Abstract
Isoflurane-induced cognitive impairments are well documented in animal models; yet, the molecular mechanisms remain largely to be determined. In the present study, 22-month-old male Sprague-Dawley rats received 2 h of 1.5 % isoflurane or 100 % oxygen daily for 3 consecutive days. For the intervention study, the rats were intraperitoneally injected with 1.2 g/kg sodium butyrate 2 h before isoflurane exposure. Our data showed that repeated isoflurane exposure significantly decreased the freezing time to context and the freezing time to tone in the fear conditioning test, which was associated with upregulated histone deacetylase 2, reduced histone acetylation, and increased inflammation and apoptosis in the hippocampus, and impairments of brain-derived neurotrophic factor (BDNF)-tyrosine kinase receptor B (TrkB) and the downstream signaling pathway phospho-calmodulin-dependent protein kinase and phospho-cAMP response element-binding protein. These results suggest that isoflurane-induced cognitive impairments are associated with the declines in chromatin histone acetylation and the resulting downregulation of BDNF-TrkB signaling pathway. Moreover, the cognitive impairments and the signaling deficits can be rescued by histone deacetylase inhibitor sodium butyrate. Therefore, epigenetic enhancement of BDNF-TrkB signaling may be a promising strategy for reversing isoflurane-induced cognitive impairments.
Keywords: Cognition, Histone acetylation, Brain-derived neurotrophic factor, Inflammation, Apoptosis
Introduction
Postoperative cognitive dysfunction (POCD) is a clinical phenomenon characterized by cognitive decline in patients after surgery and anesthesia, especially in elderly patients [1–3]. An international multicenter trial has reported that POCD occurs in 25.8%of patients 1 week and in 9.9%of patients 3 months after noncardiac surgery in patients with the age of more than 60 years old [4], which is associated with impaired daily functioning and increased morbidity and mortality [1–4]. Despite no difference was reported regarding the incidence of POCD in patients receiving general anesthesia or regional anesthesia in recent studies [5, 6], exposure to general anesthesia is still considered to be a remarkable contributor to POCD [7]. Consistently, isoflurane exposure has been reported to induce neurotoxicity associated with learning/memory impairments in neonatal [8], young [9], adult [10], and aged [11] rodents. However, the precise molecular mechanisms responsible for isoflurane-induced cognitive impairments still remain elusive.
Epigenetic mechanisms are increasingly being recognized for their importance in regulating gene transcription required for long-term memory processes [12]. One common form of epigenetic modification is histone acetylation, which is controlled by the balance between histone acetyltransferases and histone deacetylases (HDACs) [12]. Specifically, HDAC2 has been shown to negatively regulate memory formation and is likely the most relevant isoform responsible for HDAC inhibitor-induced memory enhancement [13, 14]. Accumulating evidence has suggested that dysregulation of hippocampal histone acetylation such as H3H9 and H4K12 contributes to memory decline by suppressing key learning and memory genes including brain-derived neurotrophic factor (BDNF), calmodulin-dependent protein kinase (CaMKII), and cAMP response element-binding protein (CREB) [13–15], whereas targeting histone acetylation using HDAC inhibitors leads to neuroprotective effects [16]. Our previous study has demonstrated that chronic treatment with HDAC inhibitor valproic acid can reverse the cognitive deficits by restoring hippocampal histone acetylation levels and enhancement of BDNF-tyrosine kinase receptor B (TrkB) signaling in a mouse model of sepsis-associated encephalopathy [17]. However, the role of HDAC2 and histone acetylation in isoflurane-induced cognitive impairments remains unclear.
Therefore, the present study aimed to test the hypothesis that the dysregulation of histone acetylation and BDNF-TrkB signaling might be a plausible molecular mechanism responsible for isoflurane-induced cognitive impairments.
Materials and Methods
Animals
Forty-two 22-month-old male Sprague-Dawley male rats were purchased from the Animal Center of Jinling Hospital, Nanjing, China. The animal care and experiment were performed in accordance with the Declaration of the National Institutes of Health Guide for Care and Use of Laboratory Animals, USA. The animals were housed under a 12-h light/dark cycle in a temperature-controlled room of 22–24 °C with free access to food and water.
Anesthesia and Drug Intervention
Anesthesia was induced by placing the rats in a chamber with 1.8 % isoflurane carried by 100 % oxygen for 10 min and then changed to 1.5 % isoflurane for the maintenance of anesthesia continuously for 2 h for 3 consecutive days. The inhaled and exhaled gas concentrations were monitored continuously by a Datex™ infrared analyzer (Capnomac, Helsinki, Finland). Rats in the sham group were not anesthetized but were given 100%O2 of 1.5 L/min for 2 h for 3 consecutive days in the identical chamber. All animals were recovered for 20 min in a chamber gassed with 100%O2 and at 37 °C. After recovery, the rats were returned to their home cages. Rectal temperature was measured and maintained at a physiological temperature of 37±10.5 °C in all groups. Sodium butyrate (NaB, 1.2 g/kg; Sigma, St. Louis, MO, USA) or phosphate-buffered saline (PBS) was intraperitoneally administered to the rats with a volume of 1 mL/kg 2 h before each of the 3-day isoflurane exposure or 100 % O2 inhalation. NaB is a potent HDAC inhibitor that affects neuronal histone acetylation when administered via intraperitoneal injection [18]. Immediately after the end of the 3-day isoflurane exposure, four animals per group were used to collect blood from the left heart for blood gas analysis. Blood pH, arterial oxygen tension (PaO2), and arterial carbon dioxide tension (PaCO2) were analyzed by using a GEM Premier 3000 (Instrumentation Laboratory, Lexington, MA, USA).
Open-Field Tests
On the fourth day, the rats were placed in the center of the white plastic chambers (100× 100×40 cm) and left to explore it for 5 min, while exploratory behavior was automatically recorded by a video-tracking system (XR-XZ301, Shanghai Softmaze Information Technology Co. Ltd., Shanghai, China). At the end of testing, the arena was cleaned with 75 % alcohol to avoid the presence of olfactory cues.
Fear Conditioning Tests
To measure associative memory, we employed the fear conditioning paradigm (30-cm long×26-cm wide×22-cm high, XR-XC404, Shanghai Softmaze Information Technology Co. Ltd., Shanghai, China). Briefly, the training consisted of a single exposure to a novel context for 3 min, followed by a tone for 30 s (30 s, 65 dB, 3 kHz), and a single electric foot shock (3 s, 0.75 mA). Contextual fear conditioning test was performed successively 24 h later by placing the rats back to the same test chamber for 5 min without any stimulation. Two hours after the contextual fear conditioning test, the rats were placed to a novel chamber changed in the shape, color, and smell, and the training tone was delivered for 3 min to evaluate tone fear conditioning. Freezing was defined as the total absence of body or head movement except for that associated with breathing. Freezing behavior was automatically recorded by a video-tracking system.
Western Blotting
The hippocampus was harvested 2 h after the behavioral analysis for the determination of HDAC2 (1:500; Abcam, UK), acetyl-H3K9 (1:2,000; Millipore, USA), acetyl-H4K12 (1:2,000;Millipore, USA), BDNF (1:500; Abcam, UK), TrkB (1:500; Abcam, UK), p-TrkB (1:1,000; Abcam, UK), p-CaMKII (1:500; Abcam, UK), p-CREB (1:200; Abcam, UK), caspase-3 (1:100; Abcam, UK), and anti-β-actin (1:1,000, Santa Cruz, USA) as previously described [19]. Bands were visualized by enhanced chemiluminescence and quantitated with the Image Quant Software (Syngene, Cambridge, UK).
Enzyme-Linked Immunosorbent Assay
We detected the hippocampal levels of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 by enzyme-linked immunosorbent assay kits following the protocols provided by the manufacturer (Jiancheng Biological Technology Co. Ltd., Nanjing, China). All samples were assayed in duplicate. The readings were normalized to the amount of standard protein.
Immunohistochemistry Analysis
The brain tissues, fixed by immersion in 4 % paraformaldehyde, were embedded in paraffin and cut using amicrotome to yield 5-μm thick sections that were mounted on precoated glass slides for all further steps. The sections were deparaffinized and hydrated by the following incubation steps: 10 min in xylene two times, 10 min in 100 %, 10 min in 95 %, 10 min in 75 %, and 10 min in 50 % ethanol, and 5 min two times in ddH2O at room temperature. Antigen retrieval was achieved by boiling the sections in 10 mM sodium citrate for 10 min in a microwave oven. The sections were washed with tap water, and immunostaining was performed by washing the sections two times in 0.01 M PBS followed by incubation in blocking buffer (5 % goat serum+ 0.3 % TritonX-100 in 0.01 M PBS) for 90 min at room temperature to block nonspecific sites and incubation in primary antibody (HDAC2, 1:500; Abcam, Cambridge, UK) diluted in blocking buffer at 4 °C overnight on a shaker. Thereafter, the sections were washed three times in an antibody wash buffer (1 % goat serum+0.2 % TritonX-100 in 0.01 M PBS) for 10 min at room temperature.
Statistical Analysis
Data are presented as mean ± SEM and analyzed by the Statistical Product for Social Sciences (version 16.0, SPSS Inc., Chicago, IL, USA). Normal distribution of data was analyzed using the Kolmogorov-Smirnov test. The difference between the groups was determined by independent sample t test, and among the groups, the difference was determined by one-way analysis of variance followed by the Bonferroni test. A p value<0.05 was regarded as statistical significance.
Results
Isoflurane Exposure Did Not Affect Arterial Blood Gas
As shown in Table 1, there was no significant difference in the values of pH, PaO2, and PaCO2 among the three groups at the end of the 3-day isoflurane exposure (pH F(2,9)=0.234, p= 0.723; PaO2 F(2,9)=0.380, p=0.694; PaCO2 F(2,9)=0.159, p= 0.855).
Table 1.
Arterial blood gas analysis of rats in the three groups
| Sham group | ISO group | ISO + NaB group | |
|---|---|---|---|
| pH | 7.42±0.05 | 7.40±0.06 | 7.39±0.05 |
| PaO2 (mmHg) | 118.5±7.6 | 116.5±9.8 | 109.3±5.7 |
| PaCO2 (mmHg) | 32.5±3.9 | 33.0±3.3 | 30.5±2.5 |
Values are presented as mean ± SEM. No significant difference in pH, PaO2, or PaCO2 was observed among the three groups (one-way ANOVA, p>0.05)
ISO isoflurane, NaB sodium butyrate, PaO2 arterial oxygen tension,PaCO2 arterial carbon dioxide tension
Repeated Isoflurane Exposure Induced Cognitive Impairments in Aged Rats
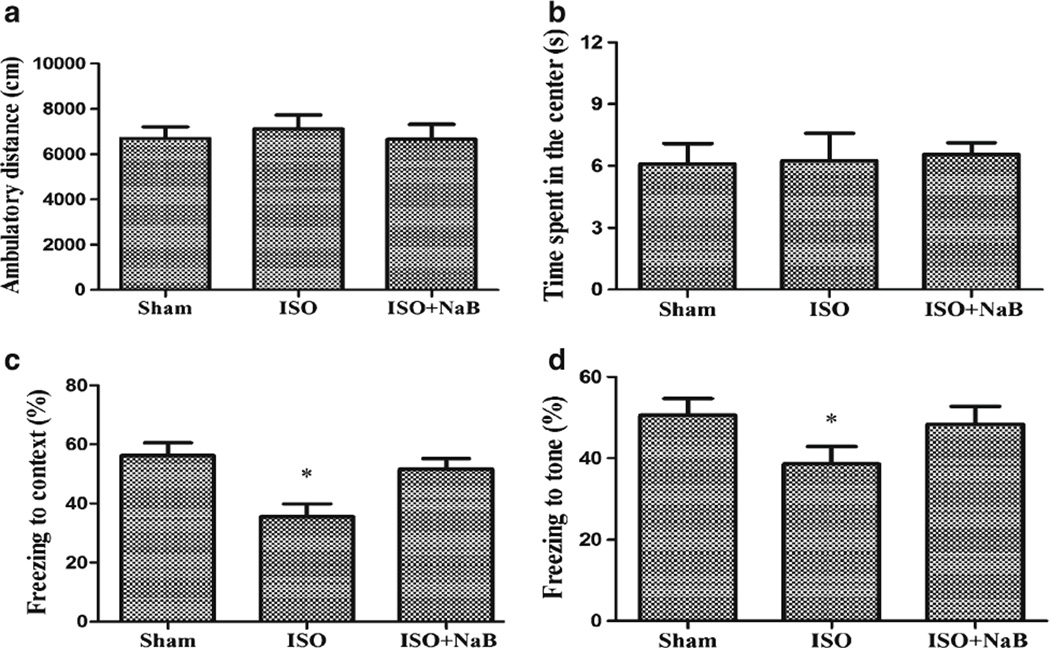
In the open-field test, no difference was observed in the total ambulatory distance and the time spent in the center of the arena among groups (F(2,27)=0.179, p=0.837; F(2,27)=0.065, p=0.938; Fig. 1a, b), suggesting neither isoflurane exposure nor NaB had no significant influence on the locomotor and exploratory activity. In the fear conditioning test, the freezing time to context and the freezing time to tone were significantly lower in the isoflurane group than those of the sham group (p=0.004 and p=0.032), which was reversed by NaB pretreatment (p=0.031 and p=0.047) (Fig. 1c, d).
Fig. 1.
Effect of repeated ISO exposure on the behavioral tests. The rats showed normal total ambulatory distance (a) and time spent in the center (b) in the open-field test. The rats showed lower percentage of freezing time to context (c) and percentage of freezing time to tone (d) in the ISO group than in the sham group, which was reversed by NaB pretreatment in the ISO + NaB group. Data are presented as mean ± SEM (n=10). *p< 0.05 versus the sham group and the ISO + NaB group by using one-way analysis of variance followed by the Bonferroni test. ISO isoflurane, NaB sodium butyrate
Repeated Isoflurane-Exposure-Induced Changes in HDAC2 and Histone Acetylation
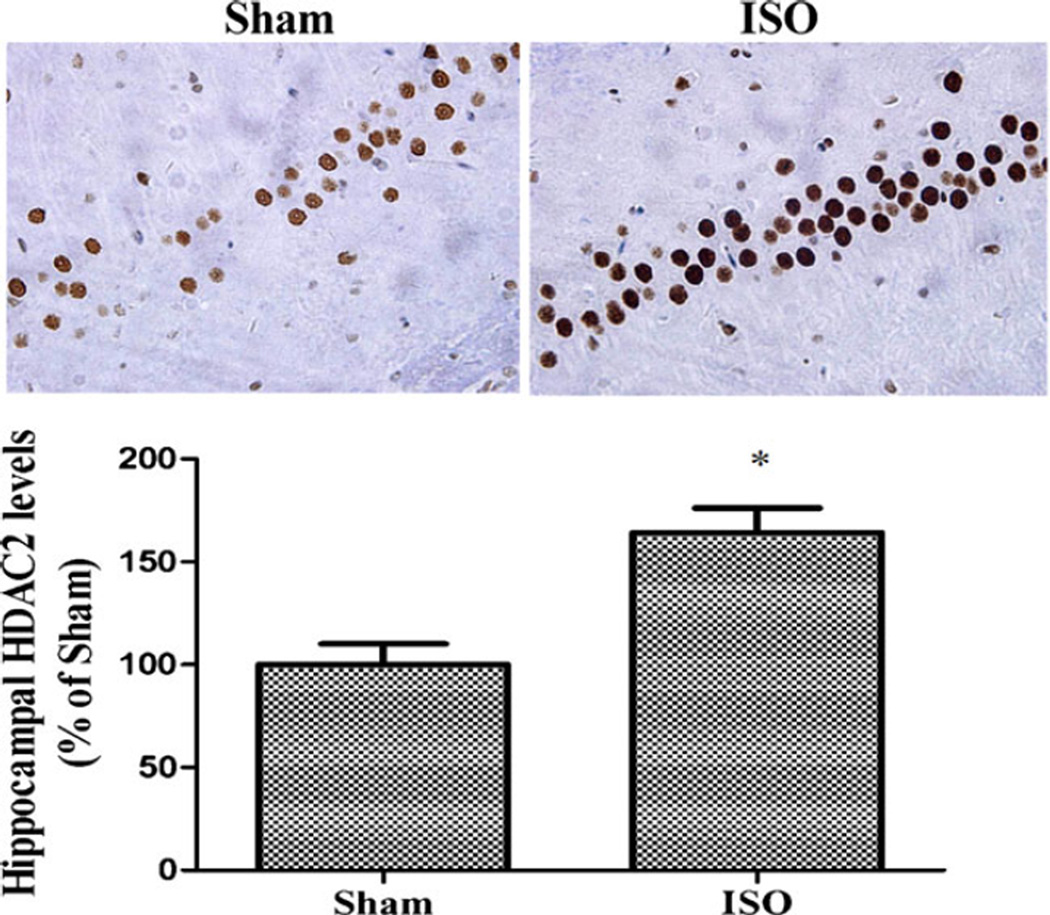
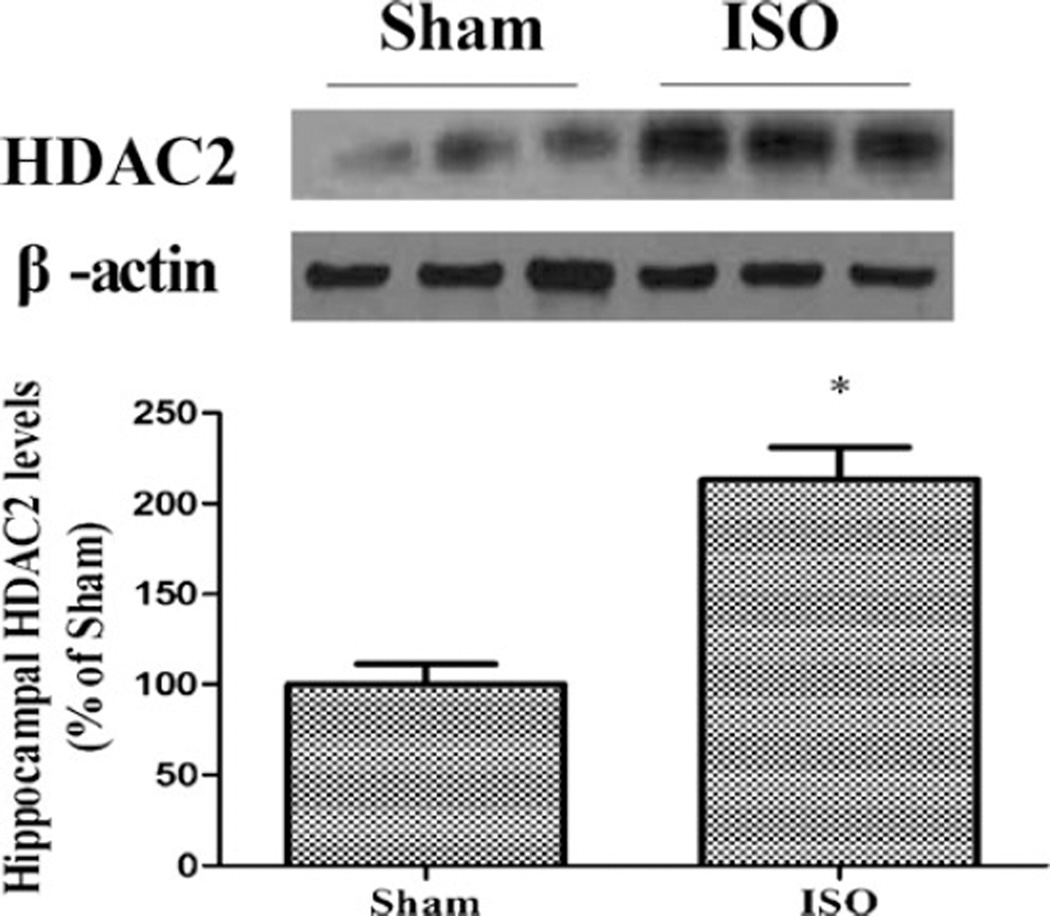
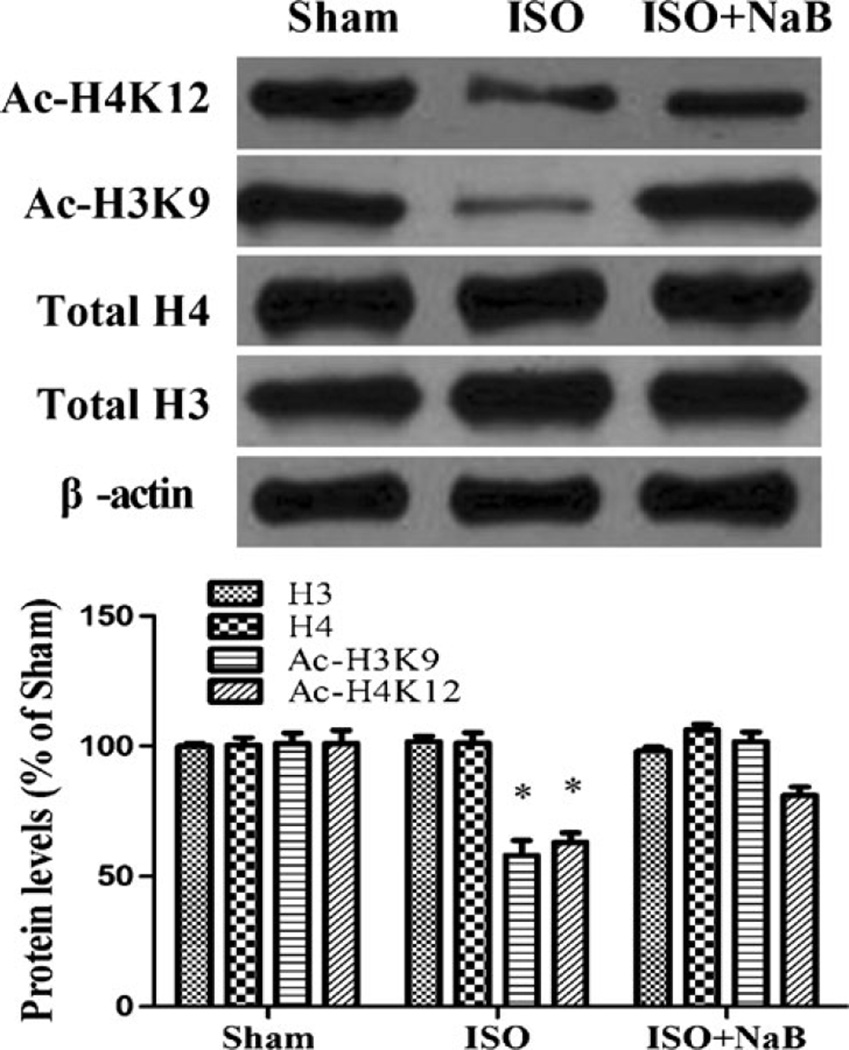
Repeated isoflurane exposure upregulated hippocampal HDAC2 expression in aged rats compared with the sham group (t=−6.945, df=6, p<0.001; Fig. 2). Consistent with the Western blotting analysis data, immunohistochemistry suggested that hippocampal HDAC2 expression increased significantly in aged rats induced by repeated isoflurane exposure (t=−3.384, df=6, p=0.015; Fig. 3). Although repeated isoflurane exposure did not alter total H3 and H4 levels, acetylation of histone H3 at lysine 9 (K9) and H4 at lysine 12 (K12) decreased significantly in the hippocampus (acetyl-H3K9 F(2,9)=30.34, p<0.001; acetyl-H4K12 F(2,9)=21.25, p<0.001). However, NaB pretreatment effectively restored acetyl-H3K9 and acetyl-H4K12 levels in aged rats induced by repeated isoflurane exposure (p<0.001 and p=0.015; Fig. 4).
Fig. 2.
Effect of repeated ISO exposure on the hippocampal level of HDAC2 determined by Western blotting. The rats showed higher hippocampal HDAC2 levels in the ISO group than in the sham group. Data are presented as mean ± SEM (n=4). *p<0.05 versus the sham group by using independent sample t test. ISO isoflurane, NaB sodium butyrate
Fig. 3.
Effect of repeated ISO exposure on the hippocampal level of HDAC2 determined by immunohistochemistry analysis. The rats showed higher hippocampal HDAC2 levels in the ISO group than in the sham group. Data are presented as mean ± SEM (n=4). *p<0.05 versus the sham group by using independent samples t test. ISO isoflurane, NaB sodium butyrate
Fig. 4.
Effect of repeated ISO exposure on the hippocampal level of histone acetylation. The rats showed lower acetylation of histone H3 at lysine 9 (K9) and H4 at lysine 12 (K12) in the ISO group than in the sham group, which was restored by NaB pretreatment in the ISO + NaB group. Data are presented as mean ± SEM (n=4). *p<0.05 versus the sham group and the ISO + NaB group by using one-way analysis of variance followed by the Bonferroni test. ISO isoflurane, NaB sodium butyrate
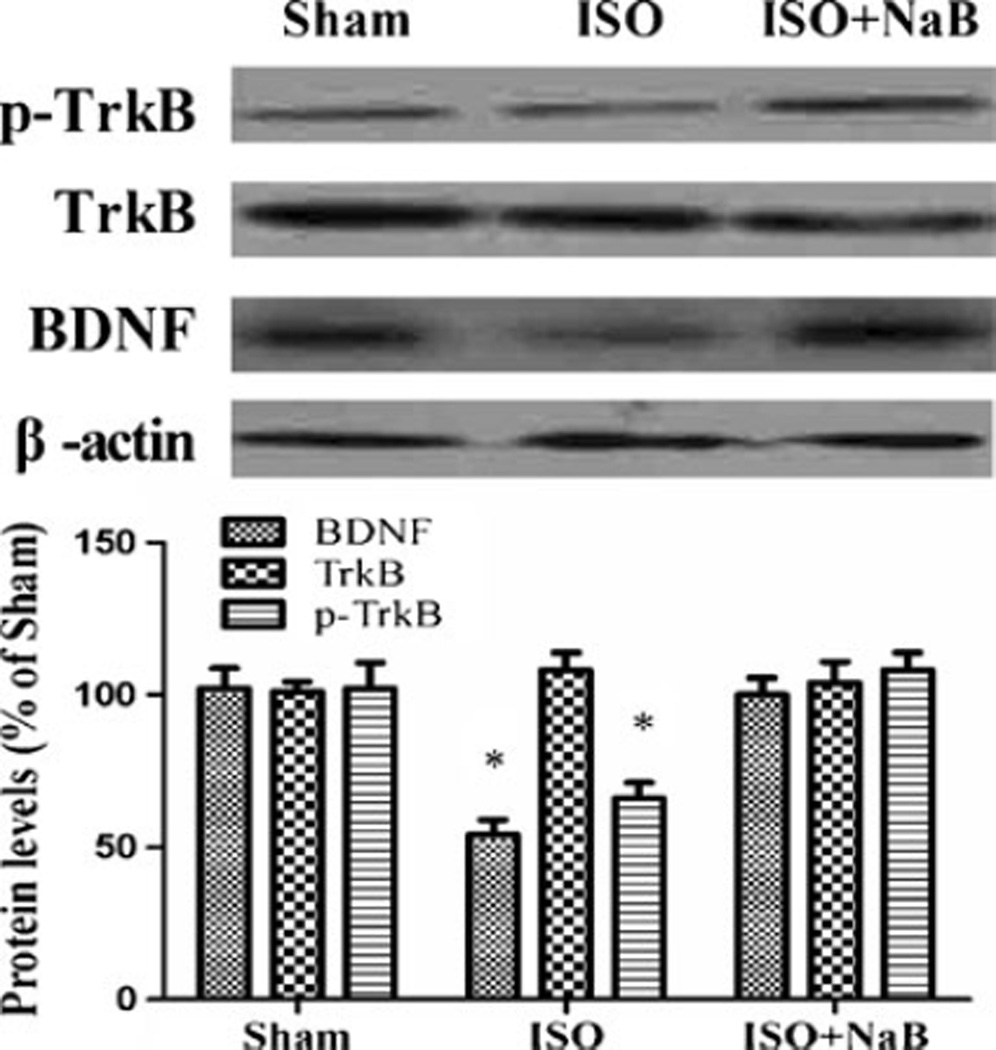
Repeated Isoflurane Exposure Led to Reduced BDNF and p-TrkB Expression
There was no difference in TrkB among groups (F(2,9)=0.407, p=0.675), and we detected that repeated isoflurane exposure reduced hippocampal BDNF and p-TrkB levels in aged rats by using Western blotting analysis (p<0.001 and p<0.001), which was reversed by NaB pretreatment (p=0.007 and p=0.002; Fig. 5).
Fig. 5.
Effect of repeated ISO exposure on the hippocampal levels of BDNF and TrkB. There was no significant difference in the hippocampal levels of TrkB among groups. However, the rats showed lower hippocampal levels of BDNF and p-TrkB in the ISO group than in the sham group, which was restored by NaB pretreatment in the ISO + NaB group. Data are presented as mean ± SEM (n=4). *p<0.05 versus the sham group and the ISO + NaB group by using one-way analysis of variance followed by the Bonferroni test. ISO isoflurane, NaB sodium butyrate
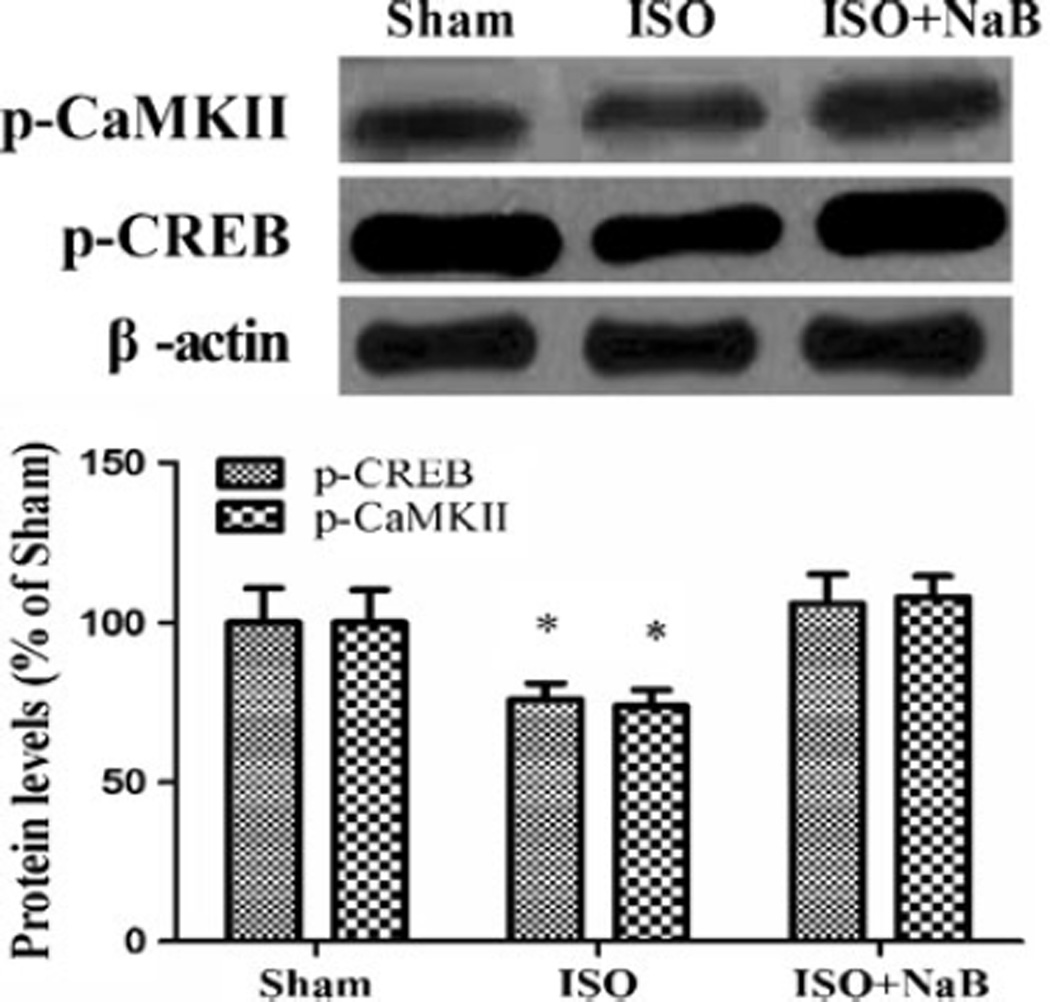
NaB Pretreatment Upregulated Proteins Related to Memory Consolidation
The levels of p-CaMKII and p-CREB were significantly reduced with repeated isoflurane exposure and could be restored by NaB pretreatment (p=0.027 and p=0.039; Fig. 6).
Fig. 6.
Effect of repeated ISO exposure on the hippocampal proteins related to memory. Repeated isoflurane exposure downregulated p-CaMKII and p-CREB in the hippocampus, which was restored by NaB pretreatment in the ISO + NaB group. Data are presented as mean ± SEM (n=4). *p<0.05 versus the sham group and the ISO + NaB group by using one-way analysis of variance followed by the Bonferroni test. ISO isoflurane, NaB sodium butyrate
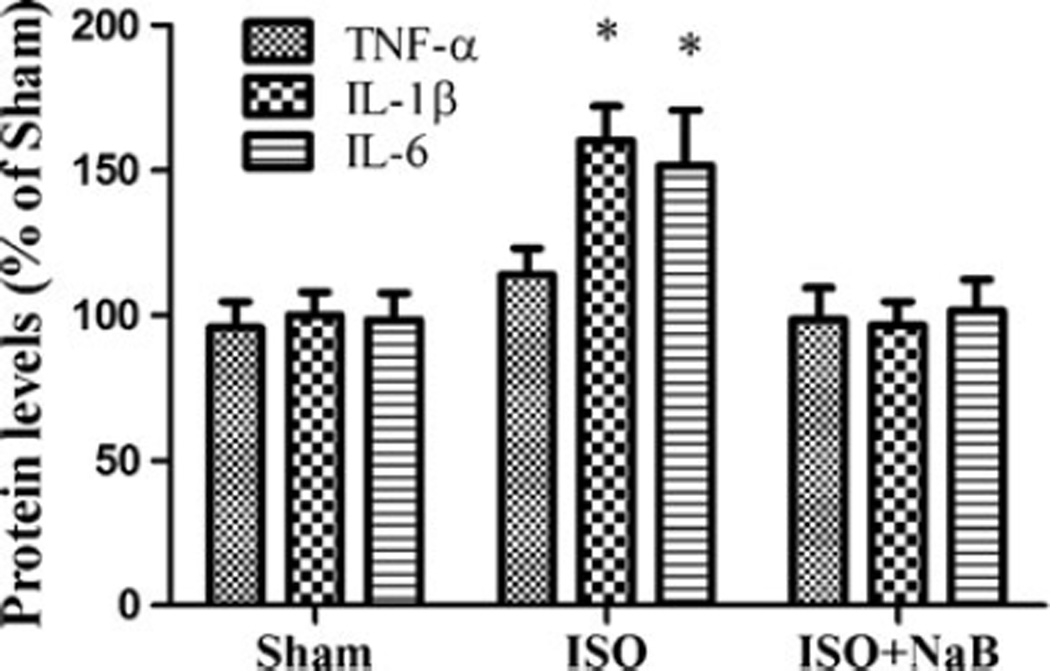
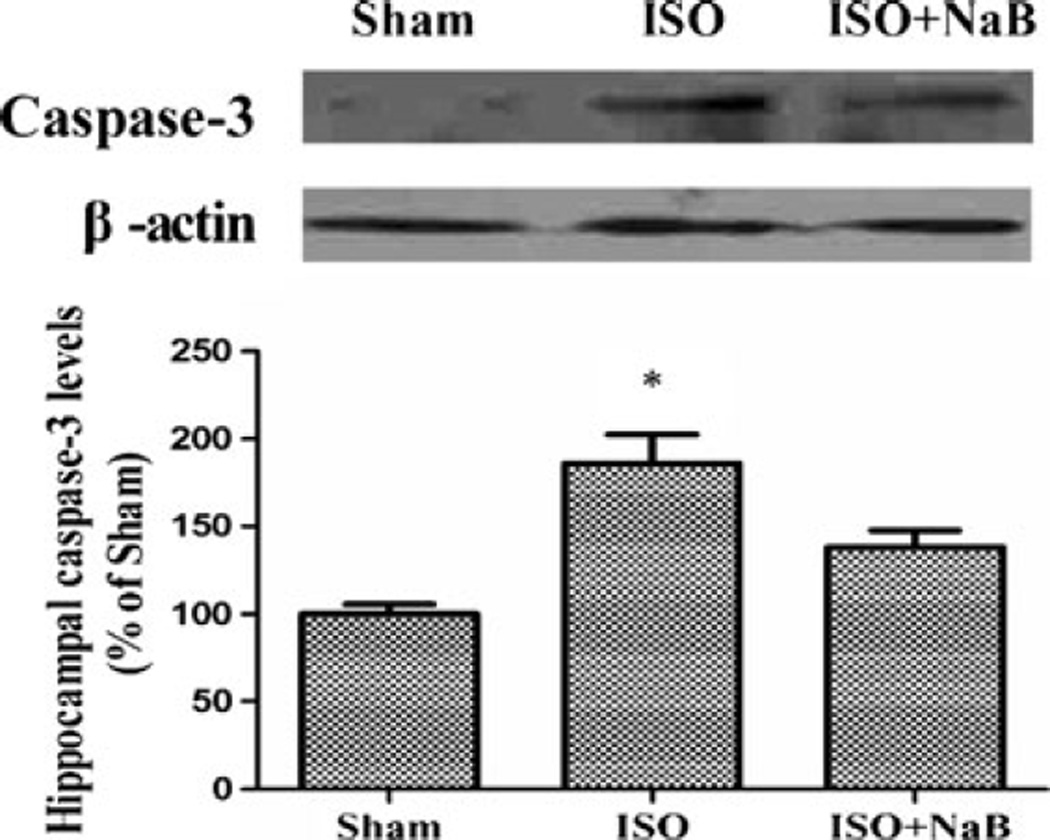
NaB Pretreatment Attenuated the Isoflurane-Induced Upregulation of Inflammation and Apoptosis in the Hippocampus
The levels of IL-1β and IL-6 increased significantly in the aged rats after the repeated isoflurane exposure when compared with those in the sham group (p=0.031 and p=0.049). NaB pretreatment reversed the upregulation of IL-1β and IL-6 after the repeated isoflurane exposure in the hippocampus (p= 0.002 and p= 0.047; Fig. 7). Furthermore, NaB pretreatment attenuated the isoflurane-induced upregulation of cleaved caspase-3 in the hippocampus (p=0.037; Fig. 8).
Fig. 7.
Effect of NaB pretreatment on hippocampal levels of proinflammatory cytokines after repeated ISO exposure. Repeated isoflurane exposure increased IL-1β and IL-6 levels in the hippocampus, which was attenuated by NaB pretreatment in the ISO + NaB group. Data are presented as mean ± SEM (n=6). *p<0.05 versus the sham group and the ISO + NaB group by using one-way analysis of variance followed by the Bonferroni test. ISO isoflurane, NaB sodium butyrate
Fig. 8.
Effect of NaB pretreatment on hippocampal neuronal apoptosis after repeated ISO exposure. Repeated isoflurane exposure increased cleaved caspase-3 expression in the hippocampus, which was attenuated by NaB pretreatment in the ISO + NaB group. Data are presented as mean ± SEM (n=4). *p<0.05 versus the sham group and the ISO + NaB group by using one-way analysis of variance followed by the Bonferroni test. ISO isoflurane, NaB sodium butyrate
Discussion
The major finding of the present study was that repeated isoflurane exposure induced cognitive impairments accompanied by reduced histone acetylation levels in the hippocampus, whereas pretreatment with NaB restored histone acetylation levels and prevented the cognitive impairments. The mechanism of these protective effects may relate to the BDNF-TrkB signaling and its downstream cascades in the hippocampus. To our knowledge, the present study is the first demonstration that isoflurane exposure is accompanied by significant reduction in epigenetic signaling, thus contributes to cognitive impairments induced by repeated isoflurane exposure in aged rats. The underlying mechanisms leading to POCD remain largely unknown but likely involve a combination of patient, surgical, and anesthetic factors [1–3]. Isoflurane, a widely used inhalation anesthetic, has been reported to impair the cognitive function in the rodent models and humans [7–10]. Consistent with these studies, we demonstrated here that repeated isoflurane exposure resulted in cognitive impairments in aged rats, supporting the role of volatile anesthetics in the development of POCD. Although neuroinflammation, neuroapoptosis, protein hyperphosphorylation, and mitochondria dysfunction in the brain have been implicated [20–22], the pathogenesis of volatile anesthetic-induced cognitive impairments is not fully understood.
Extensive research in the nervous system has supported a role for chromatin-mediated epigenetic regulation as being crucial for behavior and memory formation [12]. One common form of epigenetic modification is histone acetylation, which is dynamically altered during memory formation [12–14]. In general, increased histone acetylation leads to a more relaxed chromatin structure and promotes gene transcription required for long-term memory processes, whereas histone deacetylation negatively regulates gene transcription [12]. It has been suggested that epigenetic modifications within the brain are critical for short- and long-term behavioral adaptation to a wide range of environmental stimuli [23]. Indeed, accumulating evidence indicates that during both early development and in adult life, environmental signals may activate intracellular pathways that directly remodel the “epigenome”, which then lead to changes in gene expression and neural function [24, 25]. Thus, isoflurane exposure is likely to alter gene expression related to cognition performance involving the dysregulation of epigenetic control. In the present study, repeated isoflurane exposure induced HDAC2 upregulation, and histone acetylation deficits may represent a compromised epigenetic environment, whereas NaB restored H3H9 and H4K12 acetylation and ultimately resulted in improved cognitive dysfunction in aged rats. These results concur with one recent study showing that NaB treatment is able to reinstate associative memory in an Alzheimer’s disease mouse model when administered at an advanced stage of disease progression [18].
To clarify how the altered histone acetylation leads to isoflurane-induced cognitive impairments, we investigated the expression of proteins related to cognition performance. Multiple studies have linked increased histone acetylation to hippocampal memory, presumably reflecting the induction of chromatin modifications permissive for the transcription of learning-related plasticity genes [12]. Much of the epigenetics literature has focused on histone modifications across the BDNF gene; it has been proposed that epigenetic regulation of the BDNF gene is a transcriptional mechanism occurring in the adult brain that functions in learning and memory processes and dysregulation of BDNF has been implicated in the pathogenesis of cognitive and mental disorders [26–28]. In support with these previous findings, we demonstrated that repeated isoflurane exposure disrupted BDNF pathway. To better understand how the reduced BDNF expression contributes to the isoflurane-induced cognitive impairments, we examined the changes in two key components of BDNF-regulated signaling cascades, CaMKII and CREB. Notably, our results confirmed that disruption of the BDNF-TrkB pathway and its downstream signaling cascades contributed to isoflurane-induced cognitive impairments in aged rats.
Given the findings that the enhanced inflammation in the brain plays a key role in the pathophysiology of POCD [20, 21], our data confirmed that repeated isoflurane exposure upregulated the proinflammatory cytokine expression. Because chromatin modification is a key mechanism responsible for the regulation of gene transcription including various inflammation-related genes [29], it is not surprising that HDAC inhibitor NaB exerts its anti-inflammatory effects and hence improves isoflurane-induced cognitive impairments. It has been suggested that neuroinflammation may disrupt BDNF expression, despite that it is not clear how the exaggerated neuroinflammation impacts the levels of BDNF production [30]. Furthermore, accumulating evidence has suggested that isoflurane exposure can induce neuroapoptosis in neonatal rodents [8, 22]. The study by Zhu et al. has suggested that isoflurane exposure does not induce neuroapoptosis in rats older than 2 weeks of age [9]. In addition, another study by Stratmann et al. has reported that 2 h of isoflurane exposure leads to cell death but not neurocognitive dysfunction in postnatal day 7 rats [31]. The study protocols of these studies are different from the design of the present study. For example, the rats used are 22 months old, and the isoflurane used is 1.5 % for 2 h daily for 3 consecutive days. These differences may explain the different findings of isoflurane’s effects on neuronal apoptosis and neurocognitive outcome in the present study. Moreover, recent studies by our own and others also suggest that isoflurane exposure can induce excessive caspase-3 expression and consequent cognitive impairments in aged rodents [32, 33]. These results suggest that inhibition of hippocampal neuroapoptosis may be another mechanism involved in the neuroprotective properties of NaB against isoflurane exposure in aged rats.
HDACs are reported to interfere with acetylation of many nonhistone proteins in the nucleus and cytoplasm [18]. Therefore, it cannot be excluded that modifications on proteins other than histones could contribute to the observed effects. Furthermore, given the various HDAC enzymes expressed in the brain, more studies are needed to address the role of other individual HDACs in isoflurane-induced cognitive impairments. In addition, a previous study suggested that HDAC inhibitor such as NaB can ameliorate aging-related memory impairments [33]; it would therefore be more logical to add another group with 100%O2 plus NaB (100 % O2 + NaB group) in the study design of the present study. However, our preliminary studies suggested that 3-day NaB administration has no significant influence on histone acetylation levels or behaviors in the 100 % O2 + NaB group (n=3) when compared with that in the sham group. In order to minimize the number of rats used, we included the sham group as the negative control group, the isoflurane group as the positive control group, and the isoflurane + NaB group as the interventional study group.
In summary, our data provide evidence that isoflurane-induced cognitive impairments are associated with declines in chromatin histone acetylation and the resulting changes in BDNF expression and its downstream signaling in the aged rats. Thus, enhancement of histone acetylation by inhibiting HDACs with NaB may be a suitable therapeutic strategy for the treatment of isoflurane-induced cognitive impairments.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 81271216 and 81300946) and the Natural Science Foundation of Jiangsu Province (no. BK2012778).
Footnotes
The authors declare no conflict of interest.
Contributor Information
MuHuo Ji, Department of Anesthesiology, Jinling Hospital, School of Medicine, Nanjing University, Nanjing, China.
Lin Dong, Department of Anesthesiology, Jinling Hospital, School of Medicine, Nanjing University, Nanjing, China.
Min Jia, Department of Anesthesiology, Jinling Hospital, School of Medicine, Nanjing University, Nanjing, China.
WenXue Liu, Department of Anesthesiology, Jinling Hospital, School of Medicine, Nanjing University, Nanjing, China.
MingQiang Zhang, Department of Anesthesiology, Jinling Hospital, School of Medicine, Nanjing University, Nanjing, China.
LinSha Ju, Department of Anesthesiology, Jinling Hospital, School of Medicine, Nanjing University, Nanjing, China.
JiaoJiao Yang, Department of Anesthesiology, Jinling Hospital, School of Medicine, Nanjing University, Nanjing, China.
Zhongcong Xie, Geriatric Anesthesia Research Unit, Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA 02129-2060, USA.
JianJun Yang, Email: yjyangjj@126.com, Department of Anesthesiology, Jinling Hospital, School of Medicine, Nanjing University, Nanjing, China.
References
- 1.McDonagh DL, Mathew JP, White WD, Phillips-Bute B, Laskowitz DT, Podgoreanu MV, Newman MF. Cognitive function after major noncardiac surgery, apolipoprotein E4 genotype, and biomarkers of brain injury. Anesthesiology. 2010;112:852–859. doi: 10.1097/ALN.0b013e3181d31fd7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS ISPOCD Group. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009;110:548–555. doi: 10.1097/ALN.0b013e318195b569. [DOI] [PubMed] [Google Scholar]
- 3.Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, Gravenstein JS. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 4.Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, Langeron O, Johnson T, Lauven PM, Kristensen PA, Biedler A, van Beem H, Fraidakis O, Silverstein JH, Beneken JE, Gravenstein JS. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351:857–861. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 5.Williams-Russo P, Sharrock NE, Mattis S, Szatrowski TP, Charlson ME. Cognitive effects after epidural vs general anesthesia in older adults. A randomized trial. JAMA. 1995;274:44–50. [PubMed] [Google Scholar]
- 6.Rasmussen LS, Johnson T, Kuipers HM, Kristensen D, Siersma VD, Vila P, Jolles J, Papaioannou A, Abildstrom H, Silverstein JH, Bonal JA, Raeder J, Nielsen IK, Korttila K, Munoz L, Dodds C, Hanning CD, Moller JT ISPOCD2 (International Study of Postoperative Cognitive Dysfunction) Investigators. Does anaesthesia cause postoperative cognitive dysfunction? A randomised study of regional versus general anaesthesia in 438 elderly patients. Acta Anaesthesiol Scand. 2003;47:260–266. doi: 10.1034/j.1399-6576.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhang B, Tian M, Zhen Y, Yue Y, Sherman J, Zheng H, Li S, Tanzi RE, Marcantonio ER, Xie Z. The effects of isoflurane and desflurane on cognitive function in humans. Anesth Analg. 2012;114:410–415. doi: 10.1213/ANE.0b013e31823b2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang WY, Luo Y, Jia LJ, Hu SF, Lou XK, Shen SL, Lu H, Zhang HH, Yang R, Wang H, Ma ZW, Xue QS, Yu BW. Inhibition of aberrant cyclin-dependent kinase 5 activity attenuates isoflurane neurotoxicity in the developing brain. Neuropharmacology. 2013;77C:90–99. doi: 10.1016/j.neuropharm.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Zhu C, Gao J, Karlsson N, Li Q, Zhang Y, Huang Z, Li H, Kuhn HG, Blomgren K. Isoflurane anesthesia induced persistent, progressive memory impairment, caused a loss of neural stem cells, and reduced neurogenesis in young, but not adult, rodents. J Cereb Blood Flow Metab. 2010;30:1017–1030. doi: 10.1038/jcbfm.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin D, Zuo Z. Isoflurane induces hippocampal cell injury and cognitive impairments in adult rats. Neuropharmacology. 2011;61:1354–1359. doi: 10.1016/j.neuropharm.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckenhoff RG, Johansson JS, Wei H, Carnini A, Kang B, Wei W, Pidikiti R, Keller JM, Eckenhoff MF. Inhaled anesthetic enhancement of amyloid-beta oligomerization and cytotoxicity. Anesthesiology. 2004;101:703–709. doi: 10.1097/00000542-200409000-00019. [DOI] [PubMed] [Google Scholar]
- 12.Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Javan S, Agis-Balboa RC, Cota P, Wittnam JL, Gogol-Doering A, Opitz L, Salinas-Riester G, Dettenhofer M, Kang H, Farinelli L, Chen W, Fischer A. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science. 2010;328:753–756. doi: 10.1126/science.1186088. [DOI] [PubMed] [Google Scholar]
- 13.Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, Nieland TJ, Zhou Y, Wang X, Mazitschek R, Bradner JE, DePinho RA, Jaenisch R, Tsai LH. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gräff J, Rei D, Guan JS, Wang WY, Seo J, Hennig KM, Nieland TJ, Fass DM, Kao PF, Kahn M, Su SC, Samiei A, Joseph N, Haggarty SJ, Delalle I, Tsai LH. An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature. 2012;483:222–226. doi: 10.1038/nature10849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kazantsev AG, Thompson LM. Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat Rev Drug Discov. 2008;7:854–868. doi: 10.1038/nrd2681. [DOI] [PubMed] [Google Scholar]
- 16.Gräff J, Tsai LH. The potential of HDAC inhibitors as cognitive enhancers. Annu Rev Pharmacol Toxicol. 2013;53:311–330. doi: 10.1146/annurev-pharmtox-011112-140216. [DOI] [PubMed] [Google Scholar]
- 17.Wu J, Dong L, Zhang M, Jia M, Zhang G, Qiu L, Ji M, Yang J. Class I histone deacetylase inhibitor valproic acid reverses cognitive deficits in a mouse model of septic encephalopathy. Neurochem Res. 2013;38:2440–2449. doi: 10.1007/s11064-013-1159-0. [DOI] [PubMed] [Google Scholar]
- 18.Govindarajan N, Agis-Balboa RC, Walter J, Sananbenesi F, Fischer A. Sodium butyrate improves memory function in an Alzheimer's disease mouse model when administered at an advanced stage of disease progression. J Alzheimers Dis. 2011;26:187–197. doi: 10.3233/JAD-2011-110080. [DOI] [PubMed] [Google Scholar]
- 19.Kayser EB, Suthammarak W, Morgan PG, Sedensky MM. Isoflurane selectively inhibits distal mitochondrial complex I in Caenorhabditis elegans. Anesth Analg. 2011;112:1321–1329. doi: 10.1213/ANE.0b013e3182121d37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji MH, Yuan HM, Zhang GF, Li XM, Dong L, Li WY, Zhou ZQ, Yang JJ. Changes in plasma and cerebrospinal fluid biomarkers in aged patients with early postoperative cognitive dysfunction following total hip-replacement surgery. J Anesth. 2013;27:236–242. doi: 10.1007/s00540-012-1506-3. [DOI] [PubMed] [Google Scholar]
- 21.Cibelli M, Fidalgo AR, Terrando N, Ma D, Monaco C, Feldmann M, Takata M, Lever IJ, Nanchahal J, Fanselow MS, Maze M. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann Neurol. 2010;68:360–368. doi: 10.1002/ana.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Xu Z, Wang H, Dong Y, Shi HN, Culley DJ, Crosby G, Marcantonio ER, Tanzi RE, Xie Z. Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory. Ann Neurol. 2012;71:687–698. doi: 10.1002/ana.23536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacDonald JL, Roskams AJ. Epigenetic regulation of nervous system development by DNA methylation and histone deacetylation. Prog Neurobiol. 2009;88:170–183. doi: 10.1016/j.pneurobio.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Sun J, Sun J, Ming GL, Song H. Epigenetic regulation of neurogenesis in the adult mammalian brain. Eur J Neurosci. 2011;33:1087–1093. doi: 10.1111/j.1460-9568.2011.07607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsieh J, Eisch AJ. Epigenetics, hippocampal neurogenesis, and neuropsychiatric disorders: unraveling the genome to understand the mind. Neurobiol Dis. 2010;39:73–84. doi: 10.1016/j.nbd.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng Y, Tan M, Kohyama J, Sneddon M, Watson JB, Sun YE, Xie CW. Epigenetic enhancement of BDNF signaling rescues synaptic plasticity in aging. J Neurosci. 2011;31:17800–17810. doi: 10.1523/JNEUROSCI.3878-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Devi L, Ohno M. 7,8-dihydroxyflavone, a small-molecule TrkB agonist, reverses memory deficits and BACE1 elevation in a mouse model of Alzheimer's disease. Neuropsychopharmacology. 2012;37:434–444. doi: 10.1038/npp.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diógenes MJ, Costenla AR, Lopes LV, Jerónimo-Santos A, Sousa VC, Fontinha BM, Ribeiro JA, Sebastião AM. Enhancement of LTP in aged rats is dependent on endogenous BDNF. Neuropsychopharmacology. 2011;36:1823–1836. doi: 10.1038/npp.2011.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shakespear MR, Halili MA, Irvine KM, Fairlie DP, Sweet MJ. Histone deacetylases as regulators of inflammation and immunity. Trends Immunol. 2011;32:335–343. doi: 10.1016/j.it.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Jurgens HA, Johnson RW. Environmental enrichment attenuates hippocampal neuroinflammation and improves cognitive function during influenza infection. Brain Behav Immun. 2012;26:1006–1016. doi: 10.1016/j.bbi.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stratmann G, May LD, Sall JW, Alvi RS, Bell JS, Ormerod BK, Rau V, Hilton JF, Dai R, Lee MT, Visrodia KH, Ku B, Zusmer EJ, Guggenheim J, Firouzian A. Effect of hypercarbia and isoflurane on brain cell death and neurocognitive dysfunction in 7-day-old rats. Anesthesiology. 2009;110:849–861. doi: 10.1097/ALN.0b013e31819c7140. [DOI] [PubMed] [Google Scholar]
- 32.Li XM, Zhou MT, Wang XM, Ji MH, Zhou ZQ, Yang JJ. Resveratrol pretreatment attenuates the isoflurane-induced cognitive impairment through its anti-inflammation and -apoptosis actions in aged mice. J Mol Neurosci. 2013 Oct 15; doi: 10.1007/s12031-013-0141-2. [DOI] [PubMed] [Google Scholar]
- 33.Kong F, Chen S, Cheng Y, Ma L, Lu H, Zhang H, Hu W. Minocycline attenuates cognitive impairment induced by isoflurane anesthesia in aged rats. PLoS One. 2013;8:e61385. doi: 10.1371/journal.pone.0061385. [DOI] [PMC free article] [PubMed] [Google Scholar]