Abstract
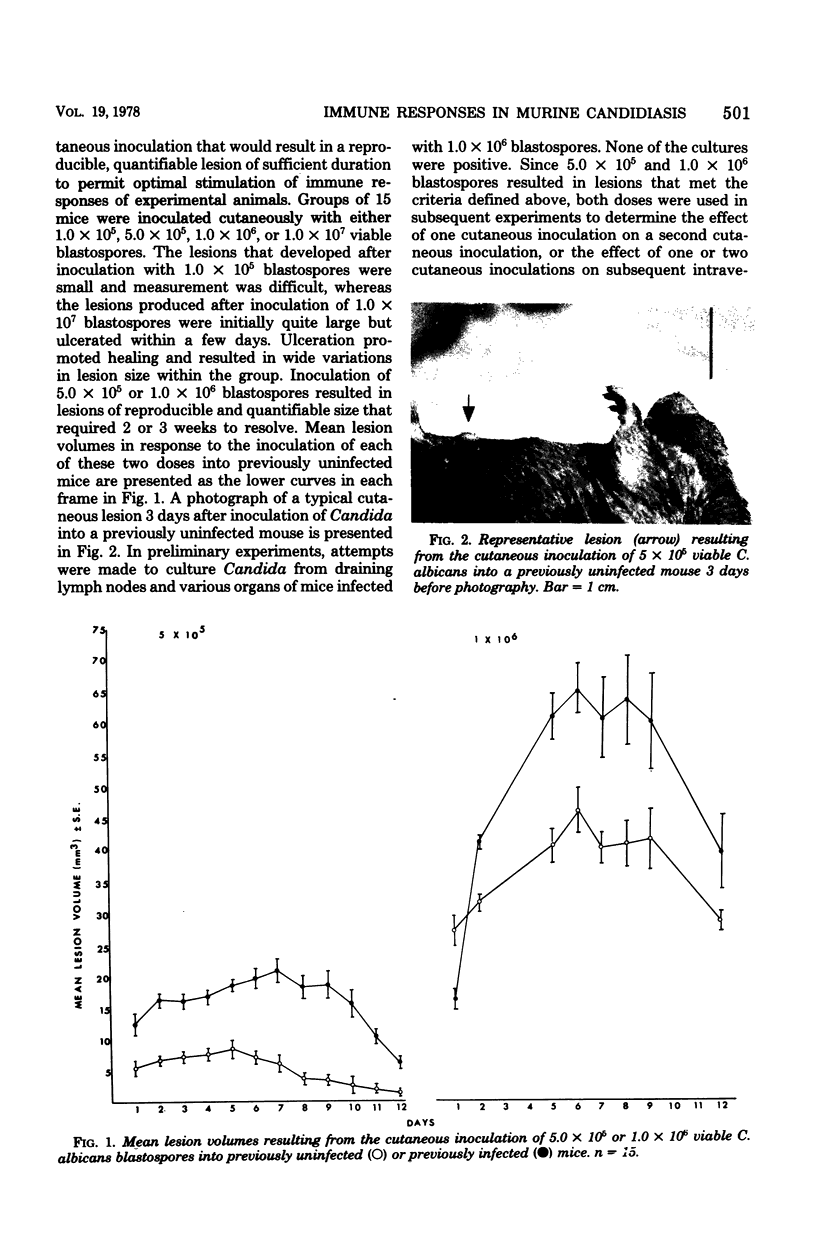
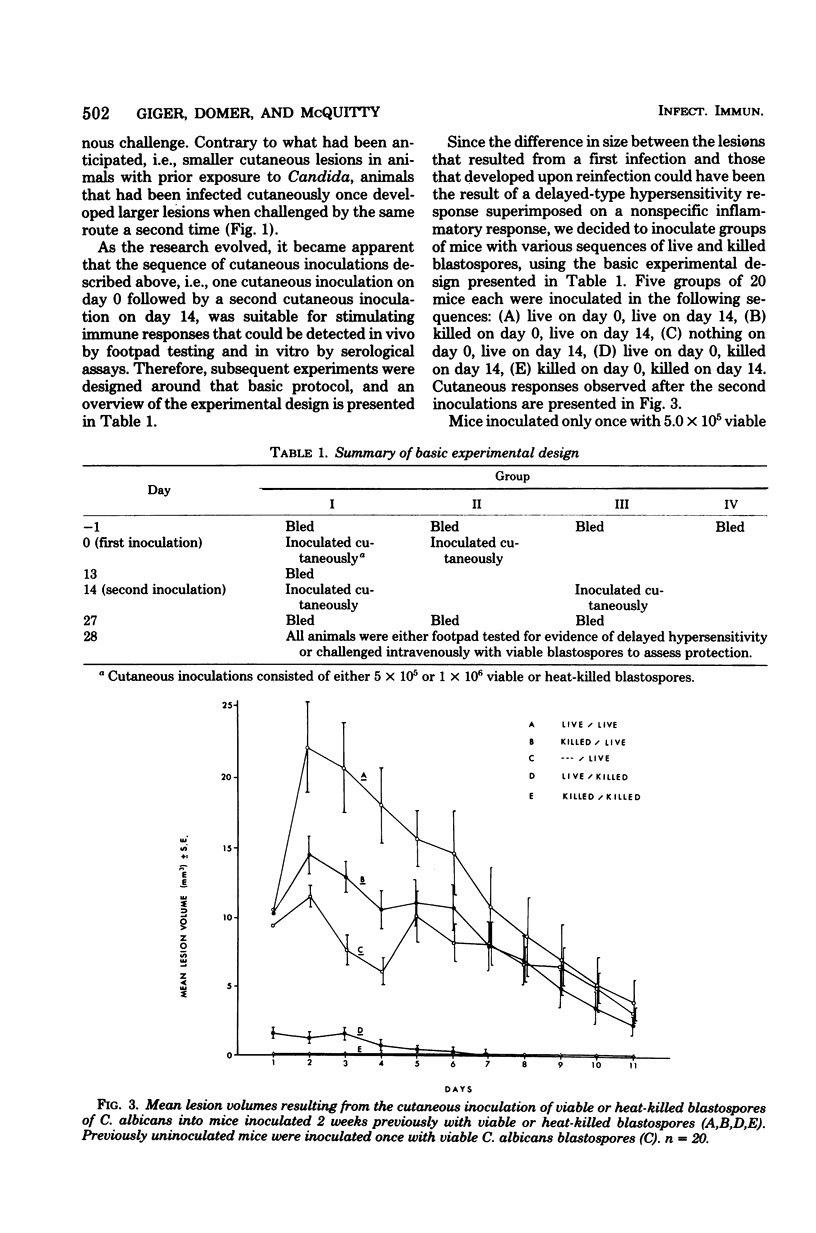
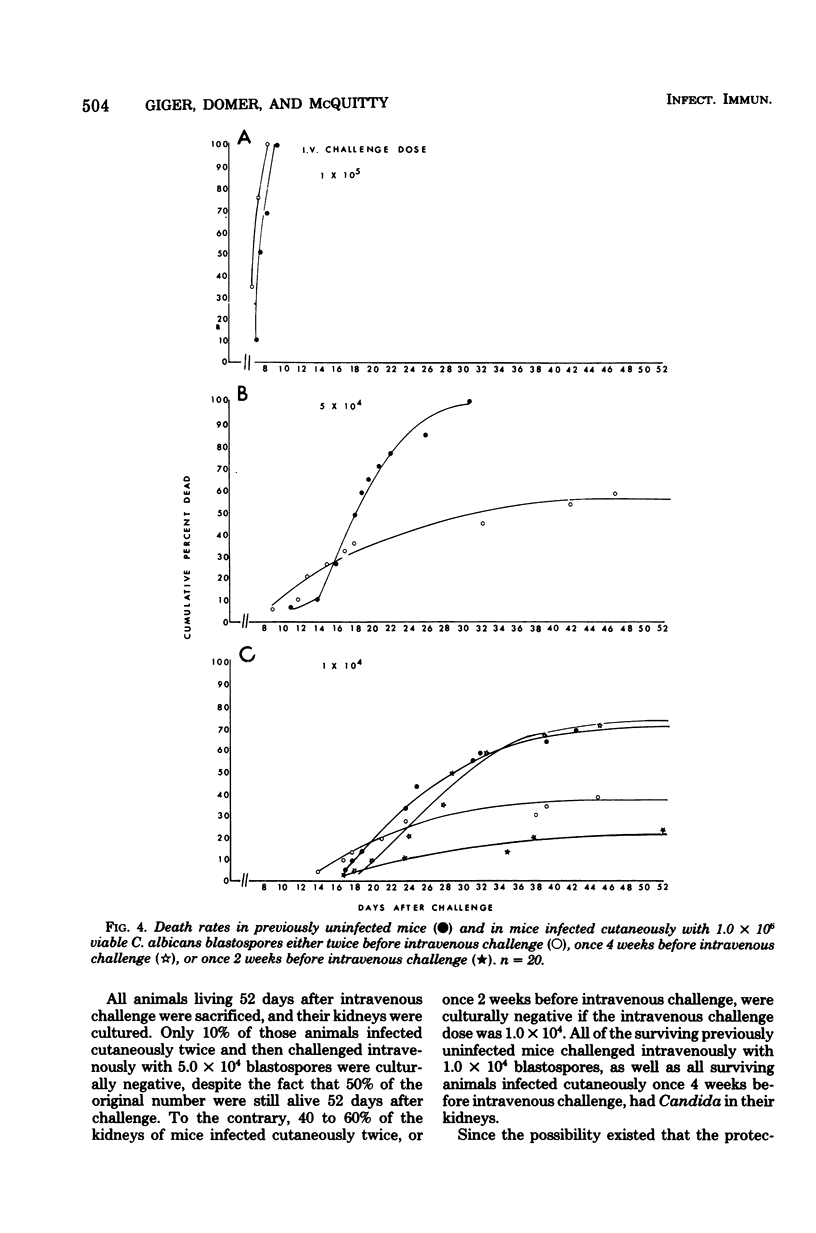
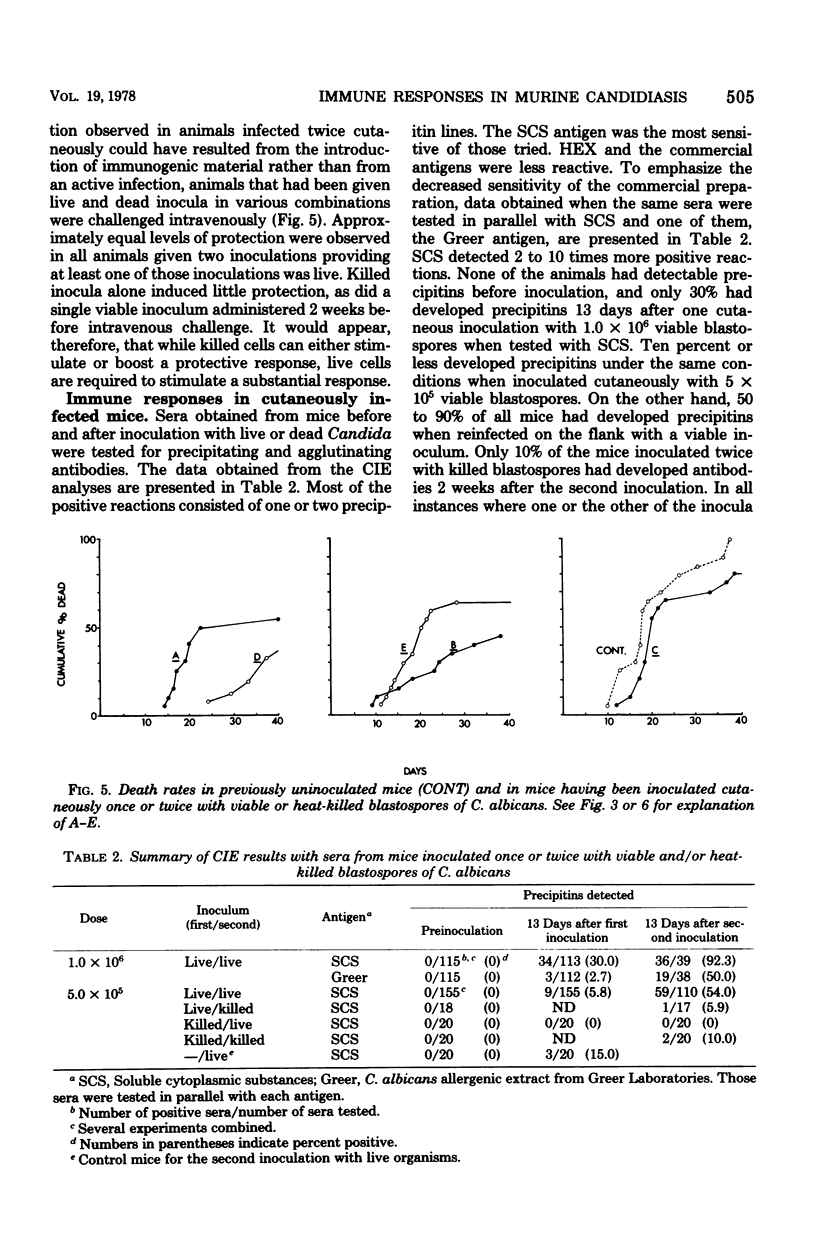
Cutaneous infection of mice with Candida albicans elicited a predominantly acute inflammatory response, stimulated the production of precipitating antibodies, and conferred protection against subsequent intravenous challenge with the same organism. The acute inflammatory skin reaction seen after cutaneous infection suggested a predominantly humoral response to Candida. Animals infected cutaneously a second time with viable C. albicans developed larger skin lesions than animals infected only once, and the twice-infected animals were more resistant to an intravenous challenge as well. The cutaneous inoculation of mice with heat-killed C. albicans was less effective in stimulating antibody production, in eliciting the inflammatory response, and in inducing a protective response demonstrable by intravenous challenge with viable Candida. This model of experimental candidiasis represents a reproducible means of studying a protective immune response to the organism.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chilgren R. A., Quie P. G., Meuwissen H. J., Hong R. Chronic mucocutaneous candidiasis, deficiency of delayed hypersensitivity, and selective local antibody defect. Lancet. 1967 Sep 30;2(7518):688–693. doi: 10.1016/s0140-6736(67)90974-9. [DOI] [PubMed] [Google Scholar]
- Cohen R., Roth F. J., Delgado E., Ahearn D. G., Kalser M. H. Fungal flora of the normal human small and large intestine. N Engl J Med. 1969 Mar 20;280(12):638–641. doi: 10.1056/NEJM196903202801204. [DOI] [PubMed] [Google Scholar]
- Cooper M. G. Delayed-type hypersensitivity in the mouse. I. Induction and elicitation by Salmonella adelaide flagellin and its derivatives. Scand J Immunol. 1972;1(2):167–178. doi: 10.1111/j.1365-3083.1972.tb00596.x. [DOI] [PubMed] [Google Scholar]
- Crowle A. J. Delayed hypersensitivity in the mouse. Adv Immunol. 1975;20:197–264. doi: 10.1016/s0065-2776(08)60209-6. [DOI] [PubMed] [Google Scholar]
- Cutler J. E. Acute systemic candidiasis in normal and congenitally thymic-deficient (nude) mice. J Reticuloendothel Soc. 1976 Feb;19(2):121–124. [PubMed] [Google Scholar]
- DeMaria A., Buckley H., von Lichtenberg F. Gastrointestinal candidiasis in rats treated with antibiotics, cortisone, and azathioprine. Infect Immun. 1976 Jun;13(6):1761–1770. doi: 10.1128/iai.13.6.1761-1770.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning T. J., Davies R. R. Candida albicans and the chemotaxis of polymorphonuclear neutrophils. Sabouraudia. 1973 Nov;11(3):210–221. [PubMed] [Google Scholar]
- Domer J. E. In vivo and in virto cellular responses to cytoplasmic and cell wall antigens of Histoplasma capsulatum in artificially immunized or infected guinea pigs. Infect Immun. 1976 Mar;13(3):790–799. doi: 10.1128/iai.13.3.790-799.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon M. A., Almy R. E., Greene C. H., Fenton J. W., 2nd Diagnostic mycoserology by immunoelectroosmophoresis: a general, rapid, and sensitive microtechnic. Am J Clin Pathol. 1971 Oct;56(4):471–474. doi: 10.1093/ajcp/56.4.471. [DOI] [PubMed] [Google Scholar]
- HASENCLEVER H. F., MITCHELL W. O. ACQUIRED IMMUNITY TO CANDIDIASIS IN MICE. J Bacteriol. 1963 Sep;86:401–406. doi: 10.1128/jb.86.3.401-406.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick C. H., Chandler J. W., Schimke R. N. Chronic mucocutaneous moniliasis with impaired delayed hypersensitivity. Clin Exp Immunol. 1970 Mar;6(3):375–385. [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick C. H., Rich R. R., Bennett J. E. Chronic mucocutaneous candidiasis: model-building in cellular immunity. Ann Intern Med. 1971 Jun;74(6):955–978. doi: 10.7326/0003-4819-74-6-955. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MOURAD S., FRIEDMAN L. Pathogenicity of Candida. J Bacteriol. 1961 Apr;81:550–556. doi: 10.1128/jb.81.4.550-556.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourad S., Friedman L. Passive immunization of mice against Candida albicans. Sabouraudia. 1968 Feb;6(2):103–105. [PubMed] [Google Scholar]
- Pearsall N. N., Lagunoff D. Immunological responses to Candida albicans. I. Mouse-thigh lesion as a model for experimental candidiasis. Infect Immun. 1974 Jun;9(6):999–1002. doi: 10.1128/iai.9.6.999-1002.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips A. W., Balish E. Growth and invasiveness of Candida albicans in the germ-free and conventional mouse after oral challenge. Appl Microbiol. 1966 Sep;14(5):737–741. doi: 10.1128/am.14.5.737-741.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston P. M., Dumonde D. C. Experimental cutaneous leishmaniasis. V. Protective immunity in subclinical and self-healing infection in the mouse. Clin Exp Immunol. 1976 Jan;23(1):126–138. [PMC free article] [PubMed] [Google Scholar]
- Ray T. L., Wuepper K. D. Experimental cutaneous candidiasis in rodents. J Invest Dermatol. 1976 Jan;66(1):29–33. doi: 10.1111/1523-1747.ep12478053. [DOI] [PubMed] [Google Scholar]
- Remington J. S., Gaines J. D., Gilmer M. A. Demonstration of Candida precipitins in human sera by counterimmunoelectrophoresis. Lancet. 1972 Feb 19;1(7747):413–413. doi: 10.1016/s0140-6736(72)90860-4. [DOI] [PubMed] [Google Scholar]
- Restrepo-Moreno A., Schneidau J. D., Jr Nature of the skin-reactive principle in culture filtrates prepared from Paracoccidioides brasiliensis. J Bacteriol. 1967 Jun;93(6):1741–1748. doi: 10.1128/jb.93.6.1741-1748.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T. J., Balish E., Manning D. D. The role of thymus-dependent cell-mediated immunity in resistance to experimental disseminated candidiasis. J Reticuloendothel Soc. 1976 Oct;20(4):291–298. [PubMed] [Google Scholar]
- Rogers T., Balish E. Experimental Candida albicans infection in conventional mice and germfree rats. Infect Immun. 1976 Jul;14(1):33–38. doi: 10.1128/iai.14.1.33-38.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdimarsson H., Holt L., Riches H. R., Hobbs J. R. Lymphocyte abnormality in chronic mucocutaneous candidiasis. Lancet. 1970 Jun 13;1(7659):1259–1261. doi: 10.1016/s0140-6736(70)91742-3. [DOI] [PubMed] [Google Scholar]
- WINNER H. I. A study of Candida albicans agglutinins in human sera. J Hyg (Lond) 1955 Dec;53(4):509–512. doi: 10.1017/s0022172400001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks B. A., Escobar M. R., Hamilton P. B., Fueston V. M. Chemotaxis of polymorphonuclear neutrophilic leukocytes by mannan-enriched preparations of Candida albicans. Adv Exp Med Biol. 1976;73(PT-A):161–169. doi: 10.1007/978-1-4684-3297-8_15. [DOI] [PubMed] [Google Scholar]



