Abstract
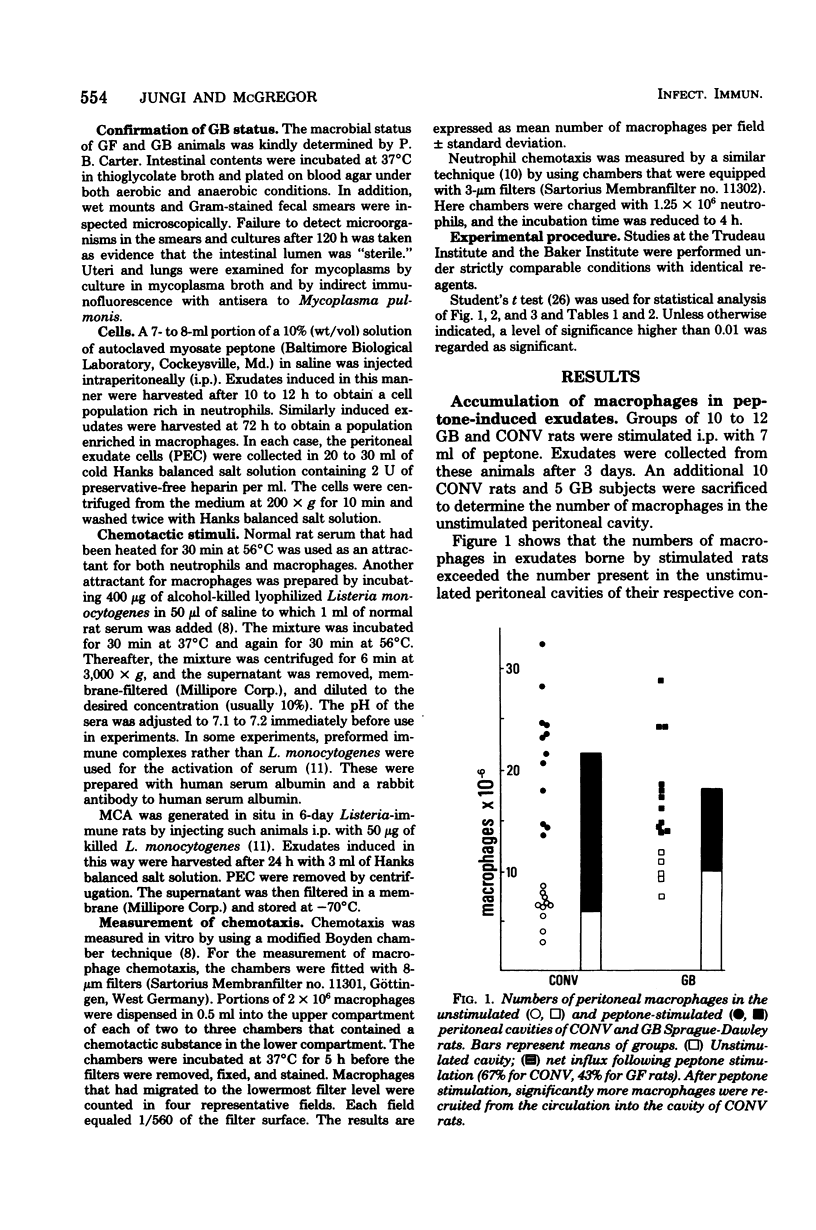
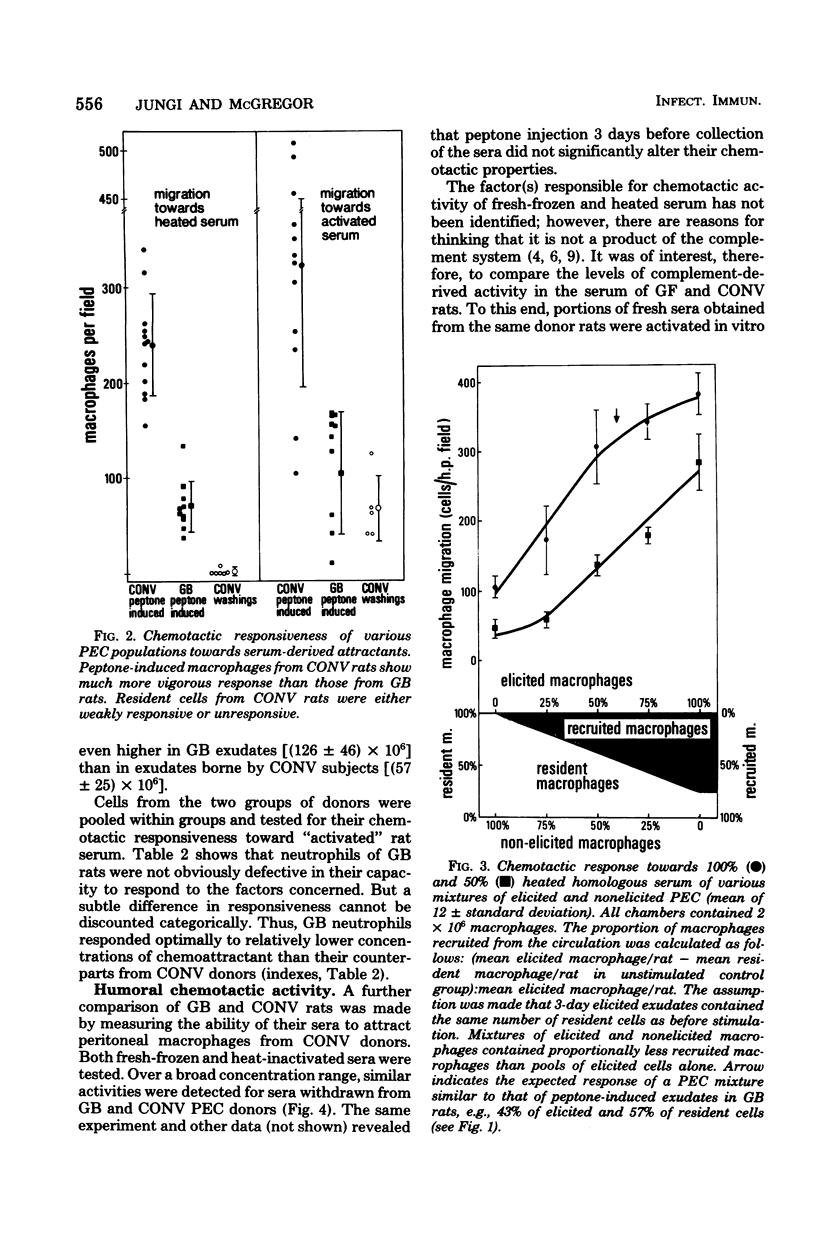
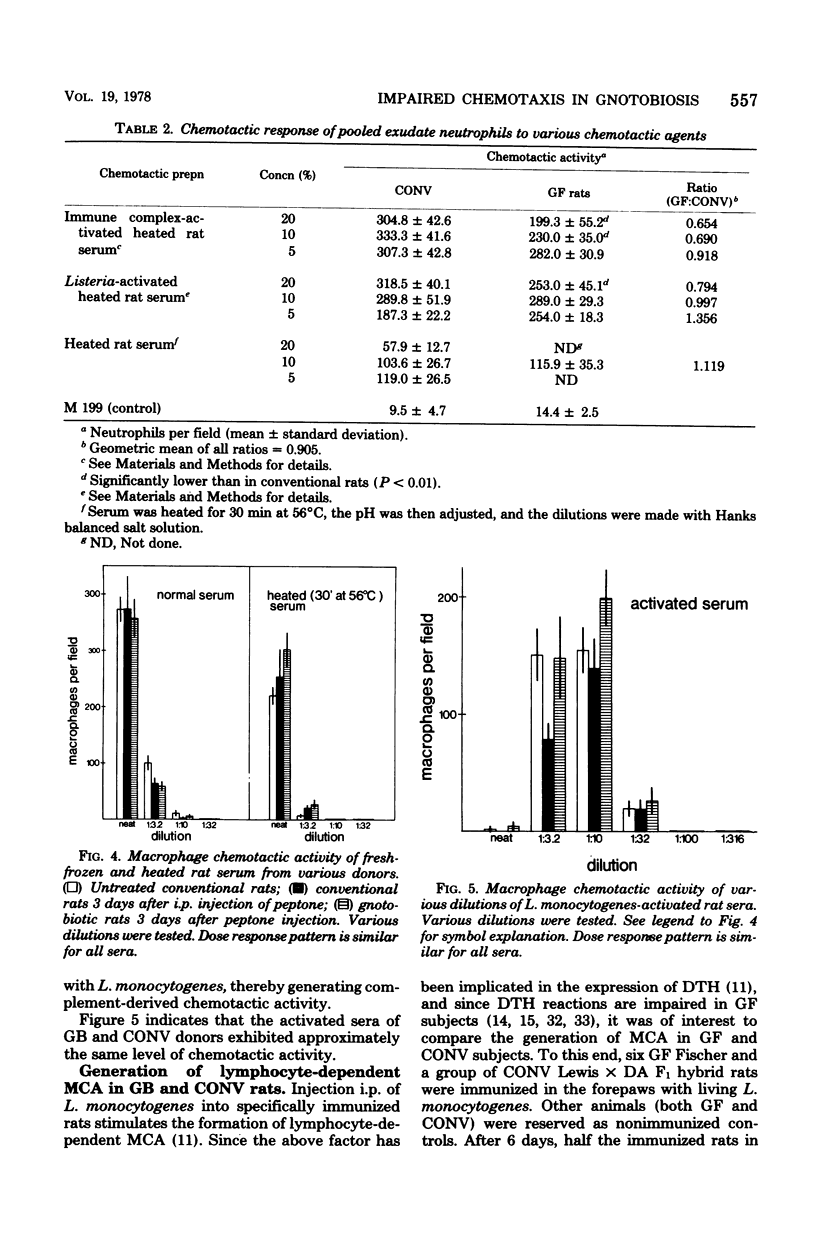
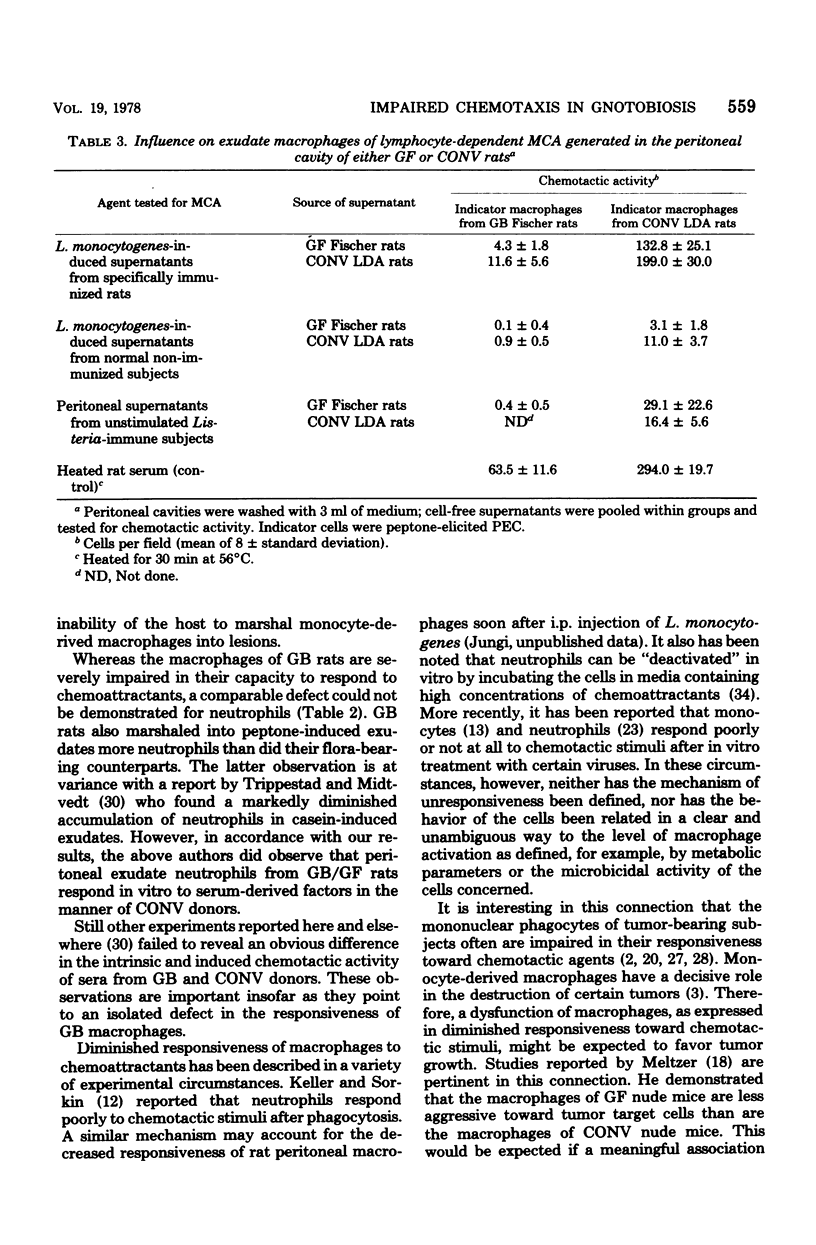
Peptone-induced macrophages obtained from gnotobiotic (GB) rats responded poorly to chemotactic stimuli that have a powerful, attractive influence upon the cells of conventional donors. Monocyte recruitment from the circulation into peptone-induced exudates also was impaired in GB subjects. Although relatively more resident cells are present in exudates borne by GB donors, their number cannot in itself account for the sluggish response of peptone-induced cells from GB rats. Neutrophil accumulation in the inflamed peritoneal cavities and their responsiveness in vitro were similar in GB and conventional rats. The levels of serum-derived chemotactic factors were similar in such animals. Furthermore, germ-free rats exhibited no obvious defects in their capacity to generate lymphocyte-dependent monocyte chemotactic activity in situ upon specific stimulation with Listeria monocytogenes. It is suggested that the diminished chemotactic responsiveness of exudate macrophages is related in some way to the level of cell activation. This state of affairs might account for the impairment of delayed-type hypersensitivity in GB animals and their inability to resist intracellular bacterial infections.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander P., Evans R. Endotoxin and double stranded RNA render macrophages cytotoxic. Nat New Biol. 1971 Jul 21;232(29):76–78. doi: 10.1038/newbio232076a0. [DOI] [PubMed] [Google Scholar]
- Boetcher D. A., Leonard E. J. Abnormal monocyte chemotactic response in cancer patients. J Natl Cancer Inst. 1974 Apr;52(4):1091–1099. doi: 10.1093/jnci/52.4.1091. [DOI] [PubMed] [Google Scholar]
- Hausman M. S., Snyderman R., Mergenhagen S. E. Humoral mediators of chemotaxis of mononuclear leukocytes. J Infect Dis. 1972 Jun;125(6):595–602. doi: 10.1093/infdis/125.6.595. [DOI] [PubMed] [Google Scholar]
- Jungi T. W. Assay of chemotaxis by a reversible Boyden chamber eliminating cell detachment. Int Arch Allergy Appl Immunol. 1975;48(3):341–352. doi: 10.1159/000231319. [DOI] [PubMed] [Google Scholar]
- Jungi T. W., Besedovsky H. O., Sorkin E., Schardt M. Hormonal control of neutrophil chemotactic activity in the rat vagina. Am J Physiol. 1977 Jul;233(1):R59–R65. doi: 10.1152/ajpregu.1977.233.1.R59. [DOI] [PubMed] [Google Scholar]
- Jungi T. W. Monocyte and neutrophil chemotactic activity of normal and diluted human serum and plasma. Int Arch Allergy Appl Immunol. 1977;53(1):18–28. doi: 10.1159/000231726. [DOI] [PubMed] [Google Scholar]
- Keller H. U., Sorkin E. Studies on chemotaxis. X. Inhibition of chemotaxis of rabbit neutrophil polymorphonuclear leucocytes. Int Arch Allergy Appl Immunol. 1968;34(5):513–520. [PubMed] [Google Scholar]
- Lev M., Battisto J. R. Impaired delayed hypersensitivity in germ-free guinea-pigs. Immunology. 1970 Jul;19(1):47–54. [PMC free article] [PubMed] [Google Scholar]
- Lubaroff D. M., Waksman B. H. Bone marrow as a source of cells in reactions of cellular hypersensitivity. I. Passive transfer of tuberculin sensitivity in syngeneic systems. J Exp Med. 1968 Dec 1;128(6):1425–1435. doi: 10.1084/jem.128.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor D. D., Logie P. S. The mediator of cellular immunity. X. Interaction of macrophages and specifically sensitized lymphocytes. Cell Immunol. 1975 Aug;18(2):454–465. doi: 10.1016/0008-8749(75)90072-6. [DOI] [PubMed] [Google Scholar]
- Meltzer M. S., Jones E. E., Boetcher D. A. Increased chemotactic responses of macrophages from BCG-infected mice. Cell Immunol. 1975 May;17(1):268–276. doi: 10.1016/s0008-8749(75)80026-8. [DOI] [PubMed] [Google Scholar]
- Meltzer M. S. Tumoricidal responses in vitro of peritoneal macrophages from conventionally housed and germ-free nude mice. Cell Immunol. 1976 Mar 1;22(1):176–181. doi: 10.1016/0008-8749(76)90018-6. [DOI] [PubMed] [Google Scholar]
- Norman S. J., Sorkin E. Cell-specific defect in monocyte function during tumor growth. J Natl Cancer Inst. 1976 Jul;57(1):135–140. doi: 10.1093/jnci/57.1.135. [DOI] [PubMed] [Google Scholar]
- North R. J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970 Sep 1;132(3):521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outzen H. C., Custer R. P., Eaton G. J., Prehn R. T. Spontaneous and induced tumor incidence in germfree "nude" mice. J Reticuloendothel Soc. 1975 Jan;17(1):1–9. [PubMed] [Google Scholar]
- ROVIN S., COSTICH E. R., FLEMING J. E., GORDON H. A. HEALING OF TONGUE WOUNDS IN GERMFREE AND CONVENTIONAL MICE. Arch Pathol. 1965 Jun;79:641–643. [PubMed] [Google Scholar]
- Smith C. S., Pilgrim H. I., Steinmuller D. The survival of skin allografts and xenografts in germ-free mice. Transplantation. 1972 Jan;13(1):38–41. doi: 10.1097/00007890-197201000-00009. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Pike M. C., Blaylock B. L., Weinstein P. Effects of neoplasms on inflammation: depression of macrophage accumulation after tumor implantation. J Immunol. 1976 Mar;116(3):585–589. [PubMed] [Google Scholar]
- Stashak P. W., Baker P. J., Roberson B. S. The serum antibody response to bacteriophage phi chi 174 in germ-free and conventionally reared mice. II. Kinetics of the serum antibody response following primary immunization. Immunology. 1970 Feb;18(2):307–317. [PMC free article] [PubMed] [Google Scholar]
- Trippestad A., Midtvedt T. Chemotaxis of polymorphonuclear leucocytes from germ-free rats and generation of chemotactic activity in germ-free rat sera. Clin Exp Immunol. 1971 Apr;8(4):639–646. [PMC free article] [PubMed] [Google Scholar]
- Ueda K., Yamazaki S., Someya S. Hyporeactivity to tuberculin in germ-free mice. J Reticuloendothel Soc. 1975 Aug;18(2):107–117. [PubMed] [Google Scholar]
- Ueda K., Yamazaki S., Someya S. Impairment and restoration of the delayed type hypersensitivity in germ-free mice. Jpn J Microbiol. 1973 Nov;17(6):533–536. doi: 10.1111/j.1348-0421.1973.tb00942.x. [DOI] [PubMed] [Google Scholar]
- Ueda K., Yamazaki S., Someya S. Studies on tubercle bacillus infection in germ-free mice. J Reticuloendothel Soc. 1972 Nov;12(5):545–563. [PubMed] [Google Scholar]
- Ward P. A., Becker E. L. The deactivation of rabbit neutrophils by chemotactic factor and the nature of the activatable esterase. J Exp Med. 1968 Apr 1;127(4):693–709. doi: 10.1084/jem.127.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]



