Abstract
Background. Low 25-hydroxyvitamin D (25(OH)D) has been associated with increased HIV mortality, but prospective studies assessing treatment outcomes after combination antiretroviral therapy (cART) initiation in resource-limited settings are lacking.
Methods. A case-cohort study (N = 411) was nested within a randomized cART trial of 1571 cART-naive adults in 8 resource-limited settings and the United States. The primary outcome (WHO stage 3/4 disease or death within 96 weeks of cART initiation) was met by 192 cases, and 152 and 29 cases met secondary outcomes of virologic and immunologic failure. We studied prevalence and risk factors for baseline low 25(OH)D (<32 ng/mL) and examined associated outcomes using proportional hazard models.
Results. Low 25(OH)D prevalence was 49% and ranged from 27% in Brazil to 78% in Thailand. Low 25(OH)D was associated with high body mass index (BMI), winter/spring season, country-race group, and lower viral load. Baseline low 25(OH)D was associated with increased risk of human immunodeficiency virus (HIV) progression and death (adjusted hazard ratio (aHR) 2.13; 95% confidence interval [CI], 1.09–4.18) and virologic failure (aHR 2.42; 95% CI, 1.33–4.41).
Conclusions. Low 25(OH)D is common in diverse HIV-infected populations and is an independent risk factor for clinical and virologic failure. Studies examining the potential benefit of vitamin D supplementation among HIV patients initiating cART are warranted.
Keywords: Vitamin D, HIV, micronutrient, ART, mortality, Africa, Asia, Americas
(See the major article by Shepherd et al on pages 234–43.)
25-Hydroxyvitamin D regulates innate and adaptive immune defenses [1–5] and its role in pathogen-specific immunity is gaining recognition [6, 7]. 25-hydroxyvitamin D insufficiency (25(OH)D > 20 to <32 ng/mL) and deficiency (25(OH) vitamin D < 20 ng/mL), or low 25(OH)D [8], is common and may be a risk factor for poor outcomes in human immunodeficiency virus (HIV) [9–13].
25-hydroxyvitamin D concentration is affected by genetics, nutritional status, and factors that affect ultraviolet (UV) light exposure and absorption, such as season, latitude, and skin pigmentation [14–16]. However, few studies on low 25(OH)D and HIV disease progression have been conducted in multiple geographic settings, and only 1 study, which found an association between 25(OH)D deficiency and mortality, examined this relationship at combination antiretroviral therapy (cART) initiation [9–12, 17]. Furthermore, no studies examined the association between 25(OH)D level and viral load at baseline and after cART initiation.
The Prospective Evaluation of Antiretrovirals in Resource Limited Settings (PEARLS) trial provided an opportunity to investigate the association between low 25(OH)D and cART outcomes in diverse settings. We used a case-cohort design in the PEARLS trial to assess risk factors for baseline low 25(OH)D in a cohort of human immunodeficiency virus type 1 (HIV-1) infected individuals at cART initiation and prospectively examined its association with HIV disease progression, virologic failure, and immunologic failure post-cART initiation in 8 low- and middle-income countries and the United States. Finding a strong association between low 25(OH)D and HIV treatment outcomes would provide a robust rationale for low 25(OH)D supplementation strategies to reduce HIV-related morbidity and mortality globally.
METHODS
Study Population
Our study population was a nested case-cohort sample of the PEARLS trial (Adult AIDS Clinical Trials Group (ACTG) A5175, ClinicalTrials.gov NCT00084136), an open-label, randomized clinical trial examining once-daily vs twice-daily dosing of 3 cART regimens. The PEARLS study has been described elsewhere [18]. In brief, between May 2005 and July 2007, 1571 HIV-infected adults (18 years or older) with a CD4+ count <300 cells/mm3 were randomized to 1 of 3 treatment arms: efavirenz plus lamivudine/zidovudine; atazanavir plus didanosine EC, and emtricitabine; or efavirenz plus emtricitabine/tenofovir-DF. Participants were enrolled in Brazil (N = 231), Haiti (N = 100), India (N = 255), Malawi (N = 221), Peru (N = 134), South Africa (N = 210), Thailand (N = 100), United States (N = 210), and Zimbabwe (N = 110). In total, 739 (47%) were women, and 787 (50%) participants were black. Exclusion criteria were pregnancy, acute illness, or acute, serious comorbidities, and specific laboratory abnormalities [18]. A physical exam, CD4+ count, and HIV viral load were scheduled at least every 8 weeks. Diagnostic criteria were standardized across sites using ACTG Appendix 60. Study follow-up ended May 2010.
We used a case-cohort design [19] to investigate the association of low 25(OH)D with outcomes after cART initiation. A case was defined as a participant who developed the primary outcome of new WHO Stage 3 or 4 events or death within 96 weeks post-cART initiation. Secondary outcomes were virologic failure (defined as 2 successive plasma HIV-1 RNA measurements ≥1000 copies/mL at or after the week 16 visit) and immunologic failure (defined as CD4+ lymphocyte count <100 cells/mm3 at >48 weeks after cART initiation). Those meeting criteria for secondary outcomes were defined as cases in separate analyses.
The full case-cohort was composed of a prespecified, randomly chosen subcohort of 270 participants (30 participants chosen randomly from each country and stratified by randomized treatment arm) and any cases meeting primary or secondary outcomes as defined above and who were not included in the random sub-cohort sample. Cases were compared to those in the random subcohort who did not develop the outcome. The case cohort included a total of N = 411 for primary outcome, n = 378 for virological failure outcome, and n = 278 for immunological failure outcome analyses (Figure 1 ).
Figure 1.
Flowchart of case-cohort study. The case cohort analysis consisted of the random subcohort (random subsample from full cohort) and any additional cases from the full cohort that met the definition. Our primary outcome (1°) was defined as WHO stage 3 or 4 event or death (N = 219 controls and N = 192 cases) within 96 weeks post-cART initiation. Secondary outcomes included virological failure (VF; N = 226 controls and N = 152 cases) and immunological failure (IF; N = 249 controls and N = 29 cases). Virologic failure was defined as 2 successive plasma HIV-1 RNA levels above 1000 copies/mL at or after 16 weeks post-ART initiation. Immunologic failure was defined as CD4+ lymphocyte count <100 cells/mm3 after 48 weeks post-ART initiation. Abbreviations: cART, combination antiretroviral therapy; HIV-1, human immunodeficiency virus type 1; WHO, World Health Organization.
Ethics Statement
This study was approved by institutional review boards and ethics committees at Johns Hopkins University and at each participating institution [18]. Written, informed consent was obtained from study participants, and human experimentation guidelines of the US Department of Health and Human Services were followed.
Laboratory Methods
Serum specimens were collected before or at entry to the PEARLS study and were stored at −70°C. Serum vitamin D concentrations were measured at the CLIA-certified Immunology Laboratory at the Johns Hopkins Hospital using the chemiluminescence immunoassay (DiaSorin, Stillwater, MN), which measures both D2 and D3 and is reported as a total serum 25(OH) vitamin D concentration [20]. Plasma HIV-1 RNA concentration was measured in real time by the Roche Amplicor Monitor assay (v1.5, Branchburg, NJ) at site laboratories participating in the DAIDS Virology Quality Assurance program.
Definitions
Low 25(OH)D was defined as 25-OH Vitamin D <32 ng/mL [8]. Severe deficiency was defined as <10 ng/mL, deficiency as 10–20 ng/mL, and insufficiency as >20 to <32 ng/mL. Sufficient vitamin D concentration was defined as ≥32 ng/mL. Currently no clear consensus exists regarding what concentration represents a clinically meaningful marker of insufficiency or deficiency [16, 21]. Thus serum vitamin D was evaluated as a categorical variable, using established clinical cutoffs, and as a continuous variable. Seasons were defined as follows, depending on the hemisphere of the country: spring (months 3–6; southern hemisphere: months 9–11), summer (months 6–8; southern hemisphere: months 12–2), autumn (months 9–11; southern hemisphere: months 3–5), winter (months 12–2; southern hemisphere: months 6–8). Race was defined as black, white, Asian, or other.
To assess weight and height, trained research assistants used standardized procedures. HIV stage was classified per World Health Organization (WHO) guidelines [22]. Patients were asked to recall any history of AIDS-defining illnesses using a standardized questionnaire. Clinical history was performed at scheduled visits at weeks 2, 4, 8, and then every 4 weeks through week 24 and then every 8 weeks. The exact week from randomization was used to calculate event and censoring times. Those not meeting case criteria were censored at the last clinic visit, which could not be >96 weeks.
Statistical Analysis
The random subcohort was used to estimate low 25(OH)D prevalence. Confidence intervals were calculated with Agresti and Coull method. Given the colinearity of race with country, a country-race variable was formulated. Fisher exact test and Wilcoxon tests were used to explore the association of baseline covariates with the outcome of low 25(OH)D. Multivariable logistic regression modeled these associations; the final model included BMI, country-race, season of sample, and viral load.
The full case-cohort sample was used to assess clinical outcomes associated with baseline low 25(OH)D. Cox proportional hazards regression was performed with partial likelihood weighting adjustment to account for the case-cohort sampling [19, 23]. The time to first qualifying event (primary outcome or 1 of 2 secondary outcomes) was calculated from cART initiation. All models were stratified by country and randomized treatment arm. The final multivariable models included season, BMI, race, CD4 count, and viral load. A history of AIDS-defining illness was a prespecified risk factor for poor clinical outcomes based on previous studies, but low 25(OH)D was hypothesized to be causally associated with both a history of AIDS-defining illness and poor outcomes. An additional multivariable model that also included history of AIDS-defining illness was included. An analysis using 25(OH)D concentration as a continuous variable was performed to assess a dose-related relationship between 25(OH)D and primary and secondary outcomes. Finally, possible nonlinear relationships between 25(OH)D and primary and secondary outcomes were explored nonparametrically using cubic splines.
RESULTS
Study Population and Comparison of Included and Excluded Persons
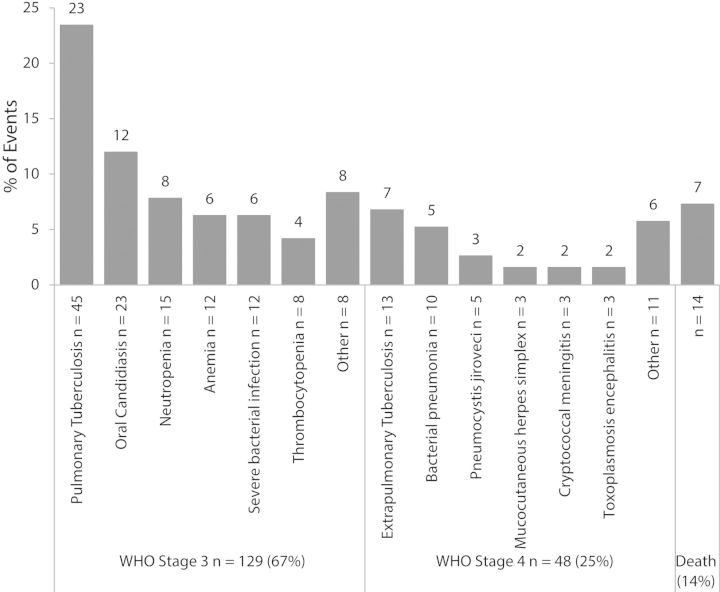
The random subcohort was comparable to the larger PEARLS parent study sample, except for country, which was the basis for the random subcohort stratification (data not shown). 20 of the 270 participants assigned to the random subcohort were excluded from analyses for missing baseline vitamin D data, as were an additional 9 subjects who met a case definition for the primary or secondary outcomes. Most missing vitamin D data were from India due to difficulties in exporting samples. Apart from country and race, participants with missing data were significantly more likely to be underweight; they were otherwise similar to the full case-cohort sample (data not shown). In the PEARLS trial, 192 (12%) of 1571 participants had incident WHO stage 3 or 4 or death by 96 weeks (Figure 2). Of these cases, 31 were already included in the random subcohort. The remaining 161 cases (Supplementary Table 1) were added to these and the 219 controls without the primary outcome to create the full case-cohort for the primary outcome (n = 411).
Figure 2.
Specific etiology of WHO stage 3, stage 4, and death primary outcome events among cases in the A5175-NWCS319 case-cohort study (N = 192). Neutropenia: Absolute neutrophil count <500 cells/µL; Anemia: Hemoglobin <8.5 g/dL; Thrombocytopenia: platelets <50 000 platelets/µL. Causes of death included hepatitis, chronic diarrhea, stroke, Stevens-Johnson syndrome, pneumonia, suicide/homicide, and unknown. Abbreviation: WHO, World Health Organization.
Baseline 25-OH Vitamin D Concentration and Associated Factors
The random subcohort (n = 250) was used to estimate prevalence of low 25(OH)D and to identify baseline characteristics associated with baseline low 25(OH)D (Table 1). Overall prevalence of low 25(OH)D was 122/250 (49%; 95% CI, 43–55); 4 (2%) subjects (all from the US) had severe deficiency, 34 (14%) had deficiency, and 84 (34%) had insufficiency.
Table 1.
Baseline Characteristics of Study Population in Cohort Random Sample
| Characteristic | All N = 250 |
Sufficient 25(OH)D (≥32 ng/mL) N = 128 n (%)*** |
Low 25(OH)D (<32 ng/mL) N = 122 n (%)*** |
P Value* |
|---|---|---|---|---|
| Median age, years (IQR) | 35 (30–41) | 34.5 (31–42) | 35 (29–41) | .81** |
| Gender | ||||
| Male | 130 (52) | 70 (54) | 60 (46) | .38 |
| Female | 120 (48) | 58 (48) | 62 (52) | |
| Country | ||||
| Brazil | 30 (12) | 23 (77) | 7 (23) | <.001 |
| White | 14 (6) | 9 (64) | 5 (36) | |
| Black | 11 (4) | 10 (91) | 1 (9) | |
| Other | 5 (2) | 4 (80) | 1 (20) | |
| Haiti | 30 (12) | 22 (73) | 8 (27) | |
| India | 18 (7) | 5 (28) | 13 (72) | |
| Malawi | 28 (11) | 19 (68) | 9 (32) | |
| Peru | 29 (12) | 19 (66) | 10 (34) | |
| South Africa | 29 (12) | 13 (45) | 16 (55) | |
| Thailand | 27 (11) | 6 (22) | 21 (78) | |
| United States | 30 (12) | 8 (27) | 22 (73) | |
| White | 14 (6) | 6 (43) | 8 (57) | |
| Black | 13 (5) | 1 (8) | 12 (92) | |
| Other | 3 (1) | 1 (33) | 2 (67) | |
| Zimbabwe | 29 (12) | 13 (45) | 16 (55) | |
| Race | ||||
| White | 29 (12) | 16 (55) | 13 (45) | .001 |
| Black | 140 (56) | 78 (56) | 62 (44) | |
| Asian | 46 (18) | 11 (24) | 35 (76) | |
| Other | 34 (14) | 23 (67) | 11 (33) | |
| Season | ||||
| Summer | 68 (27) | 42 (62) | 26 (38) | .08 |
| Winter | 99 (40) | 42 (42) | 57 (58) | |
| Spring | 57 (23) | 25 (44) | 32 (56) | |
| Autumn | 26 (10) | 13 (50) | 13 (50) | |
| BMI | ||||
| BMI < 18.5 kg/m2 | 19 (8) | 9 (47) | 10 (53) | .01 |
| BMI 18–24.9 kg/m2 | 164 (66) | 95 (58) | 69 (42) | |
| BMI ≥ 25 kg/m2 | 77 (31) | 24 (32) | 43 (56) | |
| Prior tuberculosis diagnosis | 45 (18) | 21 (47) | 24 (53) | .50 |
| Prior AIDS diagnosis | 19 (8) | 10 (53) | 9 (47) | .90 |
| Median CD4 count, cells/µL (IQR) | 180 (90, 231) | 167 (79, 235) | 185 (102, 231) | .35** |
| Median HIV RNA, log10 copies/mL (IQR) | 5.08 (4.61, 5.44) | 4.93 (4.47, 5.44) | 5.16 (4.64, 5.51) | .06** |
| Median hemoglobin g/dL (IQR) | 12.4 (10.9, 13.8) | 12.2 (11.0, 13.8) | 12.7 (10.9, 13.9) | .46** |
| Median albumin g/dL (IQR) | 3.98 (3.60, 4.30) | 4.00 (3.60, 4.30) | 3.90 (3.60, 4.30) | .70** |
Data presented as no. (%) unless otherwise indicated.
Abbreviations: BMI, body mass index, IQR: interquartile range.
* P-values calculated using Fischer's exact test unless otherwise specified.
** P-value calculated using Wilcoxon's rank sum test.
*** ( ) Indicates prevalence of sufficient and low 25-hydroxyvitamin D within baseline characteristics unless otherwise noted.
Prevalence of low 25(OH)D varied significantly by country, race and season; it ranged from 7/30 (27%) in Brazil to 21/27 (78%) in Thailand and 13/18 (72%) in India (Table 1). Only the United States and Brazil included >1 race, with blacks in the United States having a prevalence of low 25(OH)D of 12/13 (92%). In multivariable analysis that was stratified by country and included cART regimen, race, season, BMI, and plasma HIV-1 RNA, low 25(OH)D was significantly associated with BMI, country-race groups, season, and HIV viral load (Table 2). Compared to normal BMI (18–24.9 kg/m2, n = 69/164), participants with higher BMI (≥25 kg/m2, n = 43/77; adjusted odds ratio (aOR) 2.02, 95% CI, 1.02–4.06), and lower BMI (<18 kg/m2, n = 10/19; aOR 2.23, 95% CI, .73–7.07) were more likely to have low 25(OH)D, though the latter did not reach statistical significance. Most groups had lower odds of low 25(OH)D than Zimbabwe, with the exception of Thailand (n = 21/27; aOR 3.22, 95% CI, 1.00–11.20) and blacks from the United States (n = 12/13; aOR 7.16, 95% CI, 1.27–76.10). Indians (n = 13/18) and other nonwhites, nonblacks from the United States (n = 10/17) also had increased odds of low 25(OH)D but did not reach statistical significance.
Table 2.
Risk Factors for Vitamin D Insufficiency/Deficiency (Serum 25(OH) Vitamin D Levels <32 ng/mL)
| Variable | Adjusted OR (95% CI)a |
|---|---|
| Country-Race | |
| Zimbabwe (black) | 1 |
| Haiti (black) | 0.23 (.07–. 73) |
| India (Asian) | 1.87 (.52–7.21 |
| Malawi (black) | 0.27 (.08–.83) |
| Peru (Other) | 0.39 (.13–1.15) |
| South Africa (black) | 0.79 (.26–2.39) |
| Thailand (Asian) | 3.22 (1.00–11.2) |
| Brazil (white) | 0.39 (.09–1.48) |
| Brazil (black) | 0.11 (.01–.60) |
| Brazil (other) | 0.25 (.02–1.65) |
| United States (white) | 0.88 (.21–3.69) |
| United States (black) | 7.16 (1.27–76.1) |
| United States (other) | 2.85 (.30–37.4) |
| BMIb | |
| Normal | 1 |
| Underweight | 2.23 (.73–7.09) |
| Overweight | 2.02 (1.02–4.06) |
| Season | |
| Summer | 1 |
| Winter | 3.75 (1.53–9.73) |
| Spring | 2.58 (1.04–6.67) |
| Autumn | 1.17 (.49–2.84) |
| HIV viral loadc | 1.46 (.97–2.24) |
Abbreviations: BMI, body mass index; CI, confidence interval; HIV-1, human immunodeficiency virus type 1; OR, odds ratio.
a Model adjusted for race, season, body mass index, plasma HIV-1 RNA, as well as country and randomized cART regimen stratification.
b Body mass index (BMI): Normal BMI 18.5–25 kg/m2; Underweight BMI < 18.5 kg/m2; Overweight BMI ≥ 25 kg/m2.
c Each log10 decrease in viral load.
Additionally, those assessed in winter (n = 57/99; aOR 3.75, 95% CI, 1.53–9.73) or spring (n = 32/57; aOR 2.58, 95% CI, 1.04–6.67) compared to summer (n = 26/68), and those with lower baseline viral load had higher odds of low 25(OH)D (aOR 1.46 per log10 decrease, 95% CI, .97–2.24; Supplementary Figure 1). An analysis of vitamin D concentration by category (severe deficiency, deficiency, insufficiency, and sufficient) identified the same significant risk factors (data not shown).
Progression to Clinical Events
In the full case-cohort for the primary outcome (n = 411), of 192 cases who experienced a new WHO stage 3 or 4 disease or death event by 96 weeks (Figure 2), 114 (59%) had low 25(OH)D. The median time to development of the primary outcome was 16 weeks (IQR 4 to 48 weeks) in the 114 with baseline low 25(OH)D compared to 18 weeks (IQR 4 to 40 weeks) in the 78 with sufficient vitamin D who also developed the primary outcome.
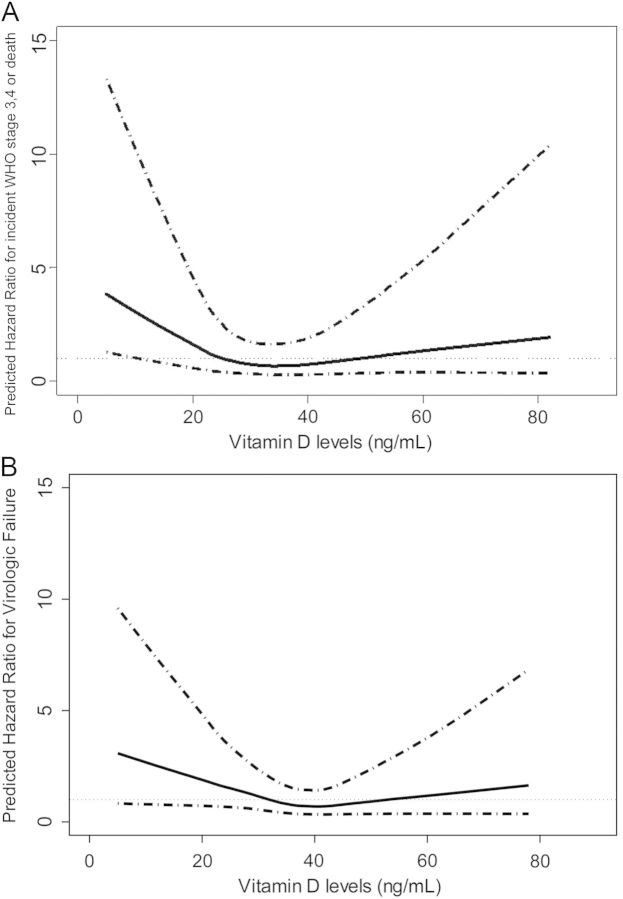
In univariate analysis, participants with low 25(OH)D had an increased risk of HIV disease progression or death compared to participants with sufficient 25(OH)D (hazard ratio (HR) 1.78, 95% CI, .98–3.26) (Table 3). In multivariate analysis, which adjusted for season, baseline CD4+ count, HIV-1 viral load, BMI, and race, the adjusted HR (aHR) was 1.84 (95% CI, .95–3.59). After adding history of AIDS-defining illness to the model, the aHR was 2.13 (95% CI, 1.09–4.18). Analysis of 25(OH)D concentration as a continuous variable showed a nonlinear relationship between increased hazard of HIV progression or death below 25 ng/mL, without any apparent benefit at much higher 25(OH)D concentrations (Figure 3A).
Table 3.
Association of Baseline Vitamin D Insufficiency/Deficiency (Serum 25(OH) Vitamin D Levels<32 ng/mL) With the Primary and Secondary Outcomes in the Full Case-Cohort
| Primary Outcome | Secondary Outcomes |
Factors Adjusted for in Model | ||
|---|---|---|---|---|
| Outcome Hazard Ratio (95% CI) | WHO Grade 3/4 Event or Death within 96 wks (n = 192)a | Virologic Failureb (n = 152) | Immunologic Failurec (n = 29) | |
| Univariable model | 1.78 (0.98–3.26) | 2.03 (1.81–3.50) | 3.78 (1.19–12.0) | |
| Multivariable model 1 | 1.84 (0.95–3.59) | 2.39 (1.31–4.36) | 2.37 (0.28–19.7) | Season of sample, race, BMI, viral load, CD4+ cell count |
| Multivariable model 2 | 2.13 (1.09–4.18) | 2.42 (1.33–4.41) | 2.28 (0.32–16.1) | All variables in Model 1, plus self-reported history of AIDS |
The conversion factor for converting nanograms per milliliter to nanomoles per liter is 2.496. All Cox models used partial likelihood with Breslow weights to accommodate case-cohort sampling design, and stratification by randomized treatment arm and country.
a N = cases with each outcome. These were compared to N = 219 controls in the analysis for the primary outcome, N = 226 controls for virologic failure and N = 249 controls for immunologic failure.
b Virologic failure: Two successive measurements of plasma HIV-1 RNA >1000 copies/mL, with the first measurement at the week 16 visit or later.
c Immunologic failure: CD4+ count <100 cells/mm3 at 48 weeks or later.
Figure 3.
A, Association of baseline serum 25-hydroxyvitamin D and the primary outcome of WHO stage 3 or 4 AIDS or death. Solid line indicates predicted hazard ratios for individual vitamin D levels, adjusted for baseline CD4, baseline log10 viral load, body mass index, and history of AIDS. Dotted lines denote 95% confidence interval. (B). Association of baseline serum 25-hydroxyvitamin D and the secondary outcome of virologic failure. Solid line indicates predicted hazard ratios for individual vitamin D levels, adjusted for baseline CD4, baseline log10 viral load, body mass index, and history of AIDS. Dotted lines denote 95% confidence interval. Abbreviation: WHO, World Health Organization.
Among the case-cohort for the secondary outcome of virologic failure (n = 378), 152 participants experienced virologic failure. The 87 with low 25(OH)D who experienced virologic failure had a shorter median time to virological failure (16 weeks, IQR 16–48 weeks) than the 65 with sufficient 25(OH)D (24 weeks, IQR 16–48 weeks), although this was not statistically significant (P-value .21); the 16-week visit was the earliest time virologic failure could be recorded. Using a similar series of models, low 25(OH)D was independently associated with a 2-fold increased hazard of virologic failure (HR 2.03 [95% CI, 1.81–3.50]; Table 3). This relationship remained statistically significant in all multivariate models and in analysis of 25(OH)D concentration as a continuous variable (Figure 3B). Finally, there were few immunologic failure events (n = 29). Although baseline low 25(OH)D was associated with immunological failure in univariate analysis (HR 3.78, CI, 1.19–12.0), this relationship was attenuated in multivariate models (Table 3).
DISCUSSION
Although several studies have examined risk factors for low 25-hydroxyvitaimin D in HIV-infected adults, we are unique in assessing this in a diverse, treatment-naive population, primarily from middle- and low-income countries where HIV burden is most prevalent. We found substantial differences in prevalence of 25(OH)D by country, race, season, and BMI. Most importantly, we found an association between baseline low 25(OH)D and risk of HIV disease progression, death, and notably virologic failure post-cART initiation. To our knowledge, this is the first time low 25(OH)D has been associated with HIV virologic failure and represents a novel risk factor for virologic failure.
Prevalence Estimates and Risk Factors Compared to Other Studies
We found that 49% of study participants in the random subcohort had low 25(OH)D, a prevalence similar to that reported in the US SUN cohort and other cohorts [24–27]. However, few studies have assessed pre-cART 25(OH)D levels, an important distinction, as cART itself can affect serum 25(OH)D levels [28–30]. In addition, we chose a higher prespecified cutoff of <32 ng/mL because, although severe vitamin D deficiency was relatively uncommon in our study, vitamin D insufficiency was relatively common in all country-race groups and has widespread clinical relevance [8]. More importantly, this is the most varied group of HIV-infected patients to have low 25(OH)D risk factors assessed. Genetics, nutrition, seasonal patterns, skin pigmentation, and sun exposure, including sun-avoidant cultural practices, affect serum 25(OH)D levels, contributing to the wide range in prevalence of low 25(OH)D across countries and racial groups studied. Our study confirms the importance of taking these factors into account in future 25-hydroxyvitamin D studies.
We also observed a significant association between low 25(OH)D and BMI, an important relationship in resource-limited populations with suboptimal nutrition. Interestingly, our study had higher proportions of overweight and obese individuals than undernourished individuals, a finding largely driven by the United States, South Africa, and Brazil. Being overweight or obese may be associated with less active lifestyle and therefore less sun exposure. Perhaps more relevant is that 25(OH)D appears to be sequestered in adipose tissue, a hormonally active tissue that plays a role in inflammation and throughout which vitamin D receptors are found. Vitamin D metabolites also influence the inflammatory response in adipose tissue in multiple ways, including modulating the release of proinflammatory factors such as MCP-1 and IL-6 by human preadipocytes [31, 32].
Low 25-hydroxyvitamin D Associated with HIV Disease Progression and Virological Failure
Our finding that low 25(OH)D was independently associated with higher rates of clinical progression and death is biologically plausible given the wealth of studies now emerging on the diverse roles of vitamin D in the immune system. Vitamin D regulates inflammatory cytokines [33], modulates adaptive immune responses, and controls T-cell activation [4], and B-cell proliferation [34]. 25(OH)D upregulation is associated with the peptide cathelicidin, important in killing intracellular pathogens such as Mycobacteiun tuberculosis which accounted for 30% of primary outcome events in this study. Studies demonstrate that polymorphisms of the vitamin D receptor are associated with faster progression to AIDS in HIV-infected patients [35, 36] and increased susceptibility to diseases such as tuberculosis in certain populations [37, 38]. Our findings are also consistent with the few studies investigating the association between 25(OH)D concentration and HIV outcomes, including the EuroSIDA cohort study and studies conducted in Tanzania. Only one of these enrolled patients initiating cART and did not assess the association between viral load and 25(OH)D levels [9–12, 17].
Our examination of the relationship between 25(OH)D and viral load indicates a mechanism that may explain the association between disease progression and low 25(OH)D. We were able to prospectively examine viral load in the PEARLS study due to frequent monitoring, close follow-up, and quality assurance guaranteeing consistency across sites. We found, paradoxically, that 25(OH)D at baseline has a non-statistically significant association with lower viral load; higher 25(OH)D levels in the face of elevated viral loads prior to cART initiation may result from increased systemic immune activation and modulation of vitamin D by activated macrophages, but this finding needs further investigation.
The novel finding that low 25(OH)D was associated with virologic failure after cART initiation is easier to explain. Studies indicate that 25(OH)D plays a role in inhibiting viral replication in multiple viruses, including respiratory viruses such as influenza and RSV, herpes simplex virus, and adenoviruses [39, 40]. Low 25(OH)D levels have recently been associated with high levels of hepatitis B viral replication [6]. Furthermore, similar to our results with HIV patients initiating cART, for HIV/HCV coinfected patients, lower 25(OH)D levels resulted in impaired virologic responses to anti-HCV treatment [41]. 25(OH)D defends against these viruses through the induction of antiviral genes including cathelicidin and β-defensin 4 and the release of reactive oxygen species [39]. 25(OH)D reduces inflammatory cytokine production, decreases T-cell activation, and shifts the T-cell profile from a Th1 response to Th2 response [42]. As HIV infections are characterized by a chronic Th1 state, sufficient levels of 25(OH)D might alleviate inflammation and tissue dysregulation seen with HIV infection. Recent in vitro studies also demonstrate the role of 25(OH)D in triggering autophagy in macrophages, inhibiting HIV replication [43, 44]. Thus low 25(OH)D may impair macrophage autophagy, leading to a loss of HIV virologic control. Whatever the mechanism, it is intriguing and novel that low 25(OH)D was observed to have a 2-fold higher hazard of virologic failure.
Our study has limitations. The study participants were enrolled in a clinical trial and were excluded if they had any major comorbidities or laboratory abnormalities. Therefore, they were relatively healthy compared to many patients initially evaluated in routine HIV care programs. Also, as immunologic failure events were infrequent, the study lacked power to detect a statistically significant increase in immunologic failure as a result of low 25(OH)D. We also did not collect data on possible 25(OH)D supplementation, but this was not common at the time this study was conducted and unlikely to have occurred in resource-limited settings. The associations found in this article raise questions of reverse causation: does advanced HIV disease cause low 25(OHD) concentrations; or, is low 25(OH)D concentration a general marker for poor health? The fact that the study was prospective and that severely ill persons were excluded from the study make this less likely. Also, 25(OH)D concentrations were comparable to those found in studies of non-HIV infected persons in similar populations [45–47].
CONCLUSION
Our study demonstrated high prevalence of low 25(OH)D among HIV-infected persons in a wide variety of settings. We showed prospectively that low 25(OH)D is a predictor of poor clinical outcomes among HIV-infected adults in diverse geographic, economic, and cultural settings. Furthermore, the novel association of low 25(OH)D with virologic failure is biologically plausible, but it remains unknown whether 25-hydroxyvitamin D supplementation could improve cART outcomes, particularly in resource-limited settings where the burden of disease lies. Our findings support the concept that 25-hydroxyvitamin D supplementation may be an inexpensive, safe therapeutic addition for HIV-infected persons, but clearly a well-designed clinical trial is needed.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. The authors thank the PEARLS study participants who volunteered their time and efforts.
The authors acknowledge the contributions of the following investigators:
F. H. contributed to the data analysis and wrote the primary version of the manuscript. B. D. conducted laboratory analysis, data interpretation, and writing of the manuscript. L. S. and N. G. contributed to the data analysis, and R. B., A. A., and P. C. contributed to data interpretation and writing of the manuscript. J. H., N. K., and J. L. contributed to cohort maintenance, data collection, and review of the manuscript. T. C. contributed to the establishment and maintenance of the cohort, design of the study, data interpretation, and writing of the manuscript. A. G. obtained funding, designed the study, and contributed to data analysis and the drafting of the manuscript.
Financial support. This work was supported by the AIDS Clinical Trials Group and the US National Institute of Allergy and Infectious Diseases [AI68636, AI069450]; and the US National Institutes of Health [R01 AI45462 to A. G., T32 AI007291 to F. H.]. This work was also supported in part by Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, and GlaxoSmithKline. The funders had no role in study design, data collection and analysis, decision to publish, or manuscript preparation.
Potential conflicts of interest. All authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE) and were fully responsible for all aspects of manuscript development. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Hewison M. Vitamin D and immune function: an overview. Proc Nutr Soc. 2011;71:50–61. doi: 10.1017/S0029665111001650. [DOI] [PubMed] [Google Scholar]
- 2.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 3.Khoo AL, Joosten I, Michels M, et al. 1,25-Dihydroxyvitamin D3 inhibits proliferation but not the suppressive function of regulatory T cells in the absence of antigen-presenting cells. Immunology. 2011;134:459–68. doi: 10.1111/j.1365-2567.2011.03507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Essen MR, Kongsbak M, Schjerling P, Olgaard K, Odum N, Geisler C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat Immunol. 2010;11:344–9. doi: 10.1038/ni.1851. [DOI] [PubMed] [Google Scholar]
- 5.Hewison M, Freeman L, Hughes SV, et al. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J Immunol. 2003;170:5382–90. doi: 10.4049/jimmunol.170.11.5382. [DOI] [PubMed] [Google Scholar]
- 6.Farnik H, Bojunga J, Berger A, et al. Low vitamin D serum concentration is associated with high levels of hepatitis B virus (HBV) replication in chronically infected patients. Hepatology. 2013;58:1270–6. doi: 10.1002/hep.26488. [DOI] [PubMed] [Google Scholar]
- 7.Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179:2060–3. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- 8.Hollis BW. Assessment of vitamin D status and definition of a normal circulating range of 25-hydroxyvitamin D. Curr Opin Endocrinol Diabetes Obes. 2008;15:489–94. doi: 10.1097/MED.0b013e328317ca6c. [DOI] [PubMed] [Google Scholar]
- 9.Viard JP, Souberbielle JC, Kirk O, et al. Vitamin D and clinical disease progression in HIV infection: results from the EuroSIDA study. AIDS. 2011;25:1305–15. doi: 10.1097/QAD.0b013e328347f6f7. [DOI] [PubMed] [Google Scholar]
- 10.Mehta S, Hunter DJ, Mugusi FM, et al. Perinatal outcomes, including mother-to-child transmission of HIV, and child mortality and their association with maternal vitamin D status in Tanzania. J Infect Dis. 2009;200:1022–30. doi: 10.1086/605699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta S, Giovannucci E, Mugusi FM, et al. Vitamin D status of HIV-infected women and its association with HIV disease progression, anemia, and mortality. PLoS One. 2010;5:e8770. doi: 10.1371/journal.pone.0008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sudfeld CR, Wang M, Aboud S, Giovannucci EL, Mugusi FM, Fawzi WW. Vitamin D and HIV progression among Tanzanian adults initiating antiretroviral therapy. PLoS One. 2012;7:e40036. doi: 10.1371/journal.pone.0040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sudfeld CR, Giovannucci EL, Isanaka S, et al. Vitamin D status and incidence of Pulmonary Tuberculosis, opportunistic infections, and wasting among HIV-Infected Tanzanian adults initiating antiretroviral therapy. J Infect Dis. 2013;207:378–85. doi: 10.1093/infdis/jis693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376:180–8. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greene-Finestone LS, Berger C, de Groh M, et al. 25-Hydroxyvitamin D in Canadian adults: biological, environmental, and behavioral correlates. Osteoporos Int. 2011;22:1389–99. doi: 10.1007/s00198-010-1362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 17.Sudfeld CR, Giovannucci EL, Isanaka S, et al. Vitamin D status and incidence of pulmonary tuberculosis, opportunistic infections, and wasting among HIV-Infected tanzanian adults initiating antiretroviral therapy. J Infect Dis. 2012;207:378–85. doi: 10.1093/infdis/jis693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell TB, Smeaton LM, Kumarasamy N, et al. Efficacy and safety of three antiretroviral regimens for initial treatment of HIV-1: a randomized clinical trial in diverse multinational settings. PLoS Med. 2012;9:e1001290. doi: 10.1371/journal.pmed.1001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73:1–11. [Google Scholar]
- 20.Ersfeld DL, Rao DS, Body JJ, et al. Analytical and clinical validation of the 25 OH vitamin D assay for the LIAISON automated analyzer. Clin Biochem. 2004;37:867–74. doi: 10.1016/j.clinbiochem.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academies Press: 2011. [PubMed] [Google Scholar]
- 22.Interim WHO Clinical Staging of HIV/AIDS and HIV/AIDS Case Definitions for Surveillance. 2005. http://www.who.int/hiv/pub/guidelines/clinicalstaging.pdf . Accessed 13 November 2013.
- 23.Breslow NE, Lumley T, Ballantyne CM, Chambless LE, Kulich M. Using the whole cohort in the analysis of case-cohort data. Am J Epidemiol. 2009;169:1398–405. doi: 10.1093/aje/kwp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiboonchutikul S, Sungkanuparph S, Kiertiburanakul S, et al. Vitamin D insufficiency and deficiency among HIV-1-Infected patients in a tropical setting. J Int Assoc Physicians AIDS Care (Chic) 2012;11:305–10. doi: 10.1177/1545109711432142. [DOI] [PubMed] [Google Scholar]
- 25.Nansera D, Graziano FM, Friedman DJ, Bobbs MK, Jones AN, Hansen KE. Vitamin D and calcium levels in Ugandan adults with human immunodeficiency virus and tuberculosis. Int J Tuberc Lung Dis. 2011;15:1522–7. doi: 10.5588/ijtld.10.0701. i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mave V, Shere D, Gupte N, et al. Vitamin D deficiency is common among HIV-infected breastfeeding mothers in Pune, India, but is not associated with mother-to-child HIV transmission. HIV Clin Trials. 2012;13:278–83. doi: 10.1310/hct1305-278. [DOI] [PubMed] [Google Scholar]
- 27.Dao CN, Patel P, Overton ET, et al. Low vitamin D among HIV-infected adults: prevalence of and risk factors for low vitamin D Levels in a cohort of HIV-infected adults and comparison to prevalence among adults in the US general population. Clin Infect Dis. 2011;52:396–405. doi: 10.1093/cid/ciq158. [DOI] [PubMed] [Google Scholar]
- 28.Brown TT, McComsey GA. Association between initiation of antiretroviral therapy with efavirenz and decreases in 25-hydroxyvitamin D. Antivir Ther. 2010;15:425–9. doi: 10.3851/IMP1502. [DOI] [PubMed] [Google Scholar]
- 29.Fox J, Peters B, Prakash M, Arribas J, Hill A, Moecklinghoff C. Improvement in vitamin D deficiency following antiretroviral regime change: Results from the MONET trial. AIDS Res Hum Retroviruses. 2011;27:29–34. doi: 10.1089/aid.2010.0081. [DOI] [PubMed] [Google Scholar]
- 30.Welz T, Childs K, Ibrahim F, et al. Efavirenz is associated with severe vitamin D deficiency and increased alkaline phosphatase. AIDS. 2010;24:1923–8. doi: 10.1097/QAD.0b013e32833c3281. [DOI] [PubMed] [Google Scholar]
- 31.Ding C, Gao D, Wilding J, Trayhurn P, Bing C. Vitamin D signalling in adipose tissue. Br J Nutr. 2012;108:1915–23. doi: 10.1017/S0007114512003285. [DOI] [PubMed] [Google Scholar]
- 32.Gao D, Trayhurn P, Bing C. 1,25-Dihydroxyvitamin D3 inhibits the cytokine-induced secretion of MCP-1 and reduces monocyte recruitment by human preadipocytes. Int J Obes (London) 2013;37:357–65. doi: 10.1038/ijo.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu Y, Mahon BD, Froicu M, Cantorna MT. Calcium and 1 alpha,25-dihydroxyvitamin D3 target the TNF-alpha pathway to suppress experimental inflammatory bowel disease. Eur J Immunol. 2005;35:217–24. doi: 10.1002/eji.200425491. [DOI] [PubMed] [Google Scholar]
- 34.Chen S, Sims GP, Chen XX, Gu YY, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;179:1634–47. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 35.Barber Y, Rubio C, Fernandez E, Rubio M, Fibla J. Host genetic background at CCR5 chemokine receptor and vitamin D receptor loci and human immunodeficiency virus (HIV) type 1 disease progression among HIV-seropositive injection drug users. J Infect Dis. 2001;184:1279–88. doi: 10.1086/324000. [DOI] [PubMed] [Google Scholar]
- 36.Nieto G, Barber Y, Rubio MC, Rubio M, Fibla J. Association between AIDS disease progression rates and the Fok-I polymorphism of the VDR gene in a cohort of HIV-1 seropositive patients. J Steroid Biochem Mol Biol. 2004;89–90:199–207. doi: 10.1016/j.jsbmb.2004.03.086. [DOI] [PubMed] [Google Scholar]
- 37.Bellamy R. Identifying genetic susceptibility factors for tuberculosis in Africans: a combined approach using a candidate gene study and a genome-wide screen. Clin Sci (London) 2000;98:245–50. [PubMed] [Google Scholar]
- 38.Roth DE, Soto G, Arenas F, et al. Association between vitamin D receptor gene polymorphisms and response to treatment of pulmonary tuberculosis. J Infect Dis. 2004;190:920–7. doi: 10.1086/423212. [DOI] [PubMed] [Google Scholar]
- 39.Beard JA, Bearden A, Striker R. Vitamin D and the anti-viral state. J Clin Virol. 2011;50:194–200. doi: 10.1016/j.jcv.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maxwell CS, Carbone ET, Wood RJ. Better newborn vitamin D status lowers RSV-associated bronchiolitis in infants. Nutr Rev. 2012;70:548–52. doi: 10.1111/j.1753-4887.2012.00517.x. [DOI] [PubMed] [Google Scholar]
- 41.Mandorfer M, Reiberger T, Payer BA, et al. Low vitamin D levels are associated with impaired virologic response to PEGIFN+RBV therapy in HIV/HCV coinfected patients. AIDS. 2012;27:227–32. doi: 10.1097/QAD.0b013e32835aa161. [DOI] [PubMed] [Google Scholar]
- 42.Lake JE, Adams JS. Vitamin D in HIV-infected patients. Curr HIV/AIDS Rep. 2011;8:133–41. doi: 10.1007/s11904-011-0082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Campbell GR, Spector SA. Toll-like receptor 8 ligands activate a vitamin D mediated autophagic response that inhibits human immunodeficiency virus type 1. PLoS Pathog. 2012;8:e1003017. doi: 10.1371/journal.ppat.1003017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spector SA. Vitamin D and HIV: letting the sun shine in. Top Antivir Med. 2011;19:6–10. [PMC free article] [PubMed] [Google Scholar]
- 45.Adeyemi OM, Agniel D, French AL, et al. Vitamin D deficiency in HIV-infected and HIV-uninfected women in the United States. J Acquir Immune Defic Syndr. 2011;57:197–204. doi: 10.1097/QAI.0b013e31821ae418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chailurkit LO, Aekplakorn W, Ongphiphadhanakul B. Regional variation and determinants of vitamin D status in sunshine-abundant Thailand. BMC Public Health. 2011;11:853. doi: 10.1186/1471-2458-11-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harinarayan CV, Ramalakshmi T, Venkataprasad U. High prevalence of low dietary calcium and low vitamin D status in healthy south Indians. Asia Pac J Clin Nutr. 2004;13:359–64. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.