Abstract
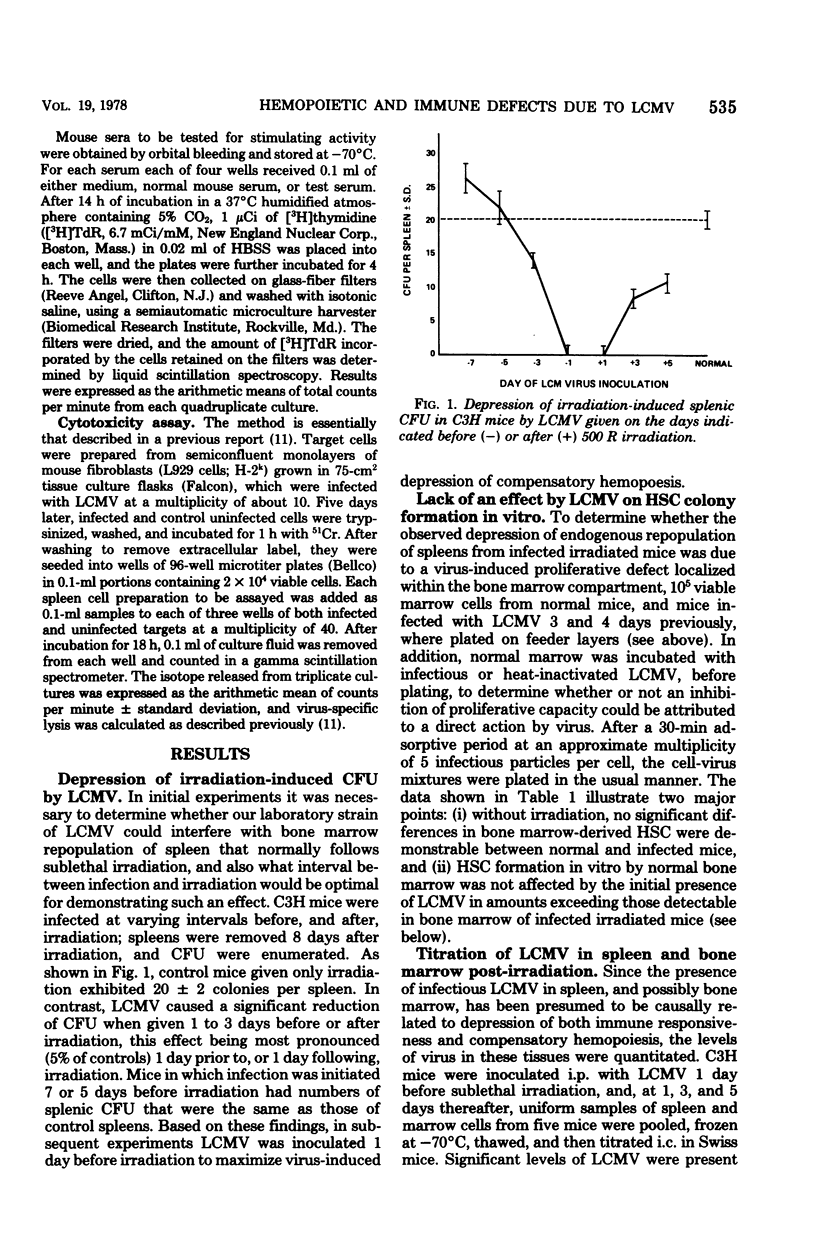
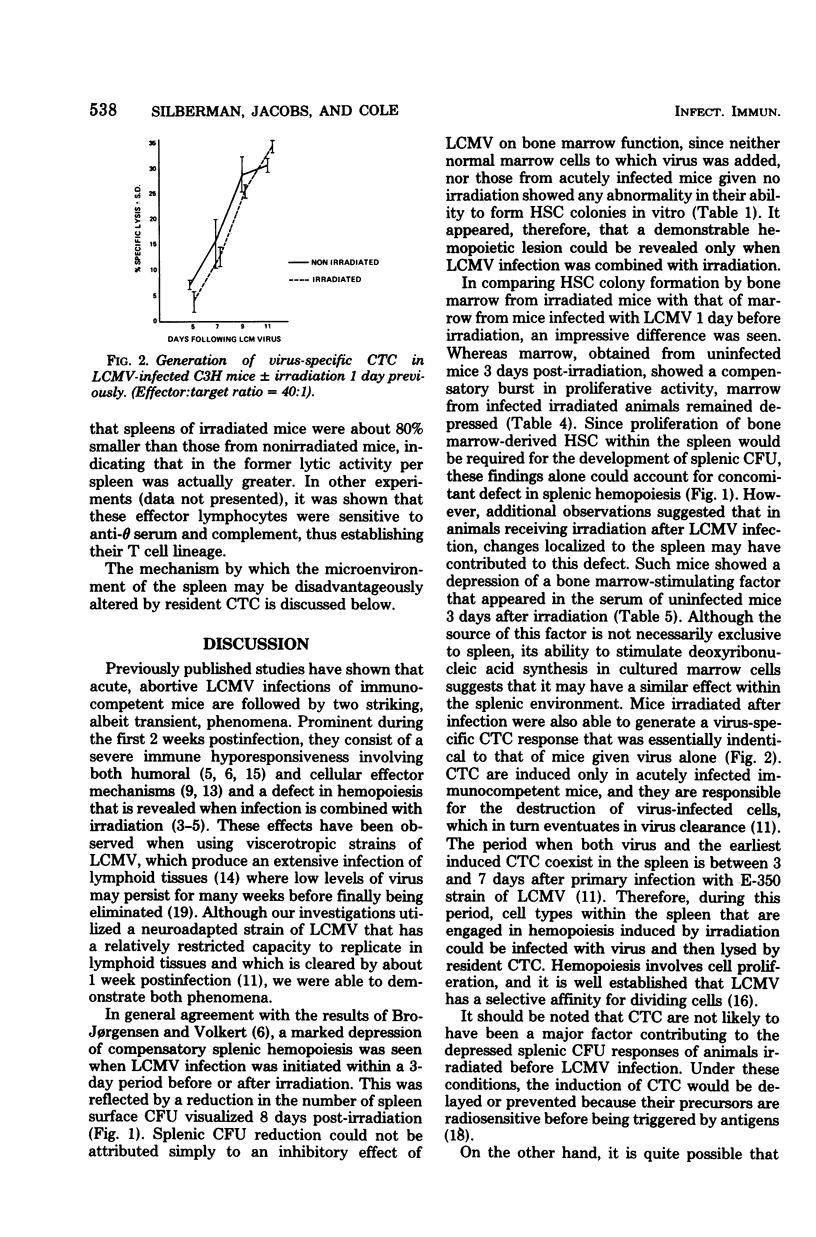
Sublethal irradiation (500 R) of C3H mice is followed by a gradual replacement of radiosensitive cells in their spleens by surviving stem cells originating in bone marrow. This compensatory hemopoiesis was quantitated by counting the numbers of stem cell-derived colonies appearing on spleen surfaces, as well as those which grew in vitro after marrow cells, suspended in soft agar, were overlaid onto syngenic mouse embryo fibroblast feeder layers. Compensatory colony formation, both in vivo and in vitro, was severely depressed when mice were infected with lymphocytic choriomeningitis virus (LCMV) 1 day before irradiation, although the induction of virus-specific cytotoxic T cells in their spleens was unimpaired. Without irradiation, mice, acutely infected with LCMV, showed a dramatic reduction in the numbers of specific antibody-forming cells generated in their spleens after priming with sheep erythrocytes during week 1 post-infection, yet the ability of their marrow cells to form colonies in vitro remained normal. Therefore, the basis of immunodepression is distinct from that of defective hemopoiesis since the latter is apparent only when LCMV infection is accompanied by irradiation. However, as discussed, both phenomena may be related to alterations induced within the splenic environment by LCMV.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley T. R., Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966 Jun;44(3):287–299. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- Bro-Jorgensen K., Güttler F., Jorgensen P. N., Volkert M. T lymphocyte function as the principal target of lymphocytic choriomeningitis virus-induced immunosuppression. Infect Immun. 1975 Apr;11(4):622–629. doi: 10.1128/iai.11.4.622-629.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bro-Jorgensen K., Knudtzon S. Changes in hemopoiesis during the course of acute LCM virus infection in mice. Blood. 1977 Jan;49(1):47–57. [PubMed] [Google Scholar]
- Bro-Jorgensen K., Volkert M. Defects in the immune system of mice infected with lymphocytic choriomeningitis virus. Infect Immun. 1974 Apr;9(4):605–614. doi: 10.1128/iai.9.4.605-614.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bro-Jorgensen K., Volkert M. Haemopoietic defects in mice infected with lymphocytic choriomeningitis virus. 1. The enhanced x-ray sensitivity of virus infected mice. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(6):845–852. doi: 10.1111/j.0365-5563.1973.tb00010.x. [DOI] [PubMed] [Google Scholar]
- Bro-Jorgensen K., Volkert M. Haemopoietic defects in mice infected with lymphocytic choriomeningitis virus. 2. The viral effect upon the function of colony-forming stem cells. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(6):853–862. [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Curry J. L., Trentin J. J. Hemopoietic spleen colony studies. I. Growth and differentiation. Dev Biol. 1967 May;15(5):395–413. doi: 10.1016/0012-1606(67)90034-6. [DOI] [PubMed] [Google Scholar]
- Güttler F., Bro-Jorgensen K., Jorgensen P. N. Transient impaired cell-mediated tumor immunity after acute infection with lymphocytic choriomeningitis virus. Scand J Immunol. 1975;4(4):327–336. doi: 10.1111/j.1365-3083.1975.tb02633.x. [DOI] [PubMed] [Google Scholar]
- Jacobs R. P., Cole G. A. Lymphocytic choriomeningitis virus-induced immunosuppression: a virus-induced macrophage defect. J Immunol. 1976 Sep;117(3):1004–1009. [PubMed] [Google Scholar]
- Johnson E. D., Cole G. A. Functional heterogeneity of lymphocytic choriomeningitis virus-specfic T lymphocytes. I. Identification of effector amd memory subsets. J Exp Med. 1975 Apr 1;141(4):866–881. [PMC free article] [PubMed] [Google Scholar]
- Lehmann-Grube F., Niemeyer I., Löhler J. Lymphocytic choriomeningitis of the mouse. IV. Depression of the allograft reaction. Med Microbiol Immunol. 1972;158(1):16–25. doi: 10.1007/BF02122004. [DOI] [PubMed] [Google Scholar]
- Mims C. A., Tosolini F. A. Pathogenesis of lesions in lymphoid tissue of mice infected with lymphocytic choriomeningitis (LCM) virus. Br J Exp Pathol. 1969 Dec;50(6):584–592. [PMC free article] [PubMed] [Google Scholar]
- Mims C. A., Wainwright S. The immunodepressive action of lymphocytic choriomeningitis virus in mice. J Immunol. 1968 Oct;101(4):717–724. [PubMed] [Google Scholar]
- Monjan A. A., Cole G. A., Gilden D. H., Nathanson N. Pathogenesis of cerebellar hypoplasia produced by lymphocytic choriomeningitis virus infection of neonatal rats. 1. Evolution of disease following infection at 4 days of age. J Neuropathol Exp Neurol. 1973 Jan;32(1):110–124. doi: 10.1097/00005072-197301000-00007. [DOI] [PubMed] [Google Scholar]
- Pluznik D. H., Sachs L. The induction of clones of normal mast cells by a substance from conditioned medium. Exp Cell Res. 1966 Oct;43(3):553–563. doi: 10.1016/0014-4827(66)90026-7. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Nomoto K., Sato M., Takeya K., Yano Y. Analysis of the developmental stage of specific and nonspecific cytotoxity. I. Separation of the developmental steps of specific cytotoxicity by susceptibility to irradiation. Cell Immunol. 1976 Aug;25(2):228–236. doi: 10.1016/0008-8749(76)90113-1. [DOI] [PubMed] [Google Scholar]
- Volkert M., Lundstedt C. The provocation of latent lymphocytic choriomeningitis virus infections in mice by treatment with antilymphocytic serum. J Exp Med. 1968 Feb 1;127(2):327–339. doi: 10.1084/jem.127.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]



