Abstract
Small conductance calcium-activated K+ (SK) channels regulate neuronal excitability. However, little is known about changes in SK channel activity of presympathetic neurons in the hypothalamic paraventricular nucleus (PVN) in essential hypertension. SK channels, calmodulin, and casein kinase II (CK2) form a molecular complex. Because CK2 is upregulated in the PVN in spontaneously hypertensive rats (SHRs), we hypothesized that CK2 increases calmodulin phosphorylation and contributes to diminished SK channel activity in PVN presympathetic neurons in SHRs. Perforated whole-cell recordings were performed on retrogradely labeled spinally projecting PVN neurons in Wistar-Kyoto (WKY) rats and SHRs. Blocking SK channels with apamin significantly increased the firing rate of PVN neurons in WKY rats but not in SHRs. CK2 inhibition restored the stimulatory effect of apamin on the firing activity of PVN neurons in SHRs. Furthermore, apamin-sensitive SK currents and depolarization-induced medium after-hyperpolarization potentials of PVN neurons were significantly larger in WKY rats than in SHRs. CK2 inhibition significantly increased the SK channel current and medium after-depolarization potential of PVN neurons in SHRs. In addition, CK2-mediated calmodulin phosphorylation level in the PVN was significantly higher in SHRs than in WKY rats. Although SK3 was detected in the PVN, its expression level did not differ significantly between SHRs and WKY rats. Our findings suggest that CK2-mediated calmodulin phosphorylation is increased and contributes to diminished SK channel function of PVN presympathetic neurons in SHRs. This information advances our understanding of the mechanisms underlying hyperactivity of PVN presympathetic neurons and increased sympathetic vasomotor tone in hypertension.
Introduction
Although hypertension is a well-recognized risk factor for stroke, coronary heart disease, and renal failure, the etiology of hypertension in most patients remains unknown. Among many proposed mechanisms, elevated sympathetic outflow is involved in the development of essential hypertension (Anderson et al. 1989; Greenwood et al. 1999). The paraventricular nucleus (PVN) of the hypothalamus is an important source of excitatory drive for increased sympathetic outflow in spontaneously hypertensive rats (SHRs) (Eilam et al. 1994; Allen 2002; Li and Pan 2007b). PVN neurons that project to the intermediolateral cell column of spinal cords and rostral ventrolateral medulla play an important role in controlling sympathetic outflow and blood pressure (Swanson and Sawchenko 1983; Kannan et al. 1989; Pyner and Coote 2000). Increased activity of synaptic N-methyl-D-aspartate (NMDA) receptors and group I metabotropic glutamate receptors augments glutamatergic inputs and contributes to the hyperactivity of PVN presympathetic neurons in SHRs (Li et al. 2008; Li and Pan 2010).
In addition to the identified synaptic plasticity, changes in other ion channels may also play a role in the hyperactivity of PVN presympathetic neurons in hypertension. Small conductance Ca2+-activated K+ (SK) channels regulate intrinsic neuronal excitability in the brain (Kohler et al. 1996; Sah 1996; Stocker et al. 1999). Three homologous SK channels have been identified in the brain tissues (SK1, SK2, and SK3); these subtypes display distinct overlapping patterns of expression and show high sensitivity to the SK channel blocker apamin (Kohler et al. 1996; Stocker et al. 1999). SK channels influence the firing activity by eliciting a medium after-hyperpolarization (mAHP), which is a prolonged period of hyperpolarization of the membrane (Sah 1996). SK channels are gated by a micromolar range of Ca2+ in the cytoplasm and sense Ca2+ through the binding of Ca2+ to calmodulin (Adelman et al. 2012). Chronic systemic infusion of angiotensin II reduces SK channel activity of PVN presympathetic neurons (Chen et al. 2010). However, it is not clear whether the SK channel activity in the PVN is altered in SHRs. Also, the mechanism underlying altered SK channel function in the PVN in hypertensive conditions remains unknown.
Native SK channels exist as a polyprotein complex, which consists of calmodulin, protein kinase CK2, and protein phosphatase 2A (Bildl et al. 2004). Calmodulin is constitutively bound to the C-terminus domain of SK channel subunits, and Ca2+-calmodulin binding initiates a rearrangement that changes the channel gating (Keen et al. 1999; Schumacher et al. 2001). Also, calmodulin can be phosphorylated by CK2 at amino acid residues Thr-79, Thr-80, Thr-117, Ser-81, and Ser-101 (Meggio and Pinna 2003; Arrigoni et al. 2004; Bildl et al. 2004). We have shown that CK2 activity in the PVN is increased and contributes to elevated sympathetic outflow in SHRs (Ye et al. 2011). An increase in CK2 activity may potentiate calmodulin phosphorylation and diminish the sensitivity of SK channel-bound calmodulin to Ca2+ (Bildl et al. 2004). We therefore designed the present study to determined (1) whether SK channel activity of spinally projecting PVN neurons is reduced in SHRs, and (2) whether CK2-mediated calmodulin phosphorylation contributes to reduced SK channel function of PVN presympathetic neurons in SHRs.
Methods and Materials
Animals
Experiments were performed on 13-week-old male SHRs (n = 64) and Wistar Kyoto (WKY, n = 62) rats purchased from Harlan Laboratories (Indianapolis, IN). All surgical procedures were approved by the Institutional Animal Care and Use Committee of The University of Texas MD Anderson Cancer Center. The experimental protocols adhere strictly to the National Institutes of Health guidelines on the ethical use of animals. Arterial blood pressure was measured by using a non-invasive tail-cuff system (IITC Life Science, Inc., Woodland Hills, CA) 1 week before performing electrophysiological experiments. Systolic blood pressure was significantly higher in SHRs (203.20 ± 2.50 mmHg) than in age-matched WKY rats (122.28 ± 2.20 mmHg).
Retrograde labeling, brain slice preparation, and electrophysiological recordings
Rats were anesthetized with isoflurane (2% in O2), and the spinal cord was exposed by laminectomy at the T2–T4 levels. FluoroSpheres (Molecular Probes, Eugene, OR) were pressure-ejected (Nanojector II; Drummond Scientific, Broomball, PA) bilaterally into the intermediolateral cell column, as described previously (Li et al. 2002, 2003), and rats were used for electrophysiological recordings 3-5 days later.
SHRs and WKY rats were rapidly decapitated under isoflurane anesthesia, and the brain was quickly removed and transferred into an ice-cold artificial cerebrospinal fluid (aCSF) solution containing (in mM) 126.0 NaCl, 3.0 KCl, 1.5 MgCl2, 2.4 CaCl2, 1.2 NaH2PO4, 11.0 glucose, and 26.0 NaHCO3 saturated with a gas mixture of 95% O2 and 5% CO2. The brain was trimmed to a tissue block containing the hypothalamus and was sectioned to 300-μm-thick slices with use of a vibratome (Leica, Buffalo Grove, IL). The brain slices were incubated in aCSF solution at 34°C for at least 1 hour before being used for electrophysiological recordings. Also, the spinal cord was sectioned at the injected level to confirm that the injection and diffusion sites of FluoroSpheres were located in the vicinity of the intermediolateral cell column.
Fluorescence-labeled neurons in brain slices were visualized with use of an upright microscope equipped with epifluorescence and infrared differential interference contrast optics. Recordings were performed at 34°C by using glass electrodes (resistance, 4-6 MΩ). The firing activity of labeled PVN neurons was recorded in current-clamp mode using perforated approach to minimize the interference with intracellular Ca2+ (Ye et al. 2012). The internal solution contained (in mM) 130.0 K-acetate, 15.0 KCl, 5.0 NaCl, 1.0 MgCl2, and 10.0 HEPES (pH adjusted to 7.2 with KOH to 296 mOsm). The tip of the recording pipette was backfilled with the internal pipette solution containing gramicidin (50 μg/ml). SK channel-mediated medium-duration after-hyperpolarization (mAHP) was recorded at −60 mV by injecting a positive current of 150 pA in a duration of 500 ms (Chen and Toney 2009). Also, tail currents were recorded with use of the perforated approach. Neurons were voltage-clamped at −60 mV, and a 100-ms depolarizing pulse to +10 mV was used to elicit an outward tail current (Spergel 2007). Apamin-sensitive tail currents were obtained by subtracting the tail currents in the presence of apamin (100 nM) from the currents recorded before apamin application.
Spontaneous excitatory synaptic currents (sEPSCs) and spontaneous inhibitory synaptic currents (sIPSCs) were recorded at a holding potential of −60 and 0 mV, respectively, with use of a conventional whole-cell configuration (Li and Pan 2006; Li et al. 2008). The recording internal solution for sEPSCs and sIPSCs contained (in mM) 110.0 Cs2SO4, 2.0 MgCl2, 0.5 CaCl2, 5.0 EGTA, 2.0 Mg-ATP, 0.3 Na2-GTP, 10.0 QX-314, and 10.0 HEPES (pH adjusted to 7.25 with CsOH; 285 mOSm). 2-Amino-5-phosphonopentanoic acid (50 μM) and 6-cyano-7-nitroquinoxaline-2,3-dione (20 μM) were added to the bath solution for the recording of sIPSCs, and gabazine (20 μM) was bath applied during the recording of sEPSCs. Signals were processed by a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA), filtered at 1-2 kHz, digitized at 20 kHz, and saved to a computer hard-drive.
2-Amino-5-phosphonopentanoic acid and 6-cyano-7-nitroquinoxaline-2,3-dione were purchased from Abcam Biochemicals (Cambridge, MA), and gabazine, gramicidin and 5’6-dichlorobenzimidazole (DRB) were purchased from Sigma Aldrich (St. Louis, MO). Apamin was obtained from American Peptide Company (Sunnyvale, CA).
Intracerebroventricular injection
To determine whether CK2 is involved in phosphorylation of calmodulin in the PVN, DRB (8 mM) or vehicle (0.1% dimethyl sulfoxide) was intracerebroventricularly injected in SHRs (Ye et al. 2012). A guide cannula was placed through a 2-mm burr hole in the skull of the rat (coordinates: 1.5 mm lateral to the midline, 1.0 mm caudal to the bregma, and 3.0 mm ventral to the dura). The tip of the injection cannula protruded 0.5 mm beyond the tip of the guide cannula and each injection consisted of 10 μl of solution (three injections) delivered over a period of 30 min each.
PCR analysis
We initially used agarose gel electrophoresis to determine which SK channel isoforms were expressed in the PVN. Total RNA was extracted from the rat PVN and hippocampal tissue by using Trizol Reagent (Invitrogen, Grand Island, NY) according to manufacturer's recommendations. One μg of total RNA was reverse-transcribed to cDNA by Super Script first strand cDNA synthesis kit (Invitrogen, Grand Island, NY) in 20 μl reaction volume. MyTaq-Extract-PCR kit (Bioline, Taunton, MA) was used to amplify SK3 cDNA with forward primer (5’-TCTCCATCACGTTCCTTTCC-3’) and reverse primer (5’-CTTGACACCCCTCAGTTG GT-3’). PCR conditions were as follows: one cycle of 3 min at 95°C 32 cycles of 95°C for 1 min, for 52°C for 30 s,72°C for 45 s, and final extension at 72°C for 15 min. PCR product was separated on 2% agarose gel with ethidium bromide. Gel images were captured by Fluorchem® FC2 gel document system (Alpha Inotech, San Leandro, CA). In addition, real-time PCR was used to quantify the mRNA level of SK3 in the PVN of WKY rats and SHRs.
Western immunoblotting
To determine the amount of phosphorylated calmodulin in the PVN tissues, rats were anesthetized with 2-3% isoflurane and decapitated. The brain was sectioned with use of a vibrating microtome in ice-cold aCSF. The PVN tissue was obtained by using the punch method, frozen in liquid nitrogen, and stored at –80EC until use. The PVN tissues were homogenized in RIPA buffer (20 mM Tris-HCl (pH7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3Vo4, and 1 μg/ml leupeptin) containing the protease inhibitor cocktail and phosphatase inhibitor cocktail (Cell Signaling, Danvers, MA). The homogenate was then centrifuged at 16,000 g for 10 min at 4EC, and the supernatant was collected. The protein sample was then heated at 98EC for 10 min, and 30 μg of protein was loaded onto a sodium dodecyl sulfate-polyacrylamide gradient (4-12%) gel. The protein was transferred to nitrocellulose membranes and probed with use of anti-phosphorylated calmodulin (phospho Thr-79 and Ser-81) antibody (Abcam Biochemicals) (Arrigoni et al. 2004), anti-calmodulin antibody (Millipore, Billerica, MA), or anti-SK3 antibody (Alomone Labs, Jerusalem, Israel). The GAPDH protein band was used as a loading control. Protein bands were detected with use of ECL Prime (GE Healthcare, Pittsburgh, PA). The density of each protein band was analyzed by using ImageJ software and normalized to GAPDH. The mean values of the protein level in the PVN in WKY rats or vehicle-treated SHRs were considered as 1.
Data analysis
Data were presented as the mean ± SEM. The action potential, SK channel current, and mAHP data were analyzed with use of pClamp10 (Molecular Devices). The sEPSCs and sIPSCs were analyzed off-line by using a peak detection program (MiniAnalysis, Synaptosoft, Leonia, NJ). For electrophysiological experiments, at least 4 rats were used in each protocol. The Student's t test was used for statistical comparison of sEPSCs, sIPSCs, and the mRNA and protein levels between SHRs and WKY rats. To determine the statistical differences in the action potential, SK channel current, and mAHP data, repeated measures ANOVA with Dunnett's post-hoc test was used. P < 0.05 was considered statistically significant.
Results
CK2 inhibition restores the contribution of SK channels to regulation of PVN presympathetic neuronal activity in SHRs
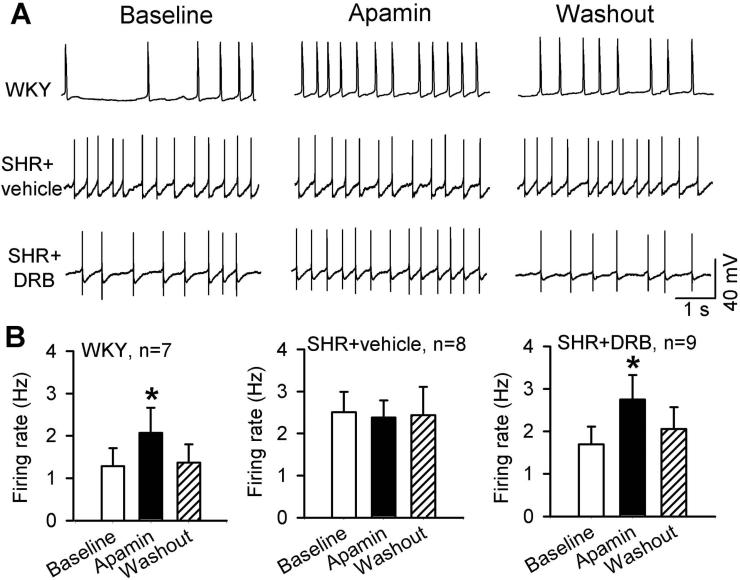
To determine whether SK channels are involved in regulating neuronal excitability of spinally projecting PVN neurons in hypertension, we determined the effect of the specific SK channel blocker apamin on the firing activity of retrogradely labeled spinally projecting PVN neurons in WKY rats and SHRs. Spontaneous firing activity was recorded by using a perforated whole-cell recording technique to minimize the interference of intracellular Ca2+ by the pipette internal solution. The basal firing rate was significantly lower in labeled PVN neurons in WKY rats (1.28 ± 0.43 Hz, n = 7 neurons) than in SHRs (2.51 ± 0.48 Hz, n = 8 neurons) (P < 0.05, Fig. 1). In WKY rats, bath application of 100 nM apamin significantly increased the firing rate of all PVN neurons tested (2.07 ± 0.59 Hz, P < 0.05). However, in SHRs, apamin failed to significantly increase the firing rate of labeled PVN neurons (Fig. 1).
Figure 1. CK2 inhibition restores the excitatory effect of apamin on the firing activity of spinally projecting PVN neurons in SHRs.
A, Original traces show gramicidin perforated recordings of the firing activity of labeled PVN neurons before and during application of 100 nM apamin in WKY rat brain slices and SHR brain slices treated with DRB (100 μM, 2-4 h) or vehicle (0.1% dimethyl sulfoxide). B, Summary data show the effects of apamin on the firing activity in labeled PVN neurons in WKY rat brain slices and SHR brain slices treated with DRB or vehicle. Data are presented as means ± SEM *P < 0.05, compared with the respective baseline control (repeated measures ANOVA with Dunnett's post-hoc test).
CK2 activity is increased in the PVN in SHRs and contributes to the hyperactivity of PVN neurons in SHRs (Ye et al. 2011). Because increased CK2 activity can reduce SK channel function (Bildl et al. 2004), we determined whether diminished SK channel activity in spinally projecting PVN neurons results from increased CK2 activity in SHRs. We treated brain slices of SHRs and WKY rats with 100 μM DRB, a highly specific CK2 inhibitor (Lieberman and Mody 1999; Ye et al. 2011), for 2-4 h. DRB pretreatment did not alter the stimulating effect of apamin on the firing rate of PVN neurons in WKY rats (from 1.31 ± 0.38 to 2.49 ± 0.36 Hz, P < 0.05, n = 8 neurons). In brain slices of SHRs, treatment with DRB restored the stimulating effect of apamin on the firing rate of labeled PVN neurons (1.69 ± 0.41 to 2.75 ± 0.57 Hz, P < 0.05, n = 9; Fig. 1) to the values seen in WKY rats. The firing activity of labeled PVN neurons recorded in the presence of apamin did not differ significantly between WKY rats, SHR plus vehicle, and SHR plus DRB groups.
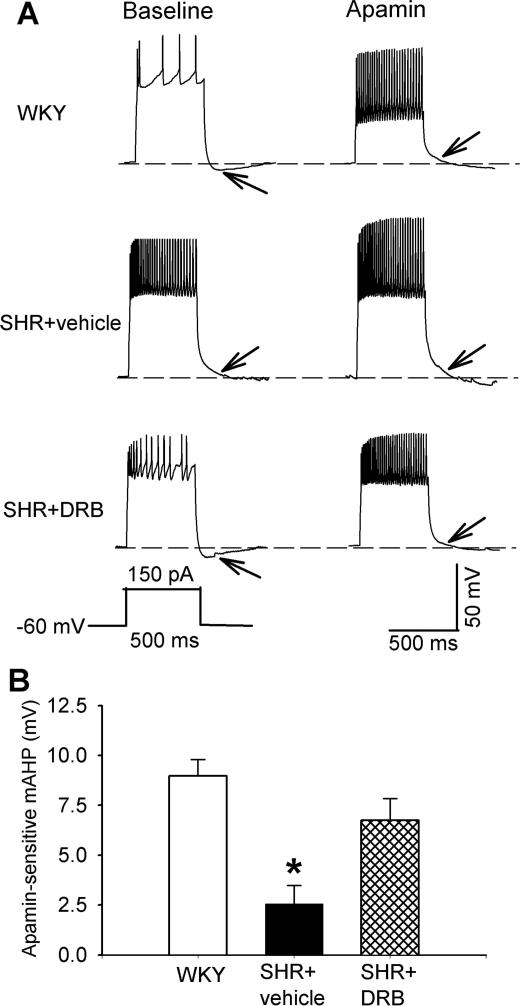
CK2 is involved in the reduction of mAHP in PVN neurons in SHRs
SK channels mediate mAHP after an action potential or a train of action potentials in neurons (Storm 1989; Stocker et al. 1999). We next used gramicidin-perforated recordings to determine whether mAHP is reduced in spinally projecting PVN neurons in SHRs. mAHP was elicited by applying a depolarizing current of 150 pA for 500 ms to the PVN neuron recorded. The mAHP of labeled PVN neurons in WKY rats was abolished by bath application of 100 nM apamin. The apamin-sensitive mAHP was determined by measuring the membrane potential difference before and during apamin application (Fig. 2). The amplitude of apamin-sensitive mAHP of labeled PVN neurons in WKY rats (8.97 ± 0.82 mV, n = 10) was significantly higher than that in SHRs (2.55 ± 0.92 mV, n = 8 neurons; P < 0.05, Fig. 2).
Figure 2. CK2 is involved in the reduction in mAHP of spinally projecting PVN neurons in SHRs.
A, Representative perforated recordings of mAHP of labeled PVN neurons before and during application of 100 nM apamin in WKY rat brain slices and SHR brain slices treated with DRB or vehicle. mAHP is indicated by the arrow, and the recording protocol is shown below the mAHP traces. B, Summary data show the amplitude of apamin-sensitive mAHP of labeled PVN neurons recorded from WKY rat brain slices (n = 10) and SHR brain slices treated with DRB (n = 6) or vehicle (n = 8). Data are presented as means ± SEM. *P < 0.05, compared with the value in WKY rats (repeated measures ANOVA with Dunnett's post-hoc test).
In WKY rats, DRB (100 μM, 2-4 h) pretreatment did not significantly alter the amplitude of apamin-sensitive mAHP of PVN neurons (8.68 ± 0.62 mV, n = 8 neurons). The amplitude of apamin-mediated mAHP in 6 labeled PVN neurons in SHR brain slices treated with DRB was significantly increased compared with that in SHR brain slices treated with vehicle (Fig. 2).
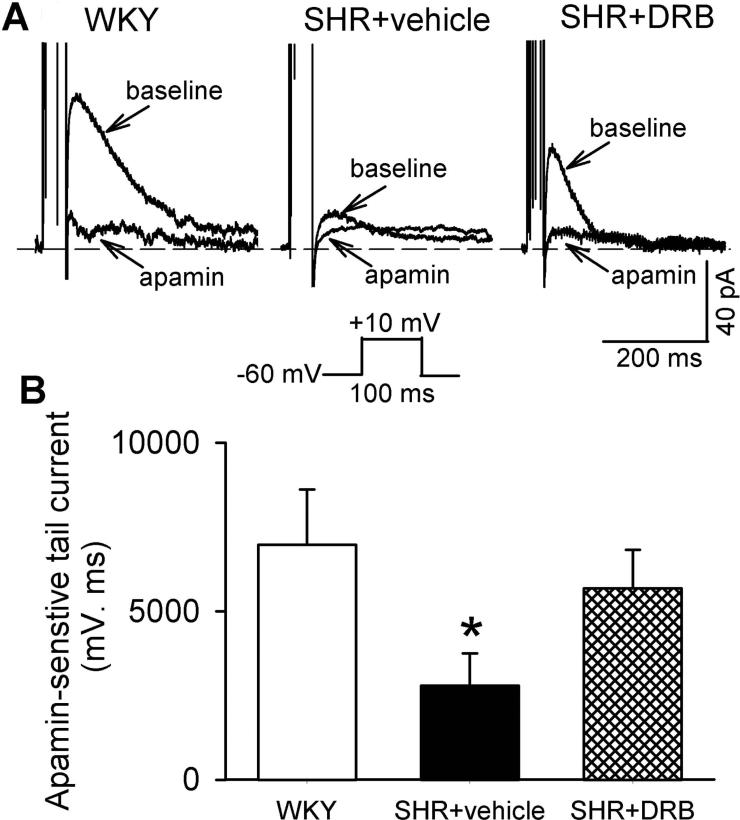
CK2 contributes to reduced SK channel currents of PVN neurons in SHRs
We then directly compared the SK channel currents in spinally projecting PVN neurons in WKY rats and SHRs. SK channel currents were also recorded by using a perforated whole-cell configuration to avoid interruption of intracellular Ca2+. Outward tail currents were elicited by a 100-ms depolarizing voltage step to 10 mV from the holding potential of −60 mV (Stocker et al. 1999; Chen and Toney 2009). Apamin-sensitive SK channel currents were obtained by subtracting the tail currents in the presence of apamin (100 nM) from currents recorded before apamin application. Because the recorded tail currents exhibit some differences in the kinetics, we used the area under the curve to quantify the size of apamin-sensitive currents. The size of apamin-sensitive SK channel currents (6,974.5 ± 1,144.4 ms.pA, n = 9 neurons) in labeled PVN neurons of WKY rats was significantly larger than that in SHRs (2,784.1 ± 962.1 ms.pA, n = 11 neurons; P < 0.05, Fig. 3).
Figure 3. CK2 contributes to diminished SK channel currents of spinally projecting PVN neurons in SHRs.
A, Original perforated recordings of tail currents in labeled PVN neurons before and during application of apamin in WKY rat brain slices and SHR brain slices treated with DRB or vehicle. The voltage protocol used to elicit tail currents is shown below the recording traces. B, Group data show the differences between apamin-sensitive tail currents of labeled PVN neurons recorded from WKY rat brain slices (n = 9) and SHR brain slices treated with DRB (n = 8) or vehicle (n = 11). Data are presented as means ± SEM. *P < 0.05, compared with the value in WKY rats (repeated measures ANOVA with Dunnett's post-hoc test).
Incubation of brain slices from WKY rats in aCSF containing 100 μM DRB for 2-4 h did not significantly change apamin-sensitive SK channel currents of labeled PVN neurons (6,918.0 ± 926.6 pA.ms, n = 8 neurons). In contrast, treatment of brain slices from SHRs with DRB largely restored apamin-sensitive SK channel currents of labeled PVN neurons (n = 9 neurons, Fig. 3). Together, these data suggest that SK channel activity is diminished in PVN presympathetic neurons in SHRs. Also, CK2 is involved in impaired function of SK channels in the PVN in SHRs.
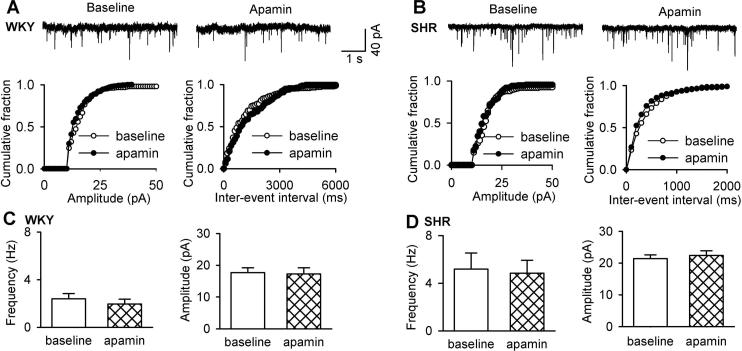
SK channels do not regulate synaptic glutamate and GABA release to PVN neurons
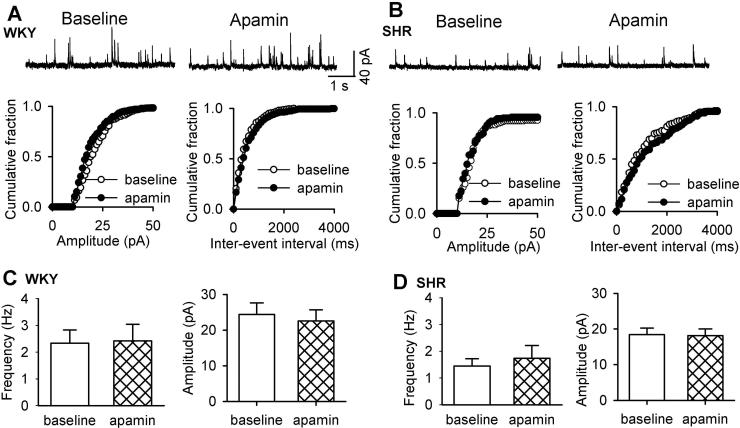
SK channels are localized in axon terminals to regulate synaptic transmission in the hippocampus (Sailer et al. 2002; Obermair et al. 2003). Also, glutamatergic input to PVN presympathetic neurons is increased in SHRs (Li and Pan 2007b; Li et al. 2008). To determine the role of SK channels in increased glutamatergic synaptic input to PVN neurons, we tested the effect of apamin on the frequency and amplitude of sEPSCs. Bath application of 100 nM apamin did not significantly alter the frequency (3.52 ± 0.42 vs. 3.46 ± 0.6 Hz, n = 7 neurons) or the amplitude (18.64 ± 1.84 vs. 19.34 ± 2.55 pA, n = 7 neurons) of sEPSCs in WKY rats (Fig. 4). The basal frequency of sEPSCs was significantly higher in SHRs than that in WKY rats. Apamin also had no significant effect on the frequency (from 5.58 ± 1.37 to 5.15 ± 1.20 Hz) or the amplitude (from 22.01 ± 1.43 to 21.18 ± 1.44 pA, n = 10 neurons) of sEPSCs in labeled PVN neurons in SHRs (Fig. 4).
Figure 4. SK channels are not involved in regulating synaptic glutamate release to spinally projecting PVN neurons in WKY rats and SHRs.
A and B, Original recordings and cumulative plot analysis of glutamatergic sEPSCs in labeled PVN neurons before and during bath application of 100 nM apamin in one WKY rat and one SHR. C and D, Group data show the frequency and amplitude of sEPSCs of labeled PVN neurons before and during apamin application in WKY rats (n = 7) and SHRs (n = 10). Data are presented as means ± SEM.
Because impaired GABAergic input in the PVN also contributes to increased sympathetic outflow in SHRs (Li and Pan 2007a), we determined the possible role of SK channels in the control of synaptic GABAergic transmission by recording sIPSCs in labeled PVN neurons. Apamin application did not significantly alter the frequency (from 2.33 ± 0.49 to 2.42 ± 0.62 Hz) or the amplitude (from 24.38 ± 3.23 to 22.57 ± 3.12 pA) of sIPSCs in WKY rats (n = 10 neurons, Fig. 5). In SHRs, apamin application also failed to significantly affect the frequency (from 1.44 ± 0.27 to 1.74 ± 0.47 Hz) or the amplitude (from 24.39 ± 1.84 to 25.63 ± 1.83 pA) of sIPSCs in labeled PVN neurons (n = 9 neurons, Fig. 5). These results indicate that SK channels are not involved in regulating glutamatergic or GABAergic synaptic inputs to spinally projecting PVN neurons.
Figure 5. SK channels do not control synaptic GABA release to spinally projecting PVN neurons in WKY rats and SHRs.
A and B, Original recordings and cumulative plot analysis of GABAergic sIPSCs in labeled PVN neurons before and during bath application of apamin in one WKY rat and one SHR. C and D, Group data show the frequency and amplitude of sIPSCs of labeled PVN neurons before and during apamin application in WKY rats (n = 10) and SHRs (n = 9). Data are presented as means ± SEM.
CK2-mediated calmodulin phosphorylation is increased in the PVN in SHRs
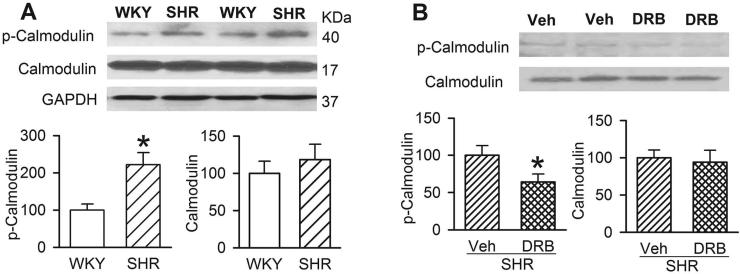
SK channels exist as a multiprotein complex containing CK2, calmodulin, and protein phosphatase 2A, and CK2-mediated calmodulin phosphorylation can reduce SK channel activity (Arrigoni et al. 2004; Bildl et al. 2004). We thus determined whether CK2-mediated phosphorylation levels of calmodulin are increased in the PVN in SHRs, which could explain diminished SK channel activity in the hypertensive condition. We used a calmodulin antibody against phosphorylated amino acid residue sites affected by CK2 as a measure of CK2-mediated calmodulin phosphorylation (Arrigoni et al. 2004). Western blot analysis of phosphorylated calmodulin showed a single protein band in PVN tissues in both WKY rats and SHRs. The protein level of phosphorylated calmodulin in the PVN was significantly higher in SHRs than in WKY rats (Fig. 6A). However, there was no significant difference in the total calmodulin protein amount in the PVN between WKY rats and SHRs (Fig. 6A).
Figure 6. CK2-mediated calmodulin phosphorylation in the PVN is increased in SHRs.
A, Original gel image and summary data show changes in phosphorylated calmodulin (p-calmodulin) and total calmodulin protein and levels in the PVN in WKY rats and SHRs (n = 6 separate experiments in each group, protein levels were normalized by GAPDH). B, Original gel image and group data show changes in phosphorylated calmodulin (p-calmodulin) in the PVN in SHRs treated with intracerebroventricular administration of DRB or vehicle (n = 6 separate experiments in each group). Data are presented as means ± SEM, * P < 0.05, compared with WKY rats or vehicle-treated SHRs.
In addition, we determined whether CK2 contributes to increased calmodulin phosphorylation in the PVN of SHRs. PVN tissues were obtained from SHRs treated with intracerebroventricular injection of DRB (8 mM, 10 μl) or vehicle (Ye et al. 2011). The total calmodulin protein level in the PVN did not differ significantly between vehicle- and DRB-treated groups in SHRs (Fig. 6B). However, DRB treatment significantly decreased the phosphorylated calmodulin level in the PVN in SHRs (Fig. 6B). These data suggest that CK2-mediated phosphorylation of calmodulin is increased in the PVN in SHRs.
SK isoform expression levels in the PVN in WKY rats and SHRs
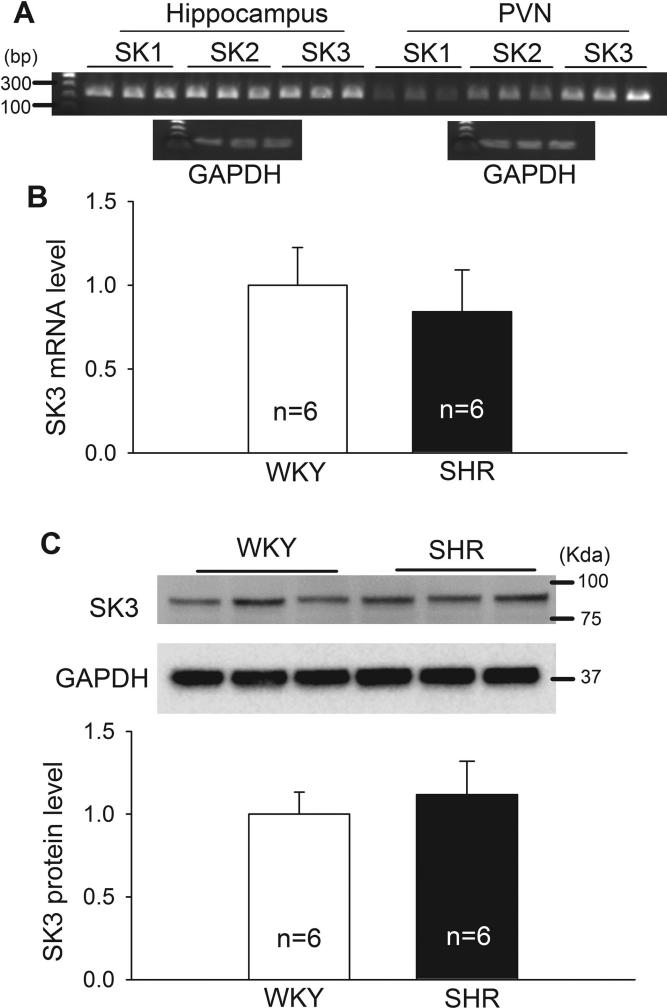
The previous in situ hybridization study indicates that only SK3 mRNA is detected in the hypothalamus (Stocker and Pedarzani 2000). As expected, conventional PCR and agarose gel analysis showed that SK3 was the predominant SK channel isoform detected in the PVN. In contrast, the SK1, SK2, and SK3 isoforms were all expressed in the hippocampus (Fig. 7A). We noted that SK1 and SK2 mRNA in the PVN can be detected only at 32 PCR cycles and that the SK1 and SK2 proteins in the PVN cannot be detected using Western blots in both WKY rats and SHRs. Real-time PCR and Western blot analysis showed that the mRNA and protein levels of SK3 channels in the PVN did not differ significantly between SHRs and WKY rats (Fig. 7B,C).
Figure 7. The expression level of SK3 in the PVN of WKY rats and SHRs.
A, Original agarose gel images show the expression of SK1, SK2, and SK3 channels in the hippocampus and PVN, analyzed by conventional PCR. PCR products (32 cycles) were loaded on 2% agarose gel containing ethidium bromide. GAPDH expression (27 PCR cycles) in both tissues was used as the control. B, Summary data show the mRNA level of SK3 channels in the PVN of WKY rats and SHRs, quantified by real-time PCR (n = 6 rats in each group). C, Original western blot gel images and quantification data show the protein levels of SK3 channels in the PVN of WKY rats and SHRs (n = 6 rats in each group). GAPDH was used as a loading control. Data are presented as means ± SEM.
Discussion
Hyperactivity of PVN presympathetic neurons can lead to elevated sympathetic vasomotor tone in SHRs (Allen 2002; Li and Pan 2007b, 2010). Blocking SK channels in the PVN increases the sympathetic outflow in anesthetized rats (Gui et al. 2012). The major finding of our study is that SK channel function of PVN presympathetic neurons is reduced through CK2-mediated calmodulin phosphorylation in SHRs, a commonly used animal model of essential hypertension. SK channels are activated by an increase in intracellular Ca2+ via calmodulin (Xia et al. 1998) and regulate neuronal excitability by influencing after-hyperpolarization (Stocker et al. 1999). SK channels are typically expressed in the postsynaptic membrane where they modulate the synaptic responses and induction of synaptic plasticity (Faber et al. 2005; Ngo-Anh et al. 2005). Some previous studies measured SK channel activity by using a conventional whole-cell configuration (Stocker et al. 1999; Chen and Toney 2009). However, because the intracellular Ca2+ is readily altered by the pipette internal solution in conventional whole-cell recordings, we selected gramicidin perforated recordings to minimize the interference of intracellular Ca2+ by the pipette solution. In the present study, blocking SK channels with apamin significantly increased the firing activity of spinally projecting PVN neurons in WKY rats but not in SHRs, suggesting that SK channel activity is probably diminished in PVN presympathetic neurons in SHRs.
SK channels are predominantly involved in mAHP after an action potential or a train of action potentials (Storm 1989; Stocker et al. 1999). Because apamin application abolished mAHP in both SHRs and WKY rats, SK channels are responsible for the generation of mAHP in spinally projecting PVN neurons. To determine whether SK channel activity is impaired in hypertension, we directly measured SK channel-mediated mAHP and apamin-sensitive tail currents in spinally projecting PVN neurons in WKY rats and SHRs. In our study, all the recording was made from the soma of labeled PVN neurons, which possess extensive dendrites and long axons. Because SK channels are expressed in the dendrites (Maciaszek et al. 2012), the recorded SK channel activity in labeled PVN neurons may reflect the SK channels present on the soma and dendrites. We found that the amplitude of mAHP of spinally projecting PVN neurons was significantly smaller in SHRs than in WKY rats. Furthermore, we showed that the apamin-sensitive tail current of spinally projecting PVN neurons was significantly reduced in SHRs compared with that in WKY rats. These data suggest that the SK channel function is impaired and contributes to increased firing activity of PVN presympathetic neurons in SHRs.
Fully functional native SK channels exist as a polyprotein complex consisting of calmodulin, CK2, and protein phosphatase 2A (Bildl et al. 2004). Calmodulin can be phosphorylated by CK2 at amino acid residues including Thr-79, Thr-80, Thr-117, Ser-81, and Ser-101 (Meggio and Pinna 2003; Arrigoni et al. 2004; Bildl et al. 2004). CK2 can lead to increased phosphorylation of SK channel-bound calmodulin causing a 5-fold decrease in its sensitivity to Ca2+, thereby resulting in decreased SK channel activity in CHO cells (Bildl et al. 2004). In this study, we found that treatment of SHR brain slices with the specific CK2 inhibitor DRB largely restored the stimulatory effect of apamin on the firing activity and apamin-sensitive mAHP of spinally projecting PVN neurons. Furthermore, treatment with DRB restored the size of SK channel currents of PVN neurons in SHRs. Our findings strongly suggest that CK2 contributes to diminished SK channel function of PVN presympathetic neurons in SHRs. Because lowering the blood pressure with celiac ganglionectomy in SHRs had no effect on increased CK2 levels in the PVN (Ye et al. 2011), it is less likely that diminished SK channel activity by CK2 is a secondary adaptive response to increased blood pressure in SHRs.
Because CK2 activity in the PVN is significantly increased in SHRs and CK2 inhibition reduces blood pressure in this hypertension model (Ye et al. 2011), it is possible that diminished SK channel activity is associated with an increase in CK2-mediated calmodulin phosphorylation in SHRs. We thus determined phosphorylated calmodulin levels in the PVN by using an antibody against phosphorylated calmodulin. We found that the level of phosphorylated calmodulin in the PVN was significantly higher in SHRs than in WKY rats, whereas the total calmodulin protein level did not differ significantly between the two groups. Also, we investigated whether CK2 is involved in increased calmodulin phosphorylation in the PVN in SHRs. We showed that DRB treatment significantly decreased the phosphorylated calmodulin level without affecting the total calmodulin protein level in the PVN in SHRs. In addition, we found that only SK3 can be readily detected in the PVN. Because the mRNA and protein levels of SK3 channels in the PVN did not differ significantly between SHRs and WKY rats, it is unlikely that diminished SK channel activity of PVN presympathetic neurons in SHRs results from reduced SK channel expression. Collectively, our findings suggest that increased CK2 activity in the PVN leads to an increased phosphorylation level of SK channel-bound calmodulin, resulting in diminished SK channel function of PVN presympathetic neurons in SHRs.
The excitatory glutamatergic synaptic input is increased, whereas the inhibitory GABAergic input is decreased in the PVN in SHRs (Li and Pan 2006, 2007b; Li et al. 2008). We thus determined whether SK channels regulate the excitability of glutamatergic and GABAergic interneurons and their terminals to influence the synaptic inputs to spinally projecting PVN neurons. We found that apamin had no effect on the frequency or amplitude of glutamatergic sEPSCs and GABAergic sIPSCs in spinally projecting PVN neurons in WKY rats and SHRs. These data suggest that SK channels either are not localized at the axon terminals of glutamatergic and GABAergic interneurons or do not play a significant role in regulating synaptic glutamate and GABA release to spinally projecting PVN neurons. Nevertheless, SK channels can influence synaptic transmission through shunting postsynaptic NMDA receptor activity (Ngo-Anh et al. 2005). In this regard, synaptic NMDA receptor-mediated transient Ca2+ increases can activate SK channels localized to dendritic spines to shape synaptic responses (Sah and Faber 2002).
The mechanisms responsible for increased CK2 activity in the PVN in SHRs are not clear. Because lowering blood pressure in SHRs does not change the increased membrane CK2α level in the PVN (Ye et al. 2011), increased CK2 activity likely contributes to the development of hypertension in SHRs. CK2 has different functions in various subcellular compartments, and its subcellular localization is tightly regulated (Faust and Montenarh 2000). Increased membrane CK2α trafficking/translocation in the PVN may increase the probability of its association with SK channel-bound calmodulin on the plasma membrane in SHRs. Interestingly, calmodulin also inhibits NMDAR activity via its binding to the NR1 subunit (Ehlers et al. 1996), and increased calmodulin phosphorylation by CK2 could reduce the calmodulin-NR1 interaction, resulting in increased NMDAR activity (Ehlers et al. 1996). Thus, CK2 likely contribute to hyperactivity of PVN presympathetic neurons in SHRs by increasing NMDA receptor activity and diminishing SK channel activity of PVN presympathetic neurons. In the present study, we limited our recordings to PVN neurons retrogradely labeled by tracer injections to the intermediolateral cell column region of the thoracic spinal cord because of the relevance of this population of PVN neurons to autonomic control (Li et al. 2002, 2003). The labeled neurons included in our study were less than 40 μm in diameter and predominantly localized in the dorsal and lateral parvocellular sections of the PVN. We recognize that the PVN is heterogeneous and contains many types of interneurons and projecting neurons. A limitation of our study is that it is unclear whether diminished SK channel activity occurs in other types of projection neurons and interneurons in the PVN, which is beyond the scope of our current work and should be addressed in future experiments.
The etiology of hypertension in the vast majority of patients remains poorly understood. Our study provides new information about the molecular mechanisms underlying hyperactivity of PVN presympathetic neurons in an animal model of essential hypertension. Our results indicate that SK channels control the intrinsic firing activity of PVN presympathetic neurons and that the activity of SK channels is diminished in SHRs. We also provide new evidence that CK2 is involved in increased calmodulin phosphorylation in the PVN and contributes to diminished SK channel activity in SHRs. Thus, in addition to NMDA receptors, the SK channel is another important target affected by increased CK2 activity in the PVN in hypertension. We have demonstrated that enhanced NMDA receptor activity by CK2 is involved in the hyperactivity of PVN presympathetic neurons and elevated sympathetic outflow (Ye et al. 2011). SK channels probably serve as a negative feedback regulator to blunt neuronal depolarization during NMDA receptor activation. Therefore, future studies are warranted to explore the relationship between diminished SK channel function and increased activity of NMDA receptors by CK2 in the PVN in hypertension. The efficacy and safety of CK2 inhibitors are currently being investigated in clinical trials. CK2 may be a potential new target for normalizing SK channel function and NMDA receptor activity to reduce sympathetic outflow in hypertension.
Acknowledgments
This study was supported by the National Institutes of Health (grant HL077400) and by the N.G. and Helen T. Hawkins Endowment (to H.-L.P.). The authors have no conflicts of interest to declare.
The abbreviations used are
- mAHP
medium after-hyperpolarization potential
- CK2
casein kinase II
- DRB
5,6-dichlorobenzimidazole
- NMDA
N-methyl-D-aspartate
- PVN
paraventricular nucleus
- SHR
spontaneous hypertensive rat
- sEPSC
spontaneous excitatory postsynaptic current
- sIPSC
spontaneous inhibitory postsynaptic current
- SK
small conductance Ca2+-activated K+ channel
- WKY
Wistar Kyoto
Footnotes
Conflicts of interest: none
The authors have no conflict of interest to declare.
References
- Adelman JP, Maylie J, Sah P. Small-conductance Ca2+-activated K+ channels: form and function. Annu Rev Physiol. 2012;74:245–269. doi: 10.1146/annurev-physiol-020911-153336. [DOI] [PubMed] [Google Scholar]
- Allen AM. Inhibition of the hypothalamic paraventricular nucleus in spontaneously hypertensive rats dramatically reduces sympathetic vasomotor tone. Hypertension. 2002;39:275–280. doi: 10.1161/hy0202.104272. [DOI] [PubMed] [Google Scholar]
- Anderson EA, Sinkey CA, Lawton WJ, Mark AL. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension. 1989;14:177–183. doi: 10.1161/01.hyp.14.2.177. [DOI] [PubMed] [Google Scholar]
- Arrigoni G, Marin O, Pagano MA, Settimo L, Paolin B, Meggio F, Pinna LA. Phosphorylation of calmodulin fragments by protein kinase CK2. Mechanistic aspects and structural consequences. Biochemistry. 2004;43:12788–12798. doi: 10.1021/bi049365c. [DOI] [PubMed] [Google Scholar]
- Bildl W, Strassmaier T, Thurm H, Andersen J, Eble S, Oliver D, Knipper M, Mann M, Schulte U, Adelman JP, Fakler B. Protein kinase CK2 is coassembled with small conductance Ca(2+)-activated K+ channels and regulates channel gating. Neuron. 2004;43:847–858. doi: 10.1016/j.neuron.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Chen QH, Toney GM. Excitability of paraventricular nucleus neurones that project to the rostral ventrolateral medulla is regulated by small-conductance Ca2+-activated K+ channels. J Physiol. 2009;587:4235–4247. doi: 10.1113/jphysiol.2009.175364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QH, Andrade MA, Calderon AS, Toney GM. Hypertension induced by angiotensin II and a high salt diet involves reduced SK current and increased excitability of RVLM projecting PVN neurons. J Neurophysiol. 2010;104:2329–2337. doi: 10.1152/jn.01013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD, Zhang S, Bernhadt JP, Huganir RL. Inactivation of NMDA receptors by direct interaction of calmodulin with the NR1 subunit. Cell. 1996;84:745–755. doi: 10.1016/s0092-8674(00)81052-1. [DOI] [PubMed] [Google Scholar]
- Eilam R, Malach R, Segal M. Selective elimination of hypothalamic neurons by grafted hypertension-inducing neural tissue. J Neurosci. 1994;14:4891–4902. doi: 10.1523/JNEUROSCI.14-08-04891.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ES, Delaney AJ, Sah P. SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala. Nat Neurosci. 2005;8:635–641. doi: 10.1038/nn1450. [DOI] [PubMed] [Google Scholar]
- Faust M, Montenarh M. Subcellular localization of protein kinase CK2. A key to its function? Cell Tissue Res. 2000;301:329–340. doi: 10.1007/s004410000256. [DOI] [PubMed] [Google Scholar]
- Greenwood JP, Stoker JB, Mary DA. Single-unit sympathetic discharge : quantitative assessment in human hypertensive disease. Circulation. 1999;100:1305–1310. doi: 10.1161/01.cir.100.12.1305. [DOI] [PubMed] [Google Scholar]
- Gui L, LaGrange LP, Larson RA, Gu M, Zhu J, Chen QH. Role of small conductance calcium-activated potassium channels expressed in PVN in regulating sympathetic nerve activity and arterial blood pressure in rats. Am J Physiol Regul Integr Comp Physiol. 2012;303:R301–310. doi: 10.1152/ajpregu.00114.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan H, Hayashida Y, Yamashita H. Increase in sympathetic outflow by paraventricular nucleus stimulation in awake rats. Am J Physiol. 1989;256:R1325–1330. doi: 10.1152/ajpregu.1989.256.6.R1325. [DOI] [PubMed] [Google Scholar]
- Keen JE, Khawaled R, Farrens DL, Neelands T, Rivard A, Bond CT, Janowsky A, Fakler B, Adelman JP, Maylie J. Domains responsible for constitutive and Ca(2+)-dependent interactions between calmodulin and small conductance Ca(2+)-activated potassium channels. J Neurosci. 1999;19:8830–8838. doi: 10.1523/JNEUROSCI.19-20-08830.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, Maylie J, Adelman JP. Small-conductance, calcium-activated potassium channels from mammalian brain. Science. 1996;273:1709–1714. doi: 10.1126/science.273.5282.1709. [DOI] [PubMed] [Google Scholar]
- Li DP, Pan HL. Plasticity of GABAergic control of hypothalamic presympathetic neurons in hypertension. Am J Physiol Heart Circ Physiol. 2006;290:H1110–1119. doi: 10.1152/ajpheart.00788.2005. [DOI] [PubMed] [Google Scholar]
- Li DP, Pan HL. Role of gamma-aminobutyric acid (GABA)A and GABAB receptors in paraventricular nucleus in control of sympathetic vasomotor tone in hypertension. J Pharmacol Exp Ther. 2007a;320:615–626. doi: 10.1124/jpet.106.109538. [DOI] [PubMed] [Google Scholar]
- Li DP, Pan HL. Glutamatergic inputs in the hypothalamic paraventricular nucleus maintain sympathetic vasomotor tone in hypertension. Hypertension. 2007b;49:916–925. doi: 10.1161/01.HYP.0000259666.99449.74. [DOI] [PubMed] [Google Scholar]
- Li DP, Pan HL. Increased group I metabotropic glutamate receptor activity in paraventricular nucleus supports elevated sympathetic vasomotor tone in hypertension. Am J Physiol Regul Integr Comp Physiol. 2010;299:R552–561. doi: 10.1152/ajpregu.00195.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DP, Chen SR, Pan HL. Nitric oxide inhibits spinally projecting paraventricular neurons through potentiation of presynaptic GABA release. J Neurophysiol. 2002;88:2664–2674. doi: 10.1152/jn.00540.2002. [DOI] [PubMed] [Google Scholar]
- Li DP, Chen SR, Pan HL. Angiotensin II stimulates spinally projecting paraventricular neurons through presynaptic disinhibition. J Neurosci. 2003;23:5041–5049. doi: 10.1523/JNEUROSCI.23-12-05041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DP, Yang Q, Pan HM, Pan HL. Pre- and postsynaptic plasticity underlying augmented glutamatergic inputs to hypothalamic presympathetic neurons in spontaneously hypertensive rats. J Physiol. 2008;586:1637–1647. doi: 10.1113/jphysiol.2007.149732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman DN, Mody I. Casein kinase-II regulates NMDA channel function in hippocampal neurons. Nat Neurosci. 1999;2:125–132. doi: 10.1038/5680. [DOI] [PubMed] [Google Scholar]
- Maciaszek JL, Soh H, Walikonis RS, Tzingounis AV, Lykotrafitis G. Topography of native SK channels revealed by force nanoscopy in living neurons. J Neurosci. 2012;32:11435–11440. doi: 10.1523/JNEUROSCI.1785-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meggio F, Pinna LA. One-thousand-and-one substrates of protein kinase CK2? Faseb J. 2003;17:349–368. doi: 10.1096/fj.02-0473rev. [DOI] [PubMed] [Google Scholar]
- Ngo-Anh TJ, Bloodgood BL, Lin M, Sabatini BL, Maylie J, Adelman JP. SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nat Neurosci. 2005;8:642–649. doi: 10.1038/nn1449. [DOI] [PubMed] [Google Scholar]
- Obermair GJ, Kaufmann WA, Knaus HG, Flucher BE. The small conductance Ca2+-activated K+ channel SK3 is localized in nerve terminals of excitatory synapses of cultured mouse hippocampal neurons. Eur J Neurosci. 2003;17:721–731. doi: 10.1046/j.1460-9568.2003.02488.x. [DOI] [PubMed] [Google Scholar]
- Pyner S, Coote JH. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience. 2000;100:549–556. doi: 10.1016/s0306-4522(00)00283-9. [DOI] [PubMed] [Google Scholar]
- Sah P. Ca(2+)-activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci. 1996;19:150–154. doi: 10.1016/s0166-2236(96)80026-9. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES. Channels underlying neuronal calcium-activated potassium currents. Prog Neurobiol. 2002;66:345–353. doi: 10.1016/s0301-0082(02)00004-7. [DOI] [PubMed] [Google Scholar]
- Sailer CA, Hu H, Kaufmann WA, Trieb M, Schwarzer C, Storm JF, Knaus HG. Regional differences in distribution and functional expression of small-conductance Ca2+-activated K+ channels in rat brain. J Neurosci. 2002;22:9698–9707. doi: 10.1523/JNEUROSCI.22-22-09698.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher MA, Rivard AF, Bachinger HP, Adelman JP. Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin. Nature. 2001;410:1120–1124. doi: 10.1038/35074145. [DOI] [PubMed] [Google Scholar]
- Spergel DJ. Calcium and small-conductance calcium-activated potassium channels in gonadotropin-releasing hormone neurons before, during, and after puberty. Endocrinology. 2007;148:2383–2390. doi: 10.1210/en.2006-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker M, Pedarzani P. Differential distribution of three Ca(2+)-activated K(+) channel subunits, SK1, SK2, and SK3, in the adult rat central nervous system. Mol Cell Neurosci. 2000;15:476–493. doi: 10.1006/mcne.2000.0842. [DOI] [PubMed] [Google Scholar]
- Stocker M, Krause M, Pedarzani P. An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons. Proc Natl Acad Sci U S A. 1999;96:4662–4667. doi: 10.1073/pnas.96.8.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm JF. An after-hyperpolarization of medium duration in rat hippocampal pyramidal cells. J Physiol. 1989;409:171–190. doi: 10.1113/jphysiol.1989.sp017491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Xia XM, Fakler B, Rivard A, Wayman G, Johnson-Pais T, Keen JE, Ishii T, Hirschberg B, Bond CT, Lutsenko S, Maylie J, Adelman JP. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature. 1998;395:503–507. doi: 10.1038/26758. [DOI] [PubMed] [Google Scholar]
- Ye ZY, Li DP, Li L, Pan HL. Protein kinase CK2 increases glutamatergic input in the hypothalamus and sympathetic vasomotor tone in hypertension. J Neurosci. 2011;31:8271–8279. doi: 10.1523/JNEUROSCI.1147-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZY, Li DP, Byun HS, Li L, Pan HL. NKCC1 upregulation disrupts chloride homeostasis in the hypothalamus and increases neuronal activity-sympathetic drive in hypertension. J Neurosci. 2012;32:8560–8568. doi: 10.1523/JNEUROSCI.1346-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]