Summary
Primarily defined by their antigen-presenting property, dendritic cells (DCs) are being implemented as cancer vaccines in immunotherapeutic interventions. DCs can also function as direct tumor cell killers. How DC cytotoxic activity can be efficiently harnessed and the mechanisms controlling this non-conventional property are not fully understood. We report here that the tumoricidal potential of mouse DCs generated from myeloid precursors with GM-CSF and IL-15 (IL-15 DCs) can be triggered with the Toll-like receptor 4 ligand lipopolysaccharide to a similar extent compared with that of their counterparts, conventionally generated with IL-4 (IL-4 DCs). The mechanism of tumor cell killing depends on the induction of iNOS expression by DCs. In contrast, interferon (IFN)-γ induces the cytotoxic activity of IL-4 but not IL-15 DCs. Although the IFN-γ-STAT1 signaling pathway is overall functional in IL-15 DCs, IFN-γ fails to induce iNOS expression in these cells. iNOS expression is negatively controlled in IFN-γ-stimulated IL-15 DCs by the cooperation between the E3 SUMO ligase PIAS1 and STAT-3, and can be partially restored with PIAS1 siRNA and STAT-3 inhibitors.
Keywords: Cytotoxic dendritic cells, iNOS, NF-κB, PIAS1, STAT3
Introduction
The unique ability of dendritic cells (DCs) to function as professional antigen presenting cells (APCs) capable of inducing and regulating immune responses has been the foundation for their development and utilization as anti-cancer vaccines [1, 2]. The potential of DCs to mediate direct killing of tumor cells has emerged in recent years as a novel non-conventional property of these cells, which may be harnessed to enhance their therapeutic efficacy [3-6]. Several populations of killer DCs, naturally occurring in vivo or generated in vitro from dedicated precursors, and multiple modalities for the induction of their cytotoxic function have been described [3, 7]. The effector mechanisms underlying DC-mediated tumoricidal function are still being explored and may differ depending on the DC subtype [3-8].
Myeloid DCs represent the most extensively used type of DCs in cancer immunotherapy clinical trials. These cells can be generated in vitro in the presence of GM-CSF (a hematopoietic growth factor widely used to differentiate and expand these cells in vitro) and IL-4. These IL-4 DCs, loaded with specific tumor antigens and stimulated with diverse maturation cocktails, are capable of priming and activating tumor-specific helper CD4+ (Th) and cytotoxic CD8+ (CTL) T lymphocytes. Beside these conventional IL-4 DCs, the possibility of generating DCs with IL-15 has also been documented in mice and humans [9, 10]. IL-15 DCs may exhibit improved potential to stimulate Th-1 and CTL responses in cancer patients [9, 10]. Evidence has been provided in the mouse, rat, and human systems that IL-4 DCs activated with LPS mediate tumor cell killing by NO− and/or peroxynitrite- dependent mechanisms [3, 8, 11-14]. However, the cytotoxic properties of IL-15 DCs and the molecular control of DC killing activity have not been fully explored [15]. How the cytotoxic activity of these cells may impact their antigen presenting function remains unclear.
In the current study, we investigated the non-conventional tumoricidal potential of mouse bone marrow-derived IL-15 DCs in response to LPS or IFN-γ and its molecular mechanisms of regulation. The ability of IL-15 DCs to present tumor-derived antigens from killed cancer cells to specific T lymphocytes and how the cytotoxic activity of these cells may affect their antigen presenting function was further evaluated.
Results
IFN-γ selectively activates the tumor killing activity of IL-4 DCs
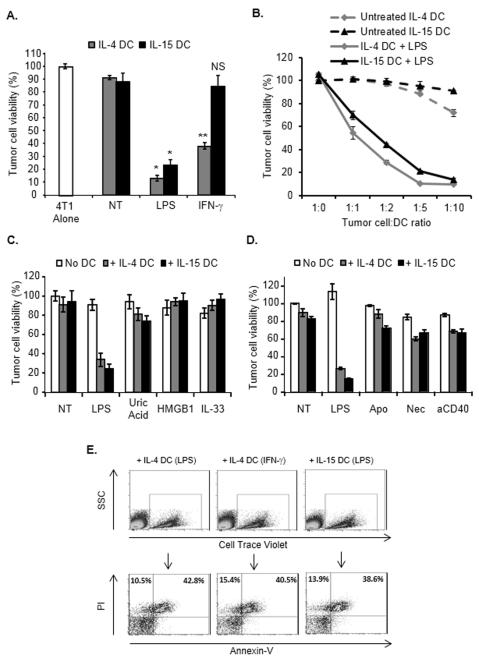
Consistent with previous reports [9, 10], IL-4 and IL-15 DCs exhibited analogous characteristics and both subsets similarly responded to LPS or IFN-γ stimulation (Supporting Information Fig. 1). At the basal level, IL-15 DCs produced reduced amounts of TNF-α compared with IL-4 DCs (Supporting Information Fig. 1). Similar to IL-4 DCs, non-activated IL-15 DCs were not capable of mediating tumor cell killing, but their cytotoxic activity could be triggered upon LPS activation (Fig.1A) and depended on the DC-to-tumor cell ratio (Fig. 1B). Interestingly, IFN-γ did not induce IL-15 DC tumoricidal activity (Fig. 1A). Consistent with our previous studies [11, 14], analysis of other typical “exogenous danger signals” [16] such as CpG, PGN or Gardiquimod, indicated that these molecules did not significantly induce DC killing potential, and as reported [11] Pam3Cys-SK4 triggered DC killing activity but to a lesser extend compared to LPS (data not shown). In addition, neither IL-4 DCs nor IL-15 DCs exposed to “endogenous danger signals”, such as High Mobility Group Box -1 (HMGB1), uric acid and IL-33 (reported to act as potent DC activators [17]), mediated significant tumor cell killing (Fig. 1C). Similarly, dead tumor cells (apoptotic or necrotic) or CD40 triggering, reported to promote DC maturation and/or activation [18-20], did not induce the cytotoxic activity of either DC type (Fig. 1D).
Figure 1.
IFN-γ induces the cytotoxic function of IL-4 DCs but not IL-15 DCs. (A) 4T1 tumor cells were cultured for 48 hours with untreated (NT), LPS- (0.1 μg/ml) or IFN-γ- (5 ng/ml) activated day 6 CD11c+ IL-4 DCs or IL-15 DCs (DC: tumor cell ratio =5:1) and tumor cell survival was determined using a Crystal Violet assay. NS, Non-significant difference when compared with tumor cells cultured with untreated (NT) IL-15 DCs; *p<0.001, **p<0.002 compared with tumor cells cultured with the corresponding untreated (NT) DC group, Student t test. (B) Tumor cells were cultured as in (A), but at the indicated tumor cell: DC ratios. (C, D) Tumor cells were cultured with DCs activated as indicated (High Mobility Goup Box-1, HMGB1; anti-CD40, aCD40; Apoptotic tumor cells, Apo; Necrotic tumor cells, Nec) and tumor cell survival was determined. (A-D) Data are shown as mean ± SD from triplicate wells and are from one representative experiment out of three performed. (E) Determination of the type of tumor cell death induced by DCs. Tumor cells were cultured as in (A) and subsequently stained with Cell Trace Violet before being co-cultured with IL-4 DCs treated with LPS (+ IL-4 DC (LPS)) or IFN-γ (+ IL-4 DC (IFN-γ)) or with IL-15 DCs treated with LPS (+ IL-15 DC (LPS)). Cells were stained with propidium iodide and Annexin-V and analyzed after gating on Cell Trace Violet-positive cells. Data shown are representative of two independent experiments.
Non-adherent tumor cells recovered after culture with LPS-activated IL-4 or IL-15 DCs or IFN-γ-activated IL-4 DCs were not able to form colonies when sub-cultured in fresh culture medium for 4 days (data not shown). Further analysis indicated that DCs induced tumor cell death by a caspase-independent necrotic-like process associating plasma membrane permeabilization as evidenced by propidium iodide and Annexin-V staining (Fig. 1E) and the absence of caspase-3 cleavage in the dead tumor cells (Supporting Information Fig. 2).
Therefore, similar to their IL-4 counterparts, DCs generated with IL-15 and stimulated with LPS can act as potent tumor cell killers. However, upon IFN-γ activation, IL-4 DCs are significantly superior tumor cell killers compared with IL-15 DCs.
The cytotoxic activity of IL-4 and IL-15 DCs depends on iNOS expression and NO secretion
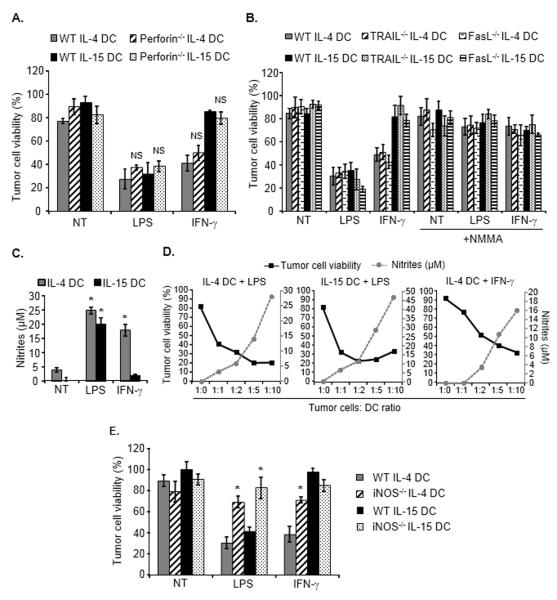
Some reports have suggested that DC cytotoxic activity may depend on the perforin/granzyme system or on death receptor ligands (TRAIL or Fas-L) [21-27]. IL-4 DCs and IL-15 DCs generated from perforin knock-out mice and activated with LPS or IFN-γ exhibited a similar tumoricidal activity compared with their counterparts generated from wild-type animals (Fig. 2A). Using TRAIL knock-out and Fas-L knock-out mice we further demonstrated that these two death ligands did not significantly contribute to the cytotoxic activity of LPS- or IFN-γ-stimulated IL-4 DCs or of LPS-activated IL-15 DCs (Fig. 2B).
Figure 2.
Mechanisms underlying DC-mediated cytotoxic activity. (A) Cytotoxic activity of DCs generated from perforin−/− mice. Untreated, LPS- or IFN-γ-activated IL-4 DCs or IL-15 DCs generated from wild-type (WT) or from perforin−/− mice were cultured with tumor cells for 48 hours and tumor cell survival was determined. NS, Non-significant difference when compared with tumor cells cultured with the corresponding treated WT DC group. (B) Tumor cells were cultured with DCs generated from wild-type, TRAIL−/− or FasL−/− mice, and treated or not as indicated with the iNOS inhibitor NMMA. Tumor cell survival was then determined. (A, B) Data are shown as mean ± SD from triplicate wells and are from one representative experiment out of two performed. (C) Nitrites production in the culture supernatants of untreated, LPS- or IFN-γ-activated IL-4 DCs or IL-15 DCs following culture with tumor cells. Nitrites concentration was detected using the Griess reagent and shown as mean + SD of duplicates from one representative experiment of three performed. (D) LPS- or IFN-γ-activated IL-4 DCs or LPS-treated IL-15 DCs were co-cultured for 48 hours with tumor cells at the indicated tumor cell:DC ratio. Tumor cell survival (black line) and associated nitrite concentration (grey dotted line) were determined. (E) Tumor cells were cultured with DCs generated from WT or iNOS−/− mice and tumor cell viability determined. Data are shown as mean ± SD of triplicate wells from a representative experiment out of two performed. *p<0.02 compared with tumor cells cultured with the corresponding treated WT DC group, Student t test.
Nitric oxide (NO) and peroxynitrites are short half-life molecules endowed with tumoricidal properties [3, 11, 12, 14, 28]. The concentration of nitrites, the main metabolites of NO, was significantly increased in the culture supernatants of LPS-activated IL-15 DCs and of LPS- or IFN-γ- treated IL-4 DCs (Fig. 2C). Interestingly, the level of nitrites correlated with the killing efficacy of the DCs and with the effector DC to target tumor cell ratio (Fig. 2D). The role of NO was further confirmed using iNOS knock-out mice. The cytotoxic function of LPS-activated IL-15 DCs and LPS- or IFN-γ- activated IL-4 DCs generated from these transgenic animals was significantly impaired (Fig. 2E). Consistent with this result, the iNOS inhibitor NMMA inhibited the tumoricidal activity of both DC types (Fig. 2B). Moreover, NMMA also inhibited the cytotoxic activity of IL-4 and IL-15 DCs generated from TRAIL knock-out or from Fas-L knock-out mice (Fig. 2B). Of note, the cancer cells used in our study were sensitive to the NO donor SNAP, indicating that NO alone is capable of killing these tumor cells (not shown).
IL-15 DCs do not overexpress iNOS following IFN-γ stimulation
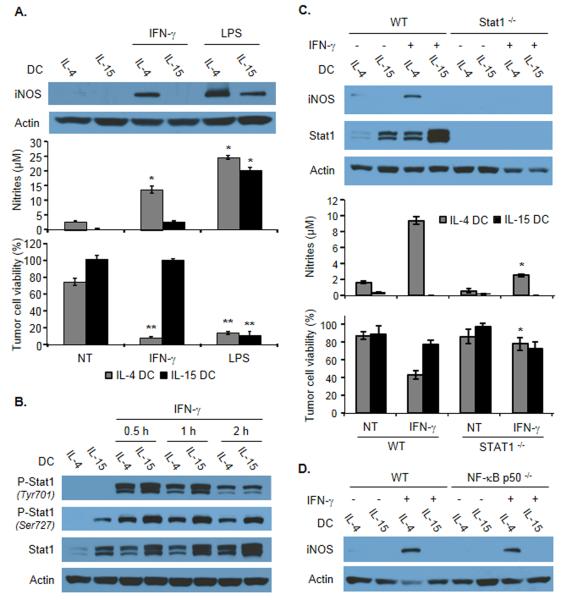
Given that iNOS played a central role in the killing activity of IL-15 DCs induced by LPS and since IFN-γ did not trigger the cytotoxic function of these cells, we reasoned that the expression of this enzyme may be impaired in IFN-γ-stimulated IL-15 DCs. Western blot analysis indicated that LPS induced a significant increase of iNOS expression in both DC types (Fig. 3A, top panels). However, up-regulation of this enzyme was observed only in IL-4 DCs but not in IL-15 DCs in response to IFN-γ stimulation (Fig. 3A, top panels). iNOS expression profile correlated with NO concentration in the culture supernatant and with DC killing function (Fig. 3A, middle and lower panels). Consistently, iNOS mRNA transcripts were not detected by real-time PCR in IL-15 DCs stimulated with IFN-γ (not shown). Further investigation after subcellular fractionation confirmed the cytosolic localization of this enzyme (Supporting Information Fig. 3A).
Figure 3.
iNOS expression and role of STAT-1 and NF-κB in IFN-γ-activated IL-4 DCs or IL-15 DCs. (A) Detection of iNOS expression by immunoblotting in total cell extracts of IL-4 DCs or IL-15 DCs treated as indicated (top), corresponding concentration of nitrites in the cultures (middle), and tumor killing activity of DCs in the indicated conditions (bottom). Data are shown as mean ± SD of duplicates (middle) or triplicate wells (bottom) from one representative experiment out of three performed. *p<0.02; **p<0.001, compared with the corresponding untreated DC group, Student t test. (B) Analysis of the phosphorylation of STAT-1 by immunoblotting in total cell extracts of IL-4 DCs or IL-15 DCs at the indicated time points following IFN-γ exposure. Data shown are representative of four experiments performed. (C) Detection of iNOS expression and STAT-1 phosphorylation by immunoblotting in total extracts of IL-4 DCs or IL-15 DCs generated from WT or from STAT-1−/− mice and treated or not with IFN-γ (top), corresponding concentration of nitrites in the cultures (middle), and tumor killing activity of the DCs in the indicated conditions (bottom). Data are shown as mean ± SD of duplicates (middle) or triplicate wells (bottom) from one representative experiment out of three performed. *p<0.02, compared with IFN-γ-treated WT IL-4 DC group, Student t test. (D) Detection of iNOS expression by immunoblotting in total extracts of IL-4 DCs or IL-15 DCs generated from WT or from NF-κBp50−/− mice and treated with IFN-γ as indicated. Data shown are representative of three experiments performed. (A-D) β-actin was used as a loading control.
iNOS expression is differentially regulated in IL-4 and IL-15 DCs exposed to IFN-γ
We next focused on identifying the intracellular signaling pathways induced in IL-4 DCs in response to IFN-γ and leading to the expression of iNOS. As expected, IFN-γ induced the phosphorylation of the transcription factor STAT-1 on Tyrosine 701 and Serine 727 (Fig. 3B). Importantly, IFN-γ-induced iNOS expression was abrogated in IL-4 DCs generated from STAT-1 knock-out mice (Fig. 3C, top panel). Consistent with this result, NO production and cytotoxic function of STAT-1−/− IL-4 DCs were impaired (Fig. 3C, middle and bottom panels). The transcription factor NF-κB has been shown to participate in iNOS expression in different other cell types [29-31]. Our results indicate that iNOS expression was not reduced in IFN-γ-stimulated IL-4 DCs generated from NF-κB p50 knock-out mice compared with wild-type mice (Fig. 3D), indicating that NF-κB is not essential for IFN-γ-mediated induction of iNOS expression in these cells.
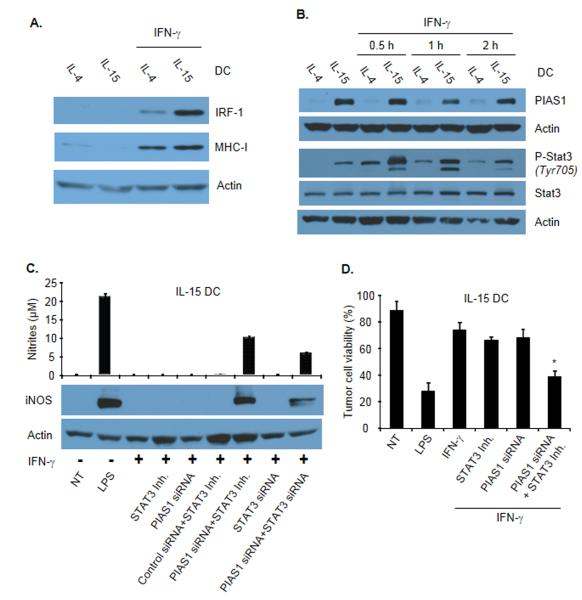
Since IFN-γ selectively induced iNOS expression in IL-4 but not in IL-15 DCs, we next sought to evaluate whether the IFN-γ-STAT-1 signaling axis may be altered in IL-15 DCs. Interestingly, STAT-1 phosphorylation was augmented in IL-15 DCs treated with IFN-γ (Fig. 3B). In line with this result, the level of expression of the STAT-1-controlled transcriptional regulator IRF-1 was increased by IFN-γ in IL-15 DCs (Fig. 4A). Consistently, the expression of MHC Class I molecules (controlled by STAT-1 and IRF-1 [32]) was increased following IFN-γ treatment (Fig. 4A). These data indicate that in IL-15 DCs the IFN-γ-STAT-1 signaling pathway is functional.
Figure 4.
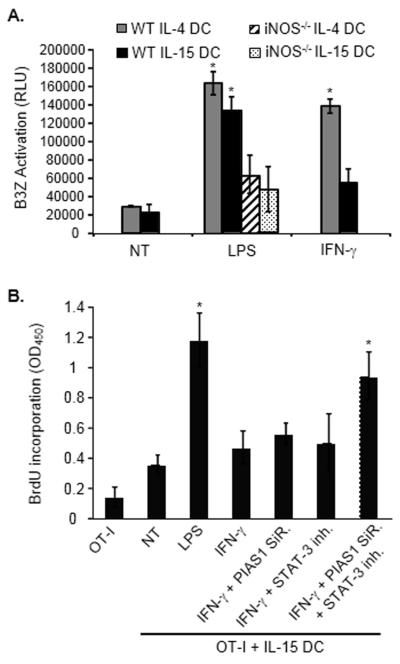
Control of iNOS expression by PIAS1 and STAT-3. (A) Expression of IRF-1 and MHC class I detected by immunoblotting in total extracts from IFN-γ-stimulated IL-4 DCs or IL-15 DCs. (B) PIAS1 expression and STAT-3 phosphorylation in total IL-4 DC or IL-15 DC extracts at the indicated time points following IFN-γ stimulation. (C) Effects of PIAS1 knockdown and STAT-3 inhibition on iNOS expression in IL-15 DCs stimulated with IFN-γ. IL-15 DCs were treated with PIAS1 siRNA, STAT-3 siRNA or the STAT-3 inhibitor JSI-124 or the combination of both before being exposed to IFN-γ. iNOS expression was determined by immunoblotting of total cell extracts (bottom) and nitrite concentration in the corresponding culture supernatants was determined (top). Data are shown as mean ± SD of duplicates and are from one representative experiment out of three performed. (A-C) β-actin was used as a loading control. (D) Experiment as in (C) except that after treatment DCs were cultured with tumor cells for 48 hours and their cytotoxic activity was evaluated using a Crystal Violet assay. Data are shown as mean ± SD of triplicates and are from one representative experiment out of two performed. *p<0.05, compared with IFN-γ-treated IL-15 DCs, Student t test.
The protein inhibitor of activated STAT (PIAS) 1 has been identified as a transcriptional repressor of STAT-1 [33]. Consistent with previous reports [34], PIAS1 expression was not detected in DCs generated with IL-4 (Fig. 4B). In contrast, the basal level of PIAS1 expression was substantially elevated in IL-15 DCs (Fig. 4B). Similarly, STAT-3 phosphorylation was enhanced in IL-15 DCs compared to IL-4 DCs (Fig. 4B). To evaluate the role of these two molecules, PIAS1 expression was blocked using siRNA and STAT-3 activation was restricted using the chemical inhibitor JSI-124 [35]. Individual elimination or inactivation of these proteins (Supporting Information Fig. 3B) had no effect on iNOS expression (Fig. 4C). Only the combination of PIAS1 with STAT-3 inhibition led to a partial restoration of iNOS expression, NO production (Fig 4C) and DC tumoricidal function (Fig. 4D).
Cytotoxic DCs are more efficient than non-cytotoxic DCs at activating T cells
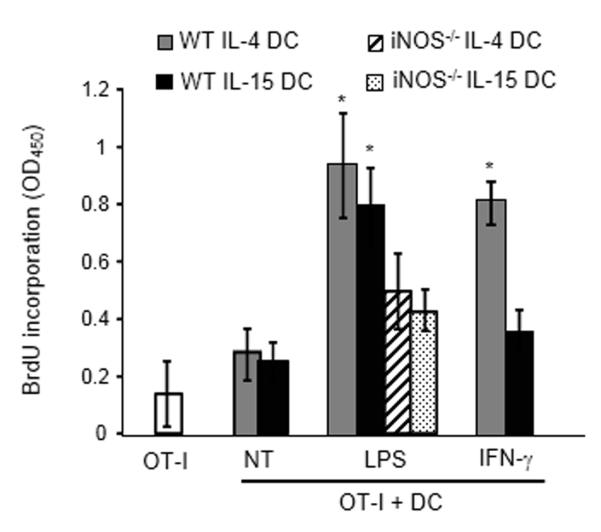
We next evaluated the functional implications of this differential regulation of IL-4 or IL-15 DC iNOS expression and thereby cytotoxic function. DCs were co-cultured with B16 melanoma cells expressing the model antigen ovalbumin (B16-OVA) and then re-isolated using CD11c microbeads. Following re-isolation from the co-culture, LPS-stimulated IL-4 or IL-15 DC or IFN-γ-treated IL-4 DC were significantly more efficient at activating the CD8+ T cell line B3Z [13] than IFN-γ-activated non-cytotoxic IL-15 DCs (Fig. 5A). Further confirming the link between the tumor killing and the antigen presenting functions of DCs, LPS-activated IL-4 DCs or IL-15 DCs generated from iNOS−/− mice (all deficient in their killing function, Fig. 2) were less potent at activating B3Z following culture with tumor cells compared to their cytotoxic wild-type counterparts (Fig. 5A). Of note, IL-15 DCs loaded with OVA and activated with IFN-γ were capable of inducing OVA257-264-specific OTI lymphocyte proliferation (not shown).
Figure 5.
Capability of IL-4 DCs and IL-15 DCs to activate antigen-specific T lymphocytes following tumor cell killing. (A) IL-4 DCs or IL-15 DCs were treated with LPS (LPS) or IFN-γ (IFN-γ) and cultured with B16-OVA. DCs were then selected using CD11c microbeads and incubated for 24 hours with B3Z cells (DC:B3Z ratio=1:10). The activity of β-galactosidase was measured by evaluating the conversion of its substrate into a chemiluminescent product (RLU, Relative Luminescence Unit). Data are shown as mean ± SD of triplicates from one experiment representative of two performed. *p<0.001, compared with the corresponding untreated DC group, Student t test. IL-4 DCs or IL-15 DCs generated from iNOS knock-out mice were also used in these experiments (iNOS−/− IL-4 DCs or IL-15 DCs). (B) Effects of PIAS1 siRNA and STAT-3 inhibitor on the ability of IFN-γ-stimulated IL-15 DCs to activate OVA-specific T cells after incubation with B16-OVA. DCs were treated with PIAS1 siRNA (PIAS1 SiR.) or/and with the STAT-3 inhibitor JSI-124 (500 nM, STAT-3 inh.) before being activated with IFN-γ, cultured with B16-OVA, selected based on CD11c expression and subsequently incubated with T lymphocytes isolated from OT-I transgenic mice. OT-I cell proliferation was evaluated by BrdU incorporation. LPS-activated DCs (LPS) were used as positive and untreated DCs (NT) as negative controls. Data are shown as mean + SD of triplicates from a single experiment representative of two performed. *p<0.02 compared to OT-I lymphocytes cultured with untreated DCs, Student t Test.
Since the combination of PIAS1 and STAT-3 inhibition restores iNOS expression and cytotoxic function of IFN-γ-activated IL-15 DCs (Fig. 4), we next evaluated the capability of IL-15 DCs treated with PIAS1 siRNA and STAT-3 inhibitor and stimulated with IFN-γ to prime OVA-specific T cells after incubation with B16-OVA. Our results indicate that these IL-15 DCs, for which the cytotoxic activity had been rescued, promoted the proliferation of OT-I lymphocytes following killing of cancer cells (Fig. 5B).
The activity of IL-15 and IL-4 DCs activated in vitro with LPS or IFN-γ was then assessed in vivo. CD11c-GFP-DTR mice were implanted with B16-OVA tumor cells and were administered with diphtheria toxin (DT) to eliminate host CD11c+ DCs when tumors became palpable. LPS- or IFN-γ- activated IL-4 or IL-15 DCs were then injected into the tumor beds and CD11c+ cells were re-isolated from the tumor draining lymph nodes after 36 hours. The ability of these purified cells to activate OVA-specific T lymphocytes was then evaluated. IL-4 DCs activated with LPS or IFN-γ and IL-15 DCs activated with LPS, but not non-cytotoxic IFN-γ-treated IL-15 DCs nor untreated DCs, induced the proliferation of OT-I lymphocytes (Fig. 6). Consistent with the results obtained in vitro (Fig. 5A), LPS-activated IL-4 and IL-15 DCs from iNOS−/− mice injected into the tumor beds and recovered from the draining lymph nodes were less potent at inducing T-cell proliferation (Fig. 6).
Figure 6.
Antigen presenting capability of DCs injected into B16-OVA tumor beds and recovered from the draining lymph nodes. CD11c-GFP-DTR mice were injected with B16-OVA cells. When tumors become palpable, endogenous DCs were depleted by diphtheria toxin (DT) administration. CD11c+ IL-4 DCs or IL-15 DCs were generated in vitro and treated with LPS or IFN-γ and then injected intratumorally (20×106 cells/tumor). LPS-activated IL-4 DCs and IL-15 DCs generated from iNOS−/− mice were also used in these experiments. After 36 hours, CD11c+ cells were re-isolated from the draining lymph nodes. The obtained DCs were then cultured with OT-I lymphocytes. T-cell proliferation was evaluated by BrdU incorporation. Data are shown as mean ± SD of triplicates from a single experiment representative of two performed. * p<0.01 compared to the corresponding untreated DC group, Student t test.
Discussion
Positioned at the center of the immune regulatory networks controlling innate and adaptive immunity, DCs have been the targets of a plethora of therapeutic strategies. The rationale for the development of DC-based anti-tumor vaccines has traditionally focused on their role as professional antigen presenting cells capable of priming and activating effector T lymphocytes [36]. More recent studies have lent support to the concept that DCs can also function as direct cytotoxic effectors against cancer [3-6, 37, 38]. However, the prospect of harnessing this less conventional aspect of DC biology has fueled intensive debates and controversy has risen as it relates to the mode of induction, the underlying mechanisms, and the physiological implications of this cytotoxic activity. The molecular regulation of DC tumoricidal function and the link between DC cytotoxic activity and antigen presenting function remain unclear.
In the current study, we provide data indicating that similar to our results obtained with IL-4 DCs [14, 25, 26, 29], LPS-activated IL-15 DCs can kill cancer cells by an iNOS-dependent mechanism. While IFN-γ acts as a potent inducer of iNOS-dependent IL-4 DC cytotoxic activity, this cytokine does not promote IL-15 DC killing function. Unexpectedly, IFN-γ-mediated phosphorylation of STAT-1 is not inhibited and IFN-γ-induced expression of MHC class I and IRF-1 is maintained in IL-15 DCs, suggesting that the IFN-γ-STAT-1 signaling axis is still functional in IL-15 DCs. No significant difference was observed between IL-4 and IL-15 DCs in the expression of suppressor of cytokine signaling (SOCS) 1, an important regulator of STAT-1 (not shown). Furthermore, since STAT-1 phosphorylation is not impaired in IL-15 DCs, the role of SOCS1 is unlikely. In an effort to further probe for negative factors that may lessen iNOS expression in IL-15 DCs, we determined for the first time that the protein inhibitor of activated STAT-1, PIAS1, is surprisingly highly expressed in IL-15 DCs (but not in IL-4 DCs). PIAS1 is a transcriptional regulator, endowed with small ubiquitin-related modifier (SUMO) E3 ligase activity, which selectively hinder the binding of transcription factors such as STAT-1 to gene promoters [33, 39, 40]. A previous report has indicated that bone-marrow derived DCs do not express PIAS1 [34]. Of note, in the aforementioned study, DCs were generated in presence of GM-CSF alone. Consistent with this result, our data indicate that PIAS1 is undetectable in IL-4 DCs. This observation opens the possibility that PIAS1 may be differentially up-regulated and thus may play a regulatory role depending on the DC subset. Transient knockdown of PIAS1 expression using siRNA, however, does not restore iNOS expression in IFNγ-stimulated IL-15 DCs. We also demonstrate that STAT-3 is phosphorylated in IL-15 DCs. Inhibition of STAT-3 by itself has no effect on iNOS expression, but the combination of PIAS1 and STAT-3 inhibition restores iNOS expression, NO production and the cytotoxic activity of IFN-γ-activated IL-15 DCs. These results therefore suggest that PIAS1 and STAT-3 cooperate to restrain iNOS-dependent killing activity of DCs.
The link between DC tumoricidal activity and DC antigen presenting function has been discussed [3]. In the current study, DCs endowed with killing abilities are more efficient at presenting tumor-derived antigenic peptides to T lymphocytes than their non-killer counterparts. This result indicates that the capability of IL-15 and IL-4 DCs to function as potent antigen presenting cells may be enhanced by their cytotoxic activity. Consistently, restoration of IFN-γ-activated IL-15 DC killing function by negatively targeting PIAS1 and STAT-3 promotes their ability to activate antigen specific T cells following culture with tumor cells. IL-15 DCs loaded with OVA and activated with IFN-γ induce the proliferation of OVA257-264-specific OTI lymphocytes, indicating that IFN-γ-stimulated IL-15 DCs are not impaired in their antigen presentating function. It is possible that the direct killing of cancer cells by DCs stimulate the acquisition of tumor-derived antigenic material in a more rapid and efficient manner. The type of tumor cell death (primarily a necrotic-like process) induced by DCs may be highly immunogenic in nature and may also contribute to foster their maturation and therefore immunostimulatory capability. Of note, PIAS1 and STAT3 may also negatively impact the expression of unidentified cytokines and cell surface ligands by IFN-γ-stimulated IL15 DCs. These results have functional consequences since LPS-activated cytotoxic IL-4 or IL-15 DCs or IFN-γ-treated IL-4 DCs are more efficient at presenting acquired tumor antigens from the killed tumor cells to T lymphocytes compared with IFN-γ-activated IL-15 DCs, following intratumoral injection of these cells.
By providing further insights into the mechanisms controlling the cytotoxic activity of DC, and by more precisely defining the relationship between DC killing and antigen-presenting functions, these findings may contribute to a better understanding of this less conventional aspect of DC biology and may also help designing improved strategies to appropriately harness this potential in DC-based cancer vaccine approaches.
Materials and Methods
Animals
Mice were housed and cared for according to the University of Arizona Institutional Animal Care and Use Committee guidelines. Six to 8 -week old female BALB/c (H2d), or C57BL6 (H2b) mice were obtained from the National Cancer Institute (Bethesda, MD). iNOS−/−(B6.129P2-Nos2tm1Lau/J), FasL−/− (B6Smn.C3-Faslgld/J), perforin−/− (CByJ.B6-Prf1tm1Sdz/J), NF-κBp50−/− (B6.129P-Nfkb1tm1Bal/J), CD11c-DTR-GFP (B6.FVB-Tg(Itgax-DTR/EGFP)57Lan/J), OT-I and OT-II mice were obtained from the Jackson Laboratory (Bar Harbor, ME). STAT-1−/− (129S6/SvEv-Stat1tm1Rds) mice were purchased from Taconic Farms (Hudson, NY). TRAIL deficient mice were obtained from Amgen (Thousand Oaks, CA).
Cell lines
The mouse melanoma cell line B16 and mammary carcinoma 4T1 cells were obtained from the American Tissue and Cell Collection (ATCC). OVA-expressing B16 (B16-OVA) were obtained as reported [13]. Cells were cultured (37°C, 5% CO2) in RPMI 1640 media (Thermo Fisher Scientific, Waltham, MA) containing 10% heat-inactivated fetal bovine serum (Thermo Fisher Scientific), 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin sulfate, 0.025 μg/ml amphotericin B, 0.5X MEM non-essential amino acids and 1 mM sodium pyruvate (complete media, CM).
Inhibitors and reagents
NG-methyl-L-arginine (NMMA), the STAT-3 inhibitor Cucurbitacin I hydrate (JSI-124) [35] and LPS were purchased from Sigma-Aldrich (St. Louis, MO). Recombinant murine IFN-γ was obtained from Peprotech (Rocky Hill, NJ).
Generation of bone marrow-derived IL-4 or IL-15 DC
DC were generated from mouse bone marrow as previously described [11, 13]. IL-4 DC were grown with granulocyte-macrophage colony-stimulating factor (GM-CSF; Peprotech) and Interleukine-4 (IL-4; Peprotech) (10 ng/ml each) [11, 13, 41, 42] and IL-15 DC were generated with GM-CSF and IL-15 (Peprotech) (20 ng/ml each) [10]. The medium was replaced on day 3 and 5. On day 6, CD11c+ cells were selected from the culture using anti-CD11c microbeads (Miltenyi Biotec, Auburn, CA) [13] and used for subsequent analyses.
Cytotoxic Assays
Purified CD11c+ DC, treated or not with IFN-γ (5 ng/ml) or LPS (0.1 μg/ml) or as indicated, were cultured with B16 melanoma or 4T1 carcinoma cells (tumor cells:DC ratio=1:5, or as indicated). Tumor cell killing by DC was then assessed after 48 h as previously reported [11-13, 43]. Briefly, the cells were rinsed with PBS and remaining adherent cells were fixed with 95% ethanol and stained with the Crystal Violet dye (100 μl in each well of a 96-well plates) for less than 10 seconds. The wells were then extensively washed with water. The dye was then eluted with acetic acid (30%). The amount of dye resuspended in the well is proportionate to the number of viable tumor cells. Plates were then read at 570 nm. Data were presented as the percentage of relative absorbance calculated from the formula: Atest/Acontrol, where Atest is the absorbance of tumor cells cultured with DCs in different conditions and Acontrol is the absorbance of DC cultured alone. DCs are very poorly stained with the dye and minimally contribute to the detected absorbance.
Flow Cytometry
Cells (~106) were washed in PBS and incubated with an Fc receptor blocking Ab (BD Biosciences, San Jose, CA), then stained with saturating amounts of the appropriate fluorochrome-conjugated antibodies [13]. Cells were then washed and analyzed using a FACS Calibur (Becton Dickinson Immunocytometry Systems, San Jose, CA), a FACSVerse (BD Biosciences) or a LSRFortessa (BD biosciences). A minimum of 10,000 events was collected for each sample, and data analysis was performed using FlowJo software (Treestar Inc., Ashland, OR). The following antibodies were purchased from eBiosciences (San Diego, CA): anti-CD4 (GK1.5), anti-CD8 (53-6.7), anti-CD70 (FR70), anti-TCR (KJ1-26), anti-CD83 (Michel-17), anti-CD205 (205yekta), anti-NKG2D (CX5), anti-F4/80 (BM8), anti-CD11c (N418), anti-CD49b (DX5), anti-CD86 (GL1), anti-MHC II (NIMR-4), anti-MHC I (SF1-1.1.1), anti-B220 (RA3-6B2) and from BD: anti-CD40 (HM40-3) and anti-CD11b (M1-70). For the analysis of tumor cell death, cancer cells were labeled with CellTrace Violet (Life Technologies, NY), incubated with DC and stained with AnnexinV/PI (eBiosciences) or with an anti-cleaved caspase-3 antibody (Cell Signaling Technology, Boston, MA). CellTrace Violet positive cells were gated and analyzed.
Real-time PCR
Total RNA from untreated or treated DC was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA) and its integrity was confirmed by denaturing agarose gel electrophoresis and calculated densitometric 28S/18S ratio. Total RNA (250 ng) were reverse-transcribed using iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Subsequently, 20 μL of the PCR reactions were set up in 96-well plates containing 10 μl 2X IQ Supermix (Bio-Rad), 1 μL of the indicated TaqMan® primer/probe set (ABI, Foster City, CA), 2 μL of the cDNA synthesis reaction (10% of RT reaction) and 7 μL of nuclease-free water. Reactions were run and analyzed on a iCycler iQ real–time PCR detection system (Bio-Rad). Cycling parameters were determined and resulting data were analyzed by using the comparative Ct method as means of relative quantification, normalized to an endogenous reference (TATA Box Binding Protein, TBP) and relative to a calibrator (normalized Ct value obtained from untreated DC) and expressed as 2−ΔΔCt (Applied Biosystems User Bulletin #2: Rev B “Relative Quantification of Gene Expression”).
Endocytosis assays
Selected CD11c+ IL-4 or IL-15 DC were incubated at 37°C or at 4°C for 1 hour with DQTM ovalbumin (DQ-OVA, Molecular Probes, 10 μg/ml). Cells were then washed and analyzed using a FACS Calibur. A minimum of 10,000 events was collected for each sample, and data analysis was performed using FlowJo software. DQ-OVA, a self-quenched conjugate of OVA, releases green fluorescent fragments when undergoing proteolytic degradation. Accumulation of degraded DQ-OVA fragments in organelles at high concentration exhibit red fluorescence.
Transfections, siRNA and luciferase reporter assays
Selected CD11c+ DC (1.5×106) were transiently transfected in 6-well plates using X-tremeGENE HP transfection reagent following the manufacturer’s instructions (Roche, Indianapolis, IN). PIAS1 or STAT-3 siRNA was combined with transfection reagent at 4:1 ratio (μl reagent:μg of plasmid). The transfection solution was added to the DC culture and cells were incubated at 37° C. For transient knockdown, transfections of siRNA were performed twice (40 and 24 hours) before additional treatment with IFN-γ or LPS. PIAS1 siRNA, STAT3 siRNA, and control siRNA were purchased from Santa Cruz Biotechnology (Santa Cruz Biotechnology, Santa Cruz, CA).
Western blot Analysis
DC were generated, purified and treated as explained above. Cells were collected, washed with cold PBS and lysed with radio-immunoprecipitation assay (RIPA) buffer containing a protease and phosphatase inhibitor cocktail (Thermo Scientific, Rockford, IL). The cell lysate was sonicated, centrifuged (8,000g, 8 min, 4°C) and protein concentration was determined (BCA Protein Assay kit, Thermo Scientific). Thirty micrograms of total cell lysate were boiled and resolved in 10% tris-glycine gel (Invitrogen, Carlsbad, CA) by electropheoresis (125 V, 105 min). Proteins were then transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, Billerica, MA). The membrane was blocked 30 min with 5% milk in Tris-buffered saline containing 0.1% Tween-20 and incubated overnight (4°C) with the indicated primary antibodies. The blot was washed and incubated with a secondary antibody. Reactive bands were visualized by exposure to film using SuperSignal Chemiluminescent Substrate (Thermo Scientific). Antibodies against iNOS, phospho-Stat1 (Tyr701), phospho-Stat1 (Ser727), Stat1, PIAS1, phospho-Stat3, Stat3, Lamin A/C were purchased from Cell Signaling Technology (Beverly, MA). Antibodies against IRF1 were from R&D Systems (Minneapolis, MN) and antibodies against Actin from Sigma-Aldrich (St. Louis, MO). The secondary antibodies, peroxidase conjugated goat anti-rabbit and peroxidase conjugated goat anti-mouse, were obtained from Jackson ImmunoResearch (West Grove, PA).
Detection of cytokine production by ELISA
The concentration of the indicated cytokines in the culture supernatants was determined using enzyme-linked immunosorbent assay (ELISA) kits (eBiosciences).
Antigen presentation assays in vitro and in vivo
IL-4 or IL-15 DC, treated as indicated, were cultured (48 hrs) with B16-OVA tumor cells. DC were selected using anti-CD11c-microbeads and co-cultured with B3Z cells (DC:B3Z ratio=1:10). B3Z is a mouse CD8+ T-cell hybridoma that contains an Escherichia coli lacZ reporter gene driven by nuclear factor of activated T cell (NF-AT) elements from the IL-2 promoter. The specific recognition of H2Kb/OVA257-264 complexes by B3Z TCR results in β-galactosidase expression. The activity of this enzyme is detected by measuring the conversion of a chemoluminescent substrate (Novagen kit, Madison, WI, USA) as previously documented [11, 13]. In similar experiments, DC ability to induce MHC-I-restricted proliferation of OVA257-264- specific OT-I T lymphocytes was determined using BrDU incorporation assays (Millipore) as previously reported [13, 44].
In other in vivo experiments, CD11c-DTR mice were subcutaneously injected with 1×106 B16 or B16-OVA tumor cells. When tumors were palpable, diphtheria toxin (DT, Sigma, 5 ng/g body weight) was administered (i.p,) on 2 consecutive days, which resulted in a significant depletion of endogenous DC as determined by flow cytometry (not shown). On the day of the second DT injection, 20×106 IL-4 or IL-15 DC, activated with LPS or IFN-γ were injected intratumorally. Thirty-six hours later, mice were euthanized and CD11c+ DC were re-isolated using CD11c microbeads (Miltenyi). The ability of these re-isolated DC to activate OVA-specific T cells was then determined.
Statistical analysis
Unless specified otherwise, all experiments were reproduced 3 times and performed in duplicate or triplicate. A two-sided student’s t test with paired samples (assuming equal variance) was used to determine significant differences between groups. For real time PCR experiments, statistical significance was determined by the analysis of variance (ANOVA) followed by Fisher protected least significant difference (PLSD) post-hoc test with StatView software package v.4.53 (SAS Institute, Cary, NC). Data are expressed as mean ± standard error of mean.
Supplementary Material
Acknowledgments
We thank Amanda Herrell, Jessica Kartchner, Jessica Stokes and Sophie Marin for technical assistance. This work was supported in part by the NIH grant R01 CA104926, the Cancer Biology Training Grant T32CA009213 (CJL and DA), the AZ Cancer Center Support Grant CA023074, the Tee Up for Tots and PANDA Funds (E.K. and N.L.).
Abbreviations
- DT
diphtheria toxin
- KDCs
killer dendritic cells
- NMMA
NG-methyl-L-arginine
- PIAS1
protein inhibitor of activated STAT-1
Footnotes
Conflict of Interest The authors declare no financial or commercial conflict of interest.
References
- 1.Adema GJ. Dendritic cells from bench to bedside and back. Immunol Lett. 2009;122:128–130. doi: 10.1016/j.imlet.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 2.Nestle FO, Farkas A, Conrad C. Dendritic-cell-based therapeutic vaccination against cancer. Curr Opin Immunol. 2005;17:163–169. doi: 10.1016/j.coi.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Larmonier N, Fraszczak J, Lakomy D, Bonnotte B, Katsanis E. Killer dendritic cells and their potential for cancer immunotherapy. Cancer Immunol Immunother. 2010;59:1–11. doi: 10.1007/s00262-009-0736-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan CW, Housseau F. The ‘kiss of death’ by dendritic cells to cancer cells. Cell Death Differ. 2008;15:58–69. doi: 10.1038/sj.cdd.4402235. [DOI] [PubMed] [Google Scholar]
- 5.Wesa AK, Storkus WJ. Killer dendritic cells: mechanisms of action and therapeutic implications for cancer. Cell Death Differ. 2008;15:51–57. doi: 10.1038/sj.cdd.4402243. [DOI] [PubMed] [Google Scholar]
- 6.Chauvin C, Josien R. Dendritic cells as killers: mechanistic aspects and potential roles. J Immunol. 2008;181:11–16. doi: 10.4049/jimmunol.181.1.11. [DOI] [PubMed] [Google Scholar]
- 7.Hanke N, Alizadeh D, Katsanis E, Larmonier N. Dendritic cell tumor killing activity and its potential applications in cancer immunotherapy. Crit Rev Immunol. 2013;33:1–21. doi: 10.1615/critrevimmunol.2013006679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larmonier N, Bonnotte B, Katsanis E. Cytotoxic and antigen presenting functions of T helper-1-activated dendritic cells. Oncoimmunology. 2012;1:566–568. doi: 10.4161/onci.19370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubsky P, Saito H, Leogier M, Dantin C, Connolly JE, Banchereau J, Palucka AK. IL-15-induced human DC efficiently prime melanoma-specific naive CD8+ T cells to differentiate into CTL. Eur J Immunol. 2007;37:1678–1690. doi: 10.1002/eji.200636329. [DOI] [PubMed] [Google Scholar]
- 10.Pulendran B, Dillon S, Joseph C, Curiel T, Banchereau J, Mohamadzadeh M. Dendritic cells generated in the presence of GM-CSF plus IL-15 prime potent CD8+ Tc1 responses in vivo. Eur J Immunol. 2004;34:66–73. doi: 10.1002/eji.200324567. [DOI] [PubMed] [Google Scholar]
- 11.Fraszczak J, Trad M, Janikashvili N, Cathelin D, Lakomy D, Granci V, Morizot A, et al. Peroxynitrite-dependent killing of cancer cells and presentation of released tumor antigens by activated dendritic cells. J Immunol. 2010;184:1876–1884. doi: 10.4049/jimmunol.0900831. [DOI] [PubMed] [Google Scholar]
- 12.Nicolas A, Cathelin D, Larmonier N, Fraszczak J, Puig PE, Bouchot A, Bateman A, et al. Dendritic cells trigger tumor cell death by a nitric oxide-dependent mechanism. J Immunol. 2007;179:812–818. doi: 10.4049/jimmunol.179.2.812. [DOI] [PubMed] [Google Scholar]
- 13.LaCasse CJ, Janikashvili N, Larmonier CB, Alizadeh D, Hanke N, Kartchner J, Situ E, et al. Th-1 lymphocytes induce dendritic cell tumor killing activity by an IFN-gamma-dependent mechanism. J Immunol. 2011;187:6310–6317. doi: 10.4049/jimmunol.1101812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lakomy D, Janikashvili N, Fraszczak J, Trad M, Audia S, Samson M, Ciudad M, et al. Cytotoxic dendritic cells generated from cancer patients. J Immunol. 2011;187:2775–2782. doi: 10.4049/jimmunol.1004146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anguille S, Lion E, Tel J, de Vries IJ, Coudere K, Fromm PD, Van Tendeloo VF, et al. Interleukin-15-induced CD56(+) myeloid dendritic cells combine potent tumor antigen presentation with direct tumoricidal potential. PloS one. 2012;7:e51851. doi: 10.1371/journal.pone.0051851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13:114–119. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 17.Miyake Y, Yamasaki S. Sensing necrotic cells. Adv Exp Med Biol. 2012;738:144–152. doi: 10.1007/978-1-4614-1680-7_9. [DOI] [PubMed] [Google Scholar]
- 18.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 19.Larmonier N, Merino D, Nicolas A, Cathelin D, Besson A, Bateman A, Solary E, et al. Apoptotic, necrotic, or fused tumor cells: an equivalent source of antigen for dendritic cell loading. Apoptosis. 2006;11:1513–1524. doi: 10.1007/s10495-006-8765-0. [DOI] [PubMed] [Google Scholar]
- 20.Ma DY, Clark EA. The role of CD40 and CD154/CD40L in dendritic cells. Semin Immunol. 2009;21:265–272. doi: 10.1016/j.smim.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janjic BM, Lu G, Pimenov A, Whiteside TL, Storkus WJ, Vujanovic NL. Innate direct anticancer effector function of human immature dendritic cells. I. Involvement of an apoptosis-inducing pathway. J Immunol. 2002;168:1823–1830. doi: 10.4049/jimmunol.168.4.1823. [DOI] [PubMed] [Google Scholar]
- 22.Liu S, Yu Y, Zhang M, Wang W, Cao X. The involvement of TNF-alpha-related apoptosis-inducing ligand in the enhanced cytotoxicity of IFN-beta-stimulated human dendritic cells to tumor cells. J Immunol. 2001;166:5407–5415. doi: 10.4049/jimmunol.166.9.5407. [DOI] [PubMed] [Google Scholar]
- 23.Lu G, Janjic BM, Janjic J, Whiteside TL, Storkus WJ, Vujanovic NL. Innate direct anticancer effector function of human immature dendritic cells. II. Role of TNF, lymphotoxin-alpha(1)beta(2), Fas ligand, and TNF-related apoptosis-inducing ligand. J Immunol. 2002;168:1831–1839. doi: 10.4049/jimmunol.168.4.1831. [DOI] [PubMed] [Google Scholar]
- 24.Stary G, Bangert C, Tauber M, Strohal R, Kopp T, Stingl G. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. J Exp Med. 2007;204:1441–1451. doi: 10.1084/jem.20070021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vanderheyde N, Aksoy E, Amraoui Z, Vandenabeele P, Goldman M, Willems F. Tumoricidal activity of monocyte-derived dendritic cells: evidence for a caspase-8-dependent, Fas-associated death domain-independent mechanism. J Immunol. 2001;167:3565–3569. doi: 10.4049/jimmunol.167.7.3565. [DOI] [PubMed] [Google Scholar]
- 26.Vidalain PO, Azocar O, Yagita H, Rabourdin-Combe C, Servet-Delprat C. Cytotoxic activity of human dendritic cells is differentially regulated by double-stranded RNA and CD40 ligand. J Immunol. 2001;167:3765–3772. doi: 10.4049/jimmunol.167.7.3765. [DOI] [PubMed] [Google Scholar]
- 27.Shi J, Ikeda K, Fujii N, Kondo E, Shinagawa K, Ishimaru F, Kaneda K, et al. Activated human umbilical cord blood dendritic cells kill tumor cells without damaging normal hematological progenitor cells. Cancer Sci. 2005;96:127–133. doi: 10.1111/j.1349-7006.2005.00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimamura H, Cumberland R, Hiroishi K, Watkins SC, Lotze MT, Baar J. Murine dendritic cell-induced tumor apoptosis is partially mediated by nitric oxide. J Immunother. 2002;25:226–234. doi: 10.1097/00002371-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Gilad E, Wong HR, Zingarelli B, Virag L, O’Connor M, Salzman AL, Szabo C. Melatonin inhibits expression of the inducible isoform of nitric oxide synthase in murine macrophages: role of inhibition of NFkappaB activation. FASEB J. 1998;12:685–693. doi: 10.1096/fasebj.12.9.685. [DOI] [PubMed] [Google Scholar]
- 30.Madrigal JL, Moro MA, Lizasoain I, Lorenzo P, Castrillo A, Bosca L, Leza JC. Inducible nitric oxide synthase expression in brain cortex after acute restraint stress is regulated by nuclear factor kappaB-mediated mechanisms. J Neurochem. 2001;76:532–538. doi: 10.1046/j.1471-4159.2001.00108.x. [DOI] [PubMed] [Google Scholar]
- 31.Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- 32.Zhou F. Molecular mechanisms of IFN-gamma to up-regulate MHC class I antigen processing and presentation. Int Rev Immunol. 2009;28:239–260. doi: 10.1080/08830180902978120. [DOI] [PubMed] [Google Scholar]
- 33.Shuai K, Liu B. Regulation of gene-activation pathways by PIAS proteins in the immune system. Nat Rev Immunol. 2005;5:593–605. doi: 10.1038/nri1667. [DOI] [PubMed] [Google Scholar]
- 34.Tahk S, Liu B, Chernishof V, Wong KA, Wu H, Shuai K. Control of specificity and magnitude of NF-kappa B and STAT1-mediated gene activation through PIASy and PIAS1 cooperation. Proc Natl Acad Sci U S A. 2007;104:11643–11648. doi: 10.1073/pnas.0701877104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishdorj G, Johnston JB, Gibson SB. Inhibition of constitutive activation of STAT3 by curcurbitacin-I (JSI-124) sensitized human B-leukemia cells to apoptosis. Mol Cancer Ther. 2010;9:3302–3314. doi: 10.1158/1535-7163.MCT-10-0550. [DOI] [PubMed] [Google Scholar]
- 36.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 37.Bonmort M, Dalod M, Mignot G, Ullrich E, Chaput N, Zitvogel L. Killer dendritic cells: IKDC and the others. Curr Opin Immunol. 2008;20:558–565. doi: 10.1016/j.coi.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 38.Ullrich E, Chaput N, Zitvogel L. Killer dendritic cells and their potential role in immunotherapy. Horm Metab Res. 2008;40:75–81. doi: 10.1055/s-2007-1022554. [DOI] [PubMed] [Google Scholar]
- 39.Liu B, Mink S, Wong KA, Stein N, Getman C, Dempsey PW, Wu H, et al. PIAS1 selectively inhibits interferon-inducible genes and is important in innate immunity. Nat Immunol. 2004;5:891–898. doi: 10.1038/ni1104. [DOI] [PubMed] [Google Scholar]
- 40.Liu B, Yang Y, Chernishof V, Loo RR, Jang H, Tahk S, Yang R, et al. Proinflammatory stimuli induce IKKalpha-mediated phosphorylation of PIAS1 to restrict inflammation and immunity. Cell. 2007;129:903–914. doi: 10.1016/j.cell.2007.03.056. [DOI] [PubMed] [Google Scholar]
- 41.Larmonier N, Janikashvili N, LaCasse CJ, Larmonier CB, Cantrell J., Situ, E., Lundeen T, et al. Imatinib mesylate inhibits CD4+ CD25+ regulatory T cell activity and enhances active immunotherapy against BCR-ABL- tumors. J Immunol. 2008;181:6955–6963. doi: 10.4049/jimmunol.181.10.6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cantrell J, Larmonier C, Janikashvili N, Bustamante S, Fraszczak J, Herrell A, Lundeen T, et al. Signaling pathways induced by a tumor-derived vaccine in antigen presenting cells. Immunobiology. 2010;215:535–544. doi: 10.1016/j.imbio.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Larmonier N, Billerey C, Rebe C, Parcellier A, Moutet M, Fromentin A, Kroemer G, et al. An atypical caspase-independent death pathway for an immunogenic cancer cell line. Oncogene. 2002;21:6091–6100. doi: 10.1038/sj.onc.1205738. [DOI] [PubMed] [Google Scholar]
- 44.Janikashvili N, LaCasse CJ, Larmonier C, Trad M, Herrell A, Bustamante S, Bonnotte B, et al. Allogeneic effector/memory Th-1 cells impair FoxP3+ regulatory T lymphocytes and synergize with chaperone-rich cell lysate vaccine to treat leukemia. Blood. 2011;117:1555–1564. doi: 10.1182/blood-2010-06-288621. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.