Abstract
Postoperative peritoneal adhesions are major concerns in abdominal surgery. In this experimental study, the effects of 4 % icodextrin and omega-3 fatty acids (ω-3 FA) on prevention of postoperative peritoneal adhesions were evaluated. Twenty-four Wistar albino rats were divided into three groups. After laparotomy, serosal abrasion was carried out by cecal brushing. Intraperitoneally 3 cm3 0.9 % NaCl, 3 cm3 4 % icodextrin, and 200 mg/kg ω-3 FAs for each group were applied, and then the abdomen was closed. All subjects sacrificed 10 days postoperatively. Macroscopic and histopathological cellular reactions as a function of giant cell, lymphocyte/plasmocyte, neutrophil, histiocyte, intracellular adhesion molecule-1 (ICAM-1), and platelet endothelial cell adhesion molecule-1 (PECAM-1) were assessed and hydroxyproline levels were measured in all three groups and compared using Kruskal–Wallis and ANOVA tests when appropriate. Macroscopically, both ω-3 FAs and 4 % icodextrin reduced adhesion formation but the difference was not statistically significant (P = 0.253). Histopathological examination revealed that there was no statistical significance in terms of giant cell, lymphocyte/plasmocyte, neutrophil, ICAM-1, and PECAM-1 scores; however, both ω-3 FAs and 4 % icodextrin were found to be prone to reduce fibrosis (P = 0.047), whereas in the ω-3 FA group, histiocytic reaction was significantly increased (P = 0.001), and hydroxyproline levels were significantly lower than other groups (P = 0.044). In this study, ω-3 FAs were found to be superior to 4 % icodextrin with the lower hydroxyproline level and greater histiocytic reaction. Considering these results, ω-3 FAs can be a promising agent in the prevention of adhesion formation.
Keywords: Peritoneum, Adhesion formation, Icodextrin, Omega-3 fatty acids, Experimental study
Introduction
Postoperative peritoneal adhesion is one of the major complications in abdominal surgery, which was reported to occur from 70 to 93 % of the cases [1]. It causes intestinal obstruction, infertility, chronic pelvic pain, and secondary surgery [1, 2]. In addition to medical problems, increasing cost is another problem. In the USA, it is estimated that 1.3 billion US$ has been spent annually for management of peritoneal adhesions [1].
The first phase of peritoneal adhesion formation is initiated by tissue damage leading to coagulation cascade in several hours. After coagulation, the inflammation phase begins during the first few days. Cell seeding, proliferation, and migration and matrix deposition occur during the first week. The last phase is matrix remodeling from weeks to months [3].
Icodextrin is a corn starch-derived, water-soluble, branched glucoside polymer linked by alpha(1-4) and less than 10 % alpha(1-6) glucoside bonds. About 4 % solution is administered intraperitoneally, and it functions as a colloid osmotic agent and its colloidal osmotic action allows the retention of fluid as a reservoir within the peritoneal cavity for 3–4 days. Its physical effect provides a temporary separation of peritoneal surfaces by hydroflotation as a result of maintaining a fluid reservoir. Subsequently, it is suggested that it minimizes tissue apposition during the critical period of fibrin formation and mesothelial regeneration following surgery, thereby providing a barrier to adhesion formation [4]. It is well known that icodextrin is absorbed only slowly, and its effect on colloid osmotic pressure creates a constant fluid volume into the abdominal cavity, allowing a protective role against peritoneal adhesion.
Omega-3 fatty acids (ω-3 FAs) are an important nutritional source for human being that have a final carbon–carbon double bond in the n-3 position (third carbon bonds from methyl terminal), and this structural characteristic confers peculiar properties on themselves as compared to other polyunsaturated fatty acids (PUFA), such as ω-6 and ω-9 PUFA. The two most important bioactive ω-3 FAs, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), are generated from an essential precursor molecule, α-linolenic acid [5].
The benefits of dietary ω-3 FAs are attributed to their capacity to regulate specific cellular metabolic functions and gene expression. ω-3 FAs from eicosanoid pathways can be substrates for both cyclooxygenases (COX) and lipooxygenases (LOX) whose activity leads to generation of prostanoid and thromboxanes on one side, and leukotrienes on the other. The products of COX are modulators of the hemostatic, inflammatory, and immune response, whereas the products of LOX have control on vasomotility and vascular extravasation [5]. Besides the altered eicosanoid production, ω-3 FAs in higher doses have anti-inflammatory effects such as the following:
A time-dependent decrease in chemotaxis of human neutrophil and monocyte
Decreased expression of some adhesion molecules on the surface of monocyte, macrophage, or endothelial cells
Decrease in the production of reactive oxygen species
Inhibition of the production of interleukin 1β (IL-1β) and tumor necrosis factor-α (TNF-α) by monocyte and the production of IL-6, IL-8 by venous endothelial cells [6]
We assume that these features of ω-3 FAs can have effects on both postsurgical peritoneal inflammation and formation of collagen matrix from stable fibrin matrix following migration of fibroblasts from capillaries.
There are some studies on the preventive role of icodextrin. However, to the best of our knowledge, no previous study has compared ω-3 FAs with icodextrin in terms of gross macroscopic view, cellular reaction, fibrin formation, and biochemical tissue repair marker in an adhesion formation model. The aim of this experimental study is to compare the macroscopic, histopathological, and biochemical changes after application of icodextrin and ω-3 FAs in a rat adhesion model assessing postoperative peritoneal adhesions.
Materials and Methods
Animals
This study was performed at the Experimental Animal Laboratory in the Medical Faculty of Marmara University by the approval of Animal Ethics Committee. All protocols were in accordance with the regulations concerning the care and use of laboratory animals as in the Declaration of Helsinki.
Twenty-four outbred female Wistar albino rats (mean weight 250 ± 30 g, mean age 7 months) were divided into three groups and kept by applying the 12-hour light/dark cycle with a stable temperature between 19 and 22 °C in standard rat cages, containing maximum 5 rats in each. They were fed with standard rat pellet and tap water.
Operation and Adhesion Model
After a 12-hour starvation, ketamine hydrochloride 40 mg/kg IM and xylazine 10 mg/kg IM were used for anesthesia. Rats were on supine position and their extremities were fixed to the operating table using medical plaster. All operations were made using powder-free, nonlatex gloves in order to prevent anticipated peritoneal adhesions due to foreign body reactions. After abdominal skin shaving, povidone iodine was used for antisepsis.
A midline incision of 3 cm in length was made. The cecum was pulled out of the abdomen, and scrubbed with a sterile toothbrush for five times in order to compose a subserosal hemorrhage on an area equivalent to the toothbrush surface. Thereafter cecum was returned into the normal position.
In the control group (n = 8) 3 ml of saline solution 0.9 % NaCl and in the icodextrin group (n = 8) 3 ml of 4 % icodextrin were instilled intraperitoneally. In the omega-3 group, a mixture of 200 mg/kg of ω-3 FA in 3 ml of saline solution 0.9 % NaCl was prepared under sterile conditions, and then was applied to the peritoneal cavity.
Immediately after the application of protocol material, the wound edges were lifted up by way of four clamps in order to prevent fluid leakage from peritoneal cavity, and then incision was closed with 4/0 polypropylene running sutures. Paracetamol, 100 mg/kg, was injected subcutaneously for analgesia. The rats were allowed to eat normally at 6 h postoperatively.
Macroscopic Assessment
All rats were sacrificed on the 10th postoperative day by giving high-dose (100–150 mg/kg) sodium thiopental. The peritoneal cavity was entered using a “reverse U” incision very carefully to avoid damaging the formed adhesions. Anterior abdominal wall, peritoneal cavity, small bowels, and cecum were examined carefully and assessed according to the staging scale as used by Blauer and Collins [7] (Table 1). After macroscopic evaluation, an ileocecal segment of 2 cm in length and its neighboring mesenteric root of 0.5 cm × 0.5 cm were resected for both histopathologic and biochemical examinations. Sacrificed animals were discarded at Marmara University Experimental Animal Laboratory’s medical waste and the study was finished.
Table 1.
Scoring system used for microscopic evaluation of the inflammatory reactions on serosal surfaces [8]
| SCORE | Macroscopic findings [7] | Cellular reactiona | Fibrosis | ICAM1 and PECAM1 staining |
|---|---|---|---|---|
| 0 | No adhesions | None | None | None |
| 1 | Thin and narrow, easily separable adhesions | Rare | Rare | < 10 % |
| 2 | Thick adhesions limited to one area | Mild | Mild | 11-50 % |
| 3 | Thick and wide adhesions | Severe | Severe | >51 % |
| 4 | Thick and wide adhesions between organs and abdominal wall | Not available | Not available | Not available |
aGiant cell, lymphocyte/plasmocyte, neutrophil, histiocyte reactions
Histopathologic Assessment
Resected adhesion model specimen was fixed in 10 % formalin solution. After dehydration, it was embedded into the paraffin at tissue follow-up equipment. Four slices of 3 μ in thickness were prepared by microtome from each intestinal segment.
As defined by the producers’ standard protocol, the first slice was stained with hematoxylin and eosin for assessing giant cell, lymphocyte/plasmocyte, neutrophil, and histiocyte reaction. The second one was stained with Masom-tricom for fibrosis assessment; the third with CD54 for inter-cellular adhesion molecule 1 (ICAM1), and finally the forth with CD31 for platelet endothelial cell adhesion molecule 1 (PECAM1).
For evaluation of giant cell, lymphocyte/plasmocyte, neutrophil, histiocyte reaction, and fibrosis, a scoring system from 0 to 3 according to criteria defined by Delaco et al. was employed [8]. The other two plates stained with CD54 and CD31 for ICAM1 and PECAM1, respectively, were scored from 0 to 3 (Table 1). Photos were taken for each plate in light microscopy under × 100 and × 200 magnification. The pathologist in the study was blinded during the interpretation of the results.
Biochemical Assessment
Mesenteric tissue samples were put into dry tubes and kept in the biochemistry laboratory for assessment. After collecting all tissues, they were homogenized by 70 ml tissue in 1 ml 0.9 % NaCl solution. Adding equal volume of HCl, homogenized tissues were incubated at 95 °C water bath for 24 h.
Acetate citrate buffer (pH 6.5), chloramines T reactive, and Erlich reactive were prepared freshly as study reactive. Hydroxyproline study standards were prepared. At the end of the study, absorbent values were quantified from samples and standards by using spectrophotometer.
Statistical Analysis
All statistical analyses were performed by a statistical software package (SPSS 16.0). Numerical data were expressed as mean and standard derivation unless otherwise stated. Kruskal–Wallis test was used for statistical analysis of giant cell, lymphocyte/plasmocyte, neutrophil, histiocyte reaction, fibrosis, ICAM1, and PECAM1 scores as their values were nonparametric, and the number of subjects in each group was less than 30. Statistical significance of hydroxyproline levels was assessed by using ANOVA test because all values obtained from three groups were parametric. Results were expressed with a confidence interval of 95 %. The P values below 0.05 were considered statistically significant.
Results
There were no postoperative complications such as bowel obstructions or peritonitis. No mortality was observed in any group during the study. At necropsy assessment, no intraperitoneal fluid was found on day 10.
Macroscopic Assessment
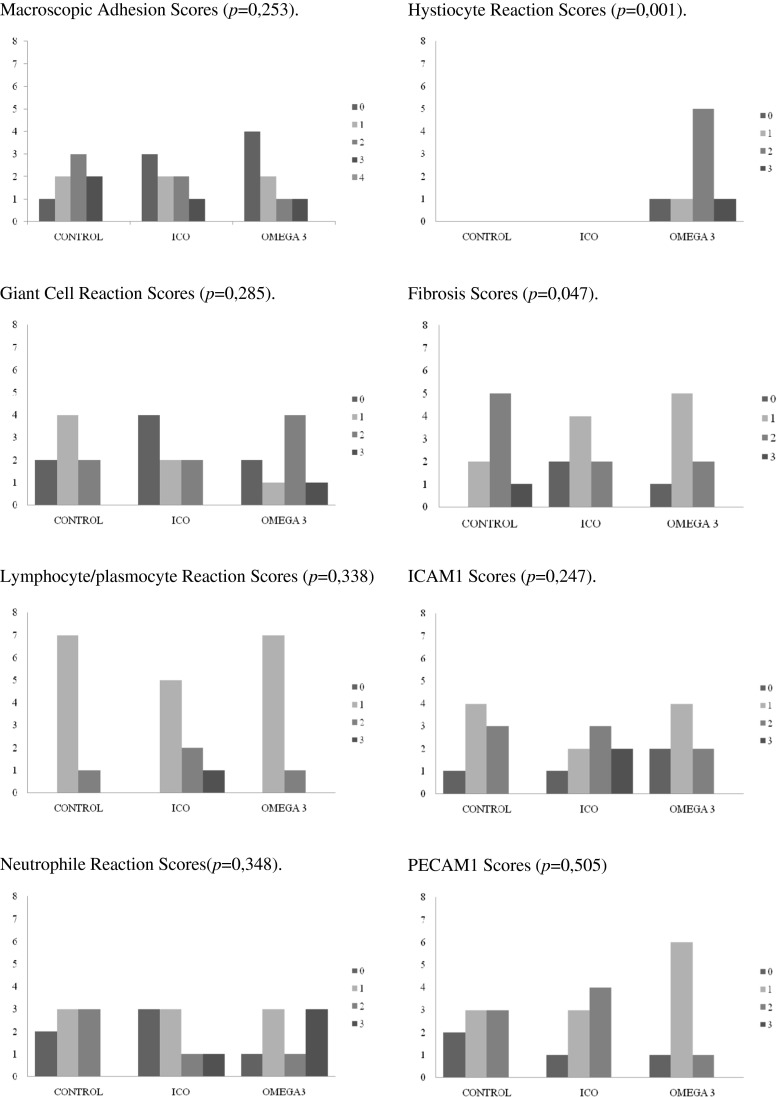
Although there was a tendency toward lower mean scores in icodextrin (1.12 ± 1.13) and ω-3 FA (0.88 ± 1.13) groups compared with the control group (1,75 ± 1,04), the differences were not statistically significant (P = 0.253) (Table 2 and Fig. 1).
Table 2.
Mean scores obtained from gross evaluation and histopathologic and biochemical assessment of each group in the study
| Scores | Control | Icodextrin | Omega 3 | p |
|---|---|---|---|---|
| Macroscopic assessment | 1,75 ± 1,04 | 1,12 ± 1,13 | 0,88 ± 1,13 | 0,253 |
| Giant cell | 1,00 ± 0,76 | 0,75 ± 0,89 | 1,50 ± 1,07 | 0,285 |
| Lymphocyte/plasmocyte | 1,12 ± 0,35 | 1,50 ± 0,76 | 1,12 ± 0,35 | 0,338 |
| Neutrophil | 1,12 ± 0,84 | 1,00 ± 1,11 | 1,75 ± 1,16 | 0,348 |
| Histiocyte | 0,00 ± 0,00 | 0,00 ± 0,00 | 1,75 ± 0,89 | 0,001 |
| Fibrosis | 1,88 ± 0,64 | 1,00 ± 0,76 | 1,12 ± 0,64 | 0,047 |
| ICAM1 | 1,25 ± 0,70 | 1,75 ± 1,04 | 1,00 ± 0,76 | 0,247 |
| PECAM1 | 1,12 ± 0,83 | 1,38 ± 0,74 | 1,00 ± 0,54 | 0,505 |
| Hydroxiproline | 302,65 ± 147,69 | 273,40 ± 118,56 | 148,59 ± 91,89 | 0,044 |
Fig. 1.
Schematic presentation of the results of macroscopic, microscopic and biochemical assessment of study groups. Macroscopic Adhesion Scores (p = 0,253), Giant Cell Reaction Scores (p = 0,285), Lymphocyte/plasmocyte Reaction Scores (p = 0,338), Neutrophile Reaction Scores(p = 0,348), Hystiocyte Reaction Scores (p = 0,001), Fibrosis Scores (p = 0,047), ICAM1 Scores (p = 0,247), PECAM1 Scores (p = 0,505)
Histopathological Assessment
Histopathological assessment of the cellular reaction of icodextrin and ω-3 FA showed that giant cell, lymphocyte/plasmocyte, and neutrophil reaction scores were not significantly different from that of the control group (P = 0.285, 0.338, and 0.348, respectively) (Table 2 and Fig. 1). On the other hand, histiocyte reaction scores were nil in the histopathologic examination of the rats in control and icodextrin groups, while the mean histiocyte reaction score was significantly higher in the ω-3 FA group (1.75 ± 0.89) (P = 0.001). Likewise, comparison of the mean fibrosis scores revealed that icodextrin and ω-3 FA showed a lower tendency to induce fibrosis than the sham group with a slight statistical significance (Table 2 and Fig. 1).
The microscopic evaluation of serosal surfaces was not significantly different among groups in terms of ICAM1 and PECAM expressions, as shown in Table 2.
Biochemical Assessment
A regular fall of mean hydroxyproline levels was detected in control, icodextrin, and ω-3 FA groups as 302.65 ± 147.69, 273.40 ± 118.56, 148.59 ± 91.89, respectively. This was found statistically significant (P = 0.044).
Discussion
Postoperative peritoneal adhesions are important causes of mortality and morbidity affecting 70–93 % of patients who have undergone abdominal surgery for any reason. Moreover, peritoneal adhesion causes infertility and pelvic pain in women [1]. Actually there is no widely agreed consensus on preventing adhesion formation except intraoperative preventive measures such as reducing retained surgical material, meticulous hemostasis, avoiding excessive dissection, and ischemia. Protection of wounded surfaces with various materials had been proposed to overcome this condition, including absorbable patches [9], gels [10], or some liquids such as icodextrin [11]. All three types of adhesion barriers have limited approval by the United States Food and Drug Administration [6, 12].
In our study, we have compared the effects of ω-3 FAs on peritoneal adhesion formation with 4 % icodextrin, another frequently studied peritoneal washing solution [4]. Icodextrin is an iso-osmolar biodegradable glucose polymer solution allowing a prolonged separation of peritoneal surfaces by hydroflotation, and being absorbed slowly. This feature gives the result of its common use for ambulatory peritoneal dialysis.
The effect of ω-3 FA on wound healing, inflammation, hemostasis, and atherosclerosis has been largely investigated by parenteral, enteral, and cutaneous applications. However, intraperitoneal route for these purposes was not previously searched. In a study by McDaniel et al., per os ω-3 FA prolonged wound healing process [13]. In another study, ω-3 FAs have produced weaker tension strength than ω-6 FAs in mice [14]. Mooney et al. stated that ω-3 FA reduced re-epithelization and contraction in dogs [15].
ω-3 FAs are found to act on peritoneal wound healing by activation of the inflammatory cascade via peroxisome proliferator-activated receptors (PPARs) in a study. This activation mediating the lipid metabolism, fatty acid oxidation, and cytokine production can lead the antiadhesive effect of ω-3 FAs by reducing the level of the type 1 collagen, vascular endothelial growth factor, and transforming growth factor β-1 [16].
The only study about the intraperitoneal usage of ω-3 FA aiming the dosage titration for seizure therapy used a dose of 40–200 mg/kg daily. In this study, a dosage from 400 mg/kg to 1,000 mg/kg was found to be toxic [17]. Thus, we suggested that 200 mg/kg of ω-3 FA was suitable as a reliable dose in our study. Further studies are required to establish dose adjustment.
In the microscopic assessment of the rats that were subjected to ω-3 FA, we found lipid deposits on peritoneum. Indeed, pure EPA and/or DHA forms can be slowly absorbed. Peritoneal absorption capacity and its rate are essential for an agent assumed to prevent peritoneal adhesions [8].
Many liquid and gel barriers have had some adverse effects such as edema and leakage from the wound edges out of the abdomen [12], but none of them has been reported in studies regarding to ω-3 FA and 4 % icodextrin.
This study showed that although it was not statistically significant, icodextrin and ω-3 FA administered intraperitoneally caused a slight decrease in peritoneal adhesion after a standardized surgical trauma. In microscopic assessment, all groups had similar foreign body reactions, acute and chronic inflammations, serosal ICAM1 and PECAM1 productions by looking at giant cell, lymphocyte/plasmocyte and PMNL reaction scores, and serosal surface (ICAM1 and PECAM1) scores.
Histiocytes, being a major component of monocyte–phagocytic system and tissue consolidating mature macrophages, are the most important cells of chronic inflammation. Histiocytic reaction was significantly higher in ω-3 FA than in other groups. The effect of ω-3 FA on leukocyte chemotaxis, reactive oxygen derivatives, adhesion molecules, and pro-inflammatory cytokines such as TNF-α, and IL-1, 6, 8, and 10 seemed to be continued on day 10. This suggests that ω-3 FA not only tries to phagocyte foreign bodies as a peritoneal defense mechanism but also activates cellular anti-inflammatory reaction into the peritoneal surfaces, and thus allows prevention of adhesion formation and incorrect tissue repairing. This feature has not been shown in 4 % icodextrin which revealed an identical result with the sham group, anticipating its probable physiological role with a different way—“hydroflotation” theory keeping anti-inflammatory cells from adhering to the peritoneal surfaces. Whatever the pharmacokinetic characteristics, the current study indicates that both ω-3 FA and icodextrin have significantly reduced fibrosis formation.
Meanwhile cellular reactions, such as giant cells, mononuclear, and polymorph nuclear leukocytes, did not differ in all groups. This may be commented as the neutral effect of both antiadhesion materials on normal wound healing. On the other hand, histiocytic reaction was very prominent in rats in the ω-3 FA group, whereas it was nil in the other two groups, suggesting that tissue macrophages were also contributed to the inflammatory process in animals under ω-3 FA treatment.
In a study by Victory et al., it was demonstrated that DHA specifically and significantly reduced markers in both normal peritoneal cells and adhesion fibroblasts. This decrease in extracellular matrix deposition can lead to reduce tissue strength and have notorious impacts on adequate wound healing as well as anastomotic integrity [16]. Meanwhile, the fact that VEGF mRNA levels in DHA-treated fibroblasts was lower compared to those in normal peritoneal fibroblasts can be commented as ω-3 FAs do not reduce the appropriate tissue healing while they have a great potential for adhesion formation. Owing to the lack of evidence whether this effect is beneficial for clinical wound or anastomotic healing, the possibility of hazardous events cannot be excluded and more experimental studies disrupting not only the peritoneal surfaces as in our study but also the bowel integrity are required.
Hydroxyproline is the basic amino acid of collagen structure. In a study carried out on cutaneous wounds, Cardoso et al. showed that ω-3 FA caused thin, low-tensile strength as a result of decrease in the hydroxyproline levels [14]. The fact that ω-3 FA inhibits pro-inflammatory cytokines like IL-10 which regulates collagen synthesis might be a cause of the decrease in hydroxyproline levels, which could not be seen in icodextrin as it has no effect on inflammation and pro-inflammatory cytokines.
In conclusion, this current study shows that ω-3 FA can be assessed as a novel agent of great value, especially in the light of its effects on decrease in chronic inflammation, and positive cellular and molecular findings in preventing adhesion formation. Further investigations are required in order to detect safer and the most effective dose of ω-3 FA, while keeping the meticulous dissection techniques always in mind.
References
- 1.Menzies D. Postoperative adhesions: their treatment and relevance in clinical practice. Ann R Coll Surg Engl. 1993;75:147–153. [PMC free article] [PubMed] [Google Scholar]
- 2.Ellis H, Moran B, Thompson J, et al. Adhesion-related hospital readmissions after abdominal and pelvic surgery: a retrospective cohort study. Lancet. 1999;353:1476–1480. doi: 10.1016/S0140-6736(98)09337-4. [DOI] [PubMed] [Google Scholar]
- 3.Boland GM, Weigel RJ. Formation and prevention of postoperative abdominal adhesions. J Surg Res. 2006;132:3–12. doi: 10.1016/j.jss.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 4.FDA (2006) Adept Adhesion Reduction Solution (4 % Icodextrin) P050011 in United States. FDA. Available at http://www.accessdata.fda.gov/cdrh_docs/pdf5/p050011a.pdf
- 5.Colussi G, Catena C, Baroselli S, Nadalini E, Lapenna R, et al. Omega 3 fatty acids: from biochemistry to their clinical use in the prevention of cardiovascular disease. Recent Pat Cardiovasc Drug Discov. 2007;2:13–21. doi: 10.2174/157489007779606158. [DOI] [PubMed] [Google Scholar]
- 6.Calder PC. n-3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83:1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 7.Blauer KL, Collins RL. The effect of intra peritoneal progesterone on postoperative adhesion formation in rabbit. Fertil Steril. 1988;49:144–149. [PubMed] [Google Scholar]
- 8.Delaco PA, Stefanetti M, Pressato D, Piana S, Dona M, et al. A novel hyaluronan-based gel in laparoscopic adhesion prevention: preclinical evaluation in an animal model. Fertil Steril. 1998;69:318–323. doi: 10.1016/S0015-0282(98)00496-8. [DOI] [PubMed] [Google Scholar]
- 9.Vrijlan WW, Tseng LN, Eijkman HJ, Hop WC, Jakimovicz JJ, et al. Fewer intraperitoneal adhesions with use of hyaluronic acid-carboxymethylcellulose membrane: a randomized clinical trial. Ann Surg. 2002;235:193–199. doi: 10.1097/00000658-200202000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.diZerega GS, Coad J, Donnez J. Clinical evaluation of endometriosis and differential response to surgical therapy with and without application of Oxiplex/AP* adhesion barrier gel. Fertil Steril. 2007;87:485–489. doi: 10.1016/j.fertnstert.2006.07.1505. [DOI] [PubMed] [Google Scholar]
- 11.diZerega GS, Verco SJ, Young P, Kettel M, Kobak W, et al. A randomized, controlled pilot study of the safety and efficacy of %4 icodextrin solution in the reduction of adhesions following laparoscopic gynaecological surgery. Hum Reprod. 2002;17:1031–1038. doi: 10.1093/humrep/17.4.1031. [DOI] [PubMed] [Google Scholar]
- 12.Ward BC, Panitch A. Abdominal adhesions: current and novel therapies. J Surg Res. 2011;165:91–111. doi: 10.1016/j.jss.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 13.McDaniel JC, Belury M, Ahijevych K, Blakely W. Omega-3 fatty acids effect on wound healing. Wound Repair Regen. 2008;16:337–345. doi: 10.1111/j.1524-475X.2008.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cardoso CR, Souza MA, Ferro EA, Favoreto S, Jr, Pena JD. Influence of topical administration of n-3 and n-6 essential and n-9 nonessential fatty acids on healing of cutaneous wounds. Wound Repair Regen. 2008;12:235–243. doi: 10.1111/j.1067-1927.2004.012216.x. [DOI] [PubMed] [Google Scholar]
- 15.Mooney MA, Vaughn DM, Reinhart GA, Powers RD, Wright JC, et al. Evaluation of the effects of omega-3 fatty acid-containing diets on the inflammatory stage of wound healing in dogs. Am J Vet Res. 1998;59:859–863. [PubMed] [Google Scholar]
- 16.Victory R, Saed GM, Diamond MP. Antiadhesion effects of docosahexaenoic acid on normal human peritoneal and adhesion fibroblasts. Fertil Steril. 2007;88(6):1657–1662. doi: 10.1016/j.fertnstert.2007.01.123. [DOI] [PubMed] [Google Scholar]
- 17.Taha AY, Filo E, Ma DW, McIntyre Burnham W. Dose-dependent anticonvulsant effects of linoleic and alpha-linolenic polyunsaturated fatty acids on pentylenetetrazol induced seizures in rats. Epilepsia. 2009;50:72–82. doi: 10.1111/j.1528-1167.2008.01731.x. [DOI] [PubMed] [Google Scholar]