Highlights
-
•
AQP9 is expressed in the upper layer of the stratum granulosum of human epidermis.
-
•
AQP9 expressed in keratinocytes facilitates glycerol and urea transportation.
-
•
Retinoic acid down-regulates AQP9 mRNA expression in differentiated keratinocytes.
Abbreviations: AQPs, aquaporins; DUOX1, dual oxidase I; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; LXR, liver X receptor; NHEK, normal human epidermal keratinocytes; PPARγ, peroxisome proliferators-activated receptor gamma; RA, retinoic acid; SG, stratum granulosum; TJs, tight junctions; VD3, 1,25-dihydroxyvitamin D3
Keywords: AQP9, AQP3, Epidermis, Differentiated keratinocytes
Abstract
Aquaporin 9 (AQP9) is a member of the aquaglyceroporin family that transports glycerol, urea and other small solutes as well as water. Compared to the expression and function in epidermal keratinocytes of AQP3, another aquaglyceroporin, our knowledge of epidermal AQP9 remains elusive. In this study, we investigated the expression of AQP9 in the human epidermis and cultured keratinocytes. Immunofluorescence studies revealed that AQP9 expression is highly restricted to the stratum granulosum of the human epidermis, where occludin is also expressed at the tight junctions. Interestingly, the AQP3 staining decreased sharply below the cell layers in which AQP9 is expressed. In cultured normal human epidermal keratinocytes (NHEK), knock-down of AQP9 expression in the differentiated cells induced by RNA interference reduced glycerol uptake, which was not as pronounced as was the case with AQP3 knock-down cells. In contrast, similar reduction of urea uptake was detected in AQP9 and AQP3 knock-down cells. These findings suggested that AQP9 expression in NHEK facilitates at least the transport of glycerol and urea. Finally, we analyzed the effect of retinoic acid (RA), a potent stimulator of keratinocyte proliferation, on AQP3 and AQP9 mRNA expression in differentiated NHEK. Stimulation with RA at 1 μM for 24 h augmented AQP3 expression and down-regulated AQP9 expression. Collectively, these results indicate that AQP9 expression in epidermal keratinocytes is regulated in a different manner from that of AQP3.
1. Introduction
The aquaporins (AQPs) are a family of small transmembrane proteins that facilitate osmotically driven water transport. To date, 13 AQPs (AQP0-12) have been cloned from humans and are categorized into distinct subgroups in terms of their amino acid sequence and molecular functions: water-selective AQPs (AQPs 1, 2, 4, and 5), aquaglyceroporins that transport water and possibly other small solutes such as glycerol and urea (AQPs 3, 7, 9 and 10), and unorthodox AQPs (AQPs 6, 8, 11 and 12) [1–3].
In the skin, AQP3 is highly expressed in the epidermis, especially in the basal layer and stratum spinosum [4,5]. AQP3 null mice show impairments in hydration, elasticity, barrier recovery and wound healing, indicating that AQP3 plays an important role in skin physiology [6–8]. These defects are accompanied by disturbed cell proliferation and migration in which AQP3-facilitated water and glycerol transport are involved [9]. In cultured normal human epidermal keratinocytes (NHEK), osmotic stress up-regulates AQP3 gene expression, suggesting that AQP3 in keratinocytes is involved in maintaining cellular osmolality [10]. In addition, several biological factors have been shown to affect AQP3 expression in keratinocytes. Retinoic acid (RA) increases AQP3 expression at both mRNA and protein levels, indicating that RAR and RXR is involved in the mediation of AQP3 gene expression [11–14]. Activation of peroxisome proliferators-activated receptor gamma (PPARγ) and liver X receptor (LXR) activators also induce AQP3 expression [14]. In contrast, tumor necrosis factor-α decreases AQP3 expression in DJM-1 keratinocytes, and nicotinamide attenuates augmented AQP3 expression in HaCat cells treated with RA [15,16]. Thus, many aspects of epidermal physiology and pathology have been considered thorough the findings of the molecular functions of AQP3 in keratinocytes and the factors that regulate its expression.
Comparing with our knowledge of AQP3 in the epidermis and keratinocytes, many features of AQP9, including its function and expression, remain elusive. Through a series of phenotype analyses of AQP9 knockout mice, AQP9 expression has been shown to be highly restricted to the stratum granulosum (SG), but no apparent defects were observed, including wound healing [17]. Our previous studies showed that AQP9 mRNA expression is only detected in differentiated NHEK [10]. However, osmotic stress did not have any effect on AQP9 expression under culture conditions. Similarly, AQP9 expression in undifferentiated NHEK cultured in a low calcium medium is not altered by treatment with PPAR, LXR, RAR or RXR activators [14]. While both AQP9 and AQP3 are members of the aquaglyceroporin family, these findings suggest that different mechanisms underlie the regulation of AQP9 and AQP3 expression in keratinocytes.
In this study, we examined AQP9 expression on human skin sections to clarify its localization within the epidermis. Double staining with occludin, a tight junction marker, was also performed to distinguish the expression site of AQP9 from that of AQP3. In order to find out whether AQP9 in NHEK possesses the molecular function of aquaglyceroporins, its permeability to glycerol and urea was investigated. Finally, we demonstrated that RA decreases AQP9 expression but increases AQP3 mRNA expression in differentiated NHEK.
2. Materials and methods
2.1. Immunofluorescence and immunohistochemical studies
Abdominal skin samples were obtained from healthy female Caucasian donors (19 and 30 years of age) through Human and Animal Bridging Research Organization (Tokyo, Japan) and BIOPREDIC International (Rennes, France). The skin tissue samples were embedded in Tissue-Tek O.C.T. compound (Sakura Finetek, Torrance, CA), cut at 5 μm with a cryostat, and fixed with ice cold ethanol or acetone. The sections were rinsed with 0.05% Tween 20 in phosphate-buffered saline (PBS-T). Nonspecific reactions were blocked by incubating the sections in 1% bovine serum albumin (BSA) in PBS (BSA/PBS) containing 10% normal goat serum for 30 min at room temperature. The sections were then incubated with the following primary antibodies in BSA/PBS overnight at 4 °C: rabbit anti- AQP3 antibody raised against a C-terminal peptide (CHLEQPPPSNEEENVKLA), rabbit anti-AQP9 antibody (Alpha Diagnostic Intl. Inc., Texas, USA). After washing in PBS-T, the sections were incubated with a Rhodamine Red-X conjugated goat anti-rabbit IgG (Jackson Immuno Research. West Grove, PA,USA) in BSA/ PBS for 1 h at room temperature, followed by rinsing in PBS-T. For double immunofluorescence studies, BSA/PBS-T containing 10% normal goat and donkey serum, rat anti-mouse occludin monoclonal antibody (MOC37, gifted from Dr. Mikio Furuse, University of Kobe, Japan), and FITC conjugated donkey anti-rat IgG (Jackson Immuno Research. West Grove, PA, USA) were used as a blocking reagent, primary antibody and secondary antibody, respectively. TO-PRO-3 iodide (Life Technologies Corporation, CA, USA) was used for nuclear counter staining. The sections were mounted using the SlowFade Antifade Kit (Molecular Probes, OR, USA). Fluorescence signals were examined with a laser scanning confocal microscope (Carl Zeiss LSM510, Jena, Germany). As control experiments for AQP9 staining, paraffin embedded skin sections were incubated with anti-AQP9 antibody preabsorbed with either AQP9 blocking peptide (ADI_AQP91-P; Alpha Diagnostic Intl. Inc., Texas, USA) or C-terminal peptide of AQP3 at 1 μg/ml. Biotin conjugated goat anti-rabbit IgG as a secondary antibody (Dako Japan, Tokyo, Japan) and VECTASTAIN Elite ABC Kit (VECTOR LABORATORIES, INC., CA, USA) were used for detection. All protocols were approved by the ethics committee of Kanebo Cosmetics Inc.
2.2. Cell culture and siRNA transfection
NHEK (Kurabo, Osaka, Japan) were cultured as described previously [18]. An siRNA solution was prepared using Lipofectamine® RNAiMAX Transfection Reagent according to the manufacturer’s instructions (Life Technologies Corporation, CA, USA). After 20 min incubation at room temperature, 200 μl of 60 nM siRNA solution was added to each well of a 12-well culture plate and then NHEK (1.6 × 105 cells in 1 ml of MCDB 153 medium) were seeded onto the well. After 2 days culturing under low Ca2+ condition (0.1 mM Ca2+) when the cells were confluent, the calcium concentration was elevated to 1.25 mM to induce cell differentiation. siRNA for non-target control, AQP3 and AQP9 were purchased from QIAGEN (Osaka, Japan). Aliquots of AQP3 and AQP9 siRNA mixtures (AQP3: Hs_AQP3_2, Hs_AQP3_5, Hs_AQP3_6, Hs_AQP3_10; AQP9: Hs_AQP9_1, Hs_AQP9_2, Hs_AQP9_4, Hs_AQP9_5) were used for AQP3 and AQP9 knock-down, respectively. AllStars Negative Control siRNA was used as the non-target control.
2.3. Measurements of [14C]-glycerol and [14C] –urea transport
[14C]-glycerol (ARC0336) and [14C] –urea (ARC0150) were obtained from American Radiolabeled Chemicals, Inc. (MO, USA). NHEK culture and siRNA transfection were performed as described above. After a 4-day culturing in the high calcium medium, the cells were rinsed with PBS and incubated for 90 seconds in 500 μL of [14C]-glycerol or [14C]-urea solution (2 μCi/ml in MCDB153 medium). The medium was removed and the cells rinsed 5 times with ice-cold PBS containing 10 mM glycerol or 6.76 mM urea. After incubation in 400 μl of 0.5N NaOH and neutralization with 100 μl of 2N HCl, the radioactivity of the cell lysates was measured.
2.4. Analysis of AQP3 and AQP9 mRNA expression
Total RNA was prepared using an RNeasy Kit (Qiagen K. K., Tokyo, Japan). The cDNA was reverse transcribed from total RNA samples using High Capacity cDNA Reverse Transcription Kits according to the manufacturer’s protocol (Life Technologies Corporation, CA, USA). Real-time PCR was performed using the TaqMan technology on an Applied Biosystems StepOnePlus system (Life Technologies Corporation, CA, USA). TaqMan probes, Hs00185020_m1 and Hs01033360_m1 were used to analyze AQP3 and AQP9 mRNA expression, respectively. The mRNA expression levels were normalized to that of 18S rRNA or glyceraldehyde 3-phosphate dehydrogenase (GAPDH). 1,25-dihydroxyvitamin D3 (VD3) and all-trans retinoic acid were purchased from Sigma-Aldrich Japan (Tokyo, Japan)
2.5. Statistical analysis
Results are expressed as mean ± SD. The statistical significance of the difference between mean values was assessed by Dunnett’s multiple comparison procedure and Student’s t-test.
3. Results
3.1. AQP9 was detected in the upper layer of the stratum granulosum
To examine the distribution of AQP9 in human skin, immunofluorescence studies on abdominal skin sections were performed. As shown in Fig. 1A, the staining for AQP9 was only detected in the upper layer of the SG, not in the cells of the stratum spinosum or basal layer. This staining pattern was consistent with that observed on mouse epidermis [17]. The AQP9 antibodies preabsorbed with antigen peptides did not show any specific staining. In contrast, the staining for AQP9 was detected using AQP9 antibodies preabsorbed with antigen peptides for AQP3 antibodies (Fig. 1A, right panels). To distinguish the localization of AQP9 from AQP3 in the human epidermis, double staining was conducted with the tight junctions (TJs) component occludin, as a marker. Occludin is highly concentrated in TJs and its pattern of expression in the human epidermis has been detected as small spots in the SG [19]. Meanwhile the AQP3 staining decreased sharply below the cell layers where occludin expression was positive, but AQP9 staining was highly restricted to the occludin-positive cell layers (Fig. 1B). The signal from AQP9 did not overlap with that from the occludin, suggesting that AQP9 is not included in the TJs. These findings indicate that AQP9 is highly localized in the upper layer of the SG and does not co-exist with AQP3 in the human epidermis.
Fig. 1.
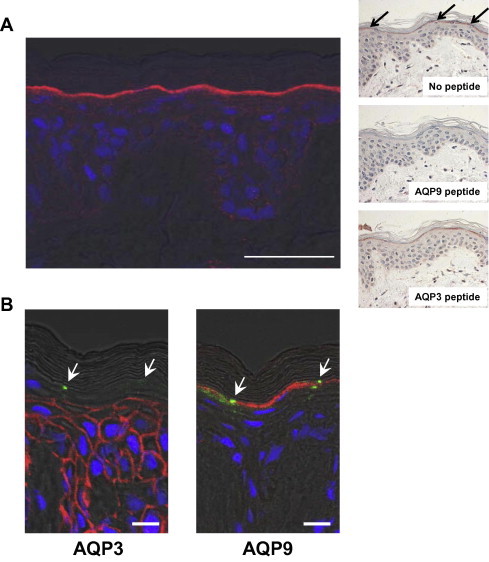
Immunofluorescence staining for AQP9, AQP3 and occludin in the human epidermis. (A) Immunofluorescence studies were performed using abdominal skin sections (19 years of age). AQP9 expression was detected in the upper layer of the SG (left panel). The nucleus was stained with TO-PRO-3 iodide. Bar = 50 μm. The results of control experiments are shown in right panels. Immunohistochemical staining resulted in very similar localization of AQP9 (top panel). The signal detected in SG was not observed by antibody preabsorbed with antigen peptide (middle panel). The AQP9 staining was not affected in the presence of AQP3 peptide (bottom panel). (B) AQP3 and AQP9 expression sites were analyzed using sequential skin sections (32 years of age). To distinguish the localization of AQP9 from AQP3 in the epidermis, the tight junction component occludin was used as the staining marker. AQP3 was highly expressed from the basal layer to the stratum spinosum. In contrast, AQP9 staining was restricted to the upper stratum granulosum. The nucleus was stained with TO-PRO-3 iodide. Arrows: occludin expression sites. Bar = 10 μm.
3.2. VD3 upregulated AQP9 mRNA expression in NHEK
As shown in Fig. 1, AQP9 was expressed in highly differentiated cells in the human epidermis. In addition, our previous study showed that AQP9 mRNA expression is only detected in differentiating NHEK by an RNase protection assay [10]. VD3, a keratinocyte differentiating agent [20,21], was added at 1 μM to NHEK at confluency and the cells were further cultured for 3 days. AQP9 mRNA expression was significantly induced by the VD3 treatment, while the increase in expression was less pronounced compared to that induced by elevated extracellular calcium (Fig. 2). This result supported the possibility that differentiation of keratinocytes triggers AQP9 expression.
Fig. 2.
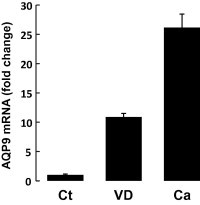
VD3 upregulated AQP9 mRNA expression in NHEK. VD3 was added at 1 μM to NHEK at confluency in a low Ca2+ medium (0.15 mM) and the cells were further cultured for 3 days. As a control inducing cell differentiation, Ca2+ concentration of medium was elevated to 1.5 mM at the point of confluency. AQP9 mRNA expression was significantly induced by VD3, a keratinocyte differentiation reagent. The mRNA expression levels were normalized to that of GAPDH.
3.3. AQP9 siRNA transfection to NHEK reduced uptake of glycerol and urea
To assess glycerol and urea permeability for AQP9 in NHEK, [14C]-glycerol and [14C]-urea uptake were examined in siRNA transfected cells. The uptake experiments were performed at 4 days after culturing in the high calcium medium, because AQP9 was highly expressed in differentiated keratinocytes. siRNA transfection of AQP9 and AQP3 noticeably suppressed their mRNA expression: AQP9 and AQP3 siRNA transfection noticeably suppressed their mRNA expression; AQP9 and AQP3 mRNA expression was 2.1% and 7.0% of non-target siRNA control, respectively (Fig. 3A). In this culture condition, AQP9 knock-down cells showed 30.6% reduction of glycerol uptake compared with the cells transfected with non-target siRNAm while for AQP3 this rate was 48.0% (Fig. 3B). A further decrease in glycerol uptake was detected for AQP3 and AQP9 double siRNA transfection. Urea uptake was also significantly reduced by both AQP9 and AQP3 siRNA transfection, but the rate of the reduction was small compared to the glycerol uptake (Fig. 3C). This reduction was augmented by AQP3/AQP9 double siRNA transfection. Thus, AQP9 expression in keratinocytes seemed to facilitate glycerol and urea transportation.
Fig. 3.
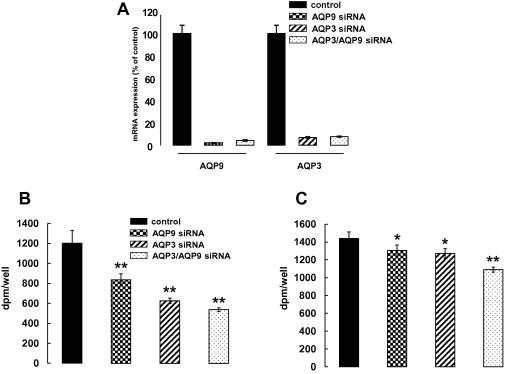
AQP9 siRNA transfection to NHEK reduced [14C]-glycerol and [14C]-urea uptake. NHEK samples mixed with AQP9 and/or AQP3 siRNA were seeded onto 12-well culture plates. On day 4 after culturing in a high Ca2+ medium, glycerol and urea permeability were analyzed. Real-time PCR analyses showed remarkable suppression of AQP9 and AQP3 mRNA expression by siRNA transfection (A). AQP9 and AQP3 siRNA transfection reduced [14C]-glycerol uptake compared to the cells transfected with non-target siRNA. Further decrease in uptake was detected with AQP3/AQP9 double siRNA transfection (B). [14C]-urea uptake was also reduced by both AQP9 and AQP3 siRNA transfection. This reduction was augmented by AQP3/AQP9 double siRNA transfection (C). The experiments were repeated three times. A representative data set is shown (n = 3; mean ± SD; ∗p < 0.05, ∗∗p < 0.01).
3.4. RA up-regulated AQP3 and down-regulated AQP9 mRNA expression in differentiated NHEK
Recent reports demonstrated that RA, a potent stimulator of keratinocyte proliferation, increases AQP3 expression in vitro and in vivo. To investigate the effect of RA on AQP9 mRNA expression in NHEK, differentiated cells were treated with RA at 1 μM for 8 and 24 h (Fig. 4A, B). As previously reported, a remarkable augmentation of AQP3 mRNA expression was detected at both 8 and 24 h after the RA stimulation (2.3- and 2.0-fold, respectively). In contrast, AQP9 expression was not altered at 8 h and was significantly reduced at 24 h after the treatment (0.7-fold). The increase in AQP3 and decrease in AQP9 mRNA expressions was detected at lower doses, 0.01 and 0.1 μM (Fig. 4C, D). These results were similar to the cases in which hypertonic stress and PPAR gamma activation were found to stimulate AQP3 mRNA expression in NHEK but have little, if any, effect on AQP9 mRNA expression [10,14].
Fig. 4.
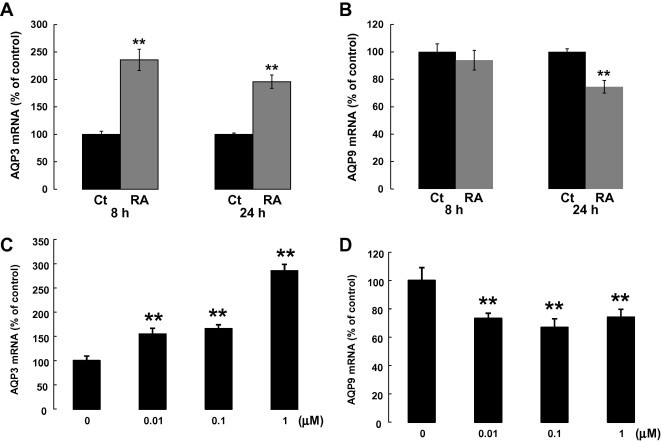
RA upregulated AQP3 and downregulated AQP9 mRNA expression in NHEK. At confluence, the cells were cultured for 4 days in a high Ca2+ medium to induce cell differentiation. After that, the cells were treated with RA at 1 μM for 8 and 24 h. AQP3 mRNA expression was increased and AQP9 decreased by RA treatment (A, B). The change in expression of AQP3 (after 8 h) and AQP9 (after 24 h) was detected at doses as low as 0.01 μM (C, D). The mRNA expression levels were normalized to that of 18S rRNA. Values are means ± SD (n = 3; ∗∗p < 0.01).
4. Discussion
AQP9 expression has been reported in many types of tissue including the leukocytes, liver, brain and lung [2]. In human skin, several studies have revealed abundant AQP3 expression from the basal layer to the stratum spinosum of the epidermis, while AQP9 distribution remains unclear. In our immunofluorescence studies, AQP9 expression was detected in the upper layers of the human epidermis. A similar staining pattern has been reported in a previous report in which AQP9 was detected specifically in the single layer of the upper SG in a mouse epidermis [17]. In the course of the wound healing process in mouse skin, AQP9 is also observed in multiple layers in the SG. Our previous study demonstrated that in cultured human keratinocytes, elevation of extracellular calcium concentration to stimulate cell differentiation triggers AQP9 mRNA expression [10]. In addition, further culturing under high calcium conditions augmented AQP9 expression. The treatment of cultured keratinocytes with VD3, a keratinocyte differentiating agent, also significantly increased AQP9 expression (Fig. 2). These findings lead us to the possibility that AQP9 plays a role in highly differentiated keratinocytes.
As in previous studies, immunofluorescence testing in this study also revealed high AQP3 expression from the basal layer to the stratum spinosum in human skin. This AQP3 staining was not detected in the cell layers where there was positive occludin expression. In contrast, AQP9 expression was highly restricted to the occludin-positive cell layers of the SG. Neither AQP3 nor AQP9 staining was observed in the portion beneath the occludin-positive layers. With regards to the localization of AQP3 and AQP9, the living cell layers of the epidermis could be divided into AQP3-positive, AQP3/AQP9- negative and AQP9-positive. Allowing that this interpretation is plausible, the biological and physiological importance of AQP3 and AQP9 distribution in the epidermis remain unclear.
Human and rat AQP9 have been independently identified from leukocytes and liver. A series of study on Xenopus oocytes demonstrated that human and rat AQP9 transport both water and non-charged small solutes such as glycerol, urea, purines and pyrimidines [22–24]. However, another study on Xenopus oocytes showed human AQP9-induced permeability to be restricted to water and urea [25]. As shown in Fig. 3B, AQP9 siRNA transfection into NHEK remarkably reduced 14C-glycerol uptake under culture conditions, which were not as pronounced as with AQP3 siRNA transfection. Since differentiated NHEK in culture express both AQP3 and AQP9, we examined glycerol uptake by conducting double transfection of AQP3 and AQP9 siRNA. The double knock-down cells revealed a further reduction in 14C-glycerol uptake, suggesting that AQP9 in NHEK functions as an uptake mechanism for glycerol and facilitates glycerol transport. In addition, although further studies are required to investigate the conflicting results for glycerol permeability in human AQP9, our findings supported the fact that human AQP9 exhibits glycerol permeability.
Recently, aquaglyceroporins have been shown in addition to urea transporters as being involved in the urea transport mechanism in NHEK [26]. In a series of inhibitor studies, treatment with divalent cations inhibiting the water and solute permeability of AQPs reduced 14C-urea uptake by NHEK. These experiments presented data that urea transport is inhibited by 34% by the urea transporter inhibitor, thiourea, and by 66% by the AQP inhibitor, mercury chloride. Our gene knock-down approaches also presented evidence that both AQP3 and AQP9 contribute to the uptake of urea by NHEK (Fig. 3C). AQP3 or AQP9 siRNA transfection revealed a reduction in 14C-urea uptake by NHEK of 11.7% and 9.4%, respectively. Further inhibition of urea uptake (24.4% reduction) was achieved by double transfection with AQP3 and AQP9 siRNA. Thus, both AQP3 and AQP9 expressed in NHEK are thought to be involved in urea transport mechanisms. Grether-Beck et al. reported that urea is a small-molecule regulator of epidermal permeability barrier function and antimicrobial peptide expression. While further studies are needed to clarify the physiological significance of urea transport through urea transporters and/or AQPs, it is considered that AQP9 localized in the upper SG may play an important role in the barrier function.
In a separate experiment, we examined the osmotic water permeability of AQP9 expressed in NHEK using a SX20 stopped-slow spectrometer. Unlike glycerol and urea permeability, AQP9 siRNA transfection did not affect water transportation under culture conditions (data not shown). Proteoliposome reconstructed with purified rat AQP9 protein exhibited high glycerol and urea permeability in a mercurial-sensitive manner, but only minimal osmotic water permeability [27]. Water permeability of erythrocytes from AQP9-null mice remained intact, whereas glycerol transport was substantially decreased [28]. AQP9 expressed in keratinocytes may play only a small role as a water channel.
RA is well known to be a critical regulator of proliferation and differentiation of keratinocytes [29,30]. RA stimulates proliferation of basal keratinocytes, leading to an accelerated turnover and thickening of the epidermis [31–33]. Several studies have shown that RA is a potent inducer of AQP3 expression in keratinocytes. Topical application of RA on mouse and human skin explants exhibited a marked increase in AQP3 expression in the epidermis [11,13]. Notably, in these studies intense AQP3 staining was observed in both basal and suprabasal keratinocytes. The up-regulation of AQP3 expression by RA has been also demonstrated in undifferentiated and differentiated keratinocytes in culture [14]. In our study, RA was added to keratinocytes 4 days after calcium-induced differentiation. This RA treatment significantly increased AQP3 mRNA expression as shown in Fig. 4A. Thus AQP3 in keratinocytes appears to be up-regulated by RA, independent of cell differentiation.
In contrast, AQP9 mRNA expression in differentiated NHEK was reduced by 24 h of RA treatment (Fig. 4B). The down-regulation of AQP9 and corresponding up-regulation of AQP3 mRNA expression in the differentiated cells was detected at doses as low as 0.01 μM (Fig. 4C, D). RA regulates many markers of epidermal differentiation. It suppresses the expression of differentiation-associated components such as keratin 1, keratin 10, loricrin, profilaggrin/filaggrin and transglutaminase I in cultured keratinocytes [29]. A similar phenomenon has been reported in NHEK transfected by dual oxidase I (DUOX1) siRNA, in which AQP9 mRNA expression as well as the differentiation markers mentioned above are inhibited [34]. DUOX1 is thought to be a major enzyme that produces H2O2 and contributes to keratinocyte differentiation in response to Ca2+. Taken together with the AQP9 expression in the upper SG of the human epidermis (Fig. 1) and the up-regulation by VD3 in culture (Fig. 2), AQP9 is thought to be one of the group of differentiation markers that plays a role in terminal differentiation of the epidermis.
In summary, AQP9 expression was highly restricted to the upper stratum granulosum of the human epidermis where AQP3 expression was diminished. RA treatment of differentiated NHEK in culture up-regulated AQP3 and down-regulated AQP9 mRNA expression. These findings suggest that the regulation of AQP9 expression is totally different from that of AQP3. Occludin, a tight junction marker, was detected in the epidermal layers expressing AQP9, indicating that AQP9 co-exists in the upper SG where the tight junctions are formed. Considering that the tight junctions function as a paracellular barrier against small molecules, the presence of AQP9 in the human epidermis may have a role to play as a conduit for inside-to-outside and/or outside-to-inside movement of glycerol and urea.
Acknowledgments
We would like to thank Prof. Mikio Furuse (Department of Cell Biology, Graduate School of Medicine, Kobe University) for the generous gift of occludin antibodies.
References
- 1.Borgnia M. Cellular and molecular biology of the aquaporin water channels. Annu. Rev. Biochem. 1999;68:425–458. doi: 10.1146/annurev.biochem.68.1.425. [DOI] [PubMed] [Google Scholar]
- 2.Takata K., Matsuzaki T., Tajika Y. Aquaporins: water channel proteins of the cell membrane. Prog. Histochem. Cytochem. 2004;39(1):1–83. doi: 10.1016/j.proghi.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Ishibashi K. The evolutionary aspects of aquaporin family. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;300(3):R566–R576. doi: 10.1152/ajpregu.90464.2008. [DOI] [PubMed] [Google Scholar]
- 4.Sougrat R. Functional expression of AQP3 in human skin epidermis and reconstructed epidermis. J. Invest. Dermatol. 2002;118(4):678–685. doi: 10.1046/j.1523-1747.2002.01710.x. [DOI] [PubMed] [Google Scholar]
- 5.Dumas M. Hydrating skin by stimulating biosynthesis of aquaporins. J. Drugs Dermatol. 2007;6(6 Suppl.):s20–s24. [PubMed] [Google Scholar]
- 6.Ma T. Impaired stratum corneum hydration in mice lacking epidermal water channel aquaporin-3. J. Biol. Chem. 2002;277(19):17147–17153. doi: 10.1074/jbc.M200925200. [DOI] [PubMed] [Google Scholar]
- 7.Hara M., Ma T., Verkman A.S. Selectively reduced glycerol in skin of aquaporin-3-deficient mice may account for impaired skin hydration, elasticity, and barrier recovery. J. Biol. Chem. 2002;277(48):46616–46621. doi: 10.1074/jbc.M209003200. [DOI] [PubMed] [Google Scholar]
- 8.Hara M., Verkman A.S. Glycerol replacement corrects defective skin hydration, elasticity, and barrier function in aquaporin-3-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 2003;100(12):7360–7365. doi: 10.1073/pnas.1230416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hara-Chikuma M., Verkman A.S. Aquaporin-3 facilitates epidermal cell migration and proliferation during wound healing. J. Mol. Med. (Berl) 2008;86(2):221–231. doi: 10.1007/s00109-007-0272-4. [DOI] [PubMed] [Google Scholar]
- 10.Sugiyama Y. Osmotic stress up-regulates aquaporin-3 gene expression in cultured human keratinocytes. Biochim. Biophys. Acta. 2001;1522(2):82–88. doi: 10.1016/s0167-4781(01)00320-7. [DOI] [PubMed] [Google Scholar]
- 11.Bellemere G., Von Stetten O., Oddos T. Retinoic acid increases aquaporin 3 expression in normal human skin. J. Invest. Dermatol. 2008;128(3):542–548. doi: 10.1038/sj.jid.5701047. [DOI] [PubMed] [Google Scholar]
- 12.Cao C. All-trans retinoic acid attenuates ultraviolet radiation-induced down-regulation of aquaporin-3 and water permeability in human keratinocytes. J. Cell. Physiol. 2008;215(2):506–516. doi: 10.1002/jcp.21336. [DOI] [PubMed] [Google Scholar]
- 13.Hara-Chikuma M. The expression of differentiation markers in aquaporin-3 deficient epidermis. Arch. Dermatol. Res. 2009;301(3):245–252. doi: 10.1007/s00403-009-0927-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang Y.J. PPARgamma activators stimulate aquaporin 3 expression in keratinocytes/epidermis. Exp. Dermatol. 2011;20(7):595–599. doi: 10.1111/j.1600-0625.2011.01269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song X. Nicotinamide attenuates aquaporin 3 overexpression induced by retinoic acid through inhibition of EGFR/ERK in cultured human skin keratinocytes. Int. J. Mol. Med. 2008;22(2):229–236. [PubMed] [Google Scholar]
- 16.Horie I. Tumor necrosis factor-alpha decreases aquaporin-3 expression in DJM-1 keratinocytes. Biochem. Biophys. Res. Commun. 2009;387(3):564–568. doi: 10.1016/j.bbrc.2009.07.077. [DOI] [PubMed] [Google Scholar]
- 17.Rojek A.M. Defective glycerol metabolism in aquaporin 9 (AQP9) knockout mice. Proc. Natl. Acad. Sci. U.S.A. 2007;104(9):3609–3614. doi: 10.1073/pnas.0610894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugiyama Y. Putative hyaluronan synthase mRNA are expressed in mouse skin and TGF-beta upregulates their expression in cultured human skin cells. J. Invest. Dermatol. 1998;110(2):116–121. doi: 10.1046/j.1523-1747.1998.00093.x. [DOI] [PubMed] [Google Scholar]
- 19.Yuki T. Characterization of tight junctions and their disruption by UVB in human epidermis and cultured keratinocytes. J. Invest. Dermatol. 2011;131(3):744–752. doi: 10.1038/jid.2010.385. [DOI] [PubMed] [Google Scholar]
- 20.Bikle D.D. Calcium- and vitamin D-regulated keratinocyte differentiation. Mol. Cell. Endocrinol. 2001;177(1–2):161–171. doi: 10.1016/s0303-7207(01)00452-x. [DOI] [PubMed] [Google Scholar]
- 21.Lehmann B., Querings K., Reichrath J. Vitamin D and skin: new aspects for dermatology. Exp. Dermatol. 2004;13(Suppl. 4):11–15. doi: 10.1111/j.1600-0625.2004.00257.x. [DOI] [PubMed] [Google Scholar]
- 22.Ko S.B. Cloning and functional expression of rAOP9L a new member of aquaporin family from rat liver. Biochem. Mol. Biol. Int. 1999;47(2):309–318. doi: 10.1080/15216549900201333. [DOI] [PubMed] [Google Scholar]
- 23.Tsukaguchi H. Molecular characterization of a broad selectivity neutral solute channel. J. Biol. Chem. 1998;273(38):24737–24743. doi: 10.1074/jbc.273.38.24737. [DOI] [PubMed] [Google Scholar]
- 24.Tsukaguchi H. Functional and molecular characterization of the human neutral solute channel aquaporin-9. Am. J. Physiol. 1999;277(5 Pt 2):F685–F696. doi: 10.1152/ajprenal.1999.277.5.F685. [DOI] [PubMed] [Google Scholar]
- 25.Ishibashi K. Cloning and functional expression of a new aquaporin (AQP9) abundantly expressed in the peripheral leukocytes permeable to water and urea, but not to glycerol. Biochem. Biophys. Res. Commun. 1998;244(1):268–274. doi: 10.1006/bbrc.1998.8252. [DOI] [PubMed] [Google Scholar]
- 26.Grether-Beck S. Urea uptake enhances barrier function and antimicrobial defense in humans by regulating epidermal gene expression. J. Invest. Dermatol. 2012;132(6):1561–1572. doi: 10.1038/jid.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carbrey J.M. Aquaglyceroporin AQP9: solute permeation and metabolic control of expression in liver. Proc. Natl. Acad. Sci. U.S.A. 2003;100(5):2945–2950. doi: 10.1073/pnas.0437994100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y. Aquaporin 9 is the major pathway for glycerol uptake by mouse erythrocytes, with implications for malarial virulence. Proc. Natl. Acad. Sci. U.S.A. 2007;104(30):12560–12564. doi: 10.1073/pnas.0705313104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fisher G.J., Voorhees J.J. Molecular mechanisms of retinoid actions in skin. FASEB J. 1996;10(9):1002–1013. doi: 10.1096/fasebj.10.9.8801161. [DOI] [PubMed] [Google Scholar]
- 30.Fisher C., Blumenberg M., Tomic-Canic M. Retinoid receptors and keratinocytes. Crit. Rev. Oral. Biol. Med. 1995;6(4):284–301. doi: 10.1177/10454411950060040201. [DOI] [PubMed] [Google Scholar]
- 31.Fisher G.J., Voorhees J.J. Molecular mechanisms of photoaging and its prevention by retinoic acid: ultraviolet irradiation induces MAP kinase signal transduction cascades that induce Ap-1-regulated matrix metalloproteinases that degrade human skin in vivo. J. Investig. Dermatol. Symp. Proc. 1998;3(1):61–68. [PubMed] [Google Scholar]
- 32.Weiss J.S. Topical tretinoin improves photoaged skin. A double-blind vehicle-controlled study. JAMA. 1988;259(4):527–532. [PubMed] [Google Scholar]
- 33.Varani J. Heparin-binding epidermal-growth-factor-like growth factor activation of keratinocyte ErbB receptors mediates epidermal hyperplasia, a prominent side-effect of retinoid therapy. J. Invest. Dermatol. 2001;117(6):1335–1341. doi: 10.1046/j.0022-202x.2001.01564.x. [DOI] [PubMed] [Google Scholar]
- 34.Choi H. Hydrogen peroxide generated by DUOX1 regulates the expression levels of specific differentiation markers in normal human keratinocytes. J. Dermatol. Sci. 2014;74(1):56–63. doi: 10.1016/j.jdermsci.2013.11.011. [DOI] [PubMed] [Google Scholar]


