Abstract
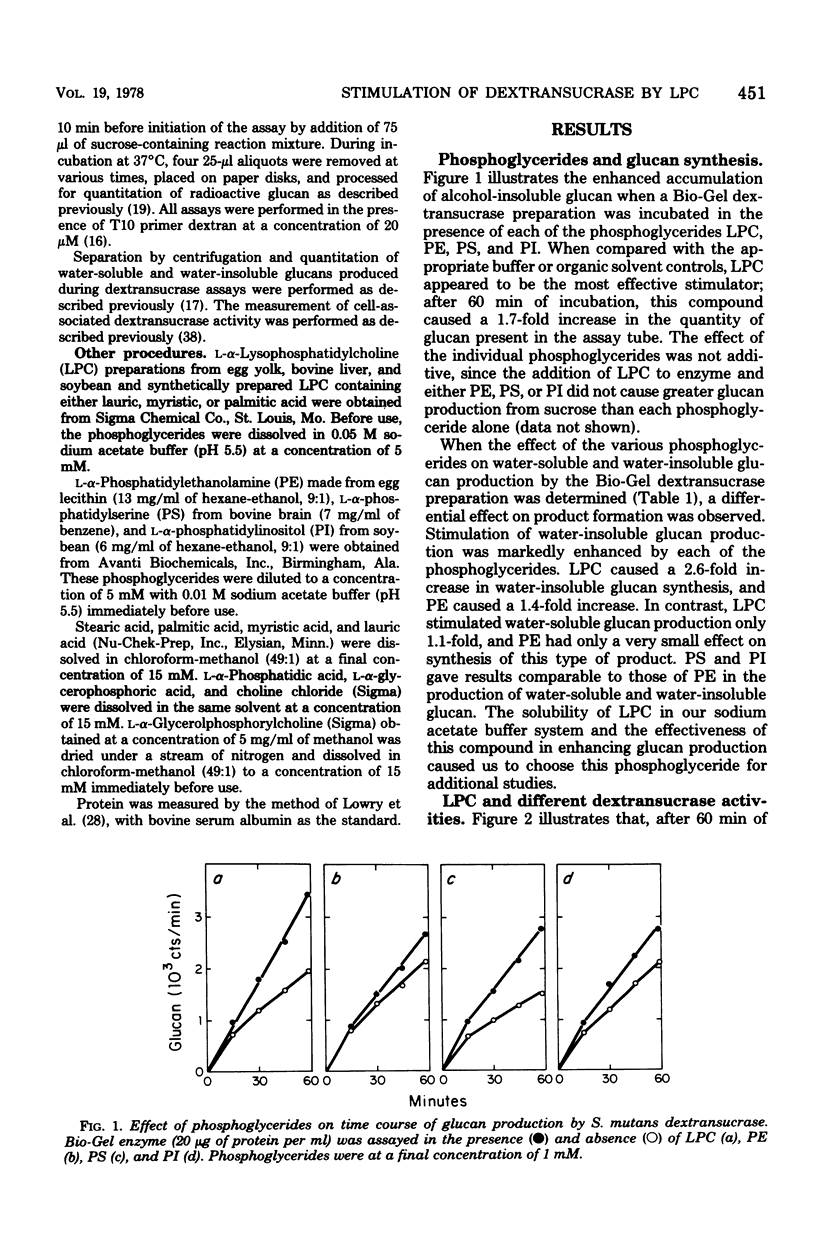
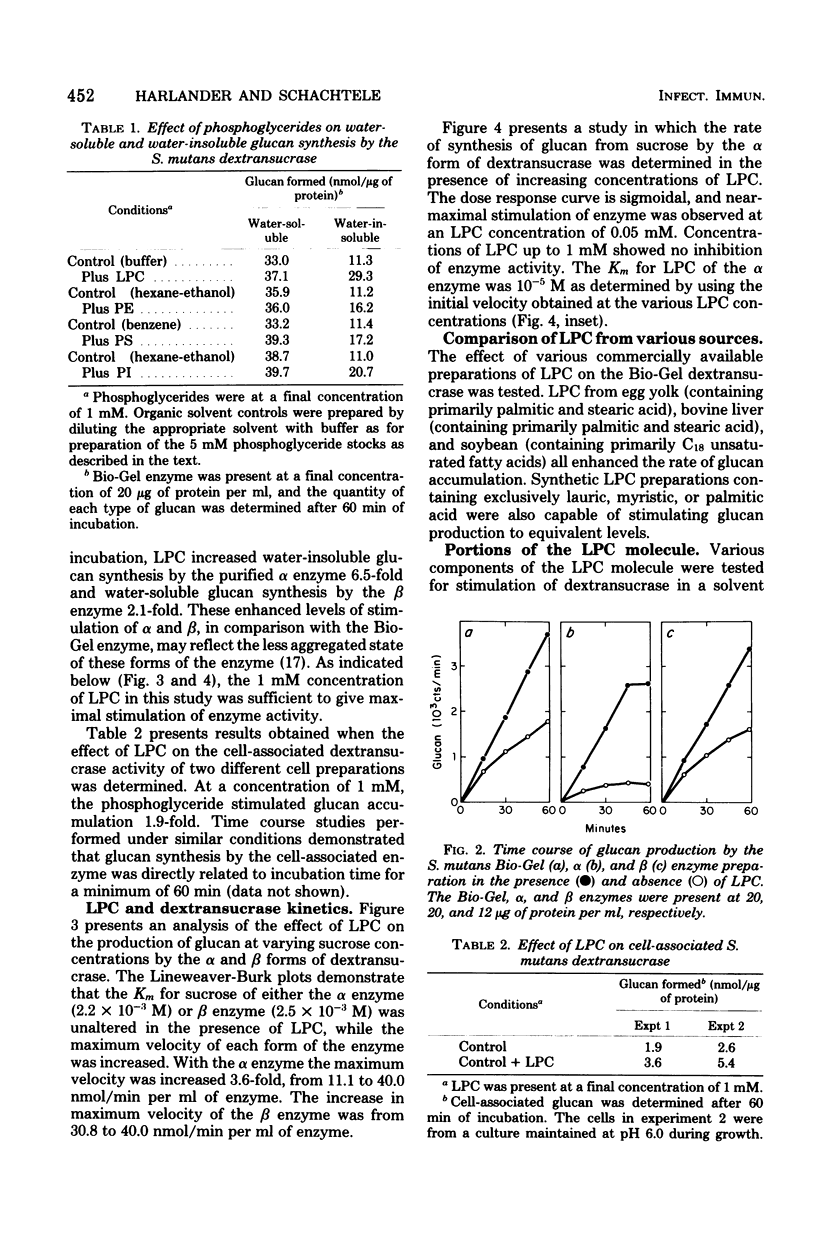
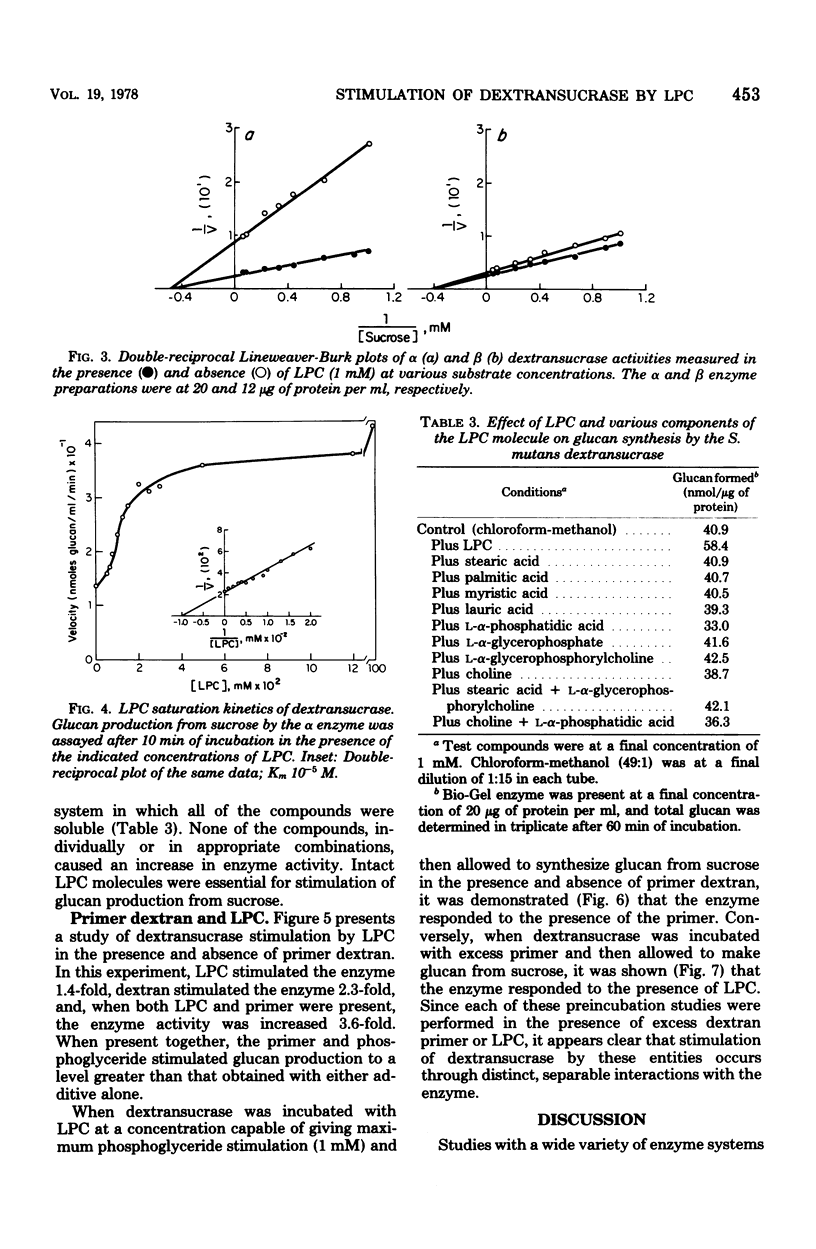
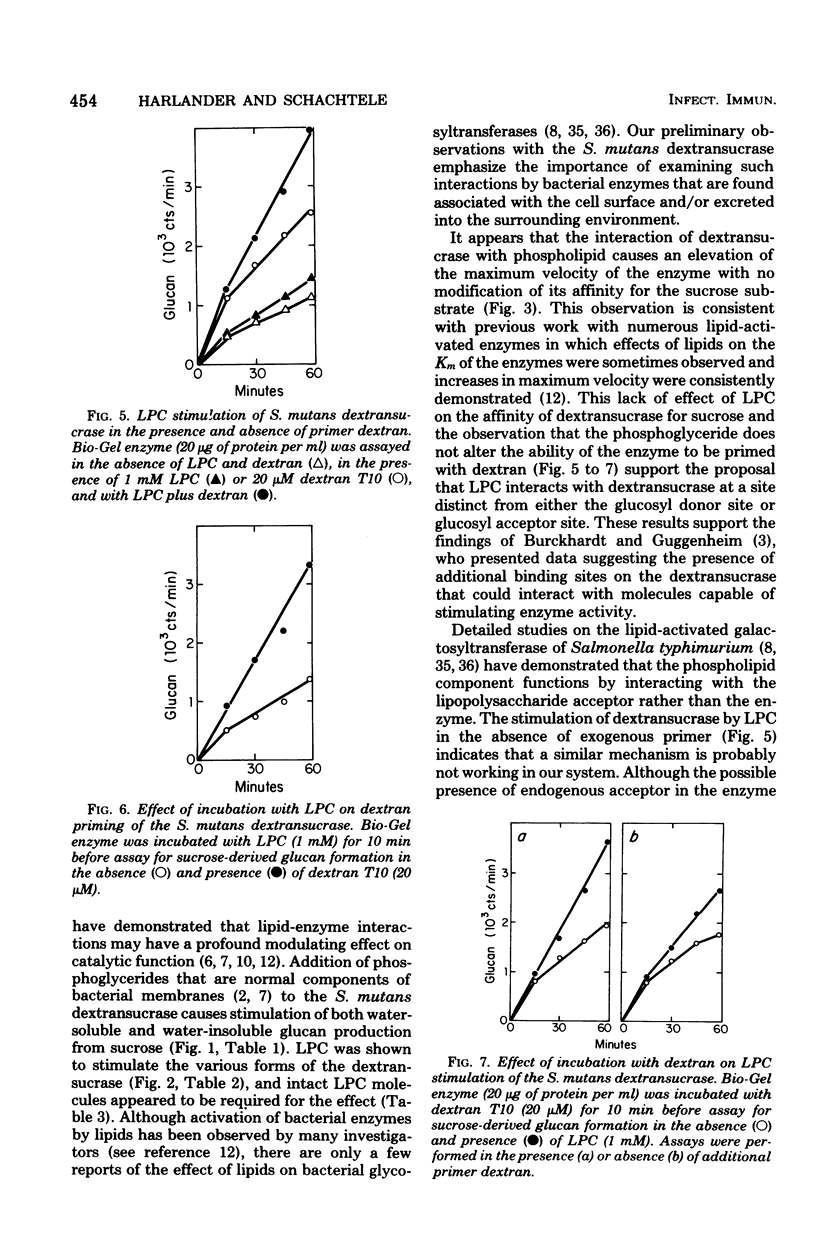
Lysophosphatidylcholine (LPC) and other phosphoglycerides stimulated water-insoluble and water-soluble glucan production by the Streptococcus mutans 6715 dextransucrase (EC 2.4.1.5). LPC stimulated crude extracellular dextransucrase 1.7-fold, the water-insoluble glucan-producing α form of the enzyme 6.5-fold, the water-soluble glucan-producing β form of the enzyme 2.1-fold, and the cell-associated dextransucrase 2.0-fold. Kinetic studies demonstrated that LPC did not change the Km for sucrose of α or β but increased the maximum velocity of the enzymes. The Km for LPC of the α enzyme was 10−5 M. LPC from various sources and synthetic preparations of lauroyl-LPC, myristoyl-LPC, and palmitoyl-LPC all stimulated glucan formation. Portions of phosphoglyceride molecules including fatty acids, phosphatidic acid, glycerophosphoric acid, glycerophos-phorylcholine, and choline, when tested individually or in combinations, did not enhance dextransucrase activity. The increased rates of glucan production caused by LPC and primer dextran were additive. Enzyme incubated with LPC before addition of sucrose was stimulated by dextran primer, and, conversely, enzyme treated with dextran was stimulated by addition of LPC with the sucrose substrate. Thus, dextransucrase can be activated by binding of intact phosphoglyceride molecules to a site on the enzyme that is distinct from either the glucosyl donor or glucosyl acceptor (primer) binding sites. Interactions between the S. mutans dextransucrase and amphipathic phosphoglycerides may explain properties of this enzyme which contribute to the cariogenicity of S. mutans.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anttinen H. Stimulation of collagen galactosyltransferase and glucosyltransferase activities by lysophosphatidylcholine. Biochem J. 1976 Oct 15;160(1):29–35. doi: 10.1042/bj1600029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burckhardt J. J., Guggenheim B. Interactions of antisera, sera, and oral fluid with glucosyltransferases. Infect Immun. 1976 Apr;13(4):1009–1022. doi: 10.1128/iai.13.4.1009-1022.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chludzinski A. M., Germaine G. R., Schachtele C. F. Purification and properties of dextransucrase from Streptococcus mutans. J Bacteriol. 1974 Apr;118(1):1–7. doi: 10.1128/jb.118.1.1-7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R. Membrane-bound enzymes and membrane ultrastructure. Biochim Biophys Acta. 1973 Apr 3;300(1):1–30. doi: 10.1016/0304-4157(73)90010-5. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Vagelos P. R. Metabolism and function of the membrane phospholipids of Escherichia coli. Biochim Biophys Acta. 1972 Feb 14;265(1):25–60. doi: 10.1016/0304-4157(72)90018-4. [DOI] [PubMed] [Google Scholar]
- Endo A., Rothfield L. Studies of a phospholipid-requiring bacterial enzyme. II. The role of phospholipid in the uridine diphosphate galactose: lipopolysaccharide alpha-3-galactosyl transferase reaction. Biochemistry. 1969 Sep;8(9):3508–3515. doi: 10.1021/bi00837a004. [DOI] [PubMed] [Google Scholar]
- Evans R. T., Genco R. J. Inhibition of glucosyltransferase activity by antisera to known serotypes of Streptococcus mutans. Infect Immun. 1973 Feb;7(2):237–241. doi: 10.1128/iai.7.2.237-241.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farías R. N., Bloj B., Morero R. D., Siñeriz F., Trucco R. E. Regulation of allosteric membrane-bound enzymes through changes in membrane lipid compostition. Biochim Biophys Acta. 1975 Jun 30;415(2):231–251. doi: 10.1016/0304-4157(75)90003-9. [DOI] [PubMed] [Google Scholar]
- Fisher D. B., Kaufman S. The stimulation of rat liver phenylalanine hydroxylase by lysolecithin and -chymotrypsin. J Biol Chem. 1973 Jun 25;248(12):4345–4353. [PubMed] [Google Scholar]
- Fourcans B., Jain M. K. Role of phospholipids in transport and enzymic reactions. Adv Lipid Res. 1974;12(0):147–226. doi: 10.1016/b978-0-12-024912-1.50011-9. [DOI] [PubMed] [Google Scholar]
- Fukui K., Fukui Y., Moriyama T. Purification and properties of dextransucrase and invertase from Streptococcus mutans. J Bacteriol. 1974 Jun;118(3):796–804. doi: 10.1128/jb.118.3.796-804.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui K., Fukui Y., Moriyama T. Some Immunochemical Properties of Dextransucrase and Invertase from Streptococcus mutans. Infect Immun. 1974 Nov;10(5):985–990. doi: 10.1128/iai.10.5.985-990.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geren L. M., Ebner K. E. Folic acid effects on glycoprotein-galactosyltransferase: a re-assessment. Biochem Biophys Res Commun. 1974 Jul 10;59(1):14–21. doi: 10.1016/s0006-291x(74)80167-1. [DOI] [PubMed] [Google Scholar]
- Germaine G. R., Chludzinski A. M., Schachtele C. F. Streptococcus mutans dextransucrase: requirement for primer dextran. J Bacteriol. 1974 Oct;120(1):287–294. doi: 10.1128/jb.120.1.287-294.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Harlander S. K., Leung W. L., Schachtele C. F. Streptococcus mutans dextransucrase: functioning of primer dextran and endogenous dextranase in water-soluble and water-insoluble glucan synthesis. Infect Immun. 1977 May;16(2):637–648. doi: 10.1128/iai.16.2.637-648.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Schachtele C. F., Chludzinski A. M. Rapid filter paper assay for the dextransucrase activity from Streptococcus mutans. J Dent Res. 1974 Nov-Dec;53(6):1355–1360. doi: 10.1177/00220345740530061101. [DOI] [PubMed] [Google Scholar]
- Germaine G. R., Schachtele C. F. Streptococcus mutans dextransucrase: mode of interaction with high-molecular-weight dextran and role in cellular aggregation. Infect Immun. 1976 Feb;13(2):365–372. doi: 10.1128/iai.13.2.365-372.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J. Presence of an invertase-like enzyme and a sucrose permeation system in strains of Streptococcus mutans. Caries Res. 1972;6(2):122–131. doi: 10.1159/000259784. [DOI] [PubMed] [Google Scholar]
- Janda W. M., Kuramitsu H. K. Regulation and extracellular glucosyltransferase production and the relationship between extracellular and cell-associated activities in Streptococcus mutans. Infect Immun. 1976 Jul;14(1):191–202. doi: 10.1128/iai.14.1.191-202.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum B. B., Bosmann H. B. Lysolecithin enhancement of glycoprotein: glycosyl transferase activity. FEBS Lett. 1973 Aug 15;34(2):129–132. doi: 10.1016/0014-5793(73)80773-2. [DOI] [PubMed] [Google Scholar]
- Kuramitsu H. K. Characterization of cell-associated dextransucrase activity from glucose-grown cells of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):227–235. doi: 10.1128/iai.10.1.227-235.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K. Characterization of extracellular glucosyltransferase activity of Steptococcus mutans. Infect Immun. 1975 Oct;12(4):738–749. doi: 10.1128/iai.12.4.738-749.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K., Ingersoll L. Differential inhibition of Streptococcus mutans in vitro adherence by anti-glucosyltransferase antibodies. Infect Immun. 1976 Jun;13(6):1775–1777. doi: 10.1128/iai.13.6.1775-1777.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linzer R., Slade H. D. Characterization of an anti-glucosyltransferase serum specific for insoluble glucan synthesis by Streptococcus mutans. Infect Immun. 1976 Feb;13(2):494–500. doi: 10.1128/iai.13.2.494-500.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe M. M., Smith E. E. Relationship between cell-bound dextransucrase and the agglutination of Streptococcus mutans. Infect Immun. 1975 Sep;12(3):512–520. doi: 10.1128/iai.12.3.512-520.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mookerjea S., Yung J. W. A study on the effect of lysolecithin and phospholipase A on membrane-bound galactosyltransferase. Can J Biochem. 1974 Nov;52(11):1053–1066. doi: 10.1139/o74-147. [DOI] [PubMed] [Google Scholar]
- Mookerjea S., Yung J. W. Stimulation of galactosyltransferase in liver microsomes by lysolecithin. Biochem Biophys Res Commun. 1974 Apr 8;57(3):815–822. doi: 10.1016/0006-291x(74)90619-6. [DOI] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of the Adherence of Streptococcus mutans to Smooth Surfaces III. Purification and Properties of the Enzyme Complex Responsible for Adherence. Infect Immun. 1974 Nov;10(5):1135–1145. doi: 10.1128/iai.10.5.1135-1145.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz J. L., Shannon I. L. Lipid changes in human male parotid saliva by stimulation. Arch Oral Biol. 1975 Jul;20(7):403–406. doi: 10.1016/0003-9969(75)90223-x. [DOI] [PubMed] [Google Scholar]
- Romeo D., Girard A., Rothfield L. Reconstitution of a functional membrane enzyme system in a monomolecular film. I. Formation of a mixed monolayer of lipopolysaccharide and phospholipid. J Mol Biol. 1970 Nov 14;53(3):475–490. doi: 10.1016/0022-2836(70)90078-1. [DOI] [PubMed] [Google Scholar]
- Romeo D., Hinckley A., Rothfield L. Reconstitution of a functional membrane enzyme system in a monomolecular film. II. Formation of a functional ternary film of lipopolysaccharide, phospholipid and transferase enzyme. J Mol Biol. 1970 Nov 14;53(3):491–501. doi: 10.1016/0022-2836(70)90079-3. [DOI] [PubMed] [Google Scholar]
- Russell M. W., Challacombe S. J., Lehner T. Serum glucosyltransferase-inhibiting antibodies and dental caries in rhesus monkeys immunized against Streptococcus mutans. Immunology. 1976 May;30(5):619–627. [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F., Germaine G. R., Harlander S. K. Production of elevated levels of dextransucrase by a mutant of Streptococcus mutans. Infect Immun. 1975 Oct;12(4):934–937. doi: 10.1128/iai.12.4.934-937.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F., Harlander S. K., Germaine G. R. Streptococcus mutans dextransucrase: availability of disaggregated enzyme after growth in a chemically defined medium. Infect Immun. 1976 May;13(5):1522–1524. doi: 10.1128/iai.13.5.1522-1524.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shier W. T., Trotter J. T., 3rd Stimulation of liver microsomal sialyltransferase activity by lysolecithin. FEBS Lett. 1976 Feb 15;62(2):165–168. doi: 10.1016/0014-5793(76)80044-0. [DOI] [PubMed] [Google Scholar]
- Spinell D. M., Gibbons R. J. Influence of culture medium on the glucosyl transferase- and dextran-binding capacity of Streptococcus mutans 6715 cells. Infect Immun. 1974 Dec;10(6):1448–1451. doi: 10.1128/iai.10.6.1448-1451.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



