Abstract
Background
Better knowledge of patient and cancer treatment factors associated with nausea/vomiting (NV) in pediatric oncology patients could enhance prophylaxis. We aimed to describe such factors in children receiving treatment for acute myeloid leukemia (AML).
Methods
Retrospective longitudinal cohort study of 1668 hospitalized children undergoing treatment for AML from the Pediatric Health Information System database (39 hospitals, 1999–2010). Antiemetic alteration, which included switch (a change in prescribed 5-HT3 receptor antagonists) and rescue (receipt of an adjunct antiemetic), were first validated and then used as surrogates of problematic NV. Logistic and negative binomial regression modeling were used to test whether patient characteristics were associated with problematic NV.
Results
Increasing age is associated with greater odds of experiencing antiemetic switch and higher relative rate of antiemetic rescue. Within a treatment cycle, each consecutive inpatient chemotherapy-day decreased the likelihood of requiring antiemetic alteration. Each consecutive inpatient day post-chemotherapy was associated with decreased need for switch, but increased need for rescue. Subsequent cycles of AML therapy were associated with lower odds of antiemetic switch on both chemotherapy and non-chemotherapy days, a lower rate of antiemetic rescue on chemotherapy days, and an increased rate of rescue on non-chemotherapy days.
Conclusion
In pediatric patients with AML, increasing age is strongly associated with greater antiemetic alteration. Antiemetic alteration occurs early in treatment overall, and early within each admission. While additional cycles of therapy are associated with less alteration overall, there is persistent rescue in the days after chemotherapy, suggesting additional etiologies of NV in pediatric cancer patients.
Keywords: antiemetics, chemotherapy, nausea, supportive care, oncology, pediatrics
BACKGROUND
Chemotherapy-induced nausea and vomiting (CINV) remain a significant challenge for children undergoing treatment for cancer. The physical and psychological consequences of nausea and vomiting (NV) are often cited as the most distressing symptoms in the cancer experience.[1] Although patients with similar cancer diagnoses receive comparable chemotherapy and antiemetic regimens, they have markedly different clinical experiences with CINV. Currently, provision of initial prophylactic antiemetics is not tailored to individual patients; instead, only the expected emetogenicity of planned chemotherapy, based largely on adult data, guides antiemetic regimens.[2–5] Furthermore, the predominant focus of most studies of CINV is the chemotherapy emetogenic potential and the burden of CINV within one cycle of therapy, with few studies assessing the longitudinal patient experience both across and between cycles of therapy.[6] Previous literature has identified heightened risk of problematic CINV in adult patients that are female, young, alcohol-naïve, or have baseline anxiety.[7, 8] While Holdsworth, et al. found that obtaining complete control of CINV was more challenging in adolescents than in younger children, other clinical and demographic factors associated with pediatric NV have yet to be elucidated.[6]
While the total number of planned chemotherapy cycles for AML has decreased over time, treatment for AML still necessitates 3–5 inpatient cycles of highly emetogenic chemotherapy, with each cycle typically delivered over 5–10 days. Once chemotherapy has been administered, some treating centers discharge patients home, while others keep patients inpatient until blood count recovery.[9] Current recommendations for prophylaxis during chemotherapy administration advocate that patients receive scheduled 5-HT3 receptor antagonists (5-HT3-RA; ondansetron, granisetron, palonosetron, dolasetron) with addition of adjunctive rescue antiemetics (antihistamines, benzodiazepines, cannabinoids, etc.) as needed.[2, 10] Even with this level of initial prophylaxis, patients often require therapeutic alteration of their antiemetic regimen to control treatment related NV. Forms of alteration can be conceptualized as including antiemetic switch (a change from one 5-HT3-RA to another within the same class) and antiemetic rescue (the provision of an adjunctive therapy to gain control of NV). Additionally, while antiemetics and rescues are critical during chemotherapy administration, some patients have prolonged nausea and or problematic emesis requiring antiemetic use far beyond the chemotherapy period, even in the absence of neoplastic agents that are known triggers of delayed CINV. Clinically, this suggests a multifactorial etiology of NV, aside from chemotherapy, in these patients.
Given the prevalence of NV in children with AML, identification of factors that lead to problematic NV is important, since such information could enable identification of patients in need of higher levels of prophylactic therapy. The current study aimed to describe the longitudinal experience of pediatric AML patients with NV and the management of NV over the treatment trajectory. We also investigated any possible association of patient socio-demographic and clinical factors with antiemetic alteration and examined if the risk of antiemetic alteration increases with increasing chemotherapy exposure. We hypothesized that there would be significant differences in antiemetic alteration based on race, sex, and age, and more alteration as the number of days with chemotherapy exposure increased.
METHODS
Study Design
We performed a retrospective cohort study to describe the inpatient longitudinal experience with antiemetic alteration (switch and rescue) in pediatric patients with new onset AML. This patient population was chosen since chemotherapy for AML is often inpatient, and available PHIS pharmacy billing data is limited to the inpatient setting.
Data Source & Analytic Sample
Our study sample was drawn from a validated cohort with new onset pediatric AML consisting of 1686 patients age ≤ 18 years old identified in the Pediatric Health Information System (PHIS, Children’s Hospital Association, Kansas City, KS) between January 1999 and March 2010.[11] PHIS represents 44 freestanding pediatric hospitals in the US and is comprised of administrative data including patient demographic characteristics, ICD-9 diagnosis and procedure codes, and billing data including pharmaceutical and other resource utilization for each patient on each inpatient day.[12] All data submitted by member hospitals are de-identified, and data quality oversight is a joint effort between the Children’s Hospital Association, Truven Health Analytics, and participating hospitals.[13] Patients entered the cohort on the first day of the hospitalization during which the AML diagnosis was first reported. Patients remained in the cohort until the first of the following events occurred: completion of 5 chemotherapy cycles, stem cell transplantation, or inpatient death.[11]
Data structure
Data regarding chemotherapy, antiemetic, and pharmaceutical utilization was abstracted for each day of each hospitalization for every patient (patient-day level data). Each inpatient day was part of a chemotherapy cycle, consisting of all inpatient days from the start of chemotherapy administration to the date of discharge for that particular admission. Within cycles, we also identified days on which chemotherapy was provided and subsequent days when no chemotherapy was provided, as described in the covariate section below.
Outcome Measures
Antiemetic alteration (antiemetic switch and antiemetic rescue) was assessed daily for each patient, both during chemotherapy administration and separately for the remaining in-hospital days following completion of chemotherapy (non-chemotherapy days). Antiemetic switch, or a change from one 5-HT3-RA to another (ondansetron, granisetron, dolasetron and palonosetron), was assessed daily beginning on day 2 of chemotherapy, allowing day 1 to reflect the initial prophylactic antiemetic regimen and serve as a baseline from which to document antiemetic switch. Antiemetic rescue, or receipt of an adjunctive medication to control NV, was assessed on all inpatient days, including the first chemotherapy day. The rescue antiemetics included: antihistamines (dimenhydrinate, meclizine), benzodiazepines (lorazepam, diazepam), cannabinoids (dronabinol, nabilone), NK1-antagonists (aprepitant), promotility (metoclopramide), phenothiazines (prochlorperazine, promethazine, thiethylperazine), anti-cholinergics (scopolamine), and trimethobenzamide. Dosage amount could not be examined in the dataset, so only the occurrence of these medications was considered evidence of an outcome having occurred. Steroids were excluded as a rescue antiemetic since they are not typically used as antiemetics in this population and diphenhydramine was excluded given its multiple indications aside from NV.
Validation of antiemetic switch and rescue
To demonstrate the validity of these surrogates, we performed a single institution, retrospective chart review at the Children’s Hospital of Philadelphia (CHOP) for all PHIS-identified AML patients at CHOP (n=41). We evaluated progress notes and flowsheets to assess the presence of nausea or emesis on each given inpatient day, scored dichotomously as yes/no. We reviewed the antiemetic therapy administered on the first 20 inpatient days (or until discharge, if earlier than 20 days) to compare the occurrence of PHIS identified switch or rescue with the patient experiencing NV as indicated in the chart. Our a priori defined threshold for positive and negative predictive values (PPV, NPV), sensitivity and specificity was 80%.
Covariates
The following were included in all models as covariates: age at diagnosis (categorized as: ≥1 month – 2 years, >2–5 years, >5–9 years, >9–12 years, >12–16 years, older than 16 years), race (White, Black, Asian, Other), and sex (male, female) and insurance status (private, public, self/pay, other). To control for possible confounding by hospital (due to prescriber variation, formulary differences, and the number of patients each center contributed), we included an indicator variable for each hospital as a covariate in the multivariable regression models. To adjust for differential risk of CINV due to type of chemotherapy received on a given day, analyses included indicator variables for specific chemotherapeutic agents received on each inpatient day. To account for effects of duration of therapy, we included categorical chemotherapy cycle (1 to 5), a cumulative count of consecutive inpatient days on chemotherapy within each cycle, and a cumulative count of consecutive inpatient days after chemotherapy administration was complete (“non-chemotherapy days”). To assess whether antiemetic switch subsequently affected the rate of antiemetic rescue, a dichotomous variable indicating whether a patient had a prior switch was incorporated in modeling antiemetic rescue.
Analysis
Multivariable regression was used to estimate the effect of demographic and clinical characteristics on occurrence of antiemetic switch (logistic model) and the rate of antiemetic rescue (negative binomial model) on a patient-day level. Models examined chemotherapy and non-chemotherapy days separately given hypothesized differences in the NV process in these distinct time periods. All models used robust variance estimation at the subject level to account for repeated observations within each patient.[14] In negative binomial models, an offset was incorporated to account for the different number of inpatient days each subject contributed to the dataset. SAS version 9.3 (Cary, NC) and Stata version 12.1 (College Station, TX) were used to perform all analyses.
RESULTS
Validation of switch and rescue
In the single institution chart review, antiemetic alteration was associated with protracted NV. Four of 41 of patients experienced antiemetic switch. Identification of switch in PHIS compared to chart review demonstrated a PPV of 100% (95% CI 39.8–100%), a NPV of 54.4% (95% CI 50.9–57.9%), a sensitivity of 1.1% (95% CI 0.29–2.72%) and a specificity of 100% (95% CI 99.2–100%) for patients experiencing NV within the first 20 days of cycle 1. All 41 patients (100%) were noted to have had antiemetic rescues. Identification of these rescues in PHIS compared to chart review demonstrated a PPV of 87% (95% CI 83.3–90.5%), a NPV of 86% (95% CI 82.8–89.3%), a sensitivity of 83% (95% CI 78.8–86.7%) and a specificity of 90% (95% CI 86.6–92.5%) for patients experiencing NV within the first 20 days of cycle 1.
Cohort
Of the 1686 patients in the source cohort, 14 patients less than 1 month old at diagnosis were excluded since the 5-HT3-RA class is not FDA approved for patients that young. Four additional patients were excluded due to incomplete data. The final cohort thus consisted of 1668 patients from 39 hospitals (Table I). 53% (n=886) were males and 47% (n=782) were female and the mean age at diagnosis was 8.15 years (range: 1.2 months – 18.91 years) for males and 8.12 years (range: 1.2 months –18.98 years) for females. 69.7% of the sample was White, 13.2% Black, 3.5% Asian, 0.7% Native American, and 12.7% were listed as other. The mean number of chemotherapy cycles was 3.8 (range: 1–5). On average, patients had 25.9 (SD ±9.6) days of chemotherapy and 91.4 (SD ±48.7) days post-chemotherapy (non-chemotherapy days).
TABLE I.
Patient Characteristics in the Acute Myeloid Leukemia Cohort (n=1668)
| n (%) | ||
|---|---|---|
| Sex | Male | 886 (53.0%) |
| Female | 782 (47.0%) | |
| Age | Average | 8.1 years (± 5.9) |
| 1 month – 2 years | 405 (24.2%) | |
| >2 – 5 years | 284 (17.0%) | |
| >5 – 9 years | 201 (12.1%) | |
| >9 – 12 years | 210 (12.6%) | |
| >12 – 16 years | 375 (22.5%) | |
| >16 years old | 193 (11.6%) | |
| Race | White | 1163 (69.7%) |
| Black | 221 (13.2%) | |
| Asian | 59 (3.5%) | |
| Native American | 13 (0.8%) | |
| Other | 212 (12.7%) | |
| Insurance | Private | 578 (34.7%) |
| Public | 670 (40.2%) | |
| Self-pay | 36 (2.2%) | |
| Other | 384 (23.0%) | |
| Cycle | 1 | 1668 (100.0%) |
| 2 | 1481 (88.8%) | |
| 3 | 1341 (80.4%) | |
| 4 | 1100 (65.9%) | |
| 5 | 806 (48.3%) | |
| Mean number of cycles | 3.8 (1–5) | |
| Induction Regimen | ADE | 924 (55.4%) |
| DAT | 241(14.5%) | |
| DCTER | 373 (22.4%) | |
| 7+3 | 83 (5.0%) | |
| Mito-AraC | 47 (2.8%) |
ADE: cytarabine, daunomycin, etoposide with or without gemtuzumab ozogamicin; DAT: daunorubicin, cytarabine, thioguanine; DCTER: dexamethasone, cytarabine, thioguanine, etoposide, rubidomycin (daunomycin) or idarubicin; 7+3: cytarabine, daunorubicin; Mito-AraC: Mitoxantrone, cytarabine.
Antiemetic utilization
98.5% of all patients in the cohort had received a 5-HT3 receptor antagonist (5-HT3-RA) antiemetic by day 4 of AML therapy, and 100% of the cohort was exposed to this antiemetic class by the start of cycle 2. Overall, 5-HT3-RA were prescribed on 86% of all chemotherapy days and 30% of inpatient days post-chemotherapy. Ondansetron constituted 88.5% of all 5-HT3-RA exposures, followed by granisetron at 11.4%. 92% of patients received at least one antiemetic rescue during their cancer treatment. The median number of distinct antiemetic rescues over patients’ entire cancer therapy was 13 (range: 0–253). Lorazepam was the most commonly used rescue accounting for 46.1% of all rescues prescribed, followed by metoclopramide (23.9%) and promethazine (17.2%).
Antiemetic Switch
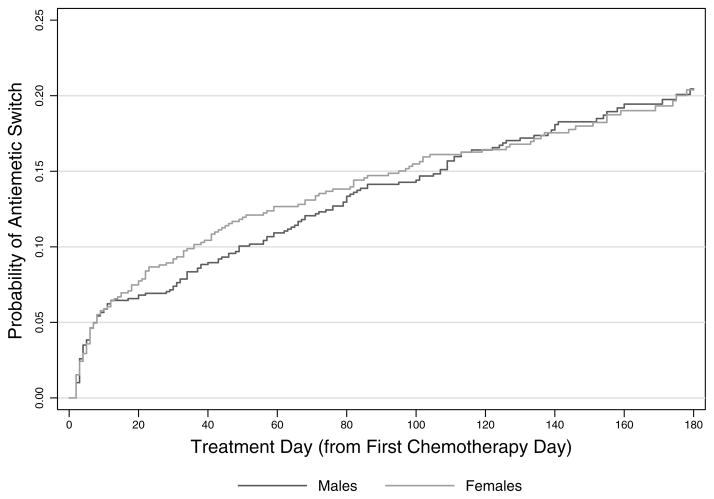
In the overall cohort, 298 patients (20.7%) experienced at least one antiemetic switch. Eleven patients (<1%) were switched twice in their treatment course. 50% of all antiemetic switches occurred within the first 40 days of therapy, and approximately 35% within the first ten days of therapy (Figure 1). Antiemetic switch occurred on both days with and without chemotherapy administration. Switches occurred in patients at 32 of the 39 treating centers and there was no association found between institution and number of switches.
FIGURE 1.
Cumulative Probability of Antiemetic Switch During AML Therapy
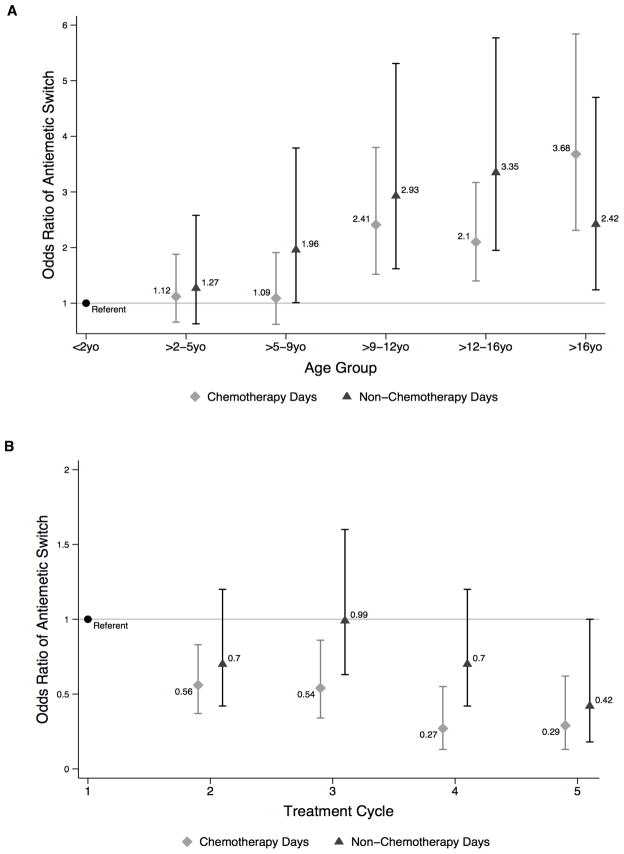
In multivariable adjusted analyses, older age groups were associated with greater antiemetic switch. As compared to the youngest patients (1 month – 2 years), antiemetic switch was significantly more likely in patients >9 years old on chemotherapy days, and in those >5 years old on non-chemotherapy days (Figure 2a). Neither sex, race nor insurance status was associated with antiemetic switch. Within a treatment cycle (Table III), each consecutive chemotherapy day was associated with a 9% decrease in the odds of switch (OR 0.91 [95% CI 0.84–0.98], P=0.009). After chemotherapy was complete, each additional inpatient day before discharge was associated with a 4% reduction in odds of experiencing an antiemetic switch (OR 0.96 [95% CI 0.93–0.98], P=0.002). With subsequent cycles of therapy, there was a decrease in the odds of antiemetic switch occurring on both chemotherapy and non-chemotherapy days as compared to cycle 1 (Figure 2b). Of note, multivariable logistic regression results above were based on data exclusively from the 32 hospitals where switch occurred.
FIGURE 2.
Odds of Antiemetic Switch by Age Group (A) and Treatment Cycle (B)
TABLE III.
Antiemetic Switch and Rescue During and Across Treatment Cycles
| Antiemetic Switch | Antiemetic Rescue | ||||
|---|---|---|---|---|---|
| OR (95% C.I.) | p-value | IRR (95% C.I.) | p-value | ||
| Subsequent Days Within a Cycle | Consecutive Days on Chemotherapy | 0.91 (0.84–0.98) | 0.009 | 0.99 (0.98–0.99) | 0.009 |
| Consecutive Days after Chemotherapy | 0.96 (0.93–0.98) | 0.002 | 1.008 (1.005–1.01) | <0.001 | |
| In Subsequent Cycles: Days on Chemotherapy | 1 | Referent | Referent | ||
| 2 | 0.56 (0.37–0.83) | 0.004 | 0.89 (0.83–0.96) | 0.002 | |
| 3 | 0.54 (0.34–0.86) | 0.01 | 0.92 (0.84–1.01) | 0.088 | |
| 4 | 0.27 (0.12–0.55) | <0.001 | 0.81 (0.73–0.90) | <0.001 | |
| 5 | 0.29 (0.13–0.62) | 0.001 | 0.77 (0.68–0.86) | <0.001 | |
| In Subsequent Cycles: Days after Chemotherapy (Non-chemo) | 1 | Referent | Referent | ||
| 2 | 0.70 (0.42–1.16) | 0.165 | 0.95 (0.83–1.08) | 0.431 | |
| 3 | 0.99 (0.63–1.57) | 0.971 | 1.06 (0.94–1.19) | 0.329 | |
| 4 | 0.70 (0.42–1.19) | 0.188 | 1.14 (0.99–1.30) | 0.051 | |
| 5 | 0.42 (0.18–1.00) | 0.051 | 0.97 (0.81–1.16) | 0.713 | |
| Prior antiemetic switch | – | – | 1.39 (1.21–1.59) | <0.001 | |
Antiemetic Rescue
92% of patients received at least one antiemetic rescue during their cancer treatment. Antiemetic rescue occurred at all 39 of the treating centers included in the cohort. On any given chemotherapy day, 37% of patients experienced antiemetic rescue versus 26% on days post-chemotherapy.
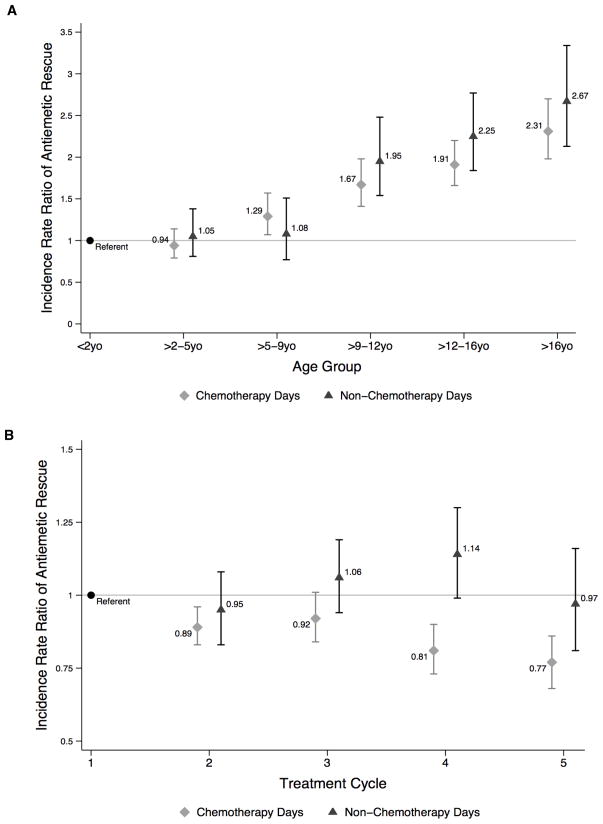
In multivariable adjusted analyses (Table II), neither sex nor race was associated with antiemetic rescue. Compared to those who were privately insured, patients with public insurance received rescues at a significantly lower rate on both chemotherapy (IRR 0.83 [95% CI 0.74–0.93], P=0.002) and post-chemotherapy (IRR 0.83 [95% CI 0.71–0.98], P=0.02) days. Like antiemetic switch, older age groups experienced greater antiemetic rescue. As compared to the youngest patients (1 month – 2 years), antiemetic rescue was significantly more likely in patients >5 years old chemotherapy days, and in those >9 years old on non-chemotherapy days (Figure 3a). Table III demonstrates that within each cycle, the likelihood of receiving an antiemetic rescue was greatest in the earliest days on chemotherapy and decreased significantly each chemotherapy day thereafter by 1% (IRR 0.99 [95% CI 0.98–0.99], P=0.009). Within each cycle, each consecutive inpatient day after the chemotherapy administration was completed, the rate of antiemetic rescue increased by 0.8% (IRR 1.008 [95% CI 1.005–1.01], P<0.001). On any given hospital day, the rate of antiemetic rescue was 39% higher if a patient had experienced a prior antiemetic switch (IRR 1.39 [95% CI 1.21–1.59], P<0.001). Across all treatment cycles, there were different patterns of antiemetic rescue for the chemotherapy days and for the non-chemotherapy days (Figure 3b).
TABLE II.
Association of Demographics with Antiemetic Switch and Rescue
| Antiemetic Switch | Antiemetic Rescue | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Days on Chemotherapy | Days after Chemotherapy | Days on Chemotherapy | Days after Chemotherapy | ||||||
| OR (95% C.I.) | p-value | OR (95% C.I.) | p-value | IRR (95% C.I.) | p-value | IRR (95% C.I.) | p-value | ||
| Sex | Female | Referent | Referent | Referent | |||||
| Male | 1.30 (0.99–1.70) | 0.053 | 0.82 (0.59–1.13) | 0.22 | 0.95 (0.86–1.05) | 0.31 | 0.93 (0.82–1.06) | 0.28 | |
| Age | 1 mo – 2y | Referent | Referent | ||||||
| >2 – 5y | 1.12 (0.66–1.88) | 0.68 | 1.27 (0.63–2.58) | 0.50 | 0.94 (0.79–1.14) | 0.55 | 1.05 (0.81–1.38) | 0.68 | |
| >5 – 9y | 1.09 (0.62–1.91) | 0.76 | 1.96 (1.01–3.79) | 0.045 | 1.29 (1.07–1.57) | 0.008 | 1.08 (0.77–1.51) | 0.66 | |
| >9 – 12y | 2.41 (1.52–3.80) | <0.001 | 2.93 (1.62–5.31) | <0.001 | 1.67 (1.41–1.98) | <0.001 | 1.95 (1.54–2.48) | <0.001 | |
| >12 – 16y | 2.10 (1.40–3.17) | <0.001 | 3.35 (1.95–5.77) | <0.001 | 1.91 (1.66–2.20) | <0.001 | 2.25 (1.84–2.77) | <0.001 | |
| >16y | 3.68 (2.31–5.84) | <0.001 | 2.42 (1.24–4.70) | 0.009 | 2.31 (1.98–2.70) | <0.001 | 2.67 (2.13–3.34) | <0.001 | |
| Race | White | Referent | Referent | ||||||
| Black | 1.22 (0.83–1.81) | 0.31 | 0.93 (0.55–1.57) | 0.79 | 0.91 (0.78–1.07) | 0.24 | 0.89 (0.70–1.14) | 0.36 | |
| Asian | 1.28 (0.56–2.94) | 0.56 | 0.75 (0.23–2.43) | 0.63 | 0.81 (0.65–1.01) | 0.056 | 0.83 (0.66–1.05) | 0.13 | |
| Other | 1.34 (0.83–2.18) | 0.23 | 0.95 (0.55–1.64) | 0.84 | 0.91 (0.79–1.07) | 0.25 | 0.86 (0.67–1.09) | 0.21 | |
| Insurance Status | Private | Referent | Referent | ||||||
| Public | 0.76 (0.54–1.08) | 0.13 | 1.01 (0.69–1.50) | 0.94 | 0.83 (0.74–0.93) | 0.002 | 0.83 (0.71–0.98) | 0.02 | |
| Other | 1.13 (0.76–1.68) | 0.54 | 1.47 (0.88–2.46) | 0.15 | 0.89 (0.77–1.02) | 0.098 | 0.86 (0.70–1.06) | 0.16 | |
FIGURE 3.
Incidence Rate Ratio of Antiemetic Rescue by Age Group (A) and Treatment Cycle (B)
DISCUSSION
In this retrospective cohort study we first validated that switch of 5-HT3-RA class and provision of rescue antiemetics are clinically relevant surrogates from which to study the antiemetic experience in pediatric cancer patients. Antiemetic switch is very specific with a reliable PPV, and antiemetic rescue is highly sensitive, specific, and has reliable NPV and PPV, for patient experienced NV. Our findings support that antiemetic alteration in pediatric patients being treated for AML is frequent, is unassociated with either sex or race, but rescue occurs less often in publically insured patients. Our study clearly demonstrated an age effect, whereby with increasing age, patients were more likely to experience alteration. With successive cycles of therapy, there is less antiemetic alteration while on chemotherapy, but successive inpatient days post-chemotherapy are associated with an increased likelihood of requiring a rescue antiemetic, suggesting two distinct phases and perhaps mechanisms of NV in these patients.
Our most striking finding was the significant association between increasing age and greater antiemetic alteration. This evidence corroborates findings in adolescent and young adult oncology patients who appear to have the highest symptom burden of all pediatric patients with cancer.[6, 15] The fact that antiemetic alteration is not associated with sex is a finding that differs from research in adults where females experience more problematic CINV.[7] The relationship between antiemetic alteration and race, although not statistically significant, suggests lower rates of rescue in patients who were non-White, specifically Asian. Finally, the finding that patients with public insurance received antiemetic rescues at a significantly lower rate than those who were privately insured, suggests that disparities in management of NV exist. There may be underreporting of symptoms in minority patients given cultural variation in perception of symptoms, and the influence of language barriers and cultural stigmas. Furthermore, there may be practice variation in response to NV between publically and privately funded hospitals.[16] The effect of race and insurance status on antiemetic need merits further investigation in other disease cohorts with more detailed data on ethnicity and insurance status.
The finding that more antiemetic switches occurred early within each cycle and in the earliest cycles overall is not surprising. Clinicians are likely to tailor the antiemetic regimen for optimal CINV control early in a patient’s care. This may also suggest that as patients progress through cycles of chemotherapy, they are experiencing less NV with subsequent chemotherapy exposure. Importantly, though, in the post-chemotherapy phase of each cycle, patients are still suffering from nausea/vomiting, as evidenced by increasing rates of rescue in that timeframe. Even in the absence of neoplastic agents that are known triggers of delayed CINV, this post-chemotherapy time highlights a period burdened with NV. Other comorbid symptoms such as pain, myelosuppression, mucositis, constipation or infection may be triggering NV, and treatment aimed at the underlying mechanism may be more effective at reducing NV during these periods. Alternatively, it is possible that nausea is an unintended side effect of supportive care medications such as opioids, anti-depressants, or anti-infectives used to manage one’s comorbid symptoms.[8, 17] Either way, providers need to address this post-chemotherapy nausea thoughtfully, and recognize that successful interventions for NV during this phase may be different than therapies prescribed during periods of chemotherapy administration.
No specific initial antiemetic regimen obviated the need for antiemetic alteration overall. Interestingly, we found the relative rate of rescue was 39% higher if a patient had experienced a prior antiemetic switch. This finding suggests that switching from one 5-HT3-RA to another in the class results in insufficient NV control.
This study should be interpreted with several limitations in mind. First, we relied upon a clinically detailed administrative database and surrogate markers of CINV to understand the patient emetic experience. As our data on receipt of medication are based on the presence of billed charges rather than on records of medication administration, there might be instances where the medication was not actually taken by the patient.[12] Additionally, we validated antiemetic switch and rescue as surrogates of patient experienced NV at a single institution, and test characteristics of antiemetic switch were limited given a small number of events. Moreover, our single institutional validation would benefit from multi-site review, both to increase the sample size and to assess generalizability. Finally, PHIS does not provide information about specific dosages, and thus we could not assess the impact of inadequate dosages or improper dosing intervals on problematic NV.
Antiemetics constitute a large proportion of medication exposure for children with cancer and understanding the pattern of antiemetic use is important. Keeping the above-mentioned limitations in mind, our findings support that in pediatric patients receiving chemotherapy for AML, there are sizeable age differences in antiemetic alteration during therapy. Our study, benefitting from a large cohort of patients who could be followed over time, highlights the unmet need in adequate control of nausea and emesis occurring post-chemotherapy. Moreover, this study demonstrates that problematic NV remains a challenge in pediatric patients, with marked rates of antiemetic alteration occurring at free-standing children’s hospitals across the United States. These results highlight the importance of continued discovery of newer antiemetics for these children, and development of pediatric specific guidelines to ensure basic prophylaxis with validated regimens.[2] Our findings also reinforce the concept that each child’s emetic profile evolves in a unique pattern, and requires meticulous attention and customization throughout therapy.
Information learned here can guide clinicians in providing evidence-based anticipatory guidance to patients and families about expected patterns of NV, as is done for other chemotherapy-related toxicities. Providers may also use the information herein to provide higher levels of prophylaxis to the oldest age groups, decreasing their symptom burden, and hopefully, improving the overall quality of life for pediatric patients with cancer. Future prospective work should focus on addressing disparities in symptom management, and identifying clinical and genomic characteristics that impact nausea and vomiting independently, especially in patients with solid tumors where burden of NV is even greater than hematologic malignancies.[18] Finally, this study demonstrates the feasibility of utilizing an administrative dataset to acquire information on pediatric supportive care issues where there is a paucity of knowledge and facilitates our ongoing work exploring the polypharmacy and potential drug-drug interactions in the provision of supportive care.
CONCLUSIONS
In pediatric patients with AML, treatment related nausea/vomiting, as evidenced by greater antiemetic alteration, is more prevalent with increasing age. Within each hospitalization, nausea/vomiting appears to improve as chemotherapy ensues, but post-chemotherapy, problematic NV is evident. Our findings suggest that problematic NV lessens with successive cycles of chemotherapy over time. These data enable provision of evidence-based anticipatory guidance to patients and families about expectations of nausea/vomiting and antiemetic usage during their AML therapy. This study highlights just one of the unmet needs in pediatric supportive oncology, and further work ought to elucidate similar issues in identifying risk factors and prevention of other symptoms faced by this patient population.
Acknowledgments
Funding/Support: This study was supported by the Agency for Healthcare Research and Quality (1RO1HS018425).
Footnotes
Authors’ contributions: JLF, JF, TK, DD, and CF participated in the design of the study; BF, YH, RA were responsible for creation of the source cohort used in this study; JLF performed the data analysis with assistance of JF; JLF, JF, and CF were involved in the interpretation of the data; JLF drafted the manuscript; all authors revised the manuscript for key intellectual content and all authors read and approved the final manuscript.
Financial Disclosures: Author BTF receives research funding from Pfizer Pharmaceuticals. This funding is paid directly to the institution and not to the author.
Role of the Sponsor: The funding organization had no role in the design of the study beyond the critique offered by the peer-review process; and had no role in the conduct of the study, including the collection, analysis, and preparation of the data or the drafting, editing, review, or approval of the manuscript. The content is solely the responsibility of the authors.
References
- 1.Hedstrom M, Haglund K, Skolin I, et al. Distressing events for children and adolescents with cancer: child, parent, and nurse perceptions. Journal of Pediatric Oncology Nursing. 2003;20:120–132. doi: 10.1053/jpon.2003.76. [DOI] [PubMed] [Google Scholar]
- 2.Dupuis LL, Boodhan S, Holdsworth M, et al. Guideline for the prevention of acute nausea and vomiting due to antineoplastic medication in pediatric cancer patients. Pediatric Blood & Cancer. 2013;60:1073–1082. doi: 10.1002/pbc.24508. [DOI] [PubMed] [Google Scholar]
- 3.Hesketh PJ. Defining the emetogenicity of cancer chemotherapy regimens: relevance to clinical practice. The Oncologist. 1999;4:191–196. [PubMed] [Google Scholar]
- 4.Hesketh PJ. Chemotherapy-induced nausea and vomiting. The New England Journal of Medicine. 2008;358:2482–2494. doi: 10.1056/NEJMra0706547. [DOI] [PubMed] [Google Scholar]
- 5.Jordan K, Roila F, Molassiotis A, et al. Antiemetics in children receiving chemotherapy: MASCC/ESMO Guideline Update 2009. Supportive Care in Cancer. 2011;19 (Suppl 1):S37–42. doi: 10.1007/s00520-010-0994-7. [DOI] [PubMed] [Google Scholar]
- 6.Holdsworth M, Raisch DW, Frost J. Acute and delayed nausea and emesis control in pediatric oncology patients. Cancer. 2006;106:931–940. doi: 10.1002/cncr.21631. [DOI] [PubMed] [Google Scholar]
- 7.Doherty KM. Closing the gap in prophylactic antiemetic therapy: patient factors in calculating the emetogenic potential of chemotherapy. Clinical Journal of Oncology Nursing. 1999;3:113–119. [PubMed] [Google Scholar]
- 8.Warr D. Prognostic factors for chemotherapy induced nausea and vomiting. European Journal of Pharmacology. 2014;722:192–196. doi: 10.1016/j.ejphar.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Lehrnbecher T, Ethier MC, Zaoutis T, et al. International variations in infection supportive care practices for paediatric patients with acute myeloid leukaemia. British Journal of Haematology. 2009;147:125–128. doi: 10.1111/j.1365-2141.2009.07844.x. [DOI] [PubMed] [Google Scholar]
- 10.Basch E, Prestrud AA, Hesketh PJ, et al. Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline Update. Journal of Clinical Oncology. 2011;29:4189–4198. doi: 10.1200/JCO.2010.34.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kavcic M, Fisher BT, Torp K, et al. Assembly of a cohort of children treated for acute myeloid leukemia at free-standing children’s hospitals in the United States using an administrative database. Pediatric Blood & Cancer. 2013;60:508–511. doi: 10.1002/pbc.24402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher BT, Lindenauer PK, Feudtner C. In-Hospital Databases. In: Strom B, Kimmel S, Hennessy S, editors. Pharmacoepidemiology. 5. Hoboken, NJ: Wiley-Blackwell; 2012. pp. 244–258. [Google Scholar]
- 13.Fisher BT, Singh S, Huang YS, et al. Induction mortality, ATRA administration, and resource utilization in a nationally representative cohort of children with acute promyelocytic leukemia in the United States from 1999 to 2009. Pediatric Blood & Cancer. 2014;61:68–73. doi: 10.1002/pbc.24585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. 2. New York: John Wiley and Sons; 2011. p. 701. [Google Scholar]
- 15.Erickson JM, Macpherson CF, Ameringer S, et al. Symptoms and symptom clusters in adolescents receiving cancer treatment: a review of the literature. International journal of nursing studies. 2013;50:847–869. doi: 10.1016/j.ijnurstu.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Green CR, Anderson KO, Baker TA, et al. The unequal burden of pain: confronting racial and ethnic disparities in pain. Pain medicine. 2003;4:277–294. doi: 10.1046/j.1526-4637.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 17.Phillips RS, Gibson F. A systematic review of treatments for constipation in children and young adults undergoing cancer treatment. Journal of Pediatric Hematology/Oncology. 2008;30:829–830. doi: 10.1097/MPH.0b013e318175898c. [DOI] [PubMed] [Google Scholar]
- 18.Olver I, Molassiotis A, Aapro M, et al. Antiemetic research: future directions. Supportive Care in Cancer. 2011;19 (Suppl 1):S49–55. doi: 10.1007/s00520-010-1036-1. [DOI] [PubMed] [Google Scholar]