Highlights
-
•
Calponin is an actin filament-associated protein and its h2 isoform inhibits cell motility.
-
•
H2-calponin expression is strong in prostate epithelial cells and diminished in cancerous cells.
-
•
Low h2-calponin metastatic prostate cancer cells had faster rates of cell proliferation and migration.
-
•
Low h2-calponin metastatic prostate cancer cells showed reduced substrate adhesion.
-
•
Low h2-calponin prostate cancer cells had a higher dependence on substrate stiffness.
Abbreviations: FBS, fetal bovine serum; mAb, monoclonal antibody; MES, 2-[N morpholino]ethanesulfonic acid; SDS–PAGE, SDS–polyacrylamide gel electrophoresis; PBS, phosphate buffered saline
Keywords: H2-calponin, Cytoskeleton, Cell motility, Mechanical regulation, Substrate stiffness
Abstract
Calponin is an actin filament-associated protein and its h2 isoform inhibits cell motility. Here we report significant expression of h2-calponin in prostate epithelial cells, which is diminished in cancerous cells. Comparison between a prostate cancer cell line PC3 and its metastatic derivative PC3-M showed lower levels of h2-calponin in PC3-M, corresponding to faster rates of cell proliferation and migration. Substrate adhesion of PC3 and PC3-M cells was positively correlated to the level of h2-calponin and the adhesion of PC3-M exhibited a higher dependence on substrate stiffness. Such effects of h2-calponin on cell proliferation, migration and substrate adhesion were also seen in normal versus cancerous primary prostate cells. Further supporting the role of h2-calponin in inhibiting cell motility, fibroblasts isolated from h2-calponin knockout mice proliferated and migrated faster than that of wild type fibroblasts. Transfective over-expression of h2-calponin in PC3-M cells effectively inhibited cell proliferation and migration. The results suggest that the diminished expression of h2-calponin in prostate cancer cells increases cell motility, decreases substrate adhesion, and promotes adhesion on high stiffness substrates.
1. Introduction
Prostate cancer is the most common malignant tumor in men in Western countries [1] and has a high frequency of bone metastasis [2]. The molecular and cellular mechanisms leading to the development of bone metastasis of prostate cancer are not fully understood. Cancer metastasis is a process involving tumor cell proliferation, detachment, intravasation, adhesion to vascular endothelial cells, extravasation, invasion, and growth in distant organs [3]. Cell motility plays a fundamental role in every step of this process, and the function of actin cytoskeleton is vital for cell division [4] and migration [5]. Alterations of actin [6,7] and actin-associated proteins, including h1-calponin [8], have been implicated in cancer metastasis.
Calponin is an actin filament-associated regulatory protein originally found in smooth muscle cells [9]. Three homologous isoforms of calponin, h1, h2 and h3 (acidic), have been identified in vertebrate species [10]. H1-calponin [11] is specifically expressed in differentiated smooth muscle cells and has been extensively studied for its role in the regulation of smooth muscle contractility [12]. Acidic calponin was first found in the brain with a possible function in neuronal regeneration and growth [13,14]. Expression of h2-calponin has been found in both smooth muscle [11] and non-muscle cells including epidermal keratinocytes [15,16], lung alveolar cells [17], endothelial cells [18,19], fibroblasts [17,20] and myeloid blood cells [21]. The function of h2-calponin includes stabilizing actin cytoskeleton [16] and inhibiting cytokinesis [22]. Biochemical properties of calponin were most extensively studied in chicken smooth muscle h1-calponin [10]. The conserved structures of h1, h2 and acidic calponins suggest conserved functional mechanisms [23].
H1-calponin was found in normal prostate tissue with a loss in prostate cancer [24]. A previous study reported that the level of h1-calponin was decreased in smooth muscle-derived cancerous tissues with a correlation to metastasis phenotypes and prognosis [8]. With the prostate cell type that expresses h1-calponin remains to be determined, the decreased level of h1-calponon in prostate cancer may indicate a destruction of smooth muscle-like stroma cells [25]. On the other hand, epithelial cells are major component in prostate gland, from which prostate carcinomas are originated. Consistent with the expression of h2-calponin in other epithelial cell types [16,17], h2-calponin is recently reported in prostate cancer cells and loss of h2-calponin induced cellular protrusions and increased cell migration [26].
In the present study, we examined the expression of h2-calponin in prostate cancer tissues and cells. Significant levels of h2-calponin were detected in the epithelia of normal prostate, which were diminished in cancerous prostate epithelial cells. Comparison between a prostate cancer cell line PC3 and its metastatic derivative PC3-M [27] showed a significantly lower level of h2-calponin in PC3-M. Correspondingly, PC3-M cells proliferated more rapidly and migrated faster. Transfective overexpression of h2-calponin in PC3-M cells effectively inhibited cell proliferation and migration. Supporting the role of h2-calponin in inhibiting cell motility, fibroblasts isolated from h2-calponin knockout mice proliferated and migrated faster than that of wild type fibroblasts. The adhesion of PC3 and PC3-M cells to culture substrate exhibited positive correlation with the level of h2-calponin, in which the adhesion of PC3-M cells was more dependent on substrate stiffness than that of PC3. These results suggest that the loss of h2-calponin in prostate cancer cells may contribute to the potency of metastasis, especially metastasis to the bone that is a high stiffness substrate.
2. Results
2.1. Presence of h2-calponin in prostate epithelial cells and its decreases in prostate cancer
A previous proteomic study reported a loss of h1-calponin in prostate cancer in comparison with non-cancerous prostate tissues [24]. Consistently, we also detected h1-calponin in non-cancerous prostate tissue and decreases in cancer tissue with immunohistochemistry and Western blotting using anti-h1-calponin monoclonal antibody (mAb) CP1 (Fig. 1A).
Fig. 1.

H2-calponin in prostate epithelia and its diminishment in cancerous prostate tissue. (A) Total protein extracts from hyperplasia or cancerous human prostate biopsies were analyzed with Western blots using anti-h1-calponin mAb CP1 and anti-h2-calponin polyclonal antibody RAH2 and mAb 1D2. The blots detected significant amounts of h1- and h2-calponins in the non-cancerous prostate tissue. Relative to the level of actin, h1-calponin was significantly decreased and h2-calponin became undetectable in the cancerous prostate tissues. (B) Thin paraffin sections of hyperplasia or cancerous human prostate tissues were examined with H&E staining and immunohistochemistry using anti-h2-calponin mAb 1D2 and SP2/0 myeloma cultural supernatant control. The results showed significant amount of h2-calponin in non-cancerous prostate gland epithelial cells but not in the cancerous tissue.
RAH2 and mAb 1D2 Western blots on total protein extract further detected h2-calponin in non-cancerous human prostate tissue. The prostate h2-calponin was also diminished in cancer (Fig. 1A). Immunohistochemistry localized h2-calponin in the epithelial cells of human prostate gland, which became undetectable in prostate cancer tissue (Fig. 1B).
The expression of h2-calponin but not h1-calponin in prostate epithelial cells was confirmed by Western blots in human prostate cancer cell line PC3 and its metastatic derivative PC3-M, together with two pairs of non-cancerous versus cancerous primary prostate epithelial cell lines 1542NPTX/1542CP3TX and 1532NPTX/1532CPTX (Fig. 2A).
Fig. 2.

Expression of h2-calponin in prostate epithelial cells. (A) RAH2, CP1 and CP21 Western blots of total protein extracts from prostate epithelial and cancer cell lines (PC3 versus PC3-M, 1542NPTX versus 1542CP3TX and 1532NPTX versus 1532CPTX) detected significant expression of h2-calponin but not h1-calponin. (B) Normalized to the level of actin, densitometry quantification of RAH2 blots showed that the metastatic PC3-M cells express significantly less h2-calponin than that in PC3 cells (∗P < 0.001). (C) The cancerous 1542CP3TX cells also expressed less h2-calponin than that in 1542NPTX cells derived from the non-cancerous portion of the same prostate (∗P < 0.001). (D) The 1532NPTX and 1532CPTX cells express similar levels of h2-calponin, providing a pair of control cells.
Normalized to the level of actin, densitometry quantification of the Western blots found that the level of h2-calponin in PC3-M cells was ∼34% lower than that in PC3 (Fig. 2B), implicating a correlation to the metastatic phenotype. Similarly, the cancerous 1542CP3TX cells had a significantly lower level of h2-calponin than that in the non-cancerous 1542NPTX cells (Fig. 2C). On the other hand, the cell pair 1532CPTX and 1532NPTX showed no significant difference in the level of h2-calponin (Fig. 2D). The different levels of h2-calponin in these primary prostate cancer cell lines indicate variations in biological characteristics among individual cancer patients, of which the 1532CPTX/1532NPTX cells expressing similar levels of h2-calponin provide a useful control pair for our study of the role of h2-calponin in prostate cancer cell motility.
Confocal microscopic images of PC3 and PC3-M cells showed that h2-calponin co-localized with F-actin (Fig. 3A), consistent with the role of h2-calponin in actin-mediated cellular functions such as migration and proliferation [21,22]. Fluorescence intensity of the confocal images confirmed that PC3-M cells contained a lower level of h2-calponin than that in PC3 (Fig. 3B). There was no qualitative difference in the cellular distribution of h2-calponin in PC3 and PC3-M cells.
Fig. 3.

Localization of h2-calponin in PC3 and PC3-M cells. (A) Monolayer cultures of PC3 and PC3-M cells were stained with anti-h2-calponin mAb 1D2 and phalloidin. The result showed that h2-calponin co-localized with F-actin in these cells. (B) Normalized to cell area, fluorescence intensity analysis of confocal microscopic images showed that PC3-M contained less h2-calponin than that in PC3 cells (∗P < 0.005).
2.2. The loss of h2-calponin in prostate cancer cells increased cell proliferation
Comparison between the growth curves of PC3 and PC3-M cells showed that PC3-M proliferated significantly faster than that of PC3 (Fig. 4A), demonstrating a correlation to the lower level of h2-calponin in PC3-M cells. This inverted correlation between the level of h2-calponin and the rate of cell proliferation was confirmed by the faster proliferation rate of 1542CP3TX versus that of 1542NPTX cells expressing low versus high levels of h2-calponin, in contrast to the lack of difference between 1532CPTX and 1532NPTX cells that express similar levels of h2-calponin (Fig. 4B).
Fig. 4.
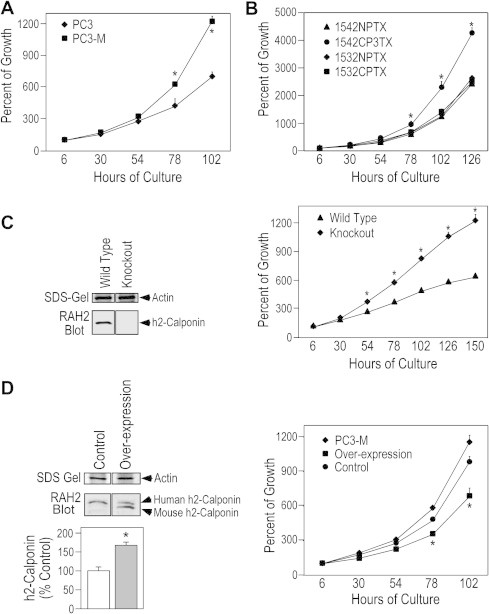
Loss of h2-calponin in prostate cancer cells increases the rate of cell proliferation. (A) The growth curves demonstrated that PC3-M cells proliferated significantly faster than that of PC3 cells (∗P < 0.001, n = 4 experiments). (B) 1542CP3TX cells also proliferated faster than that of 1542NPTX (∗P < 0.001, n = 3 experiments). Different from the two cell pairs expressing different levels of h2-calponin, there was no difference in the proliferation rates of 1532NPTX and 1532CPTX cells that express similar levels of h2-calponin (Fig. 2D). (C) The SDS–PAGE gel and RAH2 Western blot demonstrated the deletion of h2-calponin in fibroblasts isolated from h2-calponin gene knockout mice. The growth curves showed significantly higher proliferation rate of the h2-calponin-null cells as compared with the wild type control (∗P < 0.001, n = 4 experiments). (D) Western blots and densitometry quantification showed that transfective overexpression of mouse h2-calponin increased the total h2-calponin in PC3-M cells (∗P < 0.001, n = 3). The growth curves showed that the boost up of total h2-calponin to levels similar to that in PC3 cells (Table 1) effectively decreased the proliferation rate of PC3-M cells (∗P < 0.001, n = 4 experiments). Transfected G418-resistant PC3-M cells that do not overexpress mouse h2-calponin were used as control.
To demonstrate the causal effect of decreased h2-calponin on the increased proliferation of prostate cancer cells, boosting the level of h2-calponin in PC3-M cells by stable transfective overexpression (Table 1) effectively suppressed the rate of proliferation (Fig. 4D).
Table 1.
Relative level of h2-calponin expression in PC3, PC3-M and transfected PC3-M cells.
| Cell line | Level of h2-calponin (% of PC3) |
|---|---|
| PC3 | 100.00 ± 6.02 |
| PC3-M | 66.07 ± 5.91∗ |
| Transfected PC3-M (low overexpression)a | 99.93 ± 6.09†,§ |
| Transfected PC3-M (high overexpression)b | 190.46 ± 38.81¶,#,‡ |
The relative levels of total h2-calponin expressed in PC3, PC3-M and mouse h2-calponin cDNA stable transfected PC3-M cells were quantified from Western blots by normalization to the level of actin shown in parallel SDS-gel. Densitometry comparisons showed that the lower level of h2-calponin in PC3-M versus PC3 cells (∗P < 0.001) was effectively boosted up by transfective overexpression (†Low overexpression versus PC3-M: P < 0.001; ¶High overexpression versus PC3-M: P < 0.001; #High overexpression versus PC3: P < 0.001; ‡High versus low overexpression: P < 0.001. The low overexpression boosted the h2-calponin level in PC3-M cells up to levels similar to that in PC3 cells (§P = 0.493). The cell proliferation and round up experiments were carried out using transfective low overexpression PC3-M cells for comparison with non-transfected PC3-M and PC3 cells. a, 4 stable cell lines; b, 6 stable cell lines. n = 3 experiments.
2.3. The loss of h2-calponin increased prostate cancer cell migration
In vitro scratch wound healing assay showed that PC3-M cells healed the wound faster than that of PC3 (Fig. 5A), consistent with the role of h2-calponin in inhibiting cell migration [21]. Therefore, the decreased level of h2-claponin in PC3-M cells may contribute to its high motility and metastatic phenotype in comparison to its parental PC3 cells [27]. As in the proliferation assay described above, the h2-calponin-dependent inhibition of prostate cancer cell migration was further demonstrated using primary prostate cell lines 1542CP3TX (low h2-calponin) versus 1542NPTX (high h2-calponin) with no difference between the 1532NPTX/1532CPTX control pair that express similar levels of h2-calponin (Fig. 5B).
Fig. 5.

Decrease or loss of h2-calponin facilitates cell migration. (A) In vitro scratch wounding healing experiments showed that PC3-M cells started migration and closed the wound earlier than that of PC3 (∗P < 0.001, n = 3 experiments). (B) 1542CP3TX cells also migrated faster and closed the wound earlier than that of 1542NPTX (∗P < 0.001, n = 3 experiments). In contrast to the two cell pairs expressing different levels of h2-calponin, 1532CPTX and 1532NPTX cells that express similar levels of h2-calponin (Fig. 2A) migrated at similar speed in the wounding healing experiments. (C) Supporting the role of h2-calponin in inhibiting cell motility, h2-calponin-null mouse fibroblasts (Fig. 4C) started migration and closed the wound earlier than that of the h2-calponin-posirtive wild type fibroblasts (∗P < 0.001, n = 4 experiments).
Raising the level of h2-calponin in PC3-M cells by transfective over-expression (Fig. 6A) effectively inhibited the rate of cell migration in wound healing experiments as shown by the representative cell lines with high and low levels of stable transfective overexpression (Fig. 6B; Table 1). The results support a causal role of decreased h2-calponin in the high motility phenotype of metastatic prostate cancer cells.
Fig. 6.

Boosting up the level of h2-caponin inhibited the migration of prostate cancer cells. When the level of total h2-calponin in PC3-M cells was increased by transfective expression as shown by the RAH2 Western blots and densitometry quantification (A) ∗P < 0.001 versus non-transfected control; #P < 0.001 between the high and low levels of transfective expression as normalized to the amount of actin (Table 1), the rate of cell migration decreased correspondingly (B) ∗P < 0.001, n = 3 experiments.
2.4. Substrate stiffness determines the expression of h2-calponin in prostate cancer cells
We previously reported that the expression of h2-calponin is regulated by the stiffness of culture substrate [16]. In comparison to the effect of rigid or high stiffness substrates, soft substrates that produce lower traction force in the cytoskeleton [36] decreased h2-calponin expression in epidermal keratinocytes, fibroblasts [16] and lung alveolar cells [17]. The present study demonstrated that this is also true for PC3 and PC3-M prostate cancer cells. The results in Fig. 7 showed that PC3 and PC3-M cells cultured on low stiffness soft polyacrylamide gel (elastic modulus = 1 kPa) expressed significantly less h2-calponin than that in cells cultured on high stiffness hard gel (elastic modulus = 8 kPa). The high level expression of h2-calponin in cells grown on hard gel was similar to that in cells grown on plastic dish, indicating that the physical stiffness, other than the chemical property, of the cultural substrates determined the level of h2-calponin in prostate cancer cells. Together with the previous studies on other cell types, the results demonstrated a conserved regulation of h2-calponin gene expression by cytoskeleton tension in response to the stiffness of culture substrate.
Fig. 7.

Substrate stiffness-dependent expression of h2-calponin in prostate cancer cells. (A) PC3 and PC3-M cells were cultured for 3 days on thin layers of polyacrylamide gels of high (8 kPa) or low (1 kPa) stiffness and on plastic dish control. (B) The level of h2-calponin expressed in the cells was determined with Western blot using RAH2 antibody. Normalized to the level of actin, densitometry quantification showed lower expression of h2-calponin in both PC3 and PC3-M cells grown on soft polyacrylamide gel than that in cells cultured on hard gel or plastic substrates (∗P < 0.001, n = 4 experiments).
2.5. The loss of h2-calponin decreased substrate adhesion of prostate cancer cells
To demonstrate the effect of h2-calponin on cell adhesion, more PC3 cells than PC3-M cells were attached to uncoated plastic plates up to 8 h after plating (Fig. 8A). This difference was further shown in adhesion experiments using collagen-coated plastic plates. ∼53% of PC3 cells attached 10 min after seeding versus ∼20% for PC3-M cells (Fig. 8B).
Fig. 8.

Positive correlations between the level of h2-calponin and cell adhesion. (A) The time courses showed that in the first 8 h after plating, PC3 cells adhered to uncoated culture plates more than that of PC3-M cells, whereas the maximum adhesion capacity as shown at 24 h after plating was unchanged. (B) The effect of the higher level of h2-calponin in PC3 cells on facilitating adhesion was further shown in adhesion to collagen coated cultural plates in 30 min after plating (∗P < 0.05; ∗∗P < 0.001. n = 3 experiments). (C) The cell spreading area in adherent monolayer cultures was positively correlated to the level of h2-calponin as shown by the difference between PC3 and PC3-M cells. (D) Correspondingly, the speed of cell roundup during trypsin digestion of adherent cultures was negatively correlated to the level of h2-calponin, in which the roundup of PC3 cells was slower than that of PC3-M and transfective overexpression of h2-calponin in PC3-M cells to boost the level of total h2-calponin to that similar to the level in PC3 cells (Table 1) reversed the trend (∗P < 0.001, n = 4 experiments).
It was interesting to observe that the proportion of adhered cells became similar between PC3 versus PC3-M 24 h after seeding to uncoated plates and 1 h after seeding to collagen-coated plates (Fig. 8A and B). This preserved maximum adhesion suggests that h2-calponin functions in accelerating the speed, other than the capacity, of cell adhesion. The similar trends of adhesion of the cells to uncoated and collagen-coated plates suggested that the effect of h2-calponin on cell adhesion is independent of collagen receptors, in which the slower adhesion to uncoated plates reflects the effect of cell-secreted collagen on facilitating overall adhesion.
Supporting the role of h2-calponin in enhancing cell adhesion, the spreading area of PC3-M cells on plastic substrate was significantly smaller than that of PC3 cells (Fig. 8C). Also consistent with the role of h2-calponin in stabilizing actin cytoskeleton [16], the lower level of h2-calponin in PC3-M cell correlated to faster roundup during trypsin digestion than that of PC3 cells containing higher level of h2-calponin (Fig. 8D). Transfective overexpression of h2-calponin in PC3-M cells (Table 1) effectively slowed the roundup velocity (Fig. 8D).
2.6. Loss of h2-calponin increased the dependence of prostate cancer cell adhesion on substrate stiffness
We examined PC3 versus PC3-M, 1542NPTX versus 1542CP3TX prostate cells, and 1532NPTX versus 1532CPTX control prostate cells for their adhesions to hard and soft gel substrates. The results in Fig. 9A, C and E showed that all of these cell lines adhered with lower numbers to low stiffness soft substrate than that to high stiffness hard substrate. PC3-M (Fig. 9A) and 1542CP3TX (Fig. 9C) cells adhered less than that of their counterparts expressing higher levels of h2-calponin. There was no difference between 1532CP3TX and 1532NP3TX cells that express similar levels of h2-calponin (Fig. 9E).
Fig. 9.

Decreases in h2-calponin increased the dependence of prostate cancer cell adhesion on substrate stiffness. Demonstrating the positive correlation between h2-calponin and substrate stiffness dependent cell adhesion, the results of cell adhesion to polyacrylamide gel substrates showed that PC3-M (A) and 1542CP3TX (C) adhered less in comparison with that of PC3 and 1542NPTX, respectively. The data further showed that less cells adhered to soft gel substrate than that to hard gel (∗P < 0.001). In contrast, 1532CPTX and 1532NPTX cells that express similar levels of h2-calponin (Fig. 2D) showed no difference in adhesion to substrates (E). Panels B and D illustrate the ratio of cells adhered to high versus low stiffness gel substrates. Comparing with cells expressing high levels of h2-calponin, cells expressing lower level of h2-calponin had significantly higher increases in cell adhesion to high stiffness substrate versus that to low stiffness substrate (∗P < 0.001). 1532CPTX and 1532NPTX cells that express similar levels of h2-calponin showed no such difference (F). The data were summarized from four repeated experiments.
An interesting observation was that the adhesion of low h2-calponin cells was more dependent on substrate stiffness. The comparisons in Fig. 9B and D demonstrated that high stiffness substrate had significantly greater enhancement for the adhesion of PC3-M and 1542CP3TX cells than that for their counterparts expressing higher levels of h2-calponin. Such pattern was not seen between 1532CP3TX and 1532NP3TX cells that express similar level of h2-calponin (Fig. 9F). These data suggest that the decreased level of h2-calponin in prostate cancer cells may increase the preference of adhesion to high stiffness substrates. This mechanism may contribute to prostate cancer’s high tendency of metastasis to the bone that provides a uniquely high stiffness tissue environment.
3. Discussion
The present study investigated the expression and function of h2-calponin in prostate cancer cells. The following findings are of notable significance:
3.1. The expression of h2-calponin in prostate gland epithelial cells and its diminishment in prostate cancer
The Western blot and immunohistochemistry data in Fig. 1 demonstrated that prostate gland epithelial cells express significant amounts of h2-calponin, which were diminished in cancerous prostate tissues. This finding was confirmed by the significant amount of h2-calponin in the prostate cancer cell line PC3 and the decreased expression in its metastatic derivative PC3-M.
The previously reported decrease of h1-calponin in prostates cancer tissues [24] was also confirmed in our study. It is important to emphasize that h1-calponin is known to express specifically in smooth muscle cells [12,37]. Since h1-calponin is not detected in the gland epithelial cells of prostate tissue (Fig. 1) or epithelial/carcinoma-derived cell lines (Fig. 2), the detection of h1-calponin in normal prostate tissue may reflect its presence in stroma smooth muscle-like cells and its diminishment in prostate cancer may reflect lesions destroying the organ structure.
H2-calponin is expressed in smooth muscles [11] as well as in various non-muscle cells, including epithelial cells [15–17]. H2-calponin in prostate epithelial cells with a functional significance in cancer cell proliferation, migration and adhesion is a novel observation. The decreased h2-calponin in metastatic prostate cancer cells is consistent with a report that lower levels of h1-calponin in leiomyosarcoma were correlated to metastasis and poorer prognosis [8]. It is worth noting that decreased h2-calponin was not uniformly seen in all prostate cancers examined (Fig. 2), but with strong correlation to cell motility features. Therefore, decreases in h2-calponin may be explored as a potential biomarker for the evaluation of the degree of malignancy of prostate cancer in individual patients.
3.2. Decreases in h2-calponin increase prostate cancer cell proliferation and motility
H2-calponin has been shown to affect the structural organization [15,38] and stability [16] of actin cytoskeleton. The actin cytoskeleton undergoes dynamic remodeling during cell proliferation and migration, which are key steps in tumor progression and metastasis. We previously demonstrated that over-expression of h2-calponin effectively reduced the rate of cell proliferation by inhibiting cytokinesis [22]. PC3-M cells with increased metastatic ability [27,39] have decreased level of h2-calponin (Fig. 2) and proliferated and migrated faster than that of the less metastatic PC3 cells (Figs. 4 and 5). The faster proliferation and migration of PC3-M cells were effectively suppressed by transfective overexpression of h2-calpon (Figs. 4 and 6).
To demonstrate that the effect of decreased h2-calponin on the proliferation and motility of prostate cancer cells is not restricted to the PC3 versus PC3-M cells, the correlation between decreases in h2-calponin and increased rates of cell proliferation and migration was further demonstrated in the non-malignant 1542NPTX versus malignant/cancerous 1542CP3TX prostate endothelial cells from the same patient (Figs. 4 and 5). As a control, the 1532NPTX versus 1532CPTX cell pair that express similar levels of h2-calponin proliferated and migrated at similar rates (Figs. 4 and 5).
The inhibitory effect of h2-calponin on cell proliferation and migration was further confirmed by the significantly faster proliferation and migration rates of fibroblasts from h2-calponin knockout mice in comparison to that of wild type fibroblasts expressing a high level of h2-calponin (Fig. 4C and Fig. 5C). These data suggest that decreases in h2-calponin in prostate cancer cells may contribute to malignant growth and metastasis through facilitating remodeling of the actin cytoskeleton during cell proliferation and migration.
3.3. Decreases in h2-calponin alter prostate cancer cell adhesion
Extensive studies have investigated the chemical environment of bone tissue underlying prostate cancer’s high tendency of bone metastasis [2]. It has been observed that living cells dynamically change structure and function through gene regulation and posttranslational protein modification in response to mechanical environment [40–44]. A potential contribution of the high stiffness mechanical environment in bone tissue to the metastasis of prostate cancer suggests an interesting new direction of research. Based on calponin’s established role as a cytoskeleton regulatory protein [10,12] that is responsive to mechanical tension environment [16,17,45], our study of normal versus cancerous prostate epithelial and cancer cells differing in metastatic potency explored the role of h2-calponin in prostate cancer cell adhesion and the dependence on substrate stiffness.
Our results demonstrated that decreases in h2-calponin slowed prostate cancer cell adhesion to both soft and hard substrates (Fig. 7). Consistent with the role of h2-calponin in stabilizing actin cytoskeleton [16], we demonstrated that decreases in h2-calponin slowed cell adhesion, reduced cell spreading area and facilitated cell roundup during trypsin digestion (Fig. 8). On the other hand, substrate stiffness is known to up-regulate h2-calponin gene expression [16,17,45]. The high stiffness substrate environment in bone tissue would, therefore, result in higher levels of h2-calponin in adhered cells, which in turn stabilize the cell adhesion to promote metastatic anchor of arriving cancer cells.
The observation that low in h2-calponin resulted in higher dependence of prostate cancer cell adhesion on substrate stiffness (Fig. 9) suggests that diminishment of h2-calponin in prostate cancer cells may contribute to the high tendency of metastasis to bone that provides a unique high stiffness substrate environment. This proposed positive feedback effect of substrate stiffness would be more important for cancer cells that had primarily decreased expression of h2-calponin to sustain a critical level of h2-calponin protein and maintain a stable actin cytoskeleton by adhesion on high stiffness substrates. The h2-calponin-dependent effect of substrate stiffness on prostate cancer cell adhesion suggests a potentially novel therapeutic target to prevent bone metastasis and merits further investigation.
4. Materials and methods
The examination of de-identified diagnostic human tissue samples removed during surgery was determined as exempt research by the institutional Human Investigation Committee. The study of mouse cells was carried out using protocols approved by the Institutional Animal Care and Use Committee of Wayne State University.
4.1. Anti-calponin antibodies
The following previously described antibodies were used in the present study: A rabbit polyclonal anti-h2-calponin antibody RAH2 that was raised by immunization with purified mouse h2-calponin [28]; a mouse anti-h1-calponin mAb CP1 that was developed by immunization with chicken gizzard calponin [29]; mouse anti-h2-calponin mAbs CP21 [22] and 1D2 [16] that were raised by immunizations with mouse and human h2-calponin, respectively.
4.2. Immunohistochemistry
To examine the expression and distribution of h1- and h2-calponins in human prostate tissue, thin paraffin sections were stained with anti-h2-calponin mAb 1D2 followed by horseradish peroxidase-labeled anti-mouse IgG second antibody (Sigma) and H2O2-daminobenzidin substrate reactions using standard immunohistochemical method [16,17]. Hybridoma cultural supernatant was used to avoid potential autoantibody reactivity in adult animal sera or mouse ascites fluid to cytokeratins (unpublished observations). SP2/0 myeloma cultural supernatant was used as control. Counterstaining with 0.6% hematoxylin for 20 s was used to outline the tissue morphology for comparison with H&E stained sections. The results were examined using a Zeiss Axiovert 100H microscope and photographed.
4.3. SDS–polyacrylamide gel electrophoresis (PAGE) and Western blotting
Total protein was extracted from discarded anonymous human prostate tissue samples removed during surgery by homogenization in SDS–PAGE sample buffer containing 2% SDS (to rapidly inactivate endogenous proteases) using a high-speed mechanical tissue homogenizer. Total protein extracts from cultured cells were prepared by direct lysis of monolayer cells in SDS–PAGE sample buffer after washing with phosphate buffered saline (PBS).
The protein extracts were heated at 80 °C for 5 min, clarified by centrifugation at top speed in a microcentrifuge, and examined using SDS–PAGE with 12% gel in Laemmili buffers and acrylamide:bisacrylamide ratio of 29:1. Contents and integrity of the protein samples were evaluated by staining the gels with Coomassie Blue R250. Duplicate gels were electrically transferred to nitrocellulose membranes for Western blotting using anti-calponin antibodies and alkaline phosphatase-labeled anti-rabbit IgG or anti-mouse IgG second antibody (Sigma), followed by 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium chromogenic substrate reaction [16].
4.4. Cell cultures
Human prostate cancer cell lines PC3 and its metastatic derivative PC3-M [27] were cultured in RPMI-1640 containing 10% fetal bovine serum (FBS), 2 mM l-glutamine, 100 i.u./mL penicillin and 50 i.u./mL streptomycin at 37 °C in 5% CO2.
Two pairs of non-malignant versus malignant/cancerous primary human prostate epithelial cell lines 1542NPTX (non-malignant)/1542CP3TX (malignant) and 1532NPTX (non-malignant)/1532CPTX (malignant) [30] were cultured in keratinocyte media (Invitrogen) containing 5% FBS, 10 mM HEPES, 2 mM l-glutamine, 100 i.u./mL penicillin and 50 i.u./mL streptomycin at 37 °C in 5% CO2.
Primary fibroblasts were isolated as described previously [31] from leg muscles of adult wild type and h2-calponin knockout mice [21]. Briefly, muscles were isolated under sterile conditions immediately after euthanasia and minced with a pair of sharp scissors in DMEM containing 0.5% pancreatin and 0.125% trypsin. Triturated with a 10 mL pipette, incubated at 37 °C for 6 min, triturated again and incubated for 7 more min, the digestion mix was passed through a 100 μm mesh and the isolated cells were collected by centrifugation at 200×g for 5 min, re-suspended in DMEM containing 20% FBS, 2 mM l-glutamine, 100 i.u./mL penicillin and 50 i.u./mL streptomycin, and incubated in cultural dishes in 5% CO2 at 37 °C for 1 h. The non-attached cells were discarded to selectively culture the adherent fibroblasts. Second and third passages of the primary fibroblasts were used for experiments.
4.5. Immunofluorescence microscopy
Pre-cleaned glass cover slips were coated with 0.1% gelatin and dried under UV radiation before being placed in culture dish for the seeding of PC3 and PC3-M cells. Coverslips with a monolayer of PC3 and PC3-M cells were collected and washed with PBS. The cells were fixed with cold acetone for 30 min. After blocking with 1% BSA in PBS in a humidity box at room temperature for 30 min, the coverslips were incubated with anti-h2-calponin mAb 1D2 at room temperature for 2 h. After washes with PBS containing 0.05% Tween-20, the coverslips were stained with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG second antibody (Sigma) and tetramethylrhodamine isothiocyanate (TRITC)-conjugated phalloidin (Sigma) (for F-actin) at room temperature for 1 h. After final washes with PBS containing 0.05% Tween-20, the coverslips were mounted on glass slides and examined using confocal microscopy for the cellular localization of h2-calponin and the relationship to the actin cytoskeleton.
4.6. Transfective expression of h2-calponin
Transfection of PC3-M human prostate cancer cells with recombinant pcDNA3.1 plasmids encoding mouse h2-calponin [22] was carried out with Lipofectamin (Invitrogen) following the manufacturer’s protocol. 2 × 106 of PC3-M cells were seeded in a 100 mm culture dish that the transfection was carried out when the monolayer culture reached 60–80% confluence. Two microgram of supercoil recombinant plasmid DNA in 100 μL RPMI-1640 was mixed with 5 μL of Lipofectamin in 100 μL RPMI-1640 and incubated at room temperature for 20 min. The Lipofectamin-DNA complex was then gently mixed with 5 mL of RPMI-1640 and added to the culture dish to replace the cultural media. The cells were incubated in 5% CO2 at 37 °C for 6 h before adding 5 mL fresh RPMI-1640 media containing 20% FBS without antibiotics.
To establish stable transfection of PC3-M cells, the cell culture was selected by 400 μg/mL of G418. G418-resistant colonies were individually picked from the culture dish by trypsinization in small cylinders greased to the dish. The cells were expanded and samples were taken to extract DNA for verification of the transfection using polymerase chain reaction. Overexpression of mouse h2-calponin in PC3-M cells was examined on total cellular protein extracts using Western blot as above.
4.7. Cell proliferation assay
To investigate the effects of h2-calponin on the rate of cell proliferation, we employed the Crystal Violet method as described previously [22]. Cells were seeded in 96-well culture plates at 2 × 103 cells per well in 200 μL of culture media. The cultures were stopped at a series of time points by adding 20 μL per well of 11% glutaraldehyde to fix the cells. After gently shaking at room temperature for 15 min, the plates were washed three times with double distilled water and air-dried. The plates were then stained with 100 μL per well of 0.1% Crystal Violet (Sigma) in 20 mM 2-[N morpholino]ethanesulfonic acid (MES) buffer (pH 6.0). After gentle shaking at room temperature for 20 min, excess dye was removed by extensive washing with double distilled water and the plates were air-dried prior to extracting the bound dye with 100 μL per well of 10% acetic acid. Optical density of the dye extracts was measured at 595 nm using an automated microplate reader (Benchmark, BioRad Labs).
4.8. Cell culture on polyacrylamide gel substrates of different stiffness
Thin layers of polyacrylamide gel were formed to provide cell culture substrates of different stiffness [32,33]. Hard (5% acrylamide and 0.3% bisacrylamide with elastic modulus = 8 kPa) and soft (5% acrylamide and 0.03% bisacrylamide with elastic modulus = 1 kPa) gels approximately 70 μm thick were prepared and covalently coated with type I collagen as described previously [16,34]. To examine the effect of cytoskeletal tension generated from substrate stiffness on the expression of h2-calponin in prostate cancer cells, PC3 and PC3-M cells were cultured on the hard and soft gels as well as on plastic dish (as an extremely high stiffness control) for 3 days, washed extensively with PBS, and harvest by direct lysis in SDS-gel sample buffer. The level of h2-calponin was examined using Western blot as above.
4.9. In vitro wound healing assay
To examine the role of h2-calponin in the motility of prostate cancer cells, PC3 versus PC3-M, 1532NPTX versus 1532CPTX, and 1542NPTX versus 1542CP3TX cells, h2-calponin cDNA versus vector-only stable-transfected PC3-M cells, and primary fibroblasts from h2-calponin knockout versus wild type mice, were seeded on 6-well culture plates at 2 × 105 cells per well and cultured for 3 days. As described previously [35], the confluent monolayer cells were scratch-wounded with a thin pipette tip. To monitor the healing process, phase-contrast images were photographed every 2 h for the transfected PC3-M cells and every 1.5 h for the other cell types. The width of the wound was measured and plotted against time to determine the rate of cell migration.
4.10. Cell adhesion assay
Adhesion velocity of PC3 and PC3-M cells to uncoated or 0.2 μg/mL collagen type I-coated plastic culture plates was measured at a series of time points using Crystal Violet staining as above after removing non-adhered cells with PBS washes.
Cell spreading area on plastic substrate was also measured to evaluate the effect of h2-calponin on cell adhesion. PC3 and PC3-M cells were seeded on uncoated 6-well culture plates and phase-contrast images were taken after 8 h of culture. The cell spreading area was measured from the photographs using NIH Image program version 1.61.
To investigate the effect of h2-calponin on the stability of actin cytoskeleton in adherent cells, the round up velocity of PC3 versus PC3-M cell was measured during trypsinization. 4 × 105 cells per well were seeded in 6-well plates and cultured for 24 h, the monolayer cells were washed with PBS and treated with a low concentration trypsin-EDTA solution (0.025% trypsin, 0.1 mM EDTA). Phase-contrast images were taken 10 min after the treatment and the round up cells were counted in randomly selected areas against the remaining adhered cells to evaluate the round up velocity.
To examine the role of h2-calponin in cell adhesion to substrates of different stiffness, PC3, PC3-M, 1532NPTX, 1532CPTX, 1542NPTX and 1542CP3TX cells were seeded on hard (8 kPa) and soft (1 kPa) polyacrylamide gels. Phase contrast images were taken after 8 h of culture. The adhered cells were recognized by the photographic image and counted from multiple randomly selected areas.
4.11. Data analysis
Densitometry analysis of SDS-gels, Western blots and confocal microscopic images were performed using NIH Image program version 1.61 on high resolution digital images. Western blots were quantified by normalization to the amount of actin in parallel Coomassie Blue-stained gels. The quantitative data of Western blots, cell proliferation, wound healing and cell adhesion assays are presented as mean ± SD. Statistical analysis was performed with unpaired two-tail Student’s t-test using the Microsoft Excel computer program.
Author contributions
M.M.H. performed the experiments, drafted the manuscript and figures, and formatted the submission; X.W. performed the experiments; R.C.B. designed the experiments; J.P.J. made conception of the research and designed the experiments, drafted the manuscript and figures, edited and revised the submission.
Acknowledgement
This study was supported by a grant from the National Institutes of Health, USA HL-086720 to J-PJ.
References
- 1.Greenlee R.T., Murray T., Bolden S., Wingo P.A. Cancer statistics. CA Cancer J. Clin. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 2.Ye L., Kynaston H.G., Jiang W.G. Bone metastasis in prostate cancer: molecular and cellular mechanisms. Int. J. Mol. Med. 2007;20:103–111. [PubMed] [Google Scholar]
- 3.Fidler I.J. Critical factors in the biology of human cancer metastasis: twenty-eighth G.H.A. Clowes Memorial Award Lecture. Cancer Res. 1990;50:6130–6138. [PubMed] [Google Scholar]
- 4.Han E.K.H., Guadagno T.M., Dalton S.L., Assoian R.K. A cell cycle and mutational analysis of anchorage-dependent growth: cell adhesion and TGF-beta1 control G1/S transit specificity. J. Cell Biol. 1993;122:461–471. doi: 10.1083/jcb.122.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ponti A., Machacek M., Gupton S.L., Waterman-Storer C.M., Danuser G. Two distinct actin networks drive the protrusion of migrating cells. Science. 2004;305:1782–1786. doi: 10.1126/science.1100533. [DOI] [PubMed] [Google Scholar]
- 6.Shimokawa-Kuroki R., Sadano H., Taniguchi S. A variant actin (beta m) reduces metastasis of mouse B16 melanoma. Int. J. Cancer. 1994;56:689–697. doi: 10.1002/ijc.2910560514. [DOI] [PubMed] [Google Scholar]
- 7.Okamoto-Inoue M., Kamada S., Kimura G., Taniguchi S. The induction of smooth muscle alpha actin in a transformed rat cell line suppresses malignant properties in vitro and in vivo. Cancer Lett. 1999;142:173–178. doi: 10.1016/s0304-3835(99)00150-0. [DOI] [PubMed] [Google Scholar]
- 8.Horiuchi A., Nikaido T., Ito K., Zhai Y., Orii A., Taniguchi S., Toki T., Fujii S. Reduced expression of calponin h1 in leiomyosarcoma of the uterus. Lab. Invest. 1998;78:839–846. [PubMed] [Google Scholar]
- 9.Takahashi K., Hiwada K., Kokubu T. Isolation and characterization of a 34,000-dalton calmodulin- and F-actin-binding protein from chicken gizzard smooth muscle. Biochem. Biophys. Res. Commun. 1986;141:20–26. doi: 10.1016/s0006-291x(86)80328-x. [DOI] [PubMed] [Google Scholar]
- 10.Jin J.P., Zhang Z., Bautista J.A. Isoform diversity, regulation and functional adaptations of troponin and calponin. Crit. Rev. Eukaryot. Gene Expr. 2008;18:93–124. doi: 10.1615/critreveukargeneexpr.v18.i2.10. [DOI] [PubMed] [Google Scholar]
- 11.Strasser P., Gimona M., Moessler H., Herzog M., Small J.V. Mammalian calponin identification and expression of genetic variants. FEBS Lett. 1993;330:13–18. doi: 10.1016/0014-5793(93)80909-e. [DOI] [PubMed] [Google Scholar]
- 12.Morgan K.G., Gangopadhyay S.S. Cross-bridge regulation by thin filament-associated proteins. J. Appl. Physiol. 2001;91:953–962. doi: 10.1152/jappl.2001.91.2.953. [DOI] [PubMed] [Google Scholar]
- 13.Applegate D., Feng W., Green R.S., Taubman M.B. Cloning and expression of a novel acidic calponin isoform from rat aortic vascular smooth muscle. J. Biol. Chem. 1994;269:10683–10690. [PubMed] [Google Scholar]
- 14.Ferhat L., Charton G., Represa A., Ben-Ari Y., der Terossian E., Khrestchatski M. Acidic calponin cloned from neural cells is differentially expressed during rat brain development. Eur. J. Neurosci. 1996;8:1501–1509. doi: 10.1111/j.1460-9568.1996.tb01612.x. [DOI] [PubMed] [Google Scholar]
- 15.Fukui Y., Masuda H., Takagi M., Takahashi K., Kiyokane K. The presence of h2-calponin in human keratinocyte. J. Dermatol. Sci. 1997;14:29–36. doi: 10.1016/s0923-1811(96)00545-2. [DOI] [PubMed] [Google Scholar]
- 16.Hossain M.M., Crish J.F., Eckert R.L., Lin J.C., Jin J.P. H2-calponin is regulated by mechanical tension and modifies the function of actin cytoskeleton. J. Biol. Chem. 2005;280:42442–42453. doi: 10.1074/jbc.M509952200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hossain M.M., Smith P.G., Wu K., Jin J.P. Cytoskeletal tension regulates both expression and degradation of h2-calponin in lung alveolar cells. Biochemistry. 2006;45:15670–15683. doi: 10.1021/bi061718f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakihara C., Nishimura J., Kobayashi S., Takahashi S., Kanaide H. Expression of calponin mRNA in porcine aortic endothelial cells. Biochem. Biophys. Res. Commun. 1996;222:195–200. doi: 10.1006/bbrc.1996.0721. [DOI] [PubMed] [Google Scholar]
- 19.Tang J., Hu G., Hanai J., Yadlapalli G., Lin Y., Zhang B., Galloway J., Bahary N., Sinha S., Thisse B., Thisse C., Jin J.P., Zon L.I., Sukhatme V.P. A critical role for calponin 2 in vascular development. J. Biol. Chem. 2006;281:6664–6672. doi: 10.1074/jbc.M506991200. [DOI] [PubMed] [Google Scholar]
- 20.Masuda H., Tanaka K., Takagi M., Ohgami K., Sakamaki T., Shibata N., Takahashi K. Molecular cloning and characterization of human non-smooth muscle calponin. J. Biochem. 1996;120:415–424. doi: 10.1093/oxfordjournals.jbchem.a021428. [DOI] [PubMed] [Google Scholar]
- 21.Huang Q.Q., Hossain M.M., Wu K., Parai K., Pope R.M., Jin J.P. Role of h2-calponin in regulating macrophage motility and phagocytosis. J. Biol. Chem. 2008;283:25887–25899. doi: 10.1074/jbc.M801163200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hossain M.M., Hwang D.Y., Huang Q.Q., Sasaki Y., Jin J.P. Developmentally regulated expression of calponin isoforms and the effect of h2-calponin on cell proliferation. Am. J. Physiol. Cell Physiol. 2003;284:156–167. doi: 10.1152/ajpcell.00233.2002. [DOI] [PubMed] [Google Scholar]
- 23.Wu K., Jin J.P. Calponin in non-muscle cells. Cell Biochem. Biophys. 2008;52:139–148. doi: 10.1007/s12013-008-9031-6. [DOI] [PubMed] [Google Scholar]
- 24.Meehan K.L., Holland J.W., Dawkins H.J. Proteomic analysis of normal and malignant prostate tissue to identify novel proteins lost in cancer. Prostate. 2002;50:54–63. doi: 10.1002/pros.10032. [DOI] [PubMed] [Google Scholar]
- 25.Gerdes M.J., Larsen M., Dang T.D., Ressler S.J., Tuxhorn J.A., Rowley D.R. Regulation of rat prostate stromal cell myodifferentiation by androgen and TGF-beta1. Prostate. 2004;58:299–307. doi: 10.1002/pros.10327. [DOI] [PubMed] [Google Scholar]
- 26.Verone A.R., Duncan K., Godoy A., Yadav N., Bakin A., Koochekpour S., Jin J.P., Heemers H.V. Androgen-responsive serum response factor target genes regulate prostate cancer cell migration. Carcinogenesis. 2013;34:1737–1746. doi: 10.1093/carcin/bgt126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozlowski J.M., Fidler I.J., Campbell D., Xu Z.L., Kaighn M.E., Hart I.R. Metastatic behavior of human tumor cell lines grown in the nude mouse. Cancer Res. 1984;44:3522–3529. [PubMed] [Google Scholar]
- 28.Nigam R., Triggle C.R., Jin J.P. H1- and h2-calponins are not essential for norepinephrine- or sodium fluoride-induced contraction of rat aortic smooth muscle. J. Muscle Res. Cell Motil. 1998;19:695–703. doi: 10.1023/a:1005389300151. [DOI] [PubMed] [Google Scholar]
- 29.Jin J.P., Walsh M.P., Resek M.E., McMartin G.A. Expression and epitopic conservation of calponin in different smooth muscles and during development. Biochem. Cell Biol. 1996;74:187–196. doi: 10.1139/o96-019. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y., Jovanovic B., Pins M., Lee C., Bergan R.C. Over expression of endoglin in human prostate cancer suppresses cell detachment, migration and invasion. Oncogene. 2002;21:8272–8281. doi: 10.1038/sj.onc.1206117. [DOI] [PubMed] [Google Scholar]
- 31.Neville C., Rosenthal N., McGrew M., Bogdanova N., Hauschka S. Skeletal muscle cultures. Methods Cell Biol. 1997;52:85–116. [PubMed] [Google Scholar]
- 32.Pelham R.J., Wang Y.L. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. U.S.A. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeung T., Georgesm P.C., Flanagan L.A., Marg B., Ortiz M., Funaki M., Zahir N., Ming W., Weaver V., Janmey P.A. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 34.Engler A., Bacakova L., Newman C., Hategan A., Griffin M., Discher D. Substrate compliance versus ligand density in cell on gel responses. Biophys. J. 2004;86:617–628. doi: 10.1016/S0006-3495(04)74140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katoh H., Hiramoto K., Negishi M. Activation of Rac1 by RhoG regulates cell migration. J. Cell Sci. 2006;119:56–65. doi: 10.1242/jcs.02720. [DOI] [PubMed] [Google Scholar]
- 36.Polte T.R., Eichler G.S., Wang N., Ingber D.E. Extracellular matrix controls myosin light chain phosphorylation and cell contractility through modulation of cell shape and cytoskeletal prestress. Am. J. Physiol. Cell Physiol. 2004;286:518–528. doi: 10.1152/ajpcell.00280.2003. [DOI] [PubMed] [Google Scholar]
- 37.Winder S.J., Allen B.G., Clement-Chomienne O., Walsh M.P. Regulation of smooth muscle actin–myosin interaction and force by calponin. Acta Physiol. Scand. 1998;164:415–426. doi: 10.1111/j.1365-201x.1998.tb10697.x. [DOI] [PubMed] [Google Scholar]
- 38.Danninger C., Gimona M. Live dynamics of GFP-calponin: isoform-specific modulation of the actin cytoskeleton and autoregulation by C-terminal sequences. J. Cell Sci. 2000;113:3725–3736. doi: 10.1242/jcs.113.21.3725. [DOI] [PubMed] [Google Scholar]
- 39.Xu L., Ding Y., Catalona W.J., Yang X.J., Anderson W.F., Jovanovic B., Wellman K., Killmer J., Huang X., Scheidt K.A., Montgomery R.B., Bergan R.C. MEK4 function, genistein treatment, and invasion of human prostate cancer cells. J. Natl. Cancer Inst. 2009;101:1141–1155. doi: 10.1093/jnci/djp227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chicurel M.E., Chen C.S., Ingber D.E. Cellular control lies in the balance of forces. Curr. Opin. Cell Biol. 1998;10:232–239. doi: 10.1016/s0955-0674(98)80145-2. [DOI] [PubMed] [Google Scholar]
- 41.Hamill O.P., Martinac B. Molecular basis of mechanotransduction in living cells. Physiol. Rev. 2001;81:685–740. doi: 10.1152/physrev.2001.81.2.685. [DOI] [PubMed] [Google Scholar]
- 42.Lehoux S., Tedgui A. Cellular mechanics and gene expression in blood vessels. J. Biomech. 2003;36:631–643. doi: 10.1016/s0021-9290(02)00441-4. [DOI] [PubMed] [Google Scholar]
- 43.Eckes B., Krieg T. Regulation of connective tissue homeostasis in the skin by mechanical forces. Clin. Exp. Rheumatol. 2004;22:73–76. [PubMed] [Google Scholar]
- 44.Walker J.L., Fournier A.K., Assoian R.K. Regulation of growth factor signaling and cell cycle progression by cell adhesion and adhesion-dependent changes in cellular tension. Cytokine Growth Factor Rev. 2005;16:395–405. doi: 10.1016/j.cytogfr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Jiang W.R., Cady G., Hossain M.M., Huang Q.Q., Wang X., Jin J.P. Mechanoregulation of h2-calponin gene expression and the role of notch signaling. J. Biol. Chem. 2014;289:1617–1628. doi: 10.1074/jbc.M113.498147. [DOI] [PMC free article] [PubMed] [Google Scholar]


