Abstract
Context
Controversies exist in the adult literature regarding the use of kidneys from small donors into larger recipients. Little is known regarding this issue in pediatric kidney transplantation.
Objective
To assess the impact of donor/recipient size mismatch on long-term renal graft survival in pediatric patients undergoing living donor renal transplantation.
Study Design
We reviewed the United Network for Organ Sharing database from 1987 through 2010 for adolescent (11-18 years old) patients who underwent primary living donor renal transplantation. According to donor/recipient body surface area (BSA) ratio, patients were stratified into 2 categories: BSA ratio <0.9 and BSA ratio ≥0.9. Graft survival rates were compared between these 2 groups using Kaplan-Meier survival curves and Cox proportional hazards models.
Results
Of the 1880 patients identified, 116 (6.2%) had a donor/recipient BSA ratio <0.9 and 1764 (93.8%) had a donor/recipient BSA ratio ≥0.9 group. BSA ratio of <0.9 conferred an increased risk of graft loss (adjusted hazard ratio [HR] 1.61, 95% CI 1.13-2.27, p=0.008). Patients with a donor/recipient BSA ratio ≥0.9 group had a significantly longer graft survival compared to those with a donor/recipient BSA ratio <0.9, after adjustment for: donor age and gender, recipient age, gender, ethnicity, cause of renal failure as well as clinical factors: cold and warm ischemia time and HLA mismatch.
Conclusion
We conclude that low donor/recipient BSA ratio was associated with an increased risk of graft loss. Appropriate size matching confered better long-term graft survival in adolescents receiving live donor kidney transplants.
Keywords: Body Surface Area, Renal Transplantation, Pediatrics and Living donor
Introduction
Kidney transplantation is the treatment of choice for children with end stage renal disease (ESRD) due to the benefits to growth and development. Life expectancy for renal transplant recipients far exceeds those who remain on dialysis.[1] Living donor kidney transplantation offers numerous significant advantages including shorter waiting times for recipients, better quality kidneys with reduced delayed graft function, decrease hospital costs, and the opportunity for pre-emptive transplant. This translates into better long-term patient and graft survival.[2, 3] Pediatric patients with end stage renal disease (ESRD) may suffer from developmental delays, growth retardation, bone loss and higher rates of hospitalizations for catheter-associated infections.[4] Living donor renal transplantation can mitigate these side effects.
Within the adult kidney transplant literature, controversies exist regarding the impact on kidney transplant allograft survival when transplanting kidneys from small donors into larger recipients.[5, 6] Multiple studies have suggested that “small for size” renal transplants leads to poor allograft function,[7, 8] a phenomenon that has been well described in the liver transplant literature.[9] It is proposed that nephron under dosing leads to hypertrophy and hyper-filtration induced injury in the transplanted allograft.[10, 11] A larger dose of functional nephron mass has been associated with better long-term graft survival in transplant recipients.[8, 12]
Body surface area (BSA) has been validated as a surrogate marker of nephron mass. [13] The aim of this study is to evaluate the impact of donor/recipient BSA ratio on allograft survival in adolescents undergoing living donor kidney transplantation.
Results
In the United States from 1987 – 2010 there were 1880 pediatric patients that underwent primary living donor only kidney transplantation as demonstrated by the UNOS STAR files. Donor demographics are shown in Table 1. There were 1764 patients in the donor/recipient BSA ratio ≥ 0.9 and 116 in the donor/recipient BSA ratio < 0.9 group that had complete data. Comparing the 2 BSA ratio groups there were no significant differences in donor age or ethnicity of the donor. Donor/recipient pairs with BSA ratio < 0.9 had statistically significant more female donors (p <0.001). Table 2 demonstrates recipient characteristic. There were no significant differences in ethnicity of the recipients between groups. However, recipients were significantly older and more likely to be male in the group with BSA < 0.9 (p <0.001). The cause of renal failure was similar in both groups and majority of the recipients in both groups were on dialysis prior to transplant. Clinical characteristics were similar between groups Table 3. Delayed graft function occurred in less than 4% in both groups.
Table 1. Donor demographics.
| Donor Characteristics | BSA ≥ 0.9 | BSA ≤ 0.9 | P-Value |
|---|---|---|---|
| Age (years) | 39.06±8.90 | 39.01±8.60 | 0.953 |
| Gender (males) % | 44.8 | 15.5 | <0.001 |
| Ethnicity % | 0.774 | ||
| White | 68.4 | 65.5 | |
| Black | 9.5 | 13.8 | |
| Hispanic | 19.1 | 19.0 | |
| Asian | 2.0 | 1.7 | |
| American Indian | 0.3 | 0.0 | |
| Native Hawaiian | 0.2 | 0.0 | |
| Multiracial | 0.4 | 0.0 |
Table 2. Recipient Demographics.
| Recipient Chracteristics | BSA ≥ 0.9 | BSA ≤ 0.9 | P-Value |
|---|---|---|---|
| Age (years) | 15.31±1.93 | 16.67±1.40 | <0.001 |
| Gender (males) % | 56.3 | 78.4 | <0.001 |
| Ethnicity % | 0.361 | ||
| White | 67.3 | 57.8 | |
| Black | 10.4 | 15.5 | |
| Hspanic | 18.8 | 22.4 | |
| Asian | 2.2 | 2.6 | |
| American Indian | 0.5 | 0.0 | |
| Native Hawaiian | 0.3 | 0.9 | |
| Multiracial | 0.6 | 0.9 | |
| Cause of Renal Failure % | 0.057 | ||
| Cystic/Hereditary/ Congenital | 42.4 | 36.1 | |
| Glomerulonephritis (GN) | 28.4 | 44.6 | |
| Secondary GN/Vasculitis | 9.5 | 9.6 | |
| Interstitial Nephritis/Pyelonephritis | 10.2 | 6.0 | |
| Miscellaneous | 6.8 | 2.4 | |
| Neoplasms/Tumors | 0.8 | 0.0 | |
| Diabetes | 0.5 | 1.2 | |
| Hypertension | 1.3 | 0.0 | |
| Dialysis (%) | 0.473 | ||
| Unknown | 0.9 | 0.0 | |
| No | 37.4 | 34.5 | |
| Yes | 61.7 | 65.5 |
Table 3. Clinical Characteristics.
| BSA ≥ 0.9 | BSA < 0.9 | P-Value | |
|---|---|---|---|
| Cold Ischemia time (hours) | 2.28±5.08 | 1.99±4.89 | 0.617 |
| Warm Ischemia time (mins) | 39.70±23.97 | 43.31±41.96 | 0.323 |
| PRA (<50%) | 2.1 | 0.0 | 0.886 |
| Delayed Graft Function (%) | 0.848 | ||
| No | 96.2 | 96.6 | |
| Yes | 3.8 | 3.4 | |
| HLA Mismatches (%) | 0.55 | ||
| 0 or 1 | 14.2 | 13.3 | |
| 2, 3 or 4 | 75.8 | 73.5 | |
| 5 or 6 | 10.1 | 13.3 |
In an unadjusted analysis (Table 4) we estimated a hazard ratio of 1.79 (95% CI [1.29, 2.50]; p=0.001) comparing donor/recipient pairs with BSA ratio <0.9 to pairs with BSA ratio ≥0.9 (Table 4). Table 5 shows the model adjusted for age and gender of donors and recipients as well as recipient ethnicity, delayed graft function, dialysis before transplant and HLA mismatch. Donor/recipient BSA ratio < 0.9 was associated with an increased risk of graft failure, adjusted hazard ratio was 1.62 (95% CI[1.14,2.29]; p=0.007). Recipient age, recipient ethnicity, delayed graft function, dialysis before transplant and HLA mismatch of 5 or 6 were significantly associated with graft failure.
Table 4. Unadjusted model.
| Variable | HR (95% CI) | P-Value |
|---|---|---|
| BSA < 0.9 | 1.79 (1.29, 2.50) | 0.001 |
| Donor → Recipient Gender (reference female → female) | ||
| Male → female | 1.00 (0.74, 1.34) | 0.974 |
| Male → male | 0.88 (0.67, 1.14) | 0.331 |
| Female → male | 0.96 (0.74, 1.24) | 0.756 |
| Recipient age | 1.10 (1.05, 1.15) | <0.001 |
| Donor age | 0.99 (0.98, 1.01) | 0.315 |
| Recipient Ethnicity (reference white) | ||
| Black | 1.97 (1.53, 2.54) | <0.001 |
| Hispanic | 1.00 (0.76, 1.32) | 0.987 |
| Other | 0.70 (0.36, 1.36) | 0.297 |
| Dialysis the first week post transplant | 4.60 (3.24, 6.55) | <0.001 |
| Human Leukocyte Antigen Mismatch (reference 0 or 1) | ||
| 2→4 | 1.32 (0.97, 1.80) | 0.072 |
| 5→6 | 1.74 (1.17, 2.60) | 0.007 |
| Warm Ischemia time | 1.00 (1.00, 1.01) | 0.038 |
| Cold Ischemia time | 1.01 (0.99, 1.03) | 0.438 |
Table 5. Adjusted Model.
| Variable | HR (95% CI) | P-Value |
|---|---|---|
| BSA < 0.9 | 1.61 (1.13, 2.27) | 0.008 |
| Donor → Recipient Gender (reference female → female) | ||
| Male → female | 1.07 (0.79, 1.44) | 0.672 |
| Male → male | 0.84 (0.64, 1.10) | 0.216 |
| Female → male | 0.95 (0.73, 1.23) | 0.690 |
| Recipient age | 1.11 (1.05, 1.17) | <0.001 |
| Donor age | 1.00 (0.99, 1.01) | 0.716 |
| Recipient Ethnicity (reference white) | ||
| Black | 1.87 (1.43, 2.44) | <0.001 |
| Hispanic | 1.03 (0.78, 1.36) | 0.850 |
| Other | 0.77 (0.39, 1.49) | 0.433 |
| Dialysis the first week post transplant | 4.69 (3.27, 6.70) | <0.001 |
| Human Leukocyte Antigen Mismatch (reference 0 or 1) | ||
| 2→4 | 1.30 (0.95, 1.77) | 0.103 |
| 5→6 | 1.61 (1.08, 2.41) | 0.02 |
| *Warm Ischemia time | 1.00 (1.00, 1.01) | 0.038 |
| *Cold Ischemia time | 1.01 (0.99, 1.03) | 0.438 |
Discussion
This national study is the largest thus far to report on the impact of donor/recipient BSA ratio mismatch on living donor kidney transplant allograft function in adolescents. We demonstrated that donor/recipient pairs with a BSA ratio < 0.9 (smaller kidney into a larger recipient) had a increased risk of graft loss and poorer long term graft survival compared to donor/recipient pairs with a BSA ratio ≥ 0.9. Previous adult studies have shown conflicting results on the impact of using grafts from smaller donors into larger recipients in deceased and living donor kidney transplantation. [8, 14] Giral et al demonstrated from their multicenter trial that low kidney to recipient weight ratio in adult transplantation (KwRw <2.3g/kg) was an independent risk factor for post transplant graft loss by 2 years of follow up.[8] This group of patients, KwRw <2.3g/kg, also had significantly increased proteinuria at 1 year post transplant, more anti-hypertensive drug use and glomerulosclerosis compared to those with KwRw ≥2.3g/kg. According to the hyperfiltration hypothesis, when nephron mass is reduced, such as in transplanting a small size kidney into a larger recipient, the transplanted kidney is not able to adequately compensate for the metabolic demands of the individual.[10] This leads to hypertrophy, sclerosis and decreased long-term survival.
In our analysis donor gender was not a significant predictor of graft survival. Previous studies have had inconsistent results as to the role of donor gender in kidney transplant graft survival. However, one consistent conclusion is that inappropriate nephron dosing leads to worst graft survival..[15] Zeier et al retrospectively analyzed the Collaborative Transplant Study (CTS) database and, after adjusting for predictors of graft failure such as ethnicity, donor and recipient age, HLA mismatch and cold ischemia times, demonstrated that recipients of female kidneys had increase risk of graft loss. They postulated that inaddition to “nephron underdosing” other mechanisms such as immunogenicity may also play a role. McGee and colleagues retrospectively reviewed the UNOS database and suggested that immunologic and non-immunologic factors may play a role in inferior graft survival in females receiving male donor kidneys. [16] They also hypothesized that inferior graft survival could possibly be mitigated by transplanting a larger kidney into a smaller sized recipient ie increasing nephron mass. Both immunologic and non-immunologic factors have been suggested as possible mechanisms. Animal studies have suggested that female kidneys express more HLA antigens and are more antigenic. This increased antigenicity may lead to more rejection episodes and consequently poorer graft survival. The majority of the adolescent recipients in our study were males who received kidneys from female donors. In our analysis we did not assess rejection rates in order to corroborate the immunologic role gender played in worst graft survival. What we did demonstrate, as did the prior studies is that inadequate nephron mass, by transplanting a smaller donor organ into larger recipients would lead to poorer long-term graft survival.
Disparities in access to healthcare for effective surgical procedures for ethnic minorities are well described in the literature and this is thought to be a contributing factor to the poor outcomes reported in these populations.[17-19] It is well known that there is inferior post transplant patient and graft survival for African Americans undergoing renal transplantation.[20, 21] In our analysis black or African American ethnicity was an independent risk factor for graft loss. Omoloja et al recently reported on the impact of racial differences in renal allograft survival in the pediatric population.[22] They reviewed the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) database from 1987-2005 and demonstrated that blacks were less likely to be living donor recipients and were more likely to be maintained on dialysis longer prior to transplantation. African Americans were also more likely to have higher rates of delayed graft function and after adjusting for multiple factors blacks were 1.65 times more likely of graft failure compared to whites. [22] The current study found that even after adjustment for gender discrepancies, HLA mismatch, PRA, BSA and delayed graft function, black transplant recipients still had a 1.9-fold greater risk of graft loss, compared to whites. Further research needs to be conducted in order to investigate the reasons for this disparity. Despite the lower long-term graft survival However, compared with deceased donor renal transplantation living donor allografts still offer better long-term survival for all patients regardless of race/ethnicity.
The current study also found that HLA mismatch remained a potent risk factor for increased graft loss. Historically higher numbers of HLA mismatch have been associated with lower graft survival rates.[23] This is similar to the collaborative transplant study that demonstrated recipients of deceased donor renal transplants from identical donors, matched HLA chromosomes, had the best patient and graft survival compared to recipients of 1-haplotype matches. Patients who had complete mismatches had the worst survival.[24] Lee et al recently reported on the impact of five or six HLA mismatch on outcomes of patients undergoing living donor kidney transplantation. They reviewed 2687 living donor kidney transplants between 1984-2010 and found that recipients with 5-6 HLA mismatches had no difference in 5 year graft survival compared to those who had one-haplotype mismatch or well matched living donor kidney transplants. One plausible explanation for these dissimilar outcomes could be the improvements in immunosuppressive regimens over the last two decades.
The current study has several limitations that should be noted. Although this is one of the largest retrospective multicenter analysis of the UNOS STAR files, missing data on donors and recipients could have lead to unmeasured confounding. Our study was limited to only adolescents receiving living donor renal transplantation, which lacks generalization to the rest of pediatric population, including those < 11 years of age or those undergoing deceased donor renal transplantation. One strength of this study is that it proposes the use of a new measure that may be a predictor of graft outcome that could be incorporated into the clinical decision making process of transplant clinicians when allocating living donor organs in order to maximize outcomes. Another potential strength from our study is that it demonstrated that majority of the small for size donors were women and this could potentially increase awareness for donation rate from the male gender. National donor exchange programs should also be considered as an alternative, if appropriate sized donor is not available. Similar studies are needed to assess the impact of donor/recipient size mismatch in pediatric patients (≤ 11years old) undergoing living donor renal transplantation or pediatric patients (1-18 years old) undergoing deceased donor renal transplantation.
In conclusion, the current study found that donor/recipient body surface area ratio mismatch was associated with poorer long-term graft survival in adolescents undergoing living donor kidney transplantation. Despite the lower long-term graft survival in this population, living donor allografts offered superior outcomes compared to deceased donor allografts. Appropriate body surface area match may confer improved long-term kidney transplant survival among adolescents who receive a live donor kidney transplant.
Patients and Methods
After approval by Seattle Children's Hospital institutional review board, we conducted a retrospective cohort study of the United Network for Organ Sharing (UNOS) Standard Transplant Analysis and Research (STAR) files of all adolescents (11-18 years old) receiving primary living donor renal transplants from 1987 through 2010. Data were analyzed to assess the impact of donor/recipient body surface area (BSA) ratio on graft survival. The BSA ratio was calculated using the Mostellar formula: BSA (m2) =√ [weight (kg) × height (cm)/3600]. BSA ratios were calculated by dividing the recipient's BSA by the donor's BSA. Patients were stratified into two groups: low BSA (BSA < 0.9) and normal BSA (BSA ≥ 0.9). Heights and weights were obtained from the UNOS Transplant Candidate Registration Form (TCR). Patients without heights and or weights in the TCR were excluded from our study.
Donor data variables extracted from the STAR file included age, sex, ethnicity (black, white, Asian, Hispanic and other), height and weight. Recipient factors at the time of transplant that were included age, sex, ethnicity, height, weight, whether or not they were on dialysis and cause of renal failure: cystic/hereditary/congenital, secondary glomerulonephritis/vasculitis, interstitial nephritis/pyelonephritis, miscellaneous, neoplasms/tumors, diabetes and hypertension. Clinical factors included cold ischemia time in hours (time period from the excision of the donor kidney and placement on ice until the removal from ice at transplantation), warm ischemia time in minutes (time period from the removal of the donor kidney from ice at transplantation until reestablishment of blood flow), delayed graft function (defined as dialysis within the first week of transplant), human leukocyte antigen (HLA) mismatches and panel reactive antibody (PRA).
Statistical Analysis
Analyses were conducted with the use of STATA version 11.0 (College Station, Texas). Chi-square tests and t-tests were used to compare demographic and clinical characteristics between high and low BSA donor/recipient ratio groups (<0.9 vs. ≥0.9). Hazard ratios (HRs) from multivariable Cox proportional hazards models were used to estimate the relative risks (RRs) associated with low BSA ratio. The proportional hazards assumption was assessed for predictors of interest and the adjustment covariates using the Schoenfeld test. In addition, we examined Kaplan-Meier plots to look for inconsistent effects over time. No meaningful violation of the proportional hazards assumption was observed. The primary multivariable model was adjusted for recipient age, donor age, gender of donor and recipient (male to female, male to male, female to male, female to female), recipient ethnicity (white, black, Hispanic, other), dialysis within a week of surgery, HLA mismatch (0 or 1, 2-4, 5 or 6), cold ischemic time, and warm ischemic time. In the Cox proportional hazards models categorical measures were modeled with the use of indicator variables and continuous measures were modeled linearly per unit.
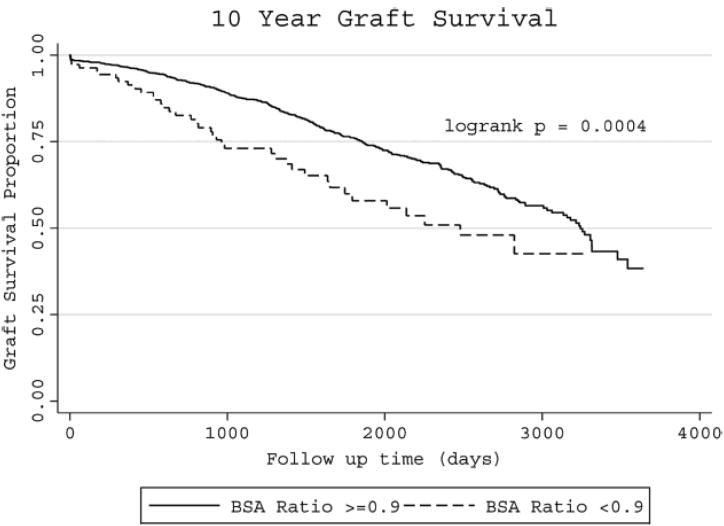
Figure 1.
Ten-year Kaplan–Meier survival estimates and results of log-rank test
Acknowledgments
None
Financial Support: This publication was funded by Seattle Children's Center for Clinical and Translational Research Faculty Research Support Fund. The Seattle Children's Core for Biomedical Statistics is supported by the Center for Clinical and Translational Research at Seattle Children's Research Institute and grant UL1RR025014 from the NIH National Center for Research Resources.
Young is supported by the VA Puget Sound and by NIH DK079745.
Footnotes
Financial disclosure: None
André Dick, the principal author, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Dick, Mercer, Young, Healey
Acquisition of data, analysis and interpretation: Dick, Mercer
Drafting of the manuscript: Dick, Mercer, Smith, McDonald, Young, Healey
Critical revision of the manuscript: Dick, Mercer, Smith, McDonald, Young, Healey
Obtained funding: Dick
Contributor Information
Laina D. Mercer, Email: mercel@uw.edu.
Jodi M. Smith, Email: Jodi.smith@seattlechildrens.org.
Ruth A. McDonald, Email: ruth.mcdonald@seattlechildrens.org.
Bessie Young, Email: youngb@uw.edu.
Patrick J. Healey, Email: Patrick.healey@seattlechilrens.org.
References
- 1.Levey AS, Danovitch G, Hou S. Living donor kidney transplantation in the United States--looking back, looking forward. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2011;58(3):343–8. doi: 10.1053/j.ajkd.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 2.Axelrod DA, et al. Kidney and pancreas transplantation in the United States, 1999-2008: the changing face of living donation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10(4 Pt 2):987–1002. doi: 10.1111/j.1600-6143.2010.03022.x. [DOI] [PubMed] [Google Scholar]
- 3.Hardy BE, et al. Kidney transplantation in children and adolescents: an analysis of United Network for Organ Sharing Database. Transplantation proceedings. 2009;41(5):1533–5. doi: 10.1016/j.transproceed.2009.01.102. [DOI] [PubMed] [Google Scholar]
- 4.Collins AJ, et al. US Renal Data System 2010 Annual Data Report. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2011;57(1 Suppl 1):A8, e1–526. doi: 10.1053/j.ajkd.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Halldorson JB, et al. Donor-recipient size matching influences early but not late graft function after pediatric en-bloc kidney transplantation. Transplantation. 2010;89(2):208–14. doi: 10.1097/TP.0b013e3181c3c17e. [DOI] [PubMed] [Google Scholar]
- 6.Kim YS, et al. Ratio of donor kidney weight to recipient bodyweight as an index of graft function. Lancet. 2001;357(9263):1180–1. doi: 10.1016/S0140-6736(00)04377-4. [DOI] [PubMed] [Google Scholar]
- 7.Poggio ED, et al. Donor kidney volume and outcomes following live donor kidney transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6(3):616–24. doi: 10.1111/j.1600-6143.2005.01225.x. [DOI] [PubMed] [Google Scholar]
- 8.Giral M, et al. Kidney and recipient weight incompatibility reduces long-term graft survival. Journal of the American Society of Nephrology : JASN. 2010;21(6):1022–9. doi: 10.1681/ASN.2009121296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahm F, Georgiev P, Clavien PA. Small-for-size syndrome after partial liver transplantation: definition, mechanisms of disease and clinical implications. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005;5(11):2605–10. doi: 10.1111/j.1600-6143.2005.01081.x. [DOI] [PubMed] [Google Scholar]
- 10.Brenner BM, Milford EL. Nephron underdosing: a programmed cause of chronic renal allograft failure. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1993;21(5 Suppl 2):66–72. doi: 10.1016/0272-6386(93)70097-i. [DOI] [PubMed] [Google Scholar]
- 11.Terasaki PI, et al. The hyperfiltration hypothesis in human renal transplantation. Transplantation. 1994;57(10):1450–4. [PubMed] [Google Scholar]
- 12.McGee J, et al. Donor-recipient gender and size mismatch affects graft success after kidney transplantation. Journal of the American College of Surgeons. 2010;210(5):718–725. e1, 725–6. doi: 10.1016/j.jamcollsurg.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan JC, et al. Validity of surrogate measures for functional nephron mass. Transplantation. 2011;92(12):1335–41. doi: 10.1097/TP.0b013e31823705ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tent H, et al. Donor kidney adapts to body dimensions of recipient: no influence of donor gender on renal function after transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(10):2173–80. doi: 10.1111/j.1600-6143.2011.03687.x. [DOI] [PubMed] [Google Scholar]
- 15.Zeier M, et al. The effect of donor gender on graft survival. Journal of the American Society of Nephrology : JASN. 2002;13(10):2570–6. doi: 10.1097/01.asn.0000030078.74889.69. [DOI] [PubMed] [Google Scholar]
- 16.Vereerstraeten P, et al. Male recipients of kidneys from female donors are at increased risk of graft loss from both rejection and technical failure. Clinical transplantation. 1999;13(2):181–6. doi: 10.1034/j.1399-0012.1999.130205.x. [DOI] [PubMed] [Google Scholar]
- 17.Epstein AM, et al. Racial disparities in access to renal transplantation--clinically appropriate or due to underuse or overuse? The New England journal of medicine. 2000;343(21):1537–44. doi: 10.1056/NEJM200011233432106. 2 p preceding 1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider EC, et al. Racial differences in cardiac revascularization rates: does “overuse” explain higher rates among white patients? Annals of internal medicine. 2001;135(5):328–37. doi: 10.7326/0003-4819-135-5-200109040-00009. [DOI] [PubMed] [Google Scholar]
- 19.Higgins RS, Fishman JA. Disparities in solid organ transplantation for ethnic minorities: facts and solutions. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6(11):2556–62. doi: 10.1111/j.1600-6143.2006.01514.x. [DOI] [PubMed] [Google Scholar]
- 20.Fan PY, et al. Access and outcomes among minority transplant patients, 1999-2008, with a focus on determinants of kidney graft survival. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10(4 Pt 2):1090–107. doi: 10.1111/j.1600-6143.2009.03009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young CJ, Kew C. Health disparities in transplantation: focus on the complexity and challenge of renal transplantation in African Americans. The Medical clinics of North America. 2005;89(5):1003–31. ix. doi: 10.1016/j.mcna.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Omoloja A, et al. Racial differences in graft survival: a report from the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) Clinical journal of the American Society of Nephrology : CJASN. 2007;2(3):524–8. doi: 10.2215/CJN.03100906. [DOI] [PubMed] [Google Scholar]
- 23.Takiff H, et al. Dominant effect of histocompatibility on ten-year kidney transplant survival. Transplantation. 1988;45(2):410–5. doi: 10.1097/00007890-198802000-00033. [DOI] [PubMed] [Google Scholar]
- 24.Opelz G, et al. HLA compatibility and organ transplant survival. Collaborative Transplant Study. Reviews in immunogenetics. 1999;1(3):334–42. [PubMed] [Google Scholar]