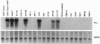Abstract
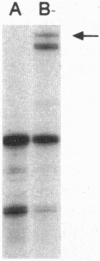
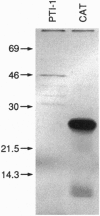
Elucidating the relevant genomic changes mediating development and evolution of prostate cancer is paramount for effective diagnosis and therapy. A putative dominant-acting nude mouse prostatic carcinoma tumor-inducing gene, PTI-1, has been cloned that is expressed in patient-derived human prostatic carcinomas but not in benign prostatic hypertrophy or normal prostate tissue. PTI-1 was detected by cotransfecting human prostate carcinoma DNA into CREF-Trans 6 cells, inducing tumors in nude mice, and isolating genes displaying increased expression in tumor-derived cells by using differential RNA display (DD). Screening a human prostatic carcinoma (LNCaP) cDNA library with a 214-bp DNA fragment found by DD permitted the cloning of a full-length 2.0-kb PTI-1 cDNA. Sequence analysis indicates that PTI-1 is a gene containing a 630-bp 5' sequence and a 3' sequence homologous to a truncated and mutated form of human elongation factor 1 alpha. In vitro translation demonstrates that the PTI-1 cDNA encodes a predominant approximately 46-kDa protein. Probing Northern blots with a DNA fragment corresponding to the 5' region of PTI-1 identifies multiple PTI-1 transcripts in RNAs from human carcinoma cell lines derived from the prostate, lung, breast, and colon. In contrast, PTI-1 RNA is not detected in human melanoma, neuroblastoma, osteosarcoma, normal cerebellum, or glioblastoma multiforme cell lines. By using a pair of primers recognizing a 280-bp region within the 630-bp 5' PTI-1 sequence, reverse transcription-PCR detects PTI-1 expression in patient-derived prostate carcinomas but not in normal prostate or benign hypertrophic prostate tissue. In contrast, reverse transcription-PCR detects prostate-specific antigen expression in all of the prostate tissues. These results indicate that PTI-1 may be a member of a class of oncogenes that could affect protein translation and contribute to carcinoma development in human prostate and other tissues. The approaches used, rapid expression cloning with the CREF-Trans 6 system and the DD strategy, should prove widely applicable for identifying and cloning additional human oncogenes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991 Jan 10;349(6305):117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- Cavallius J., Rattan S. I., Clark B. F. Changes in activity and amount of active elongation factor 1 alpha in aging and immortal human fibroblast cultures. Exp Gerontol. 1986;21(3):149–157. doi: 10.1016/0531-5565(86)90068-9. [DOI] [PubMed] [Google Scholar]
- Chi K., Jones D. V., Frazier M. L. Expression of an elongation factor 1 gamma-related sequence in adenocarcinomas of the colon. Gastroenterology. 1992 Jul;103(1):98–102. doi: 10.1016/0016-5085(92)91101-9. [DOI] [PubMed] [Google Scholar]
- Ender B., Lynch P., Kim Y. H., Inamdar N. V., Cleary K. R., Frazier M. L. Overexpression of an elongation factor-1 gamma-hybridizing RNA in colorectal adenomas. Mol Carcinog. 1993;7(1):18–20. doi: 10.1002/mc.2940070104. [DOI] [PubMed] [Google Scholar]
- Epstein J. I., Pizov G., Walsh P. C. Correlation of pathologic findings with progression after radical retropubic prostatectomy. Cancer. 1993 Jun 1;71(11):3582–3593. doi: 10.1002/1097-0142(19930601)71:11<3582::aid-cncr2820711120>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Fasano O., Birnbaum D., Edlund L., Fogh J., Wigler M. New human transforming genes detected by a tumorigenicity assay. Mol Cell Biol. 1984 Sep;4(9):1695–1705. doi: 10.1128/mcb.4.9.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A. G., Flomen R. M., Tizard M. L., Grant D. A. Differential screening of a human pancreatic adenocarcinoma lambda gt11 expression library has identified increased transcription of elongation factor EF-1 alpha in tumour cells. Int J Cancer. 1992 Mar 12;50(5):740–745. doi: 10.1002/ijc.2910500513. [DOI] [PubMed] [Google Scholar]
- Guarini L., Temponi M., Bruce J. N., Bollon A. P., Duigou G. J., Moulton T. A., Ferrone S., Fisher P. B. Expression and modulation by cytokines of the intercellular adhesion molecule-1 (ICAM-1) in human central nervous system tumor cell cultures. Int J Cancer. 1990 Dec 15;46(6):1041–1047. doi: 10.1002/ijc.2910460616. [DOI] [PubMed] [Google Scholar]
- Horoszewicz J. S., Leong S. S., Kawinski E., Karr J. P., Rosenthal H., Chu T. M., Mirand E. A., Murphy G. P. LNCaP model of human prostatic carcinoma. Cancer Res. 1983 Apr;43(4):1809–1818. [PubMed] [Google Scholar]
- Hughes D., Atkins J. F., Thompson S. Mutants of elongation factor Tu promote ribosomal frameshifting and nonsense readthrough. EMBO J. 1987 Dec 20;6(13):4235–4239. doi: 10.1002/j.1460-2075.1987.tb02772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang Y. W., Sanchez A., Miller D. L. Mutagenesis of bacterial elongation factor Tu at lysine 136. A conserved amino acid in GTP regulatory proteins. J Biol Chem. 1989 May 15;264(14):8304–8309. [PubMed] [Google Scholar]
- Jiang H., Lin J., Su Z. Z., Herlyn M., Kerbel R. S., Weissman B. E., Welch D. R., Fisher P. B. The melanoma differentiation-associated gene mda-6, which encodes the cyclin-dependent kinase inhibitor p21, is differentially expressed during growth, differentiation and progression in human melanoma cells. Oncogene. 1995 May 4;10(9):1855–1864. [PubMed] [Google Scholar]
- Lazaris-Karatzas A., Montine K. S., Sonenberg N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5' cap. Nature. 1990 Jun 7;345(6275):544–547. doi: 10.1038/345544a0. [DOI] [PubMed] [Google Scholar]
- Lazaris-Karatzas A., Sonenberg N. The mRNA 5' cap-binding protein, eIF-4E, cooperates with v-myc or E1A in the transformation of primary rodent fibroblasts. Mol Cell Biol. 1992 Mar;12(3):1234–1238. doi: 10.1128/mcb.12.3.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew Y., Jones D. V., Mars W. M., Evans D., Byrd D., Frazier M. L. Expression of elongation factor-1 gamma-related sequence in human pancreatic cancer. Pancreas. 1992;7(2):144–152. doi: 10.1097/00006676-199203000-00003. [DOI] [PubMed] [Google Scholar]
- Liang P., Pardee A. B. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992 Aug 14;257(5072):967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- Lisitsyn N., Lisitsyn N., Wigler M. Cloning the differences between two complex genomes. Science. 1993 Feb 12;259(5097):946–951. doi: 10.1126/science.8438152. [DOI] [PubMed] [Google Scholar]
- Loeb L. A. Microsatellite instability: marker of a mutator phenotype in cancer. Cancer Res. 1994 Oct 1;54(19):5059–5063. [PubMed] [Google Scholar]
- Lu-Yao G. L., McLerran D., Wasson J., Wennberg J. E. An assessment of radical prostatectomy. Time trends, geographic variation, and outcomes. The Prostate Patient Outcomes Research Team. JAMA. 1993 May 26;269(20):2633–2636. doi: 10.1001/jama.269.20.2633. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Ngo N. In vitro assembly of multiprotein complexes containing alpha, beta, and gamma tubulin, heat shock protein HSP70, and elongation factor 1 alpha. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):3028–3032. doi: 10.1073/pnas.90.7.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick W. C. Mechanism and regulation of eukaryotic protein synthesis. Microbiol Rev. 1992 Jun;56(2):291–315. doi: 10.1128/mr.56.2.291-315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukamel E., Hanna J., deKernion J. B. Pitfalls in preoperative staging in prostate cancer. Urology. 1987 Oct;30(4):318–321. doi: 10.1016/0090-4295(87)90292-5. [DOI] [PubMed] [Google Scholar]
- Rabbitts T. H. Chromosomal translocations in human cancer. Nature. 1994 Nov 10;372(6502):143–149. doi: 10.1038/372143a0. [DOI] [PubMed] [Google Scholar]
- Riis B., Rattan S. I., Clark B. F., Merrick W. C. Eukaryotic protein elongation factors. Trends Biochem Sci. 1990 Nov;15(11):420–424. doi: 10.1016/0968-0004(90)90279-k. [DOI] [PubMed] [Google Scholar]
- Salo J. O., Kivisaari L., Rannikko S., Lehtonen T. Computerized tomography and transrectal ultrasound in the assessment of local extension of prostatic cancer before radical retropubic prostatectomy. J Urol. 1987 Mar;137(3):435–438. doi: 10.1016/s0022-5347(17)44059-6. [DOI] [PubMed] [Google Scholar]
- Sandbaken M. G., Culbertson M. R. Mutations in elongation factor EF-1 alpha affect the frequency of frameshifting and amino acid misincorporation in Saccharomyces cerevisiae. Genetics. 1988 Dec;120(4):923–934. doi: 10.1093/genetics/120.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd J. C., Walldorf U., Hug P., Gehring W. J. Fruit flies with additional expression of the elongation factor EF-1 alpha live longer. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7520–7521. doi: 10.1073/pnas.86.19.7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N. Translation factors as effectors of cell growth and tumorigenesis. Curr Opin Cell Biol. 1993 Dec;5(6):955–960. doi: 10.1016/0955-0674(93)90076-3. [DOI] [PubMed] [Google Scholar]
- Song J. M., Picologlou S., Grant C. M., Firoozan M., Tuite M. F., Liebman S. Elongation factor EF-1 alpha gene dosage alters translational fidelity in Saccharomyces cerevisiae. Mol Cell Biol. 1989 Oct;9(10):4571–4575. doi: 10.1128/mcb.9.10.4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z. Z., Austin V. N., Zimmer S. G., Fisher P. B. Defining the critical gene expression changes associated with expression and suppression of the tumorigenic and metastatic phenotype in Ha-ras-transformed cloned rat embryo fibroblast cells. Oncogene. 1993 May;8(5):1211–1219. [PubMed] [Google Scholar]
- Su Z. Z., Olsson C. A., Zimmer S. G., Fisher P. B. Transfer of a dominant-acting tumor-inducing oncogene from human prostatic carcinoma cells to cloned rat embryo fibroblast cells by DNA-transfection. Anticancer Res. 1992 Mar-Apr;12(2):297–304. [PubMed] [Google Scholar]
- Tapio S., Kurland C. G. Mutant EF-Tu increases missense error in vitro. Mol Gen Genet. 1986 Oct;205(1):186–188. doi: 10.1007/BF02428051. [DOI] [PubMed] [Google Scholar]
- Tatsuka M., Mitsui H., Wada M., Nagata A., Nojima H., Okayama H. Elongation factor-1 alpha gene determines susceptibility to transformation. Nature. 1992 Sep 24;359(6393):333–336. doi: 10.1038/359333a0. [DOI] [PubMed] [Google Scholar]
- Vita J. R., Edwalds G. M., Gorey T., Housepian E. M., Fetell M. R., Guarini L., Langer J. A., Fisher P. B. Enhanced in vitro growth suppression of human glioblastoma cultures treated with the combination of recombinant fibroblast and immune interferons. Anticancer Res. 1988 May-Jun;8(3):297–302. [PubMed] [Google Scholar]