2, suppressed NMDA-evoked increases in [Ca2+]i below control in fully-adapted, Tat-treated cells, although it had no effect on NMDA-evoked responses in naive cells, suggesting that Src remained active even in cells in which NMDA-evoked responses had returned to control levels. Sustained Src-mediated phosphorylation of Tyr 1472 on the C-terminal tail of GluN2B could disrupt AP-2 binding and prevent clathrin-mediated endocytosis (Lavezzari et al. 2003), which might explain the sustained NMDAR surface expression indicated by the biotinylation experiments. Biotinylation experiments indicated that adaptation was not due to internalization of GluN2B-containing NMDARs. Thus, an additional process was activated to counteract the sustained Src-mediated potentiation of the NMDA-evoked increase in [Ca2+]i. One possibility that could account for the reduction in NMDA-evoked responses without a concomitant decrease in GluN2B surface expression is Ca2+-induced cytoskeletal changes. Depolymerization of actin protects from excitotoxicity by reducing glutamate receptor-mediated Ca2+ influx (Furukawa et al. 1995). PKG is known to alter actin polymerization via Rho-associated kinase (Sunico et al. 2010) and such cytoskeletal changes reduce Ca2+ influx through the NMDAR (Rosenmund & Westbrook 1993, Lei et al. 2001). Perhaps prolonged exposure to Tat alters the gating or ligand binding properties of the NMDAR by affecting the physical interaction between the actin cytoskeleton and NMDARs resulting in attenuated NMDA-evoked responses.
The biphasic change in NMDA-evoked [Ca2+]i responses induced by Tat raises interesting questions about the balance between optimal NMDAR function and neuronal survival. Pharmacologic inhibition of NMDARs protects neurons from Tat-induced cell death (Shin et al. 2012); thus, the adaptation described here would seem likely to improve neuronal survival. However, Tat-induced cell death and NMDAR adaptation are both prevented by inhibition of NOS with L-NAME (Eugenin et al. 2007). Does the timing, duration, and amount of NO production distinguish protective from toxic effects? Intriguingly, NO can be neuroprotective or neurotoxic (Lipton et al. 1993). NO confers neuroprotection by several mechanisms. It directly attenuates NMDAR-mediated Ca2+ influx via s-nitrosylation resulting in improved neuronal survival (Choi et al. 2000, Jaffrey et al. 2001). Furthermore, NO can stimulate the sGC/cGMP/PKG pathway to produce neuroprotective proteins such as the transcription factor CREB and the kinase Akt (Contestabile & Ciani 2004). Alternatively, the neuroprotective effects of NO may be overwhelmed by nitrosative stress following prolonged, persistent, and excessive production of NO, ultimately resulting in neurotoxicity and cell death.
Although NO production is necessary for Tat-induced cell death to occur (Eugenin et al. 2007), impaired brain function from HIV initially results from synaptodendritic injury that precedes overt neuronal death. Proper NMDAR function is essential for normal cognition. For example, overexpression of GluN2B-containing NMDARs enhances learning and memory function (Tang et al. 1999) while inhibition of NMDARs impairs these processes (Malhotra et al. 1996, Newcomer & Krystal 2001). Does the cognitive decline observed in patients with HAND result, in part, from excessive attenuation of NMDAR function? If so, then the pathways that potentiate and attenuate NMDARs during a neurotoxic challenge may prove useful targets for the pharmacological treatment of HAND. Additionally, if the complex time course of NMDAR modulation observed during in vitro exposure to Tat is extrapolated to HAND, then different therapeutic approaches may be required for different stages of the disease.
In summary, we have shown that exposure to Tat evokes a biphasic modulation of NMDA-evoked [Ca2+]i responses. An LRP/Src pathway initially potentiates NMDA-evoked increases in [Ca2+]i and then activation of the NOS/sGC/PKG signaling pathway attenuates NMDA-evoked responses. Future experiments to determine how these pathways balance NMDAR-dependent cognitive functions with NMDAR-dependent toxicity in vivo may inform the targeting and timing of neuroprotective agents of potential use in treating HAND.
Supplementary Material
Acknowledgements
Funding institution: NIH (grant # DA07304, DA034696, MH061933, DA007097).
Footnotes
The authors have no conflict of interest to declare.
References
- Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold WP, Mittal CK, Katsuki S, Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3':5'-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci U S A. 1977;74:3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachani M, Sacktor N, McArthur JC, Nath A, Rumbaugh J. Detection of anti-tat antibodies in CSF of individuals with HIV-associated neurocognitive disorders. J Neurovirol. 2013;19:82–88. doi: 10.1007/s13365-012-0144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain J, Plater L, Elliott M, et al. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock HH, Herz J. Reelin activates SRC family tyrosine kinases in neurons. Current biology : CB. 2003;13:18–26. doi: 10.1016/s0960-9822(02)01403-3. [DOI] [PubMed] [Google Scholar]
- Browning DD, Mc Shane M, Marty C, Ye RD. Functional analysis of type 1alpha cGMP-dependent protein kinase using green fluorescent fusion proteins. The Journal of biological chemistry. 2001;276:13039–13048. doi: 10.1074/jbc.M009187200. [DOI] [PubMed] [Google Scholar]
- Bu G. The roles of receptor-associated protein (RAP) as a molecular chaperone for members of the LDL receptor family. Int Rev Cytol. 2001;209:79–116. doi: 10.1016/s0074-7696(01)09011-8. [DOI] [PubMed] [Google Scholar]
- Butt E, Abel K, Krieger M, Palm D, Hoppe V, Hoppe J, Walter U. cAMP- and cGMP-dependent protein kinase phosphorylation sites of the focal adhesion vasodilator-stimulated phosphoprotein (VASP) in vitro and in intact human platelets. The Journal of biological chemistry. 1994;269:14509–14517. [PubMed] [Google Scholar]
- Carey AN, Sypek EI, Singh HD, Kaufman MJ, McLaughlin JP. Expression of HIV-Tat protein is associated with learning and memory deficits in the mouse. Behav Brain Res. 2012;229:48–56. doi: 10.1016/j.bbr.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra T, Maier W, Konig HG, Hirzel K, Kogel D, Schuler T, Chandra A, Demirhan I, Laube B. Molecular interactions of the type 1 human immunodeficiency virus transregulatory protein Tat with N-methyl-d-aspartate receptor subunits. Neuroscience. 2005;134:145–153. doi: 10.1016/j.neuroscience.2005.02.049. [DOI] [PubMed] [Google Scholar]
- Chang HC, Samaniego F, Nair BC, Buonaguro L, Ensoli B. HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. AIDS. 1997;11:1421–1431. doi: 10.1097/00002030-199712000-00006. [DOI] [PubMed] [Google Scholar]
- Choi YB, Tenneti L, Le DA, Ortiz J, Bai G, Chen HSV, Lipton SA. Molecular basis of NMDA receptor-coupled ion channel modulation by S-nitrosylation. Nature Neuroscience. 2000;3:15–21. doi: 10.1038/71090. [DOI] [PubMed] [Google Scholar]
- Contestabile A, Ciani E. Role of nitric oxide in the regulation of neuronal proliferation, survival and differentiation. Neurochem Int. 2004;45:903–914. doi: 10.1016/j.neuint.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Crump FT, Dillman KS, Craig AM. cAMP-dependent protein kinase mediates activity-regulated synaptic targeting of NMDA receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:5079–5088. doi: 10.1523/JNEUROSCI.21-14-05079.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Maruff P, Brew BJ. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: a combined study of two cohorts. J Neurovirol. 2004;10:350–357. doi: 10.1080/13550280490521078. [DOI] [PubMed] [Google Scholar]
- Del Valle L, Croul S, Morgello S, Amini S, Rappaport J, Khalili K. Detection of HIV-1 Tat and JCV capsid protein, VP1, in AIDS brain with progressive multifocal leukoencephalopathy. J Neurovirol. 2000;6:221–228. doi: 10.3109/13550280009015824. [DOI] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, King JE, Nath A, Calderon TM, Zukin RS, Bennett MV, Berman JW. HIV-tat induces formation of an LRP-PSD-95- NMDAR-nNOS complex that promotes apoptosis in neurons and astrocytes. Proc Natl Acad Sci U S A. 2007;104:3438–3443. doi: 10.1073/pnas.0611699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Ignatowska-Jankowska BM, Bull C, et al. Synaptic dysfunction in the hippocampus accompanies learning and memory deficits in human immunodeficiency virus type-1 tat transgenic mice. Biol Psychiatry. 2012;73:443–453. doi: 10.1016/j.biopsych.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K, Mattson MP. Secreted amyloid precursor protein alpha selectively suppresses N-methyl-D-aspartate currents in hippocampal neurons: involvement of cyclic GMP. Neuroscience. 1998;83:429–438. doi: 10.1016/s0306-4522(97)00398-9. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Smithswintosky VL, Mattson MP. Evidence that actin depolymerization protects hippocampal neurons against excitotoxicity by stabilizing [ca2+](i). Experimental Neurology. 1995;133:153–163. doi: 10.1006/exnr.1995.1018. [DOI] [PubMed] [Google Scholar]
- Gallo EF, Iadecola C. Neuronal nitric oxide contributes to neuroplasticity-associated protein expression through cGMP, protein kinase G, and extracellular signal-regulated kinase. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:6947–6955. doi: 10.1523/JNEUROSCI.0374-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genis P, Jett M, Bernton EW, et al. Cytokines and arachidonic metabolites produced during human immunodeficiency virus (HIV)-infected macrophage interactions: implications for the neuropathogenesis of HIV disease. J. Exp. Med. 1992;176:1703–1718. doi: 10.1084/jem.176.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc L, Heine M, Cousins SL, Stephenson FA, Lounis B, Cognet L, Choquet D. NMDA receptor surface mobility depends on NR2A-2B subunits. Proc Natl Acad Sci U S A. 2006;103:18769–18774. doi: 10.1073/pnas.0605238103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of Biological Chemistry. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hargus NJ, Thayer SA. Human Immunodeficiency Virus-1 Tat Protein Increases the Number of Inhibitory Synapses between Hippocampal Neurons in Culture. J Neurosci. 2013;33:17908–17920. doi: 10.1523/JNEUROSCI.1312-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey NJ, Nath A, Mattson MP, Slevin JT, Geiger JD. HIV-1 Tat through phosphorylation of NMDA receptors potentiates glutamate excitotoxicity. Journal of neurochemistry. 2001;78:457–467. doi: 10.1046/j.1471-4159.2001.00396.x. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr., et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, McCutchan JA, Reicks C, Grant I. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10:317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- Herz J, Strickland DK. LRP: a multifunctional scavenger and signaling receptor. J Clin Invest. 2001;108:779–784. doi: 10.1172/JCI13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson L, Liu J, Nath A, Jones M, Raghavan R, Narayan O, Male D, Everall I. Detection of the human immunodeficiency virus regulatory protein tat in CNS tissues. J Neurovirol. 2000;6:145–155. doi: 10.3109/13550280009013158. [DOI] [PubMed] [Google Scholar]
- Hui L, Chen X, Haughey NJ, Geiger JD. Role of endolysosomes in HIV-1 Tat-induced neurotoxicity. ASN neuro. 2012;4:243–252. doi: 10.1042/AN20120017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH. Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nature Cell Biology. 2001;3:193–197. doi: 10.1038/35055104. [DOI] [PubMed] [Google Scholar]
- Jiang ZG, Piggee C, Heyes MP, Murphy C, Quearry B, Bauer M, Zheng J, Gendelman HE, Markey SP. Glutamate is a mediator of neurotoxicity in secretions of activated HIV-1-infected macrophages. Journal of Neuroimmunology. 2001;117:97–107. doi: 10.1016/s0165-5728(01)00315-0. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Martemyanov KA, Thayer SA. Human immunodeficiency virus protein Tat induces synapse loss via a reversible process that is distinct from cell death. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:12604–12613. doi: 10.1523/JNEUROSCI.2958-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Shin AH, Thayer SA. Activation of Cannabinoid Type 2 Receptors Inhibits HIV-1 Envelope Glycoprotein gp120-Induced Synapse Loss. Mol Pharmacol. 2011;80:357–366. doi: 10.1124/mol.111.071647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JE, Eugenin EA, Buckner CM, Berman JW. HIV tat and neurotoxicity. Microbes Infect. 2006;8:1347–1357. doi: 10.1016/j.micinf.2005.11.014. [DOI] [PubMed] [Google Scholar]
- King JE, Eugenin EA, Hazleton JE, Morgello S, Berman JW. Mechanisms of HIV-tat-induced phosphorylation of N-methyl-D-aspartate receptor subunit 2A in human primary neurons: implications for neuroAIDS pathogenesis. Am J Pathol. 2010;176:2819–2830. doi: 10.2353/ajpath.2010.090642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohr G, Seeburg PH. Subtype-specific regulation of recombinant NMDA receptor-channels by protein tyrosine kinases of the src family. J Physiol. 1996;492(Pt 2):445–452. doi: 10.1113/jphysiol.1996.sp021320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo JF, Greengard P. An assay method for cyclic AMP and cyclic GMP based upon their abilities to activate cyclic AMP-dependent and cyclic GMP-dependent protein kinases. Adv Cyclic Nucleotide Res. 1972;2:41–50. [PubMed] [Google Scholar]
- Lavezzari G, McCallum J, Dewey CM, Roche KW. Subunit-specific regulation of NMDA receptor endocytosis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:6383–6391. doi: 10.1523/JNEUROSCI.1890-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzari G, McCallum J, Lee R, Roche KW. Differential binding of the AP-2 adaptor complex and PSD-95 to the C-terminus of the NMDA receptor subunit NR2B regulates surface expression. Neuropharmacology. 2003;45:729–737. doi: 10.1016/s0028-3908(03)00308-3. [DOI] [PubMed] [Google Scholar]
- Lei S, Czerwinska E, Czerwinski W, Walsh MP, MacDonald JF. Regulation of NMDA receptor activity by F-actin and myosin light chain kinase. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:8464–8472. doi: 10.1523/JNEUROSCI.21-21-08464.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei SZ, Pan ZH, Aggarwal SK, Chen HS, Hartman J, Sucher NJ, Lipton SA. Effect of nitric oxide production on the redox modulatory site of the NMDA receptor-channel complex. Neuron. 1992;8:1087–1099. doi: 10.1016/0896-6273(92)90130-6. [DOI] [PubMed] [Google Scholar]
- Li W, Huang Y, Reid R, et al. NMDA receptor activation by HIV-Tat protein is clade dependent. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:12190–12198. doi: 10.1523/JNEUROSCI.3019-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Li G, Steiner J, Nath A. Role of Tat Protein in HIV Neuropathogenesis. Neurotox Res. 2009;16:205–220. doi: 10.1007/s12640-009-9047-8. [DOI] [PubMed] [Google Scholar]
- Li Y, Krogh KA, Thayer SA. Epileptic stimulus increases Homer 1a expression to modulate endocannabinoid signaling in cultured hippocampal neurons. Neuropharmacol. 2012;63:1140–1149. doi: 10.1016/j.neuropharm.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Popko J, Krogh KA, Thayer SA. Epileptiform stimulus increases Homer 1a expression to modulate synapse number and activity in hippocampal cultures. J Neurophysiol. 2013 doi: 10.1152/jn.00580.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA, Choi YB, Pan ZH, Lei SZ, Chen HS, Sucher NJ, Loscalzo J, Singel DJ, Stamler JS. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993;364:626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Stamler JS. Actions of redox-related congeners of nitric oxide at the NMDA receptor. Neuropharmacology. 1994;33:1229–1233. doi: 10.1016/0028-3908(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Liu T, Jiang CY, Fujita T, Luo SW, Kumamoto E. Enhancement by interleukin-1beta of AMPA and NMDA receptor-mediated currents in adult rat spinal superficial dorsal horn neurons. Molecular pain. 2013;9:16. doi: 10.1186/1744-8069-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Jones M, Hingtgen CM, Bu G, Laribee N, Tanzi RE, Moir RD, Nath A, He JJ. Uptake of HIV-1 tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands. Nat Med. 2000;6:1380–1387. doi: 10.1038/82199. [DOI] [PubMed] [Google Scholar]
- Maier W, Bednorz M, Meister S, Roebroek A, Weggen S, Schmitt U, Pietrzik CU. LRP1 is critical for the surface distribution and internalization of the NR2B NMDA receptor subtype. Mol Neurodegener. 2013;8:25. doi: 10.1186/1750-1326-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra AK, Pinals DA, Weingartner H, Sirocco K, Missar CD, Pickar D, Breier A. NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology. 1996;14:301–307. doi: 10.1016/0893-133X(95)00137-3. [DOI] [PubMed] [Google Scholar]
- Manzoni O, Prezeau L, Marin P, Deshager S, Bockaert J, Fagni L. Nitric oxide-induced blockade of NMDA receptors. Neuron. 1992;8:653–662. doi: 10.1016/0896-6273(92)90087-t. [DOI] [PubMed] [Google Scholar]
- Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis. 2002;186(Suppl 2):S193–198. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Krystal JH. NMDA receptor regulation of memory and behavior in humans. Hippocampus. 2001;11:529–542. doi: 10.1002/hipo.1069. [DOI] [PubMed] [Google Scholar]
- Nong Y, Huang YQ, Ju W, Kalia LV, Ahmadian G, Wang YT, Salter MW. Glycine binding primes NMDA receptor internalization. Nature. 2003;422:302–307. doi: 10.1038/nature01497. [DOI] [PubMed] [Google Scholar]
- Passiatore G, Rom S, Eletto D, Peruzzi F. HIV-1 Tat C-terminus is cleaved by calpain 1: implication for Tat-mediated neurotoxicity. Biochim Biophys Acta. 2009;1793:378–387. doi: 10.1016/j.bbamcr.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter MC, Figuera-Losada M, Rojas C, Slusher BS. Targeting the glutamatergic system for the treatment of HIV-associated neurocognitive disorders. J Neuroimmune Pharmacol. 2013;8:594–607. doi: 10.1007/s11481-013-9442-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Z, Crutcher KA, Hyman BT, Rebeck GW. ApoE isoforms affect neuronal N-methyl-D-aspartate calcium responses and toxicity via receptor-mediated processes. Neuroscience. 2003;122:291–303. doi: 10.1016/j.neuroscience.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Qiu Z, Strickland DK, Hyman BT, Rebeck GW. alpha 2-Macroglobulin exposure reduces calcium responses to N-methyl-D- aspartate via low density lipoprotein receptor-related protein in cultured hippocampal neurons. The Journal of biological chemistry. 2002;277:14458–14466. doi: 10.1074/jbc.M112066200. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Westbrook GL. Calcium-induced actin depolymerization reduces NMDA channel activity. Neuron. 1993;10:805–814. doi: 10.1016/0896-6273(93)90197-y. [DOI] [PubMed] [Google Scholar]
- Rossi S, Studer V, Moscatelli A, et al. Opposite roles of NMDA receptors in relapsing and primary progressive multiple sclerosis. PLoS One. 2013;8:e67357. doi: 10.1371/journal.pone.0067357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- She K, Ferreira JS, Carvalho AL, Craig AM. Glutamate binding to the GluN2B subunit controls surface trafficking of N-methyl-D-aspartate (NMDA) receptors. The Journal of biological chemistry. 2012;287:27432–27445. doi: 10.1074/jbc.M112.345108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen G, Tietz EI. Down-regulation of synaptic GluN2B subunit-containing N-methyl-D-aspartate receptors: a physiological brake on CA1 neuron alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid hyperexcitability during benzodiazepine withdrawal. J Pharmacol Exp Ther. 2011;336:265–273. doi: 10.1124/jpet.110.174235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Mantuano E, Inoue G, Campana WM, Gonias SL. Ligand binding to LRP1 transactivates Trk receptors by a Src family kinase-dependent pathway. Science signaling. 2009;2:ra18. doi: 10.1126/scisignal.2000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin AH, Kim HJ, Thayer SA. Subtype selective NMDA receptor antagonists induce recovery of synapses lost following exposure to HIV-1 Tat. Br J Pharmacol. 2012;166:1002–1017. doi: 10.1111/j.1476-5381.2011.01805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin & Thayer Recovery of synapses lost during exposure to HIV-1 Tat protein is induced by inhibition of a protein kinase G signaling pathway downstream of GluN2B-containing NMDARs. 2014. submitted.
- Snyder EM, Nong Y, Almeida CG, et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Song L, Nath A, Geiger JD, Moore A, Hochman S. Human Immunodeficiency Virus Type 1 Tat Protein Directly Activates Neuronal N -methyl- D -aspartate Receptors at an Allosteric Zinc-Sensitive Site. Journal of Neurovirology. 2003;9:399–403. doi: 10.1080/13550280390201704. [DOI] [PubMed] [Google Scholar]
- Spalloni A, Nutini M, Longone P. Role of the N-methyl-d-aspartate receptors complex in amyotrophic lateral sclerosis. Biochim Biophys Acta. 2013;1832:312–322. doi: 10.1016/j.bbadis.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Steinaa L, Sorensen AM, Nielsen JO, Hansen JE. Antibody to HIV-1 Tat protein inhibits the replication of virus in culture. Arch Virol. 1994;139:263–271. doi: 10.1007/BF01310790. [DOI] [PubMed] [Google Scholar]
- Sunico CR, Gonzalez-Forero D, Dominguez G, Garcia-Verdugo JM, Moreno-Lopez B. Nitric Oxide Induces Pathological Synapse Loss by a Protein Kinase G-, Rho Kinase-Dependent Mechanism Preceded by Myosin Light Chain Phosphorylation. J. Neurosci. 2010;30:973–984. doi: 10.1523/JNEUROSCI.3911-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi V, Balestra P, Serraino D, et al. Neurocognitive impairment and survival in a cohort of HIV-infected patients treated with HAART. AIDS Res Hum Retroviruses. 2005;21:706–713. doi: 10.1089/aid.2005.21.706. [DOI] [PubMed] [Google Scholar]
- Viviani B, Bartesaghi S, Gardoni F, et al. Interleukin-1b Enhances NMDA Receptor-Mediated Intracellular Calcium Increase through Activation of the Src Family of Kinases. J. Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Robinson PJ. Cyclic GMP-dependent protein kinase and cellular signaling in the nervous system. Journal of neurochemistry. 1997;68:443–456. doi: 10.1046/j.1471-4159.1997.68020443.x. [DOI] [PubMed] [Google Scholar]
- Wang YT, Salter MW. Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature. 1994;369:233–235. doi: 10.1038/369233a0. [DOI] [PubMed] [Google Scholar]
- Watkins BA, Dorn HH, Kelly WB, Armstrong RC, Potts BJ, Michaels R, Kufta CV, Dubois-Dalcq M. Specific tropism of HIV-1 for microglial cells in primary human brain cultures. Science. 1990;249:549–553. doi: 10.1126/science.2200125. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Baldwin M, Achim CL. Expression of regulatory and structural mRNA in the central nervous system. AIDS. 1996;10:943–947. doi: 10.1097/00002030-199607000-00007. [DOI] [PubMed] [Google Scholar]
- Williams K. Ifenprodil Discriminates Subtypes of the N-Methyl-D-aspartate Receptor - Selectivity and Mechanisms at Recombinant Heteromeric Receptors. Mol Pharmacol. 1993;44:851–859. [PubMed] [Google Scholar]
- Wu PJ, Coultrap SJ, Browning MD, Proctor WR. Functional adaptation of the N-methyl-D-aspartate receptor to inhibition by ethanol is modulated by striatal-enriched protein tyrosine phosphatase and p38 mitogen-activated protein kinase. Mol Pharm. 2011;80:529–537. doi: 10.1124/mol.110.068643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H, Neuveut C, Lee H, Benkirane M, Rich EA, Murphy PM, Jeang K-T. Selective CXCR4 antagonism by Tat: implications for in vivo expansion of coreceptor use by HIV-1. Proc Natl Acad Sci U S A. 2000;97:11466–11471. doi: 10.1073/pnas.97.21.11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AB, Greenamyre JT, Hollingsworth Z, Albin R, D'Amato C, Shoulson I, Penney JB. NMDA receptor losses in putamen from patients with Huntington's disease. Science. 1988;241:981–983. doi: 10.1126/science.2841762. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.