Highlights
-
•
Oxidative stress in 3T3-L1 adipocytes was elevated by silencing of FABP4.
-
•
FABP4 silencing did not alter levels of glutathione or superoxide dismutase.
-
•
The recombinant FABP4 significantly reduced levels of hydrogen peroxide.
-
•
The resistance of adipocytes to oxidative stress was decreased by FABP4 knockdown.
-
•
Silencing of FABP4 elevated the endoplasmic reticulum stress in adipocytes.
Abbreviations: ER, endoplasmic reticulum; Ern1, endoplasmic reticulum to nucleus signaling 1; FABP, fatty acid binding protein; GSH, reduced glutathione; GSTA4, glutathione S-transferase A4; H2O2, hydrogen peroxide; Ormdl3, ORM1-like 3; ROS, reactive oxygen species; siRNA, small interfering RNA; SOD, superoxide dismutase; Ssr1, signal sequence receptor α; UPR, unfolded protein response; VEGF, vascular endothelial growth factor; Xbp1, X-box binding protein 1.
Keywords: FABP4, Adipocyte, Antioxidant, Oxidative stress, ER stress
Abstract
The fatty acid binding protein 4 (FABP4), one of the most abundant proteins in adipocytes, has been reported to have a proinflammatory function in macrophages. However, the physiological role of FABP4, which is constitutively expressed in adipocytes, has not been fully elucidated. Previously, we demonstrated that FABP4 was involved in the regulation of interleukin-6 (IL-6) and vascular endothelial growth factor (VEGF) production in 3T3-L1 adipocytes. In this study, we examined the effects of FABP4 silencing on the oxidative and endoplasmic reticulum (ER) stress in 3T3-L1 adipocytes. We found that the cellular reactive oxygen species (ROS) and 8-nitro-cyclic GMP levels were significantly elevated in the differentiated 3T3-L1 adipocytes transfected with a small interfering RNA (siRNA) against Fabp4, although the intracellular levels or enzyme activities of antioxidants including reduced glutathione (GSH), superoxide dismutase (SOD) and glutathione S-transferase A4 (GSTA4) were not altered. An in vitro evaluation using the recombinant protein revealed that FABP4 itself functions as a scavenger protein against hydrogen peroxide (H2O2). FABP4-knockdown resulted in a significant lowering of cell viability of 3T3-L1 adipocytes against H2O2 treatment. Moreover, four kinds of markers related to the ER stress response including the endoplasmic reticulum to nucleus signaling 1 (Ern1), the signal sequence receptor α (Ssr1), the ORM1-like 3 (Ormdl3), and the spliced X-box binding protein 1 (Xbp1s), were all elevated as the result of the knockdown of FABP4. Consequently, FABP4 might have a new role as an antioxidant protein against H2O2 and contribute to cytoprotection against oxidative and ER stress in adipocytes.
1. Introduction
Fatty acid binding protein 4 (FABP4), also known as adipocyte FABP (A-FABP) or aP2, is a member of the FABP family, which is comprised of at least nine isoforms [1]. FABP4 is expressed in mature adipocytes during its differentiation from preadipocytes [2]. Animal studies have reported that FABP4-deficient mice were protected against obesity-mediated insulin resistance, impaired glucose tolerance and atherosclerosis [3,4]. In macrophages, the expression of FABP4 is induced by inflammatory stimuli such as lipopolysaccharides (LPS) [5], oxidized low-density lipoprotein (oxLDL) [6] and advanced glycation end products [7]. On the contrary, in FABP4-knockout macrophages, the production of inflammatory cytokines and the activation of the inflammatory signaling pathway are suppressed [8]. Furthermore, in vascular endothelial cells, FABP4 expression is induced by pro-angiogenic stimuli, such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) [9]. In the aortic endothelium, FABP4 expression is up-regulated during the progression of atherosclerosis in apolipoprotein E (ApoE)-knockout mice [10]. These findings strongly indicate that the pathological induction of FABP4 may well contribute to the pathogenesis of inflammatory disorders and vascular dysfunction. However, the physiological role of FABP4 in adipocytes as well as macrophages and endothelial cells is not currently well understood.
It was recently reported that reactive oxygen species (ROS) regulate mitotic clonal expansion through activation of the CCAAT/enhancer binding protein (C/EBP) β during adipogenesis [11]. In addition, the C/EBPβ-mediated activation of the signaling pathway, including the endoplasmic reticulum to nucleus signaling 1 (Ern1), which is a homolog of the yeast Ire1, and X-box binding protein 1 (Xbp1), for the elevation of unfolded protein response (UPR) to alleviate endoplasmic reticulum (ER) stress has been shown to be required for adipogenesis [12]. It has also been reported that the production of ROS is directly linked to ER stress [13–15], and that antioxidants reduce ER stress [16,17]. To maintain the primary function of adipocytes, i.e., fat accumulation and endocrine function, the appropriate machinery may be necessary to mitigate the increased cellular stress associated with lipid metabolism and protein biosynthesis. Thus, we hypothesized that FABP4 may play a cytoprotective role against an increase in oxidative and ER stress in adipocytes, since it is well known that the expression of FABP4 is induced during adipogenesis [2].
It has been reported that FABP1, known as liver FABP (L-FABP), functions as an antioxidant protein [18–20] through the inactivation of free radicals by its methionine and cysteine amino acids [21]. However, no direct evidence for associating FABP4 with the antioxidant function is available. Although a recent study demonstrated that FABP4 mediates lipid-induced ER stress in macrophages [22], how it impacts ER stress in adipocytes has not been explored.
In a previous study, we reported on a simple method for preparing differentiated 3T3-L1 adipocytes in the form of a monolayer denoted as density-based separation followed by re-plating of enriched adipocytes in monolayer (DREAM) and succeeded in the efficient and significant silencing of FABP4 in the differentiated adipocytes [23]. By this method, we were able to elucidate a new role of FABP4 in the regulation of interleukin-6 (IL-6) and VEGF-A production in adipocytes through modulation of the thrombin receptor. Thus, in the current study, we investigated the further importance of FABP4 in the modulation of oxidative and ER stress in adipocytes via loss-of-function analyses.
2. Results
2.1. The knockdown of FABP4 elevates intracellular ROS and 8-nitro-cyclic GMP levels in 3T3-L1 adipocytes
We first confirmed the effective silencing of the Fabp4 mRNA and FABP4 protein at 48 h after the transfection of siFabp4 into the differentiated 3T3-L1 adipocytes prepared by the DREAM protocol [23]. RT-PCR and Western blotting analyses indicated that the knockdown of the mRNA and protein was successful (Fig. 1A and B). Thus, under these experimental conditions, we assessed intracellular ROS levels using a fluorogenic probe (CellROX). As a result, the geometric mean value of CellROX fluorescence in the siFabp4-transfected adipocytes was approximately 11% higher than that in the control cells (Fig. 1C). The difference was statistically significant (n = 6, P = 0.002), but the elevation was not drastic. Therefore, to evaluate the induction of oxidative stress by FABP4 knockdown in adipocytes, we also examined the intracellular level of 8-nitro-cGMP, which is generated from nitric oxide (NO), ROS and cGMP and is involved in the adaptive response to oxidative stress [24–27]. The immunocytochemical analysis revealed that the intracellular 8-nitro-cGMP was substantially increased by the down-regulation of FABP4 expression in the differentiated 3T3-L1 adipocytes (Fig. 1D and E). These results strongly suggest that the 11% elevation in intracellular ROS levels caused by FABP4 knockdown may be sufficient to induce oxidative stress in adipocytes.
Fig. 1.
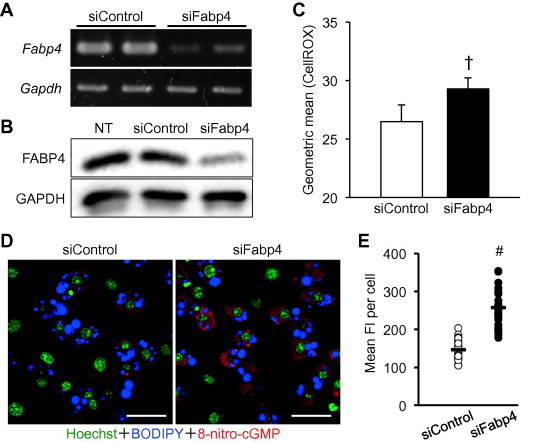
Increased oxidative stress mediated by the knockdown of FABP4 in differentiated 3T3-L1 adipocytes. (A and B) RT-PCR and Western blotting analyses for confirmation of FABP4 knockdown. At 48 h incubation of the differentiated 3T3-L1 adipocytes with 50 nM siFabp4 or siControl, the total RNA samples and cell lysates were subjected to RT-PCR (a) and Western blotting (b), respectively. Typical images obtained in three independent experiments are shown. NT: non-treatment. (C) Measurement of the intracellular ROS level. At 48 h transfection of siFabp4 or siControl, the 3T3-L1 adipocytes were fluorescently stained with CellROX Deep Red reagent and BODIPY493/503 for detection of cellular ROS and lipid droplets, and then subjected to flow cytometric analysis. The geometric mean values of CellROX fluorescence in the BODIPY-stained (fat accumulated) cells were acquired as the intracellular ROS levels. Data represent as mean ± SD (n = 6). †P < 0.005 (student’s t-test). (D) Immunocytochemical detection of 8-nitro-cGMP. At 48 h after transfection, the cells were fixed and immunostained with a pair of anti-nitroguanosine and Alexa568-labeled secondary antibodies (Red). Cell nuclei and lipid droplets were stained with Hoechst33342 (Green) and BODIPY (Blue). Typical CLSM images of 3 independent experiments were shown. Scale bars represent 50 μm. (E) Quantification of intracellular 8-nitro-cGMP contents. Mean fluorescent intensity (FI) (average intensity of pixels per cell) for 30 adipocytes per condition was measured. Open and closed circles represent the mean FI values in each cell, and black bars indicate the average values of mean FI in 30 cells. #P < 0.0001 (Student’s t-test). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.2. Knockdown of FABP4 does not alter the cellular GSH level, SOD activity and glutathione S-transferase A4 (GSTA4) expression in 3T3-L1 adipocytes
We next examined the issue of whether the cellular GSH level and SOD activity are affected by FABP4 knockdown. The antioxidant level and enzyme activity in the siFabp4-transfected adipocytes were not significantly different from those in the control cells (Fig. 2A and B). Curtis et al. recently reported that the down-regulation of GSTA4 in adipocytes leads to increased protein carbonylation, oxidative stress and mitochondrial dysfunction [28]. Thus, we assessed the expression of the GSTA4 protein in the siFabp4-transfected adipocytes. However, it was also not altered by FABP4 knockdown (Fig. 2C). From these results, we concluded that the elevation of intracellular ROS levels observed in this study was mainly directed to a decrease in FABP4 expression.
Fig. 2.
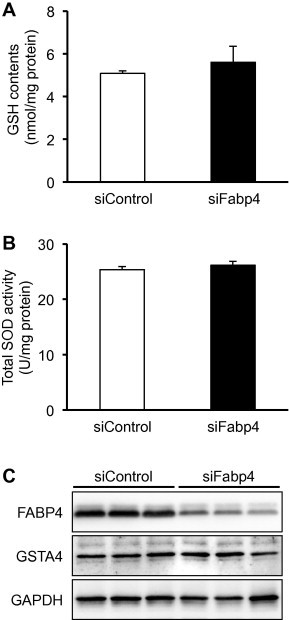
No alteration in intracellular GSH content, SOD activity and GSTA4 expression in 3T3-L1 adipocytes was found as the result of FABP4 knockdown. (A and B) Intracellular GSH content and total SOD activity. At 48 h after siRNA transfection, cell lysates were prepared as described in Section 4, and GSH contents (a) and total SOD activity (b) were measured. The intracellular GSH contents and SOD activity per mg protein were calculated. Data represents mean ± SD (n = 3). (C) Western blotting of GSTA4. The protein expression of FABP4, GSTA4 and glyceraldehyde-3-dehydrogenase (GAPDH) was assessed. The chemiluminescent signals were detected using an Image Analyzer (LAS-4000mini).
2.3. The recombinant FABP4 protein has antioxidant activity to H2O2, but not to superoxide
In order to confirm the above hypothesis, we assessed the activity of FABP4 as an antioxidant protein in vitro. We initially examined whether FABP4 is able to dismutate superoxide, which is the primary ROS generated during oxygen metabolism. A His6 peptide was used as a negative control. As shown in Fig. 3A, the residual superoxide levels in the FABP4 and His6-containing samples were approximately 10% lower than that in the buffer sample that did not contain FABP4 and His6, while the difference between the FABP4 and His6-containing samples was not statistically significant, indicating that the 10% reduction of superoxide in the recombinant FABP4 might be attributed to the N-terminal His6-tag, and that FABP4 itself has no scavenger activity for superoxide. Thus, we next examined the antioxidant ability of FABP4 for H2O2, which is formed by conversion from superoxide by SOD and is known as a major mediator of cellular oxidative stress. The recombinant FABP4 significantly scavenged H2O2 in a concentration-dependent manner, whereas the His6 peptide did not (Fig. 3B). In addition, an unrelated protein (bovine serum albumin, BSA) also showed no alteration in H2O2 levels (Fig. S1). Furthermore, mass spectrometry and SDS–PAGE analyses showed that the H2O2 treatment resulted in a marked increase in the molecular mass of the recombinant FABP4 protein (Fig. S2). These results suggest that FABP4, which is constitutively expressed in the differentiated adipocytes, may function as an intracellular antioxidant protein and plays a crucial role in the resistance to the oxidative stress induced by H2O2.
Fig. 3.
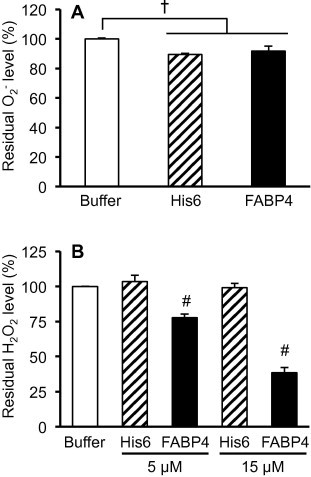
Antioxidant activity of the recombinant FABP4 protein. (A) Measurement of superoxide anion. 1.5 μM of recombinant FABP4 protein and His6 peptide were subjected to quantification of the scavenger activity against superoxide anion. The percentages of the residual superoxide levels against the negative control are shown (n = 3). (B) Quantification of H2O2. 5 or 15 μM of recombinant FABP4 control peptide were incubated with H2O2, and then, the residual H2O2 level was measured. The relative H2O2 level in each sample against the negative control (buffer alone) was calculated. Data represent mean ± SD (n = 3). †P < 0.005, #P < 0.0001 (one-way ANOVA followed by Turkey-Kramer’s HSD test).
2.4. The knockdown of FABP4 reduces resistance to oxidative stress induced by exogenous H2O2 in 3T3-L1 adipocytes
We next examined the antioxidant function of FABP4 in the differentiated 3T3-L1 adipocytes. At 48 h after the transfection of siFabp4 or siControl, 3T3-L1 adipocytes were treated with 300 mM H2O2 or vehicle for 1 h. As shown in Figs. 4A and S3, although cell viability was not significantly different between the siFabp4- and siControl-transfected cells when they were treated with vehicle, cell viability in the siFabp4-treated cells was significantly lower than that in the siControl-treated cells after exposure to 300 mM H2O2. This result indicates that FABP4 has the ability to protect adipocytes from H2O2-induced oxidative stress.
Fig. 4.
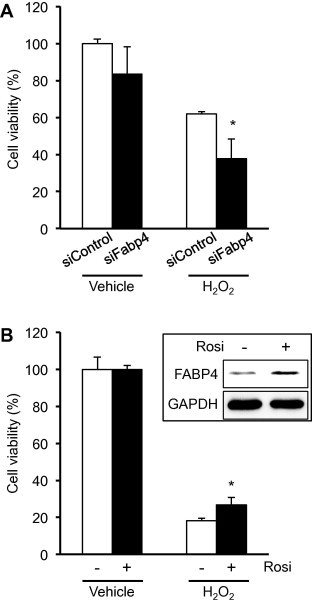
Reduced resistance to oxidative stress caused by FABP4 knockdown in the 3T3-L1 adipocytes and Raw264.7 macrophages. (A) Adipocyte viability. At 48 h after siRNA transfection, the cells were exposed with 300 mM H2O2 or vehicle for 1 h and then stained with 0.5% crystal violet. After extraction of the dye, the absorbance was measured at 540 nm to determine the cell viability. (B) Western blotting and macrophage viability. Raw264.7 cells were treated with 2 μM of Rosi or vehicle (DMSO) for 24 h. FABP4 expression was then assessed by Western blotting. After treatment with Rosi, the cells were incubated with 90 mM H2O2 or vehicle (water) for 1 h, and then cell viability was evaluated using crystal violet staining. Data represent mean ± SD (n = 3). ∗P < 0.05 (Student’s t-test).
Moreover, it is known that FABP4 expression in macrophages is induced during differentiation from monocytes and by treatment with various reagents including phorbol 12-myristate 13-acetate (PMA), an activator of protein kinase C, peroxisome proliferator-activated receptor (PPAR) γ agonists, LPS, and oxLDL [5,6,29–31]. Thus, we next examined the issue of whether or not the elevated FABP4 levels also exhibited an antioxidant function in macrophages. The elevation of FABP4 was induced by treating Raw264.7 cells, a mouse monocyte/macrophage cell line, with 2 μM rosiglitazone (Rosi), a synthesized PPARγ agonist (Fig. 4B). Under this condition, the Raw264.7 cells were treated with 90 mM H2O2 or vehicle for 1 h, and cell viability was then measured. As a result, cell viability in the Rosi-treated cells was significantly higher than that in the vehicle-treated cells (Fig. 4B). These findings suggest that FABP4 might also function as an antioxidant protein in macrophages as well as in adipocytes.
2.5. Knockdown of FABP4 induces endoplasmic reticulum (ER) stress and elevation of intracellular Ca2+ levels in 3T3-L1 adipocytes
Some reports indicate that oxidative stress and ER stress are closely linked events and that H2O2-mediated oxidative stress also induces ER stress. Thus, we next assessed whether the ER stress response was promoted by knockdown of FABP4 in 3T3-L1 adipocytes. We analyzed the expression of several ER stress markers including Ern1, Xbp1, signal sequence receptor α (Ssr1) and ORM1-like 3 (Ormdl3) in siFabp4-transfected adipocytes. As shown in Fig. 5A, while the unspliced Xbp1 (Xbp1u) and total Xbp1 mRNA levels were not altered, four types of ER stress markers were up-regulated as the result of the knockdown of FABP4, strongly suggesting that FABP4 plays an inhibitory role in ER stress associated with oxidative stress in adipocytes. To explore the mechanisms responsible for the elevated ER stress in FABP4-silenced adipocytes, we examined intracellular Ca2+ levels using a fluorescent Ca2+ probe Fluo-8. As a result, the fluorescence intensity of Fluo-8 was significantly increased by the knockdown of FABP4 in the 3T3-L1 adipocytes (Fig. 5B and C), suggesting that the impaired Ca2+ homeostasis caused by FABP4 knockdown might be attributed to the induction of ER stress in adipocytes.
Fig. 5.
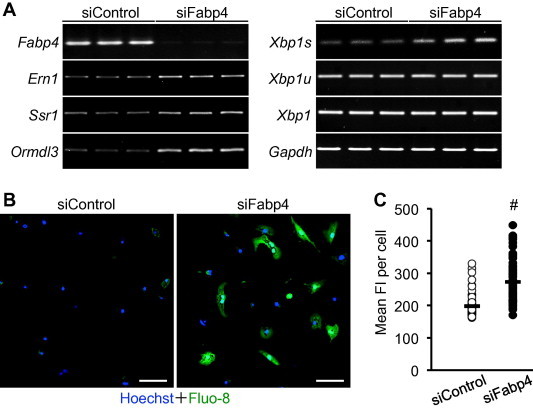
Elevation of ER stress-related genes and intracellular Ca2+ level by FABP4 knockdown in the 3T3-L1 adipocytes. (A) RT-PCR analyses for ER stress-associated genes. At 48 h after siRNA transfection, the expression of several genes related to ER stress and/or UPR were analyzed by RT-PCR. Three independent samples transfected with siFabp4 or siControl were used in this evaluation. (B) Live cell calcium imaging. At 48 h after transfection of siFabp4 or siControl, the cells were stained with Fluo-8-AM (Green). Cell nuclei were counterstained with Hoechst33342 (Blue). Typical CLSM images of 3 independent experiments were shown. Scale bars represent 100 μm. (C) Quantification of intracellular Ca2+ level. Mean fluorescent intensity (FI) (average intensity of pixels per cell) for 67–73 adipocytes per condition was measured. Open and closed circles represent the mean FI values in each cell, and the black bars indicate the average values of the mean FI in each condition. #P < 0.0001 (Student’s t-test). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3. Discussion
FABP4 is thought to be an important pathological mediator in chronic inflammation and vascular injury. The findings reported herein demonstrate that FABP4, which is expressed in adipocytes, has a role in alleviating oxidative and ER stress. The attenuation of these types of cellular stress via FABP4 might play a key role in the maintenance of adipocyte homeostasis, since the excess level of cellular oxidative and ER stress leads to adipocyte dysfunction, to include an impaired glucose/lipid metabolism and endocrine capacity [32,33].
The findings show that FABP4 expressed in the differentiated 3T3-L1 adipocytes is associated with lowering the level of intracellular ROS. The knockdown of FABP4 in adipocytes caused a 11% increase in cellular ROS levels without any other stimulation, compared to control cells (Fig. 1B). It was assumed that this change was not drastic due to the incomplete depletion of FABP4. Indeed, 30–40% of the FABP4 protein remained, even after a 48 h treatment of siFabp4 (Fig. 1A). Since the concentration of FABP4 in adipocytes was estimated to be as high as 250 μM [34,35], the residual FABP4 could suppress the surplus elevation in ROS levels. However, a marked increase in 8-nitro-cGMP was induced by FABP4 knockdown (Fig. 1C). 8-nitro-cGMP is an endogenous nucleotide that was first discovered under inflammation conditions and functions as a cytoprotective mediator of NO signaling [24–27]. Although the role of 8-nitro-cGMP in adipocytes has been not investigated, the possibility that it might be increased by the elevated cellular ROS due to FABP4 knockdown cannot be excluded, based on these data. Peroxynitrite (ONOO−), formed by increased NO and ROS, is a potent and nitrating species, and causes for the increase in the protein nitration as well as the formation of 8-nitro-cGMP. The accumulation of 3-nitrotyrosine is also known as an oxidative stress marker [36]. Therefore, in order to fully elucidate the cytoprotective function of FABP4 in adipocytes, further investigations such as the quantitative assessment for protein nitration still remain.
It has been reported that the intracellular GSH content and SOD activity increase during adipocyte differentiation in 3T3-L1 cells [37,38], indicating that the adequate regulation of the cellular redox state may be responsible for normal adipocyte function. We found that the knockdown of FABP4 in the differentiated 3T3-L1 adipocytes caused cellular ROS levels to become elevated without any alteration in intracellular GSH content and SOD activity (Fig. 2A and B). In addition, the expression of GSTA4 was also not altered by FABP4 knockdown (Fig. 2C). GSTA4 catalyzes the glutathionylation of α,β-unsaturated aldehydes such as 4-hydroxy-2-nonenal (4-HNE) to generate a conjugation product that is eliminated from the cell [39]. In a previous study, it was reported that the mitochondrial ROS was increased in GSTA4-silenced 3T3-L1 adipocytes and mitochondrial function in adipocytes from GSTA4-null mice was significantly compromised [28]. However, our results indicate that the elevation of cellular ROS induced by FABP4 knockdown in the 3T3-L1 adipocytes did not result from the modulation of GSTA4 expression. These findings suggest that adipocytes might have alternate machineries to protect themselves against oxidative stress. In this regard, we demonstrated the existence of FABP4 as alternate antioxidant protein against H2O2 (Fig. 3B). Some reports have concluded that FABP1 has the ability to inactivate the free radicals by virtue of its methionine and cysteine amino acids [18,21]. It has been also reported that the increased FABP1 levels are associated with reduced cellular ROS levels without any alteration in the levels of antioxidant enzymes including SODs, glutathione peroxidase and catalase [19,20]. Although the homology of the amino acid sequence is below 30% between FABP1 and FABP4, FABP4 also contains two cysteine and five methionine residues in both the mouse and human forms. It has been also shown that the cysteine residue (Cys117) of FABP4 is a molecular target of protein modification by 4-HNE [35], which is a reactive aldehyde formed from lipid peroxidation and significantly contributes to oxidative disease due to its potent reactivity [40,41]. In the in vitro evaluation for the reduction of H2O2 by FABP4, we also assessed the scavenging effect of an unrelated protein (BSA) for H2O2, and found no reduction in H2O2 levels (Fig. S1). It was previously reported that BSA showed the reduction of H2O2 in a concentration-dependent manner, with an IC50 of 7.86 mg/ml (118.26 μM) [42]. Therefore, the 5 and 15 μM of BSA utilized in this study might be too low to permit scavenging effect for H2O2 to be measured. From these findings, FABP4 might effectively react with H2O2 and is likely involved in the cellular antioxidant mechanism in adipocytes. This interpretation was strongly supported by the finding that FABP4 knockdown in the differentiated 3T3-L1 adipocytes significantly decreased the resistance to exogenous oxidative stress induced by H2O2 (Figs. 4A and S3). In addition, we also found a significant increase in resistance to oxidative stress in the Raw264.7 macrophages, when they were pre-treated with Rosi for the induction of FABP4 expression (Fig. 4B). These findings suggest that FABP4 can function as an antioxidant protein, and this would not be specific to adipocytes. However, in macrophages, further examinations are still needed to exclude any other possibilities, since the elevation of FABP4 in macrophages is only one of the many actions of Rosi. Furthermore, we found that the molecular mass of the recombinant FABP4 was changed from 14.4 to 19.6 kDa as the result of the H2O2 treatment (Fig. S2). In the SDS–PAGE analysis, the upper-shift of the FABP4 band by H2O2 was not recovered by treatment with dithiothreitol (DTT), a reducing agent, indicating that the increase in molecular mass of FABP4 might not be caused by the formation of S–S bonds. However, the molecular mechanisms for the effective reduction of H2O2 by FABP4 are still unclear. Further studies will be needed to clarify what specific amino acid residues are oxidized.
Moreover, we evaluated the expression of several ER stress-related genes in FABP4-silenced 3T3-L1 adipocytes. Ern1 encodes an intrinsic endoribonuclease ERN1 (also known as IRE1α), which is activated by ER stress, splices the Xbp1 mRNA and produces the spliced XBP1 protein (XBP1s). XBP1s is a transcription factor and induces a subset of UPR target genes that are responsible for the ER stress response [43,44]. Ssr1, encoding signal sequence receptor α (also known as translocon-associated protein (TRAP) α) is simultaneously induced by the IRE1α/XBP1 pathway in response to ER stress [45]. Ormdl3 encodes a transmembrane protein that is localized in the ER and may be involved in ER stress via calcium signaling [46]. RT-PCR analyses revealed that these ER stress/UPR-associated genes were up-regulated by FABP4 knockdown in differentiated 3T3-L1 adipocytes (Fig. 5A), indicating that a loss of FABP4 may induce the ER stress in adipocytes. In addition, we also found that intracellular Ca2+ levels were significantly increased as the result of FABP4 knockdown (Fig. 5B and C). It was previously reported that cellular oxidative stress induced an increase in intracellular Ca2+ levels [47]. Although the molecular mechanism responsible for the elevation of Ormdl3 expression through FABP4 knockdown is currently unclear, it is possible that the ER stress observed in this study may be secondarily induced by oxidative stress and impaired Ca2+ homeostasis as a result of FABP4 knockdown. In a previous study, we reported that the knockdown of FABP4 resulted in an alteration in the post-translational modification process of the VEGF-A protein in differentiated 3T3-L1 adipocytes [23]. A dysregulation of protein processing such as glycosylation may impair the effective secretion of VEGF-A [48] and thus cause ER stress [49]. From these findings, it is possible that FABP4 could physiologically play an important role in the quality control of protein biosynthesis and processing through its ability to control the level of ER stress in adipocytes. To the contrary, it is also known that FABP4 is necessary for ER stress associated with lipotoxicity in macrophages under pathological conditions [22]. In order to clarify the inconsistent findings under the physiological and pathological conditions, further investigations will be needed. Furthermore, it is known that adipocytes express FABP5, epidermal-FABP (E-FABP), as well as FABP4, and that FABP5 also functions as an antioxidant protein by scavenging reactive lipids including 4-hydroxynonenal (4-HNE) [50] and leukotriene A4 [51]. Therefore, it is possible that FABP5 silencing in the 3T3-L1 adipocytes leads to the elevation of oxidative stress, as is the case of FABP4. However, it has been also reported that FABP5 constitutes a minor fraction of FABPs in adipocytes, the amount being about 100-fold smaller than that of FABP4 in adipocytes [52]. Thus, in adipocytes, the contribution of FABP5 to the protection against oxidative stress might be less than that of FABP4. This issue would be addressed by the silencing of FABP5 in adipocytes.
In conclusion, we demonstrate, for the first time, that FABP4, which is constitutively expressed in adipocytes, has a new role in cytoprotection against oxidative stress and, at least partially, ER stress. Since the chronic accumulation of cellular stress in adipocytes contributes to the development of metabolic disorders such as diabetes and cardiovascular diseases, a more complete understanding of how FABP4 functions as an endogenous stress inhibitor would be helpful for the prevention of obesity-related diseases.
4. Materials and methods
4.1. Materials
Dulbecco’s modified eagle’s medium (DMEM) was obtained from Wako Pure Chemical (Osaka, Japan). 3-Isobutyl-1-methyl-xanthine (IBMX), dexamethasone (DEX) and insulin (bovine pancreas) (INS) were purchased from SIGMA (St. Louis, MO, USA). The siTrio Full Set, Mouse (Fabp4, NM_024406) and negative control were obtained from Cosmo Bio (Tokyo, Japan).
4.2. Cell cultures
3T3-L1 cells were obtained from the Human Science Research Resources Bank (JCRB9014). The cells were propagated in growth medium as described previously [23,53]. At 2 days post-confluence, differentiation was induced by adding IBMX (0.5 mM), DEX (1 μM), and INS (1.7 μM) (designated as “day 0”). At day 2, the medium was changed to growth medium supplemented with 16.4 μM d-biotin and 1.7 μM INS (maturation medium). The differentiated 3T3-L1 cells at day 8 were detached and re-plated as described previously [23] with minor modifications. In the current study, cell detachment process was carried out using 0.5 mg/ml type II collagenase (SIGMA) without trypsin. At 24 h after re-plating, the adherent cells were used for the following examinations.
Raw264.7 cells were obtained from the American Type Culture Collection (ATCC). The cells were propagated and maintained in DMEM (high glucose) supplemented with 10% FBS.
4.3. Transfection of the differentiated 3T3-L1 cells with siRNA
The siRNA transfection to the re-plated 3T3-L1 adipocytes was carried out with Lipofectamine RNAiMAX (Life Technologies, Carlsbad, CA, USA) according to the manufacture’s protocol.
4.4. RT-PCR analysis
Total RNA samples were prepared using the TRI Reagent (SIGMA), according to the manufacturer’s recommended procedure. Reverse transcription and PCR amplification were carried out as described previously [54]. The gene specific primers used in current study are summarized in Table S1. PCR products were subjected to 2% agarose gel electrophoresis and the gels were viewed by means of an Image Analyzer (model LAS-4000mini, GE Healthcare, Tokyo, Japan) after staining with ethidium bromide.
4.5. Western blotting
The cells were lysed with buffer containing 1% Triton X-100, 50 mM Tris–HCl (pH 7.5), 200 mM NaCl, 2 mM phenylmethylsulfonyl fluoride (PMSF), 1 × protease inhibitor cocktail (Nacalai tesque, Kyoto, Japan), and 1 × PhosSTOP Phosphatase inhibitor cocktail (Roche, Mannheim, Germany). The protein concentrations in cell lysates were determined with a BCA Protein Assay Kit (Pierce, Rockford, IL, USA). The cell lysates were subjected to immunodetection, as described previously [55]. The antibodies utilized in this study are listed in Table S2. Chemiluminescent signals were detected with an Image Analyzer (LAS-4000mini).
4.6. Flow cytometric analyses of intracellular reactive oxygen species (ROS) production
The re-plated 3T3-L1 adipocytes were transfected with siFabp4 or siControl as mentioned above. At 48 h after transfection, the cells were washed twice with Hank’s balanced salt solution (HBSS) (Wako), and then stained with 5 μM CellROX Deep Red reagent (Life Technologies) and 5 μM BODIPY493/503 (Life Technologies) for 30 min at 37 °C. The fluorescently stained cells were washed twice with HBSS, and then incubated in 5 mM EDTA in PBS for 5 min. The detached cells were collected in new tubes, washed with phosphate buffered saline (PBS) containing 0.5% BSA (SIGMA) and 2 mM EDTA, and then analyzed using a FACS Calibur flow cytometer (BD Biosciences, San Jose, CA, USA). The geometric mean values of CellROX fluorescence in the BODIPY-stained adipocytes were acquired as the intracellular ROS levels.
4.7. Immunofluorescent detection of 8-nitro-cGMP
The differentiated 3T3-L1 adipocytes were re-plated onto a gelatin-coated 35 mm glass-based culture dish. At 24 h after re-plating, the cells were transfected with 50 nM of siControl or siFabp4 for 48 h. The cells were then washed with PBS, fixed with Bouin’s solution (SIGMA) for 15 min at room temperature. After washing with PBS three times, the fixed cells were permeabilized with 1% Triton-X100 in PBS for 15 min, and then blocked with 1% BSA in PBS for 1 h at room temperature. After blocking, the cells were incubated with an anti-nitroguanosine mAb (clone NO2G52, Cosmo Bio) for 16 h at 4 °C. After washing three times with PBS, the cells were incubated with Alexa568-conjugated anti-mouse IgG (Life Technologies) for 2 h at room temperature. Cell nuclei and lipid droplets were fluorescently stained with 40 μM Hoechst33342 (Nacalai tesque, Kyoto, Japan) and 5 μM BODIPY493/503 for 30 min at room temperature. After washing with PBS three times, the cells were observed by confocal laser-scanning microscopy (CLSM) (model A1, Nikon, Tokyo, Japan). Mean fluorescent intensity (average intensity of pixels per cell) for 30 cells per condition was determined using the ImageJ software (http://rsb.info.nih.gov/ij/).
4.8. Quantification of intracellular reduced glutathione (GSH) level
The re-plated 3T3-L1 adipocytes were transfected with siFabp4 or siControl. At 48 h after transfection, the cells were washed with PBS and then scraped. A small part of the samples was utilized to determine protein content by the BCA method as described above. An equal volume of 5% meta-phosphoric acid (SIGMA) was added to each sample. After vortexing and sonication, the samples were centrifuged at 3000×g for 10 min at 4 °C. GSH content was determined using a BIOXYTECH GSH-400 kit (OXISResearch, Portland, CA, USA) according to the manufacture’s protocol. The standard curve was prepared using the purified GSH (Enzo Life Sciences, Farmingdale, NY, USA). The results were shown as an average GSH content per mg protein in three independent examinations.
4.9. Determination of superoxide dismutases (SODs) activity
The re-plated 3T3-L1 adipocytes were transfected with siFabp4 or siControl. At 48 h after transfection, the cells were washed with PBS twice, lysed with ice-cold 20 mM HEPES buffer (pH 7.2) containing 1 mM EGTA, 210 mM mannitol and 70 mM sucrose, and centrifuged at 1500×g for 5 min at 4 °C. SOD activity in the supernatant was determined using a Superoxide Dismutase Assay Kit (Cayman, Ann Arbor, MI, USA). A small part of the cell lysates was utilized to determine protein content by the BCA method. The results are shown as average SOD activity per mg of protein in three independent examinations.
In addition, the in vitro scavenger activity of FABP4 against superoxide was also assessed using the recombinant mouse FABP4 protein (Cayman). Since the recombinant FABP4 is a N-terminal hexahistidine (His6)-tagged protein, the synthesized His6 peptide (Abbiotec, San Diego, CA, USA) was also subjected to the assay as a control. The recombinant FABP4 and His6 peptide were dissolved in 50 mM sodium phosphate buffer (pH 7.2) containing 20% glycerol and 150 mM NaCl, and 1.5 μM of FABP4 and His6 was then incubated with a radical detection reagent and xanthine oxidase supplied in the SOD assay kit according to the manufacture’s protocol. The relative level of residual superoxide in each sample was calculated as a percentage against that in the negative control (buffer alone).
4.10. In vitro hydrogen peroxide (H2O2) measurement
For assessing the antioxidant activity of FABP4 against H2O2, 5 or 15 μM of recombinant FABP4 and His6 peptide, as mentioned above, were incubated with 44 μM of H2O2 at 37 °C for 30 min. Fatty acid-free BSA (SIGMA) was also utilized as an unrelated protein control. The residual H2O2 concentration was then measured using a Hydrogen Peroxide (urinary) Assay Kit (Cayman). The relative H2O2 level in each sample was calculated as a percentage against that in the negative control (buffer alone).
4.11. Mass spectral and SDS–PAGE analyses of the recombinant FABP4 protein
After incubation of the recombinant FABP4 (15 μM) with H2O2 or vehicle (water) as described above, the samples were analyzed by MALDI-TOF mass spectrometry.
Moreover, the samples were also subjected to 20% SDS–PAGE with or without treatment with 50 mM dithiothreitol (DTT). After electrophoresis, the gel was stained with EzStain AQua (ATTO, Tokyo, Japan).
4.12. Cell viability assay
The differentiated 3T3-L1 adipocytes were re-plated into 6 well culture plate at the density of 1.5 × 105 cells/well. At 24 h after re-plating, the cells were transfected with 50 nM siFabp4 or siControl as mentioned above. After 48 h incubation, the transfected cells were washed with HBSS, and then treated with 300 mM H2O2 or vehicle (H2O) in DMEM for 1 h at 37 °C. After washing with HBSS twice, the cells were fixed with 10% formalin in PBS and stained with 0.5% crystal violet (SIGMA) in 20% MeOH for 10 min. After washing four times, the dye was extracted with 0.2% Triton X-100, and the absorbance was then measured at 540 nm.
The Raw264.7 cells were seeded into 6 well culture plates at a density of 1 × 105 cells/well. At 24 h after inoculation, the cells were treated with 2 μM Rosi or vehicle (DMSO) for 24 h at 37 °C. After washing with HBSS, the cells were treated with 90 mM H2O2 or vehicle (H2O) for 1 h at 37 °C and cell viability was then assessed as described above.
4.13. Detection of intracellular calcium in living cells
The differentiated 3T3-L1 adipocytes were re-plated on a gelatin-coated 35 mm glass-based culture dish. At 24 h after re-plating, the cells were transfected with 50 nM of siControl or siFabp4 for 48 h. The cells were washed twice with HBSS and then incubated with 5 μM Fluo-8-AM (ABD Bioquest, Sunnyvale, CA) for 20 min at 37 °C. After washing with HBSS, the cells were observed by CLSM. The mean fluorescent intensity (average intensity of pixels per cell) for 67–73 cells per condition was determined using the ImageJ software.
4.14. Statistical analyses
All statistical analyses were performed using the JMP6 statistical package (SAS Institute, Cary, NC, USA). Student’s t-test and one-way ANOVA followed by Tukey–Kramer’s honestly significant difference (HSD) tests were used to evaluate statistical significance. A P value of <0.05 was considered to be significant.
Author contributions
KK and YM mainly contributed to the experimental design, all experiments, and data analyses. KK and HH collaborated in the preparation of the manuscript. All authors discussed the results and implications at all stages. HH was involved in the study design and supervised the overall project.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
This work was supported by grants from the Special Education and Research Expenses of the Ministry of Education, Culture, Sports, Science and Technology of Japan. We thank Dr. Milton Feather for editing this manuscript.
Appendix A. Supplementary data
Supplementary material.
References
- 1.Furuhashi M., Hotamisligil G.S. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 2008;7:489–503. doi: 10.1038/nrd2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spiegelman B.M., Frank M., Green H. Molecular cloning of mRNA from 3T3 adipocytes. Regulation of mRNA content for glycerophosphate dehydrogenase and other differentiation-dependent proteins during adipocyte development. J. Biol. Chem. 1983;258:10083–10089. [PubMed] [Google Scholar]
- 3.Hotamisligil G.S., Johnson R.S., Distel R.J., Ellis R., Papaioannou V.E., Spiegelman B.M. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science. 1996;274:1377–1379. doi: 10.1126/science.274.5291.1377. [DOI] [PubMed] [Google Scholar]
- 4.Boord J.B., Maeda K., Makowski L., Babaev V.R., Fazio S., Linton M.F., Hotamisligil G.S. Adipocyte fatty acid-binding protein, aP2, alters late atherosclerotic lesion formation in severe hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 2002;22:1686–1691. doi: 10.1161/01.atv.0000033090.81345.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kazemi M.R., McDonald C.M., Shigenaga J.K., Grunfeld C., Feingold K.R. Adipocyte fatty acid-binding protein expression and lipid accumulation are increased during activation of murine macrophages by toll-like receptor agonists. Arterioscler. Thromb. Vasc. Biol. 2005;25:1220–1224. doi: 10.1161/01.ATV.0000159163.52632.1b. [DOI] [PubMed] [Google Scholar]
- 6.Fu Y., Luo N., Lopes-Virella M.F. Oxidized LDL induces the expression of ALBP/aP2 mRNA and protein in human THP-1 macrophages. J. Lipid Res. 2000;41:2017–2023. [PubMed] [Google Scholar]
- 7.Wang X.Q., Yang K., He Y.S., Lu L., Shen W.F. Receptor mediated elevation in FABP4 levels by advanced glycation end products induces cholesterol and triacylglycerol accumulation in THP-1 macrophages. Lipids. 2011;46:479–486. doi: 10.1007/s11745-011-3542-4. [DOI] [PubMed] [Google Scholar]
- 8.Makowski L., Brittingham K.C., Reynolds J.M., Suttles J., Hotamisligil G.S. The fatty acid-binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity. Macrophage expression of aP2 impacts peroxisome proliferator-activated receptor gamma and IkappaB kinase activities. J. Biol. Chem. 2005;280:12888–12895. doi: 10.1074/jbc.M413788200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elmasri H., Karaaslan C., Teper Y., Ghelfi E., Weng M., Ince T.A., Kozakewich H., Bischoff J., Cataltepe S. Fatty acid binding protein 4 is a target of VEGF and a regulator of cell proliferation in endothelial cells. FASEB J. 2009;23:3865–3873. doi: 10.1096/fj.09-134882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee M.Y., Li H., Xiao Y., Zhou Z., Xu A., Vanhoutte P.M. Chronic administration of BMS309403 improves endothelial function in apolipoprotein E-deficient mice and in cultured human endothelial cells. Br. J. Pharmacol. 2011;162:1564–1576. doi: 10.1111/j.1476-5381.2010.01158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee H., Lee Y.J., Choi H., Ko E.H., Kim J.W. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J. Biol. Chem. 2009;284:10601–10609. doi: 10.1074/jbc.M808742200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sha H., He Y., Chen H., Wang C., Zenno A., Shi H., Yang X., Zhang X., Qi L. The IRE1alpha-XBP1 pathway of the unfolded protein response is required for adipogenesis. Cell Metab. 2009;9:556–564. doi: 10.1016/j.cmet.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhandary B., Marahatta A., Kim H.R., Chae H.J. An involvement of oxidative stress in endoplasmic reticulum stress and its associated diseases. Int. J. Mol. Sci. 2013;14:434–456. doi: 10.3390/ijms14010434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higa A., Chevet E. Redox signaling loops in the unfolded protein response. Cell Signal. 2012;24:1548–1555. doi: 10.1016/j.cellsig.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 15.Malhotra J.D., Kaufman R.J. The endoplasmic reticulum and the unfolded protein response. Semin. Cell Dev. Biol. 2007;18:716–731. doi: 10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malhotra J.D., Miao H., Zhang K., Wolfson A., Pennathur S., Pipe S.W., Kaufman R.J. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc. Natl. Acad. Sci. U.S.A. 2008;105:18525–18530. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimazaki H., Watanabe K., Veeraveedu P.T., Harima M., Thandavarayan R.A., Arozal W., Tachikawa H., Kodama M., Aizawa Y. The antioxidant edaravone attenuates ER-stress-mediated cardiac apoptosis and dysfunction in rats with autoimmune myocarditis. Free Radic. Res. 2010;44:1082–1090. doi: 10.3109/10715762.2010.499904. [DOI] [PubMed] [Google Scholar]
- 18.Wang G., Gong Y., Anderson J., Sun D., Minuk G., Roberts M.S., Burczynski F.J. Antioxidative function of L-FABP in L-FABP stably transfected Chang liver cells. Hepatology. 2005;42:871–879. doi: 10.1002/hep.20857. [DOI] [PubMed] [Google Scholar]
- 19.Yan J., Gong Y., Wang G., Gong Y., Burczynski F.J. Regulation of liver fatty acid binding protein expression by clofibrate in hepatoma cells. Biochem. Cell Biol. 2010;88:957–967. doi: 10.1139/O10-151. [DOI] [PubMed] [Google Scholar]
- 20.Rajaraman G., Wang G.Q., Yan J., Jiang P., Gong Y., Burczynski F.J. Role of cytosolic liver fatty acid binding protein in hepatocellular oxidative stress: effect of dexamethasone and clofibrate treatment. Mol. Cell Biochem. 2007;295:27–34. doi: 10.1007/s11010-006-9268-6. [DOI] [PubMed] [Google Scholar]
- 21.Yan J., Gong Y., She Y.M., Wang G., Roberts M.S., Burczynski F.J. Molecular mechanism of recombinant liver fatty acid binding protein’s antioxidant activity. J. Lipid Res. 2009;50:2445–2454. doi: 10.1194/jlr.M900177-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erbay E., Babaev V.R., Mayers J.R., Makowski L., Charles K.N., Snitow M.E., Fazio S., Wiest M.M., Watkins S.M., Linton M.F., Hotamisligil G.S. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat. Med. 2009;15:1383–1391. doi: 10.1038/nm.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kajimoto K., Takayanagi S., Sasaki S., Akita H., Harashima H. RNA interference-based silencing reveals the regulatory role of fatty acid-binding protein 4 in the production of IL-6 and vascular endothelial growth factor in 3T3-L1 adipocytes. Endocrinology. 2012;153:5629–5636. doi: 10.1210/en.2012-1456. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed K.A., Sawa T., Ihara H., Kasamatsu S., Yoshitake J., Rahaman M.M., Okamoto T., Fujii S., Akaike T. Regulation by mitochondrial superoxide and NADPH oxidase of cellular formation of nitrated cyclic GMP: potential implications for ROS signalling. Biochem. J. 2012;441:719–730. doi: 10.1042/BJ20111130. [DOI] [PubMed] [Google Scholar]
- 25.Fujii S., Sawa T., Ihara H., Tong K.I., Ida T., Okamoto T., Ahtesham A.K., Ishima Y., Motohashi H., Yamamoto M., Akaike T. The critical role of nitric oxide signaling, via protein S-guanylation and nitrated cyclic GMP, in the antioxidant adaptive response. J. Biol. Chem. 2010;285:23970–23984. doi: 10.1074/jbc.M110.145441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sawa T., Arimoto H., Akaike T. Regulation of redox signaling involving chemical conjugation of protein thiols by nitric oxide and electrophiles. Bioconjug. Chem. 2010;21:1121–1129. doi: 10.1021/bc900396u. [DOI] [PubMed] [Google Scholar]
- 27.Sawa T., Zaki M.H., Okamoto T., Akuta T., Tokutomi Y., Kim-Mitsuyama S., Ihara H., Kobayashi A., Yamamoto M., Fujii S., Arimoto H., Akaike T. Protein S-guanylation by the biological signal 8-nitroguanosine 3′,5′-cyclic monophosphate. Nat. Chem. Biol. 2007;3:727–735. doi: 10.1038/nchembio.2007.33. [DOI] [PubMed] [Google Scholar]
- 28.Curtis J.M., Grimsrud P.A., Wright W.S., Xu X., Foncea R.E., Graham D.W., Brestoff J.R., Wiczer B.M., Ilkayeva O., Cianflone K., Muoio D.E., Arriaga E.A., Bernlohr D.A. Downregulation of adipose glutathione S-transferase A4 leads to increased protein carbonylation, oxidative stress, and mitochondrial dysfunction. Diabetes. 2010;59:1132–1142. doi: 10.2337/db09-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makowski L., Boord J.B., Maeda K., Babaev V.R., Uysal K.T., Morgan M.A., Parker R.A., Suttles J., Fazio S., Hotamisligil G.S., Linton M.F. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat. Med. 2001;7:699–705. doi: 10.1038/89076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu Y., Luo N., Lopes-Virella M.F., Garvey W.T. The adipocyte lipid binding protein (ALBP/aP2) gene facilitates foam cell formation in human THP-1 macrophages. Atherosclerosis. 2002;165:259–269. doi: 10.1016/s0021-9150(02)00305-2. [DOI] [PubMed] [Google Scholar]
- 31.Pelton P.D., Zhou L., Demarest K.T., Burris T.P. PPARgamma activation induces the expression of the adipocyte fatty acid binding protein gene in human monocytes. Biochem. Biophys. Res. Commun. 1999;261:456–458. doi: 10.1006/bbrc.1999.1071. [DOI] [PubMed] [Google Scholar]
- 32.Zha B.S., Zhou H. ER stress and lipid metabolism in adipocytes. Biochem. Res. Int. 2012;2012:312943. doi: 10.1155/2012/312943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huh J.Y., Kim Y., Jeong J., Park J., Kim I., Huh K.H., Kim Y.S., Woo H.A., Rhee S.G., Lee K.J., Ha H. Peroxiredoxin 3 is a key molecule regulating adipocyte oxidative stress, mitochondrial biogenesis, and adipokine expression. Antioxid. Redox Signal. 2012;16:229–243. doi: 10.1089/ars.2010.3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matarese V., Buelt M.K., Chinander L.L., Bernlohr D.A. Purification of adipocyte lipid-binding protein from human and murine cells. Methods Enzymol. 1990;189:363–369. doi: 10.1016/0076-6879(90)89309-6. [DOI] [PubMed] [Google Scholar]
- 35.Grimsrud P.A., Picklo M.J., Sr., Griffin T.J., Bernlohr D.A. Carbonylation of adipose proteins in obesity and insulin resistance: identification of adipocyte fatty acid-binding protein as a cellular target of 4-hydroxynonenal. Mol. Cell. Proteomics. 2007;6:624–637. doi: 10.1074/mcp.M600120-MCP200. [DOI] [PubMed] [Google Scholar]
- 36.Ischiropoulos H. Biological tyrosine nitration: a pathophysiological function of nitric oxide and reactive oxygen species. Arch. Biochem. Biophys. 1998;356:1–11. doi: 10.1006/abbi.1998.0755. [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi H., Matsuda M., Fukuhara A., Komuro R., Shimomura I. Dysregulated glutathione metabolism links to impaired insulin action in adipocytes. Am. J. Physiol. Endocrinol. Metab. 2009;296:E1326–E1334. doi: 10.1152/ajpendo.90921.2008. [DOI] [PubMed] [Google Scholar]
- 38.Calzadilla P., Sapochnik D., Cosentino S., Diz V., Dicelio L., Calvo J.C., Guerra L.N. N-acetylcysteine reduces markers of differentiation in 3T3-L1 adipocytes. Int. J. Mol. Sci. 2011;12:6936–6951. doi: 10.3390/ijms12106936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He N.G., Singhal S.S., Srivastava S.K., Zimniak P., Awasthi Y.C., Awasthi S. Transfection of a 4-hydroxynonenal metabolizing glutathione S-transferase isozyme, mouse GSTA4-4, confers doxorubicin resistance to Chinese hamster ovary cells. Arch. Biochem. Biophys. 1996;333:214–220. doi: 10.1006/abbi.1996.0383. [DOI] [PubMed] [Google Scholar]
- 40.Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog. Lipid Res. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- 41.Benedetti A., Comporti M., Esterbauer H. Identification of 4-hydroxynonenal as a cytotoxic product originating from the peroxidation of liver microsomal lipids. Biochim. Biophys. Acta. 1980;620:281–296. doi: 10.1016/0005-2760(80)90209-x. [DOI] [PubMed] [Google Scholar]
- 42.Kouoh F., Gressier B., Luyckx M., Brunet C., Dine T., Cazin M., Cazin J.C. Antioxidant properties of albumin: effect on oxidative metabolism of human neutrophil granulocytes. Farmaco. 1999;54:695–699. doi: 10.1016/s0014-827x(99)00082-8. [DOI] [PubMed] [Google Scholar]
- 43.Ron D., Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 44.Lee A.H., Iwakoshi N.N., Glimcher L.H. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell Biol. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagasawa K., Higashi T., Hosokawa N., Kaufman R.J., Nagata K. Simultaneous induction of the four subunits of the TRAP complex by ER stress accelerates ER degradation. EMBO Rep. 2007;8:483–489. doi: 10.1038/sj.embor.7400933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cantero-Recasens G., Fandos C., Rubio-Moscardo F., Valverde M.A., Vicente R. The asthma-associated ORMDL3 gene product regulates endoplasmic reticulum-mediated calcium signaling and cellular stress. Hum. Mol. Genet. 2010;19:111–121. doi: 10.1093/hmg/ddp471. [DOI] [PubMed] [Google Scholar]
- 47.Oyama Y., Okazaki E., Chikahisa L., Nagano T., Sadakata C. Oxidative stress-induced increase in intracellular Ca2+ and Ca(2+)-induced increase in oxidative stress: an experimental model using dissociated rat brain neurons. Jpn. J. Pharmacol. 1996;72:381–385. doi: 10.1254/jjp.72.381. [DOI] [PubMed] [Google Scholar]
- 48.Yeo T.K., Senger D.R., Dvorak H.F., Freter L., Yeo K.T. Glycosylation is essential for efficient secretion but not for permeability-enhancing activity of vascular permeability factor (vascular endothelial growth factor) Biochem. Biophys. Res. Commun. 1991;179:1568–1575. doi: 10.1016/0006-291x(91)91752-x. [DOI] [PubMed] [Google Scholar]
- 49.Glembotski C.C. Endoplasmic reticulum stress in the heart. Circ. Res. 2007;101:975–984. doi: 10.1161/CIRCRESAHA.107.161273. [DOI] [PubMed] [Google Scholar]
- 50.Bennaars-Eiden A., Higgins L., Hertzel A.V., Kapphahn R.J., Ferrington D.A., Bernlohr D.A. Covalent modification of epithelial fatty acid-binding protein by 4-hydroxynonenal in vitro and in vivo. Evidence for a role in antioxidant biology. J. Biol. Chem. 2002;277:50693–50702. doi: 10.1074/jbc.M209493200. [DOI] [PubMed] [Google Scholar]
- 51.Dickinson Zimmer J.S., Voelker D.R., Bernlohr D.A., Murphy R.C. Stabilization of leukotriene A4 by epithelial fatty acid-binding protein in the rat basophilic leukemia cell. J. Biol. Chem. 2004;279:7420–7426. doi: 10.1074/jbc.M311404200. [DOI] [PubMed] [Google Scholar]
- 52.Simpson M.A., LiCata V.J., Ribarik Coe N., Bernlohr D.A. Biochemical and biophysical analysis of the intracellular lipid binding proteins of adipocytes. Mol. Cell Biochem. 1999;192:33–40. [PubMed] [Google Scholar]
- 53.Kajimoto K., Terada H., Baba Y., Shinohara Y. Essential role of citrate export from mitochondria at early differentiation stage of 3T3-L1 cells for their effective differentiation into fat cells, as revealed by studies using specific inhibitors of mitochondrial di- and tricarboxylate carriers. Mol. Genet. Metab. 2005;85:46–53. doi: 10.1016/j.ymgme.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 54.Kajimoto K., Hossen M.N., Hida K., Ohga N., Akita H., Hyodo M., Hida Y., Harashima H. Isolation and culture of microvascular endothelial cells from murine inguinal and epididymal adipose tissues. J. Immunol. Methods. 2010;357:43–50. doi: 10.1016/j.jim.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 55.Kajimoto K., Hiura Y., Sumiya T., Yasui N., Okuda T., Iwai N. Exclusion of the catechol-o-methyltransferase gene from genes contributing to salt-sensitive hypertension in dahl salt-sensitive rats. Hypertens. Res. 2007;30:459–467. doi: 10.1291/hypres.30.459. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.


