Abstract
Thus far, three related natriuretic peptides (NPs) and three distinct sub-types of cognate NP receptors have been identified and characterized based on the specific ligand binding affinities, guanylyl cyclase activity, and generation of intracellular cGMP. Atrial and brain natriuretic peptides (ANP and BNP) specifically bind and activate guanylyl cyclase/natriuretic peptide receptor-A (GC-A/NPRA), and C-type natriuretic peptide (CNP) shows specificity to activate guanylyl cyclase/natriuretic peptide receptor-B (GC-B/NPRB). All three NPs bind to natriuretic peptide receptor-C (NPRC), which is also known as clearance or silent receptor. The NPRA is considered the principal biologically active receptor of NP family; however, the molecular signaling mechanisms of NP receptors are not well understood. The activation of NPRA and NPRB produces the intracellular second messenger cGMP, which serves as the major signaling molecule of all three NPs. The activation of NPRB in response to CNP also produces the intracellular cGMP; however, at lower magnitude than that of NPRA, which is activated by ANP and BNP. In addition to enhanced accumulation of intracellular cGMP in response to all three NPs, the levels of cAMP, Ca2+ and inositol triphosphate (IP3) have also been reported to be altered in different cells and tissue types. Interestingly, ANP has been found to lower the concentrations of cAMP, Ca2+, and IP3; however, NPRC has been proposed to increase the levels of these metabolic signaling molecules. The mechanistic studies of decreased and/or increased levels of cAMP, Ca2+, and IP3 in response to NPs and their receptors have not yet been clearly established. This review focuses on the signaling mechanisms of ANP/NPRA and their biological effects involving an increased level of intracellular accumulation of cGMP and a decreased level of cAMP, Ca2+, and IP3 in different cells and tissue systems.
Keywords: natriuretic peptides, natriuretic peptide receptors, membrane guanylyl cyclases, cGMP, cAMP, Ca2+, inositol triphosphate
INTRODUCTION
Atrial natriuretic factor/peptide (ANF/ANP) is produced and secreted in the specific granules of cardiac atrial myocytes, which participates in the control of extracellular fluid volume, electrolyte balance, and mean arterial pressure, thus, it plays a central role in the maintenance and regulation of cardiovascular homeostasis (de Bold et al., 1981; de Bold, 1985; Brenner et al., 1990; Anand-Srivastava and Trachte, 1993; Pandey, 2005, 2011). In addition to its natriuretic, diuretic, vasorelaxant, antimitogenic, antihypertrophic, and anti-inflammatory activities, ANP inhibits the release of renin from the kidneys, aldosterone from the adrenal glands, vasopressin from posterior pituitary, and progesterone from Leydig tumor (MA-10) cells, while stimulating the synthesis and release of testosterone from normal Leydig cells in the testes, progesterone from granulosa-leuteal cells, and luteinizing hormone from anterior pituitary gland (Inagami, 1989; Brenner et al., 1990; Levin et al., 1998; Pandey, 2005). A number of studies have documented that ANP has always been found to increase the intracellular accumulation of cGMP, however, to decrease the levels of cAMP; Ca2+, and inositol triphosphate (IP3) in agonist hormone-treated cells and tissues (Waldman et al., 1984; Pandey et al., 1985, 1988; Khurana and Pandey, 1993, 1996; Pandey, 2005; Turovsky et al., 2013). It has also been suggested that ANP decreases the cAMP levels by stimulating the cGMP-specific phosphodiesterases; however, in certain cells and tissue types, ANP did not decrease or change the cAMP concentrations. Several studies have indicated that ANP diminishes the Ca2+ signals probably by activating the Ca2+ extrusion processes by protein kinase-G (PKG) specifically in endothelial and vascular smooth muscle cells (VSMCs; Rashatwar et al., 1987; Zolle et al., 2000; Pandey, 2005).
Among the natriuretic peptides (NPs) hormone family, ANP is the first described member, later, two other members of NP family; brain natriuretic peptide (BNP) and C-type natriuretic peptide (CNP) were identified and characterized, which also exhibited biochemical and structural properties similar to ANP; however, each prepro NP hormone is encoded from a separate gene (Rosenzweig and Seidman, 1991; Levin et al., 1998). Although, all three NPs (ANP, BNP, and CNP) have highly homologous structure, they bind to specific NP receptors and elicit discrete biological and physiological functions (Brenner et al., 1990). There have been three subtypes of NP receptors, namely; NP receptor-A, NP receptor-B, and NP peptide receptor-C (NPRA, NPRB, and NPRC, respectively), which were identified and characterized by molecular cloning (Pandey and Singh, 1990; Garbers, 1992). Interestingly, both ANP and BNP bind to NPRA, which produces the intracellular second messenger cGMP; however, CNP binds NPRB, which also generates cGMP, but all three NPs indiscriminately bind and activate NPRC, which lacks GC catalytic domain (Fuller et al., 1988; Drewett and Garbers, 1994; Lucas et al., 2000; Sharma, 2002; Pandey, 2005). The cellular, biochemical, and pharmacological aspects of NPs and their cognate receptors have revealed classic hallmark of physiological and pathophysiological functional significance, including; renal, cardiac, vascular, neuronal, and immunological aspects in health and disease (Pandey, 2005, 2011; Kishimoto et al., 2011). It has been suggested that ANP suppresses Na+-reabsorption at the collecting duct of the kidneys, inhibits renin synthesis and release, and stimulates natriuresis and diuresis, thereby, lowers blood pressure and blood volume and maintains cardiovascular homeostasis (de Bold, 1985; Brenner et al., 1990; Levin et al., 1998; Pandey, 2005). In the vasculature, ANP relaxes VSMCs thus causing the immediate vasorelaxant effect in the vascular bed (Levin et al., 1998; Pandey, 2005).
The expression and activity of NPRA is regulated by various hormonal agents, including its ligand ANP (Pandey, 1993, 2005; Cao et al., 1995; Pandey et al., 2002). The studies detailing the Npr1 (coding for NPRA) gene-disruption in mice have revealed the functional significance of NPRA in the control of blood pressure and cardiovascular disease states (Oliver et al., 1997; Shi et al., 2001; Holtwick et al., 2002; Vellaichamy et al., 2005; Kishimoto et al., 2011; Pandey, 2011; Yoshihara et al., 2014). Mice lacking NPRA develop high blood pressure and severe cardiac hypertrophy, fibrosis, and disorders that are reminiscent of heart disease as seen in untreated human hypertensive patients (Vellaichamy et al., 2007, 2014; Zhao et al., 2013). The regulated expression of CNP is derived from endothelial cells, which targets NPRB on the adjacent smooth muscle cells (Suga et al., 1992). Thus, the principal role of CNP is considered as a direct vasodilator involved in the regulation of vascular tone through activation of GC-B/NPRB on smooth muscle cells in the vascular beds (Hama et al., 1994). The objective of this current review is to summarize and document the findings and discoveries with particular emphasis of cellular signaling and physiological and pathological significance of ANP/NPRA in relation to the increased production of intracellular second messenger cGMP and inhibition of the phosphoinositide (IP3) hydrolysis, Ca2+ release, and protein kinase C (PKC) activity in target cells.
HISTORICAL BACKGROUND
Thirty-three years ago, the pioneer discovery by de Bold and his coworkers established that atrial extracts contained natriuretic and diuretic activity which led to the isolation and nomenclature of ANF, usually referred to as ANP (de Bold et al., 1981; de Bold, 1985). Now, it is considered that ANP is primarily synthesized and secreted in the granules of heart atrium and BNP is largely synthesized in the heart ventricle and displays most variability in the primary structure. Although, the atrium is the primary site of synthesis for ANP, however, ventricle also produces ANP but at the levels of 100-fold to 1000-fold lower than that of the atrium, respectively (Kojima et al., 1989). CNP was isolated from the porcine brain, however, is mostly present in the endothelial cells of the vasculature and is highly conserved among the mammalian species (Rosenzweig and Seidman, 1991). The primary structure deduced from the cDNA synthesis, suggested that ANP is synthesized first as the 152-amino acid prepro-ANP molecule that contains sequences of active peptide in its carboxyl-terminal region (Maki et al., 1984). The biologically active ANP is released by proteolytic cleavage of pro-ANP molecule into predominantly 28-amino acid active (residues 99–126) and the 98 amino acid inactive (residues 1–98) molecules. The active form of ANP has a disulfide-bonded loop between cysteine 105 and 121, which seems to be essential for the biological activity (Brenner et al., 1990). Initially, different lengths of sequences of ANP were identified and synthesized for the studies of structure-activity relationship, and it was suggested that the ring structure of ANP with a disulfide-bonded loop is essential for its biological activities (Rosenzweig and Seidman, 1991). All three NPs contain highly conserved amino acid sequences with a 17-residues disulfide-bonded ring but deviate from each other in the N-terminal and C-terminal flanking amino acid sequences. Furthermore, the C-terminal sequence extending from the ring structure to Asn-Phe-Arg-Tyr is essential for the biological activity of ANP. The amino acid sequence of ANP is almost identical across the mammalian species, except at the position 10, which is substituted with isoleucine in rat, mouse, and rabbit, however, in human, dog, and bovine, ANPs have methionine in this position (Misono et al., 1984). Subsequently, BNP and CNP were both isolated and characterized from the porcine brain extracts (Sudoh et al., 1988, 1990). BNP is predominantly synthesized and secreted from the heart ventricle (Phillips et al., 1991). Similarly, CNP is predominantly localized in the central nervous system and endothelial cells and is considered as a non-circulatory peptide hormone (Suga et al., 1992).
NATRIURETIC PEPTIDES SYNTHESIS AND SECRETION
It has been suggested that the processing of preprohormone to prohormone molecule and the cleavage and secretion of biologically active mature 28-residue ANP molecule occurs predominantly in response to atrial distension (de Bold, 1985; Brenner et al., 1990). Usually, ANP concentration ranges from 50 to 100-fold higher than BNP; however, the expression of both ANP and BNP increases dramatically in the atrium and ventricle in the condition of cardiac disorders and heart failure (Mukoyama et al., 1991). During the disease states, the ventricle becomes the primary site of synthesis and release for BNP. In congestive heart failure (CHF) patients, the concentrations of both ANP and BNP increase greater than the control values, however, the BNP concentration increases 10-fold to 50-fold higher than a comparative increases in the ANP levels (Mukoyama et al., 1991). Those previous findings indicated that ANP and BNP elicit distinct physiological and pathophysiological effects, nevertheless, both hormones show similar hemodynamic responses, but BNP exerts a longer duration of action and causes enhanced natriuretic responses as compared with ANP (Yoshimura et al., 1991; Omland et al., 1996). It has been suggested that the cardiac atrium expresses almost 50-fold to 100-fold or even higher levels of ANP mRNA as compared with extra -cardiac tissues (Gardner et al., 1986). Interestingly, higher ventricular ANP levels have been found in the developing embryos and fetuses; however, both mRNA and peptide levels of ANP decline rapidly during the prenatal period (Cameron et al., 1996). On the other hand, CNP does not seem to behave as a cardiac hormone and its concentration is extremely low in the circulation (Igaki et al., 1996). It is believed that CNP is largely localized in the central nervous system and in the vascular endothelial cells (Ogawa et al., 1992; Suga et al., 1992, 1993; Tamura et al., 1996; Chen and Burnett, 1998). Another class of NPs is the D-type natriuretic peptide (DNP) that represents an additional member in the NP hormone family and is largely present in the venom of the green mamba (Dendroaspis angusticeps) as a 38-amino acid peptide molecule (Schweitz et al., 1992; Lisy et al., 1999). In addition, a 32-amino acid peptide termed as urodilatin (URO) is identical to C-terminal sequence of pro-ANP, which is largely present only in the urine (Schulz-Knappe et al., 1988). Initially, URO was purified from the human urine and is considered to be synthesized only in the kidneys (Saxenhofer et al., 1990). The immunohistochemical staining indicated that URO is largely present in the cortical tubules around the collecting ducts of the kidneys (Meyer et al., 1996; Doust et al., 2005).
In the circulation, the half-life of BNP is greater than ANP, thus the evaluation of the diagnostic importance of the NPs have mostly favored BNP. The inactive N-terminal fragment of BNP (NT-proBNP) has even a greater half-life than the BNP. The plasma levels of both BNP and NT-proBNP are markedly elevated under the pathophysiological conditions of cardiac dysfunction, including diastolic dysfunction, CHF, and pulmonary embolism (Felker et al., 2006; Jaffe et al., 2006). The basal plasma levels of BNP vary from 5 to 50 pg/ml and NT-proBNP levels range from 10 to 150 pg/ml. An abnormal range is considered as 100 pg/ml for BNP and 125 pg/ml for NT-proBNP (Felker et al., 2006). Nevertheless, the secretion of both ANP and BNP from the ventricular myocytes increases proportionally in relation to the magnitude of cardiac dysfunction or heart failure condition (Yoshimura et al., 1993). It has been suggested that BNP acts as an important prognostic indicator in the CHF patients, however, NT-proBNP is considered to be a stronger risk bio-indicator for cardiovascular events (Doust et al., 2005). Both BNP and NT-proBNP seem to provide an ideal tool to be utilized as blood tests to diagnose cardiac disorders in patients with high risk of heart failure, diabetes, chronic kidney disease, and coronary artery disease (Khan et al., 2006; Freestone et al., 2008; Czucz et al., 2011; Ganem et al., 2011). The BNP level is increased to almost 200 pg/ml and NT-proBNP levels reaches to approximately 1200 pg/ml in patients with reduced creatinin clearance (McCullough et al., 2003; Anwaruddin et al., 2006).
IDENTIFICATION AND CHARACTERIZATION OF RECEPTOR MEMBRANE GUANYLYL CYCLASE
The previous studies using cross-linking and photoaffinity labeling procedures, have shown the existence of NP receptors with a wide range of molecular weight (Mr) of the 60–180 kDa (Misono et al., 1985; Schenk et al., 1985; Vandlen et al., 1985; Meloche et al., 1986; Pandey et al., 1986). Initially, NP receptors were identified with varying receptor density in different cells and tissue types (Table 1). Subsequently, high affinity ANP binding sites were with GC activity were co-purified (Kuno et al., 1986; Paul et al., 1987; Takayanagi et al., 1987; Meloche et al., 1988) On the basis of biological activity of different ANP analogs, NP receptors were classified and characterized as biologically active and clearance or silent receptors (Maack et al., 1987). Subsequently, three distinct subtypes of NP receptors were identified, which appeared to be specific to different cells and tissues (Pandey et al., 1988). Based on the cellular, biochemical, and molecular biological studies, the NPs and their receptors are quite widespread in cell and tissue distributions (Leitman et al., 1988; Pandey et al., 1988; Brenner et al., 1990; Marala et al., 1992; Levin et al., 1998; Pandey, 2005). Molecular cloning and expression of cDNA from mouse, rate, and human, led to identify and characterize the primary structure of three distinct subtypes of NP receptors, which are currently designated as GC-A/NPRA, GC-B/NPRB, and NPRC (Fuller et al., 1988; Chinkers et al., 1989; Schulz et al., 1989; Pandey and Singh, 1990; Duda et al., 1991). The general topological structures of GC-A/NPRA and GC-B/NPRB are consistent with at least four distinct domains, including extracellular ligand-binding domain, a single transmembrane spanning region, and intracellular protein kinase-like homology domain (protein-KHD), and GC catalytic domain. The transmembrane GC receptors contain a single cyclase catalytic site per protein molecule, however, based on the structural modeling data two polypeptide chains seem to be required to activate GC-A/NPRA (Wilson and Chinkers, 1995; Labrecque et al., 1999; van den Akker et al., 2000). It has been indicated that the dimerization region of the receptor is located between the KHD and GC catalytic domain that has been predicted to form an amphipathic alpha helix structure. The GC-B/NPRB has the overall domain structure similar to that of GC-A/NPRA with binding affinity to CNP also produces the intracellular second messenger cGMP (Schulz et al., 1989; Koller et al., 1991; Lucas et al., 2000). NPRA is considered as the dominant subtype of the NP receptors found in peripheral organs and mediates most of the known functions of ANP and BNP hormones. Nevertheless, NPRB is localized mainly in the central nervous system and vascular tissues, which is thought to mediate the actions of CNP in the brain and also in the vascular bed. There are increasing numbers of other GC receptors; however, the specific ligands for these receptors are still being identified (Table 2). The third member of the NP receptor family, NPRC, constitutes a large extracellular domain of 496-amino acids, a single transmembrane domain, and a very short 37-amino acid cytoplasmic tail that contains no sequence homology with any other known membrane receptor proteins and has been given the name by default as clearance receptor (Fuller et al., 1988). The extracellular region of NPRC is approximately 30% identical to both GC-A/NPRA and GC-B/NPRB. Studies using the ligand receptor binding as a criterion, have shown that NPRC has much less stringent specificity and affinity for structural variants of ANP than does NPRA (Bovy, 1990). The extracellular domain of NPRC possesses two pairs of cysteine residues along with one isolated cysteine near the transmembrane domain of the receptor. However, three potential signals for N-glycosylation and several serine and threonine for O-linked glycosylation sites are known to be present in the extracellular domain of NPRC (Fuller et al., 1988). Previously, it has been suggested that NPRC may function as a clearance receptor to remove and clear NPs from the circulation, however, a number of studies have provided the evidence that NPRC plays roles in the biological actions of NPs (Anand-Srivastava and Trachte, 1993; Matsukawa et al., 1999; Zhou and Murthy, 2003). Thus, it is evident that the clearance name carries only by a default nomenclature to NPRC.
Table 1.
ANP-dependent binding parameters of GC-A/NPRA and intracellular accumulation of cGMP in different cell types.
| Cell type | ANP-dependent Intracellular cGMP (fold stimulation) | Ligand binding parameters of NPRA |
|
|---|---|---|---|
| kd value (Molar) | Bmax (receptor site/cell) | ||
| Endothelial cells | 15 | 10–100 pM | 0.5 × 105 |
| Granulosa cells | 30 | 10–100 pM | 0.5 × 105 |
| Glomerulosa cells | 50 | 100–1 pM | 2 × 105 |
| MA-10 cells | 1,500 | 100–1 nM | 1 × 106 |
| MDCK cells | 50 | 10–100 pM | 0.5 × 105 |
| N4TG1 cells | 30 | 1–100 pM | 0.5 × 105 |
| Primary Ledig cells | 60 | 10–100 pM | 0. 5 × 105 |
| RTASM cells | 10 | 1–100 pM | 0.2 × 105 |
HEK-293 cells, human embryonic Kidney-293 cells; kd, dissociation constant; Bmax, receptor density; MA-10 cells, Leydig tumor cells; MDCK cells, Maiden-Darby kidney epithelial cells; N4TG1 cells, neuroblastoma cells; RTASM, rat thoracic aortic smooth muscle cells.
Table 2.
The distribution of natriuretic peptide receptors (NPRA, NPRB, and NPRC) and their gene-knockout phenotype.
| Receptor | Ligand | Tissue-specific distribution | Cell-specific distribution | Gene-knockout phenotype |
|---|---|---|---|---|
| NPRA (Npr1) | ANP/BNP | Kidney, adrenal glands, brain, heart, liver, lung, olfactory, ovary, pituitary gland, placenta, testis, thymus, vascular beds, liver, ileum | Renal epithelial and mesangial cells, vascular smooth muscle cells, endothelial cells, Leydig cells, granulosa cells, fibroblasts, Neuroblastoma, LLCPk-1, MDCK cells | High blood pressure, hypertension, cardiac hypertrophy and fibrosis, inflammation, volume overload, reduced testosterone |
| NPRB (Npr2) | CNP | Adrenal glands, brain, cartilage, fibroblast, heart, lung, ovary, pituitary gland, placenta, testis, thymus, vascular beds | Vascular smooth muscle cells, fibroblasts, chondrocytes | Dwarfism, decreased adiposity, female sterility, seizures, vascular complication |
| NPRC (Npr3) | ANP, BNP, CNP | Kidney, heart, brain liver, vascular bed, intestine | Vascular smooth muscle cells, endothelial cells, mesangial cells, fibroblasts | Bone deformation, skeletal over-growth, long bone overgrowth |
| GC-D | Guanylyn/uroguanylyn | Olfactory neuroepithelium | ||
| GC-E/(ROS-GC-1) | Ca2+-binding proteins | Retina, pineal gland | ||
| GC-F/(ROS-GC-2) | Ca2+-binding proteins | Retina, rod outer segment | ||
| GC-G | Orphan | Skeletal muscle, lung, intestine, and kidney | ||
| GC-Y-X1 | Orphan | Sensory neurons of C. elegans |
NPRA, natriuretic peptide receptor-A; Npr1, coding for guanylyl cyclase/natriuretic peptide receptor-A; NPRB, natriuretic peptide receptor-B; Npr2, coding for guanylyl cyclase/natriuretic peptide receptor-B; NPRC, natriuretic peptide receptor-C; Npr3, coding for natriuretic peptide clearance receptor.
INTERNALIZATION AND DOWN-REGULATION OF GC-A/NPRA
The ligand-dependent internalization plays important role in the receptor down-regulation and signaling process. Down-regulation of GC-A/NPRA has been reported in a number of cells, including PC-12 cells containing endogenous receptors and transfected COS-7 and HEK-293 cells harboring recombinant receptors (Rathinavelu and Isom, 1991; Pandey, 1993; Pandey et al., 2000a, 2002). The carboxyl-terminal deletion mutation has shown that the specific sites in the GC catalytic domain and KHD of NPRA, play critical roles in the endocytosis and sequestration of the receptor (Pandey et al., 2000a). Previous studies have also indicated that after prolonged treatment of cultured cells with ANP, both the receptor density and GC activity were decreased with simultaneous reduction in mRNA of levels NPRA (Fujio et al., 1994; Cao et al., 1995, 1998; Hum et al., 2004). In addition, transforming growth factor-β1 (TGF-β1), angiotensin II (ANG II), and endothelin (ET-1) have also been shown to reduce mRNA levels of NPRA in various types of cultured cells (Fujio et al., 1994; Chen and Gardner, 2003; Garg and Pandey, 2003; Arise and Pandey, 2006). Those previous studies demonstrated that a decrease in mRNA levels of GC-A/NPRA correlated with the repressed transcriptional activity of the receptor. On the other hand, mRNA levels of NPRA are greatly increased by retinoic acid and histonedeacetylase inhibitor treatments (Kumar et al., 2010, 2014a,b). It has been suggested that NPRA exists in the phosphorylated state and the addition of ANP causes a decrease in the phosphate contents as well as reduction or desensitization of the ANP-dependent GC catalytic activity of NPRA Potter and Garbers, 1992). The apparent mechanism of desensitization of NPRA is in contrast to many other cell-surface hormone receptors, which appear to be desensitized by phosphorylation (Sibley et al., 1987; Hugnir and Greengard, 1990; Lefkowitz et al., 1998; Sorkin and von Zastrow, 2002). The initial findings have also indicated that ANP stimulates phosphorylation of NPRA (Ballerman et al., 1988; Pandey, 1989; Duda and Sharma, 1990; Larose et al., 1992). Later, it was suggested that cGMP-dependent PKG, a serine/threonine kinase is also phosphorylates NPRA (Airhart et al., 2003).
The down-regulation of NPRC also seems to be associated with increased internalization of the ligand-receptor complexes involving receptor-mediated endocytosis and trafficking mechanisms of this receptor protein (Pandey, 1992). The phenomenon of down-regulation of NPRC has been largely documented in cultured VSMCs, which predominantly contain a high density of NPRC (Neuser and Bellermann, 1986; Hirata et al., 1987; Hughes et al., 1987; Pandey, 1992; Anand-Srivastava, 2000). The metabolic processing of ANP involving NPRC has been reported by several investigators utilizing VSMCs (Hirata et al., 1985; Napier et al., 1986; Murthy et al., 1989; Nussenzveig et al., 1990; Pandey, 1992, 2005; Cohen et al., 1996; Anand-Srivastava, 2000). It has been suggested that a population of the internalized NPRC also recycles back to the plasma membrane (Pandey, 1992).
ACTIVATION OF GC-A/NPRA GENERATES INTRACELLULAR SECOND MESSENGER cGMP
It is believed that cGMP is generated as a result of ANP binding to the extracellular domain of GC-A/NPRA, which probably allosterically regulates an increased activity of the receptor protein (Pandey, 1993, 2005, 2011; Drewett and Garbers, 1994; Pandey et al., 2002; Sharma, 2002; Sharma and Duda, 2010). The initial findings showed that ANP markedly increases cGMP in target tissues in a dose-related manner (Hamet et al., 1984; Waldman et al., 1984; Pandey et al., 1985). Previous studies have also indicated that the binding of ANP to GC-A/NPRA by itself is probably not sufficient to stimulate GC catalytic activity and the production of cGMP, however, it requires ATP (Kurose et al., 1987; Chinkers et al., 1991; Goraczniak et al., 1992). Because the non-hydrolyzable analogs of ATP mimicked ANP effect, it was suggested that ATP acts directly by allosteric regulation of GC catalytic activity of NPRA. Both the ligand binding and the interaction of ATP with the KHD of the receptor increase the cGMP production without affecting the affinity for the substrate (Kurose et al., 1987; Chang et al., 1990; Duda et al., 1991). Molecular cloning and overexpression of NPRA demonstrated that GC catalytic domain cannot be activated by ANP alone without ATP-binding to KHD region of the receptor (Chinkers et al., 1991; Larose et al., 1991; Wong et al., 1995). Further studies provided the evidence that ATP binding to KHD of NPRA is important for the effectors coupling of GC family of receptors (Goraczniak et al., 1992; Sharma, 2002).
Deletion of the KHD of GC-A/NPRA and GC-B/NPRB has been suggested that KHD represses the GC catalytic activity of these receptors (Chinkers et al., 1989). At the same time, another model was proposed indicating that KHD was not a repressor; however, ATP was required to activate the catalytic domain of NPRA (Goraczniak et al., 1992; Sharma, 2002). Both NPRA and NPRB contain a glycine-rich ATP binding motif within the KHD, which is known as glycine-rich consensus sequence (Duda et al., 1991, 1993; Goraczniak et al., 1992). The juxtamembrane hinge structure of NPRA undergoes a significant conformational change in response to ligand binding, and it may play an important role in transmembrane signaling process (Huo et al., 1999). The amino acid sequence near the transmembrane region is well conserved in GC-A/NPRA that contains several closely located proline residues and a pair of cysteine residues. The mutation of one of the proline in this region renders the receptor to bind the ligand but blocks GC catalytic activity (Huo et al., 1999). Similarly, in the juxtamembrane hinge region, the elimination of disulfide bond of cysteine residues resulted in constitutive activation of NPRA. Those previous findings suggested that juxtamembrane hinge region of NPRA may play a critical role in receptor activation and signal transduction mechanisms of GC-coupled receptors.
The glycosylation of the receptor seems to be essential for ligand binding activity of GC-A/NPRA (Lowe and Fendly, 1992; Fenrick et al., 1997). However, it has also been suggested that glycosylation may not be required for ligand binding of NPRA (Miyagi et al., 2000). The mutational analyses of N-linked glycosylation consensus sites in guanylyl cyclase-C (GC-C) have indicated that certain amino acid residues might be important for receptor stability (Hesegawa et al., 1999). The glycosylation sites onto the GC-A/NPRA binding domain have been found to be scattered on the surface of the receptor with the exception of the hormone binding site and dimer interface (van den Akker, 2001). The glycosylation sites have been implicated to function in proper folding and stability of NPRA (Lowe and Fendly, 1992; Koller et al., 1993; Heim et al., 1996). Nevertheless, the glycosylation of the extracellular domain of NPRA can be considered of significant importance for receptor orientation and packaging on the cell surface similar to that of other plasma membrane receptor proteins (Wormald and Dwek, 1999). Nevertheless, it should be noted that there is no appreciable conservation of the precise position of the glycosylation sites within the members of GC-receptor family. Clearly, more studies are needed to confirm the functional roles of glycosylation in the transmembrane signaling processes of both GC-A/NPRA and GC-B/NPRB protein molecules.
ANP/NPRA SIGNALING INHIBITS PHOSPHOINOSITIDE HYDROLYSIS, Ca2+ RELEASE, AND PKC ACTIVITY
Previous studies have demonstrated that ANP significantly decreased the hydrolysis of phosphoinositide in murine Leydig tumor (MA-10) cells in a dose-dependent manner and the H-8, a specific inhibitor of PKG, reversed the inhibitory effect of ANP on the generation of inositol phosphates, supporting the involvement of PKG in this process (Khurana and Pandey, 1995). ANP has also been shown to inhibit both autophosphorylation and enzymatic activity of PKC in different cell systems (Pandey, 1989, 1994a,b; Kumar et al., 1997). It is not yet clear if the ANP-dependent inhibitory effects on the phosphoinositide metabolism and PKC autophosphorylation and/or enzyme activity are exerted in a composite manner to negatively regulate the phosphoinositide, Ca2+, and PKC involving ANP/NPRA/cGMP/PKG cascade. It is also possible that the effect of ANP is transmitted to block the IP3 and Ca2+ signaling pathways independently in response to particular agonist stimulation. It has been suggested that potassium channels can be stimulated by ANP through the activation of PKGs, which require ATP and G-proteins (White et al., 1993). However, the possible involvements of potassium channels in the ANP-dependent inhibitory responses on the generation of inositol phosphates are not yet clearly understood. ANP has also been shown to stimulate the formation of inositol phosphates in cultured VSMCs, however, in the inner medullary collecting duct cells and smooth muscle tissues, ANP stimulated the production of inositol phosphates at lower dosages, and inhibited the formation of these metabolites at higher dosages, which increase intracellular generation of cGMP (Resink et al., 1988; Hirata et al., 1989; Teitelbaum et al., 1990; Berl et al., 1991). Thus the heterogeneity of NP receptors and their diverse cellular distribution suggest that different mechanisms might be involved in the cellular action of ANP/NPRA/cGMP (Anand-Srivastava and Trachte, 1993; Pandey, 2001, 2002, 2011). It has also been shown that ANP inhibits the thrombin-induced synthesis and release of endothelin in cultured rat aortic endothelial cells by blocking the phosphoinositide breakdown (Emori et al., 1993).
In addition to the stimulatory effect of ANP on GC activity, it has also been shown to reduce adenylyl cyclase and phospholipase C activities, sodium influx, and Ca2+ concentrations (Brenner et al., 1990; Anand-Srivastava and Trachte, 1993; Pandey, 2005). The increased production of cGMP in response to ANP correlates with the effects of dibutyryl-cGMP. The most compelling evidence supporting a role for cGMP effects was obtained with selective NPRA antagonists, A71915 and HS-121-1 in the kidneys (von Geldern et al., 1990; Sano et al., 1992). Those previous studies established that ANP effect is largely mediated by cGMP through the activation of GC-A/NPRA. In general, evidence suggests that biological activity of ANP/NPRA enhances the generation of the intracellular second messenger cGMP and decreases the levels of cAMP, Ca2+, and IP3 along with the antagonistic effects on PKC and mitogen-activated protein kinases (MAPKs) in target cells (Figure 1). ANP has been reported to induce cGMP-dependent acrosomal reaction in both capacitated and non-capacitated spermatozoa (Anderson et al., 1994). Furthermore, the acrosome reaction was essentially equal in magnitude when induced with ANP or Ca2+ ionophore A23187. However, higher concentrations of ANP were required to induce acrosomal reaction in capacitated as compared with non-capacitated spermatozoa. Those previous findings indicated that ANP-induced human acrosomal reaction does not require physiological concentrations of extracellular Ca2+. Acrosomal reaction is known to involve various extracellular signals, including cAMP (Anderson et al., 1992), cGMP (Komatsu et al., 1990), prostaglandins, Ca2+ and IP3 (Thomas and Meizel, 1989), and diacylglycerol (Breitbart et al., 1992).
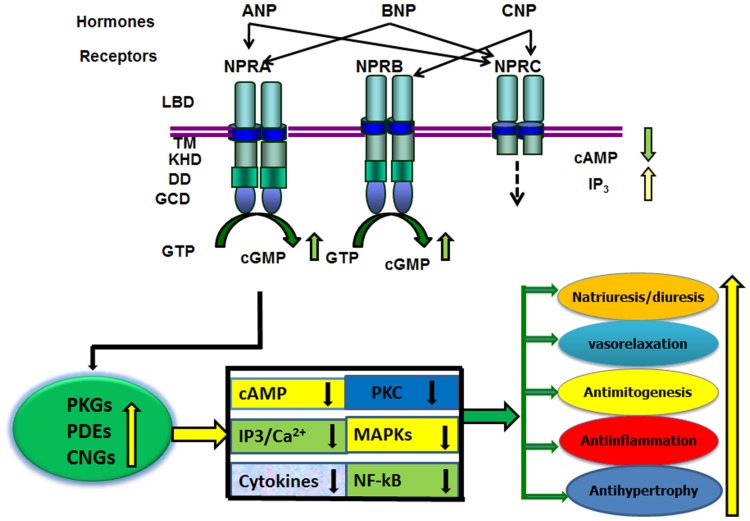
FIGURE 1.
Diagram represents the ligand specificity and physiological function(s) of GC-A/NPRA. The ligand-binding to NPRA generates second messenger cGMP from the hydrolysis of GTP. An increased level of intracellular cGMP is produced, which activates three known cGMP effecter molecules namely; cGMP-dependent protein kinases (PKGs), cGMP-dependent phosphodiesterases (PDEs), and cGMP-dependent ion-gated channels (CNGs). The ANP/NPRA/cGMP signaling may antagonize a number of pathways including; intracellular formation of cAMP, Ca2+, IP3; cytokine expression; and the activation of protein kinase C (PKC) and mitogen-activated protein kinases (MAPKs). The resulting signaling cascade can mimic the physiological responses of ANP/NPRA. LBD, ligand binding domain; TM transmembrane region; protein-KHD, protein kinase-like homology domain; and GCD, guanylyl cyclase catalytic domain; DD, dimerization domain of NPRA and NPRB. The ligand binding region, transmembrane domain, and small intracellular tail region of NPRC are indicated.
The established biochemical and cellular effects of ANP in the adrenal glomerulosa cells showed the activation of GC activity and K+ channel conductance; whereas T-type Ca2+ channels conductance and adenylyl cyclase activity are suppressed (Anand-Srivastava and Trachte, 1993). The correlative evidence between ANP-induced cGMP accumulation and vasodilation has suggested the role of cGMP as the intracellular second messenger of dilator responses to ANP (Brenner et al., 1990; Anand-Srivastava and Trachte, 1993; Cao et al., 1995; Pandey, 2005). ANP as well as cGMP analogs have been found to reduce the agonist-induced increases in cytosolic Ca2+ concentrations (Hassid, 1986; Lincoln et al., 1994; Pandey, 2005). It has been suggested that cGMP activates sarcolemmal Ca2+-ATPase, and this mechanism seems to be important in the ANP-induced decreases in cytosolic Ca2+ in VSMCs (Rashatwar et al., 1987; Cornwell and Lincoln, 1989; Levin et al., 1998; Pandey, 2005). Nevertheless, it is anticipated that the ultimate effect of ANP in VSMCs could be due to production of cGMP and the activation of PKG (Lincoln et al., 1994; Kumar et al., 1997). However, more studies are needed to define the biochemical and molecular basis of NP actions in vasculature, including VSMCs and endothelial cells.
Initial studies from our laboratory and data published from others have also shown that both ANP and cGMP inhibited the autophosphorylation and enzymatic activity of PKC in the plasma membrane preparations of various target cells (Rogers et al., 1988; Sauro and Fitzpatrick, 1990; Pandey, 1994a,b; Kumar et al., 1997). The activation of PKC triggers the agonist-dependent phosphorylation and activity of numerous cellular proteins causing alteration in many physiological and pathophysiological conditions, including hypertension, cardiac hypertrophy, ischemia, atherosclerosis, stroke, and neurological disorders (Louis et al., 1988; Turla et al., 1990; Komuro et al., 1991; Kumar et al., 1997). PKC is believed to be a multigene family, consisting of at least 12 isoenzymes that can be classified into classical, novel, and atypical forms (Hug and Sarre, 1993; Dekker and Parker, 1994). These PKC isoenzymes are multifunctional serine/threonine kinases that are largely activated by Ca2+/phospholipids and phorbol esters. However, some of these isoforms (e, δ, ή, and φ) do not require Ca2+, while other isoforms (ζ and 𝜀) do not require Ca2+ or phospholipid for PKC enzymatic activity. Previous studies have indicated that vasoconstrictive agents, including ANG II and ET-1, were able to activate several-fold PKC activity in cultured VSMCs, however, ANP potently antagonized the ANG II- and ET-1-stimulated PKC activity in the ANP/NPRA-dependent manner (Kumar et al., 1997; Pandey, 2005). The inhibitory effect of ANP was greatly amplified if cell were transfected with both PKC-α and NPRA cDNAs. The pretreatment of cells with NPRA antagonist A-71915, significantly blocked the production of cGMP as well as the inhibitory effect of ANP on PKC activity (Kumar et al., 1997). The results of those previous studies provided strong evidence that ANP antagonizes the PKC activation involving ANP/NPRA/cGMP signaling cascade. Agonists that activate PKC also produce two distinct second messengers, IP3, which activates cytosolic free Ca2+ and diacylglycesol, which stimulates PKC activity (Berridge and Irvine, 1989; Exton, 1990; Rasmussen et al., 1995; Kumar et al., 1997). Our previous studies have suggested that ANP inhibits the formation of IP3 in a cGMP-dependent manner in the intact cells, suggesting that the inhibitory effect of ANP on PKC activity might be linked with its antagonistic action on IP3 formation, however, more studies are needed to support these observations in various ANP-responsive cell and tissues systems.
EFFECT OF NPRA ON THE INHIBITION OF MAPKs ACTIVITY AND CELL PROLIFERATION
It has been shown that cGMP analogs mimicked the antiproliferative action of ANP, indicating that it exerts the antimitogenic effects largely through the intracellular second messenger cGMP (Lincoln et al., 1994; Hutchinson et al., 1997; Pandey et al., 2000b; Sharma et al., 2002). ANP has been shown to inhibit collagen synthesis in cardiac fibroblasts and also it inhibits hypertrophy of cardiac myocytes (Calderone et al., 1998; Masciotra et al., 1999; Silberbach et al., 1999; Horio et al., 2000; Gopi et al., 2013). Similarly, PKG has been shown to suppress extracellular matrix production in VSMCs (Dey et al., 1998). Both NPRA and NPRC, have been suggested to play a role in ANP-dependent antimitogenic responses (Prins et al., 1996; Hutchinson et al., 1997; Pandey et al., 2000b; Sharma et al., 2002; Tripathi and Pandey, 2012). ANP has been shown to act as a growth suppressor in a variety of cell types including; kidney, heart, neurons, thymus, vasculature, and fibroblasts (Levin et al., 1998; Pandey, 2005). Previous studies have demonstrated that ANP inhibits ANGII- and platelet-derived growth factor (PDGF) -dependent MAPK activity in different tissues and cell types (Sugimoto et al., 1993; Prins et al., 1996; Pandey et al., 2000b; Sharma et al., 2002; Tripathi and Pandey, 2012). However, in astroglial cells, ANP was shown to inhibit extracellular-regulated MAPK (Erk1/2) activity through NPRC (Prins et al., 1996). In contrast, recent findings have indicated that des- (Cys105–Cys121) -ANP, a ligand selective to NPRC, did not inhibit basal or serum-stimulated MAPK, however, CNP, which acts through NPRB, potently inhibited MAPK activity in fibroblasts in a cGMP-dependent manner (Chrisman and Garbers, 1999).
It has been postulated that cGMP-dependent signaling mechanisms of GC-A/NPRA are initiated probably at the level of gene transcription; however, the exact mechanism of this activation remains to be elucidated. A previous report also indicated that cGMP/PKG signaling was able to increase the MAPK activity in contractile rat VSMCs (Komalavilas et al., 1999). However, the process by which cGMP/PKG leads to the activation of MAPKs is unclear. Similarly, cAMP- and PKG have also been shown to inhibit as well as to activate MAPKs pathways, depending on the cell types and culture conditions (Bornfildt and Krebs, 1999). However, the involvement of specific ANP receptor subtypes in the inhibitory effects of ANP on the agonist-stimulated MAPKs activity is controversial. Indeed, more studies are needed to establish the underlying mechanisms of the antiproliferative effect of ANP in target cells. ANP has also been shown to induce apoptosis in cultured VSMCs and in neonatal rat cardiac myocytes (Trindade et al., 1995; Wu et al., 1997). The apoptotic effect of ANP was mimicked by 8-bromo-cGMP, a membrane-permeable analog of cGMP, and also by nitroprusside, an activator of soluble guanylyl cyclase. Furthermore, the effect of ANP was greatly potentiated by a cGMP-specific phosphodiesterase inhibitor zaprinast. It has been indicated that norepinephrine, a myocyte growth and proliferative effector molecule, inhibited ANP-induced apoptosis via activation of β-adrenergic receptor and elevation of cAMP (Wu et al., 1997). The existence of a complementary ANP-mediated mechanism to inhibit cell growth and proliferation is not anticipated. Nevertheless, the inhibition of cell proliferation is often accompanied by an increased probability of apoptosis, whereas, growth-promoting agents and agonist hormones tend to promote cell growth and proliferation. For instance, ANG II inhibits apoptosis, in contrast, ANP and nitric oxide, both potently inhibit cell growth and proliferation and induce apoptosis (Pollman et al., 1996; Wu et al., 1997). It has been suggested that the anti-apoptotic molecule Bcl-2 homolog Mcl-1 might serve as an important target in ANP-induced apoptosis. Intriguing was the finding that the Bcl-2 homolog Mcl-1 was initially identified as a protein marker, which was up-regulated during the differentiation of the monocytoid cell line ML-1 cells (Kozopas et al., 1993; Kiefer et al., 1995; Wu et al., 1997).
GENE-TARGETING OF Nppa AND Npr1
Genetic-targeting strategies in mice have provided novel approaches to study the physiological responses corresponding to gene-dosage in vivo (Takahashi and Smithies, 1999; Kim et al., 2002). Genetically modified mice carrying Npr1 gene-disruption or gene-duplication have provided strong support for the physiological roles of NPs and their receptors in the intact animals (John et al., 1995; Lopez et al., 1995; Kishimoto et al., 1996; Oliver et al., 1997, 1998; Matsukawa et al., 1999; Pandey et al., 1999; Shi et al., 2001, 2003; Holtwick et al., 2002; Vellaichamy et al., 2005; Das et al., 2012; Zhao et al., 2013). Numerous studies have examined the quantitative contributions and possible mechanisms mediating the responses of Npr1 gene copies by determining the renal plasma flow (RPF), glomerular filtration rate (GFR), urine flow, and sodium excretion following blood volume expansion in Npr1 homozygous null mutant (Npr1-/-; 0-copy), wild-type (Npr1+/+; 2-copy), and gene-duplicated (Npr1++/++; 4-copy) mice in a Npr1 gene-dose-dependent manner (Shi et al., 2003). Although, the blood volume expansion stimulated the release of ANP in all three Npr1 genotypes of mice, significant functional responses (RPF, GFR, and sodium excretion) occurred only in Npr1-/- and Npr1++/++ mice but not in Npr1-/- mice. These findings demonstrated that the ANP/NPRA axis is primarily responsible for mediating the renal hemodynamic and sodium excretory responses to intravascular blood volume expansion. ANP responses to volume expansion led to the significantly lesser excretion of Na+ and water in 0-copy null mutant mice and significantly greater excretory responses along with reduced tubular reabsorption in 4-copy mice as compared with 2-copy wild-type mice. Similarly, during the volume expansion, urinary cGMP concentration was significantly lower in null mutant mice and greater in gene-duplicated mice. Our previous findings have established that NPRA is a hallmark receptor, which plays a critical role in mediating the natriuresis, diuresis, and renal hemodynamic responses to acute blood volume expansion (Shi et al., 2003).
Genetic mouse models with disruption of both Nppa and Npr1 genes have provided strong support for the role of this hormone-receptor system in the regulation of blood pressure, cardiac hypertrophy, and other physiological functions (John et al., 1995; Lopez et al., 1995; Oliver et al., 1997, 1998; Melo et al., 1999; Pandey et al., 1999; Shi et al., 2001, 2003; Holtwick et al., 2002; Vellaichamy et al., 2005; Kishimoto et al., 2011; Pandey, 2011). Therefore, the genetic defects that reduce the activity of ANP and its receptor system can be considered as candidate contributors to essential hypertension and CHF (John et al., 1995; Pandey et al., 1999; Zhao et al., 1999; Knowles et al., 2001; Holtwick et al., 2002; Shi et al., 2003; Vellaichamy et al., 2005). Interestingly, complete absence of NPRA causes hypertension in mice and leads to altered renin and ANG II levels, cardiac hypertrophy, and lethal vascular events similar to those seen in untreated human hypertensive patients (Oliver et al., 1997; Shi et al., 2001, 2003; Zhao et al., 2007). In contrast, increased expression of Npr1 reduces the blood pressures and inflammatory responses, protects heart, and increases the intracellular second messenger cGMP concentrations corresponding to the increasing number of Npr1 gene copies (Oliver et al., 1998; Pandey et al., 1999; Shi et al., 2003; Vellaichamy et al., 2007, 2014; Zhao et al., 2013). Recent evidence also indicates that CNP and its receptor NPRB can play important role in regulating the cardiac hypertrophy and remodeling as a potential drug target for the treatment of cardiovascular diseases (Del Ry, 2013).
CONCLUSION
The field of NPs has been advanced to examine the function and signaling mechanisms of their receptors and the role of second messenger cGMP in physiology and pathophysiology of hypertension, renal hemodynamics, cardiovascular functions, and neural plasticity. The development of gene-knockout and gene-duplication mouse models along with transgenic mice have provided a framework for understanding both the physiological and pathophysiological functions of NPs and their receptors in the intact animals in vivo. Although, a considerable progress has been made, the transmembrane signal transduction mechanisms of NPs and their receptors remain unresolved. Future investigations should include; the identification and characterization of cellular targets of intracellular second messenger cGMP produced by NPs, including cytosolic and nuclear proteins, role in gene transcription, cell growth and proliferation, apoptosis, and differentiation. A more vigorous studies of the crosstalk with other signaling mechanisms namely, PKC, MAPKs, cAMP, Ca2+, and IP3 needs to be pursued systematically. NPs are considered as circulating markers of CHF, however, their therapeutic potential for the treatment of cardiovascular diseases such as hypertension, renal insufficiency, cardiac hypertrophy, CHF, and stroke is still lacking. The ultimate goal of the investigations is this field is to fully appreciate the mechanisms of cGMP generation after ligand binding to GC-coupled receptors and the pathways leading to elicit cellular and physiological functions in relation to other signaling molecules with special emphasis to Ca2+, IP3, and cAMP levels. Identification of the discrete switch points in signal transmission of NPs and their cognate receptors that specify unique directional responses need to be vigorously pursued.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
I thank my wife Kamala Pandey for her generous help in the preparation of this manuscript. The research in the author’s laboratory is supported by the grants from the National Institutes of Health (HL 57531 and HL 62147).
REFERENCES
- Airhart N., Yang Y. F., Roberts C. T., Jr., Silberbach M. (2003). Atrial natriuretic peptide induces natriuretic peptide receptor-cGMP-dependent protein kinase interaction. J. Biol. Chem. 278 38693–38698 10.1074/jbc.M304098200 [DOI] [PubMed] [Google Scholar]
- Anand-Srivastava M. B. (2000). Down-regulation of atrial natriuretic peptide ANP-C receptor is associated with alteration in G-protein expression in A10 smooth muscle cells. Biochemistry 39 6503–6513 10.1021/bi992660q [DOI] [PubMed] [Google Scholar]
- Anand-Srivastava M. B., Trachte G. J. (1993). Atrial natriuretic factor receptors and signal transduction mechanisms. Pharmacol. Rev. 45 455–497 10.1007/s11010-009-0335-7 [DOI] [PubMed] [Google Scholar]
- Anderson R. A., Feathergill K. A., De Jonge C., Mack S. R., Zaneveld L. J. D. (1992). Facilitative effect of pulsed addition of dibutyryl-cAMP on the acrosome reaction of noncapacitated human spermotazoa. J. Androl. 13 398–408 [PubMed] [Google Scholar]
- Anderson R. A., Feathergill K. A., Drisdel R. C., Rawlins R. G., Mack S. R., Zaneveld L. J. D. (1994). Atrial natriuretic peptide (ANP) as a stimulus of the human acrosome reaction and a component of ovarian follicular fluid: correlation of follicular ANP content with in vitro fertilization outcome. J. Androl. 15 61–70 10.1002/j.1939-4640.1994.tb01685.x [DOI] [PubMed] [Google Scholar]
- Anwaruddin S., Lloyd-Jones D. M., Baggish A., Chen A., Krauser D., Tung R., et al. (2006). Renal function, congestive heart failure, and amino-terminal pro-brain natriuretic petpide measurement: results from the proBNP investigation of dyspnea in the emergency Department (PRIDE) study. J. Am. Coll. Cardiol. 47 91–97 10.1016/j.jacc.2005.08.051 [DOI] [PubMed] [Google Scholar]
- Arise K. K., Pandey K. N. (2006). Inhibition and down-regulation of gene transcription and guanylyl cyclase activity of NPRA by angiotensin II involving protein kinase C. Biochem. Biophys. Res. Commun. 349 131–135 10.1016/j.bbrc.2006.08.003 [DOI] [PubMed] [Google Scholar]
- Ballerman B. J., Marala R. B., Sharma R. K. (1988). Characterization and regulation by protein kinase C of renal glomerular atrial natriuretic peptide receptor-coupled guanylate cyclase. Biochem. Biophys. Res. Commun. 157 755–761 10.1016/S0006-291X(88)80314-0 [DOI] [PubMed] [Google Scholar]
- Berl T., Mansour J., Teitelbaum I. (1991). ANP stimulates phospholipase C in cultured RIMCT cells: roles of protein kinases and G proteins. Am. J. Physiol. 260 F590–F595 [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. (1989). Inositol phosphates adn cel signaling. Nature 341 197–205 10.1038/341197a0 [DOI] [PubMed] [Google Scholar]
- Bornfildt K. E., Krebs E. G. (1999). Crosstalk between protein kinase A and growth factor receptor signaling pathways in arterial smooth muscle. Cell. Signal. 11 465–477 10.1016/S0898-6568(99)00020-0 [DOI] [PubMed] [Google Scholar]
- Bovy P. R. (1990). Structure activity in the atrial natriuretic peptide (ANP) family. Med. Res. Rev. 10 115–142 10.1002/med.2610100105 [DOI] [PubMed] [Google Scholar]
- Breitbart H., Lax J., Rotem R., Naor J. (1992). Role of protein kinase C in the acrosome reaction of mammalian spermatozoa. Biochem. J. 281 473–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. M., Ballermann B. J., Gunning M. E., Zeidel M. L. (1990). Diverse biological actions of atrial natriuretic peptide. Physiol. Rev. 70 665–699 [DOI] [PubMed] [Google Scholar]
- Calderone A., Thaik C. M., Takahashi N., Chang D. L. F., Colucci W. S. (1998). Nitric oxide, atrial natriuretic peptide, and cyclic GMP inhibit the growth-promoting effects of norepinephrine in cardiac myocytes and fibroblasts. J. Clin. Invest. 101 812–818 10.1172/JCI119883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron V. A., Aitken G. D., Ellmers L. J., Kennedy M. A., Espiner E. A. (1996). The sites of gene expression of atrial, brain, and C-type natriuretic peptides in mouse fetal development: temporal changes in embryos and placenta. Endocrinology 137 817–824 [DOI] [PubMed] [Google Scholar]
- Cao L., Chen S. C., Humphreys M. H., Gardner D. G. (1998). Ligand-dependent regulation of NPR-A gene expression in inner medullary collecting duct cells. Am. J. Physiol. 275 F119–F125 [DOI] [PubMed] [Google Scholar]
- Cao L., Wu J., Gardner D. G. (1995). Atrial natriuretic peptide suppresses the transcription of its guanylyl cyclase-linked receptor. J. Biol. Chem. 270 24891–24897 10.1074/jbc.270.42.24891 [DOI] [PubMed] [Google Scholar]
- Chang C.-H., Kohse K. P., Chang B., Hirata M., Jiang B., Douglas J. E., et al. (1990). Characterization of ATP-stimulated guanylyl cyclase activation in rat lung membranes. Biochim. Biophys. Acta 1052 159–165 10.1016/0167-4889(90)90071-K [DOI] [PubMed] [Google Scholar]
- Chen H. H., Burnett J. C., Jr. (1998). C-type natriuretic peptide: the endothelial component of the natriuretic peptide system. J. Cardiovasc. Pharmacol. 32(Suppl. 3), S22–S28 10.1038/jcbfm.2013.234 [DOI] [PubMed] [Google Scholar]
- Chen Y. O., Gardner D. G. (2003). Endothelin inhibits NPR-A and stimulates eNOS gene expression in rat IMCD cells. Hypertension 41 675–681 10.1161/01.HYP.0000047204.72286.34 [DOI] [PubMed] [Google Scholar]
- Chinkers M., Garbers D. L., Chang M. S., Lowe D. G., Chin H. M., Goeddel D. V., et al. (1989). A membrane form of guanylate cyclase is an atrial natriuretic peptide receptor. Nature 338 78–83 10.1038/338078a0 [DOI] [PubMed] [Google Scholar]
- Chinkers M., Singh S., Garbers D. L. (1991). Adenine nucleotides are required for activation of rat atrial natriuretic peptide receptor/guanylyl cyclase expressed in a baculovirus system. J. Biol. Chem. 266 4088–4093 [PubMed] [Google Scholar]
- Chrisman T. D., Garbers D. L. (1999). Reciprocal antagonism coordinates C-type natriuretic peptide and mitogen-signaling pathways in fibroblasts. J. Biol. Chem. 274 4293–4299 10.1074/jbc.274.7.4293 [DOI] [PubMed] [Google Scholar]
- Cohen D., Koh G. Y., Nikonova L. N., Porter J. G., Maack T. (1996). Molecular determinants of the clearance function of type-C receptor of natriuretic peptides. J. Biol. Chem. 271 9863–9869 10.1074/jbc.271.16.9863 [DOI] [PubMed] [Google Scholar]
- Cornwell T. L., Lincoln T. M. (1989). Regulation of intracellular Ca2+ levels in cultured vascular smooth muscle cells. Reduction of Ca2+ by atriopeptin and 8-bromo-cyclic GMP is mediated by cyclic GMP-dependent protein kinase. J. Biol. Chem. 264 1146–1155 [PubMed] [Google Scholar]
- Czucz J., Cervenak L., Forhecz Z., Gombos T., Pozsonyi Z., Kunde J., et al. (2011). Serum soluble E-selectin and NT-proBNP levels additively predict mortality in diabetic patients with chronic heart failure. Clin. Res. Cardiol. 100 587–594 10.1007/s00392-011-0283-6 [DOI] [PubMed] [Google Scholar]
- Das S., Periyasamy R., Pandey K. N. (2012). Activation of IKK/NF-kappaB provokes renal inflammatory responses in guanylyl cyclase/natriuretic peptide receptor-A gene-knockout mice. Physiol. Genomics 44 430–442 10.1152/physiolgenomics.00147.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bold A. J. (1985). Atrial natriuretic factor: a hormone produced by the heart. Science 230 767–770 10.1126/science.2932797 [DOI] [PubMed] [Google Scholar]
- de Bold A. J., Borenstein H. B., Veress A. T., Sonnenberg H. (1981). A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 28 89–94 10.1016/0024-3205(81)90370-2 [DOI] [PubMed] [Google Scholar]
- Dekker L. V., Parker P. J. (1994). Protein kinase C: a question of specficicit. Trends Biochem. Sci. 19 73–77 10.1016/0968-0004(94)90038-8 [DOI] [PubMed] [Google Scholar]
- Del Ry S. (2013). C-type natriuretic peptide: a new cardiac mediator. Peptides 40 93–98 10.1016/j.peptides.2012.12.010 [DOI] [PubMed] [Google Scholar]
- Dey N. B., Boerth N. J., Murphy-Ullrich J. E., Chang P. L., Prince C. W., Lincoln T. M. (1998). Cyclic GMP-dependent protein kinase inhibits osteopontin and thrombospondin production in rat aortic smooth muscle cells. Circ. Res. 82 139–146 10.1161/01.RES.82.2.139 [DOI] [PubMed] [Google Scholar]
- Doust J. A., Pietrzak E., Dobson A., Glasziou P. (2005). How well does B-type natriuretic peptide predict death and cardiac events in patients with heart failure: systematic review. BMJ 330 625 10.1136/bmj.330.7492.625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewett J. G., Garbers D. L. (1994). The family of guanylyl cyclase receptors and their ligands. Endocr. Rev. 15 135–162 10.1210/edrv-15-2-135 [DOI] [PubMed] [Google Scholar]
- Duda T., Goraczniak R. M., Sharma R. K. (1991). Site-directed mutational analysis of a membrane guanylate cyclase cDNA reveals the atrial natriuretic factor signaling site. Proc. Natl. Acad. Sci. U.S.A. 88 7882–7886 10.1073/pnas.88.17.7882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda T., Goraczniak R. M., Sharma R. K. (1993). Core sequence of ATP regulatory module in receptor guanylate cyclases. FEBS Lett. 315 143–148 10.1016/0014-5793(93)81151-O [DOI] [PubMed] [Google Scholar]
- Duda T., Sharma R. K. (1990). Regulation of guanylate cyclase activity by atrial natriuretic factor and protein kinase C. Mol. Cell. Biochem. 93 179–184 10.1007/BF00226190 [DOI] [PubMed] [Google Scholar]
- Emori T., Hirata Y., Imai T., Eguchi S., Kanno K., Marumos F. (1993). Cellular mechanism of natriuretic peptides-induced inhibition of endothelin-1 biosynthesis in rat endothelial cells. Endocrinology 133 2474–2480 [DOI] [PubMed] [Google Scholar]
- Exton J. H. (1990). Signaling through phosphatidylcholine breakdow. J. Biol. Chem. 265 1–4 [PubMed] [Google Scholar]
- Felker G. M., Petersen J. W., Mark D. W. (2006). Natriuretic peptides in the diagnosis and management of heart failure. CMAJ 175 611–617 10.1503/cmaj.060236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenrick R., Bouchard N., Mcnicoll N., De Lean A. (1997). Glycosylation of asparagines 24 of the natriuretic peptide receptor-B is crucial for the formation of a competent ligand binding domain. Mol. Cell. Biochem. 173 25–32 10.1023/A:1006855522272 [DOI] [PubMed] [Google Scholar]
- Freestone B., Gustafsson F., Chong A. Y., Corell P., Kistorp C., Hildebrandt P., et al. (2008). Influence of atrial fibrillation on plasma von willebrand factor, soluble E-selectin, and N-terminal pro B-type natriuretic peptide levels in systolic heart failure. Chest 133 1203–1208 10.1378/chest.07-2557 [DOI] [PubMed] [Google Scholar]
- Fujio N., Gossard F., Bayard F., Tremblay J. (1994). Regulation of natriuretic peptide receptor A and B expression by transforming growth factor-beta 1 in cultured aortic smooth muscle cells. Hypertension 23 908–913 10.1161/01.HYP.23.6.908 [DOI] [PubMed] [Google Scholar]
- Fuller F., Porter J. G., Arfsten A. E., Miller J., Schilling J. W., Scarborough R. M., et al. (1988). Atrial natriuretic peptide clearance receptor. Complete sequence and functional expression of cDNA clones. J. Biol. Chem. 263 9395–9401 [PubMed] [Google Scholar]
- Ganem F., Serrano C. V., Jr., Fernandes J. L., Blotta M. H., Souza J. A., Nicolau J. C. (2011). Preoperative B-type natriuretic peptide, and not the inflammation status, predicts an adverse outcome for patients undergoing heart surgery. Interact. Cardiovasc. Thorac. Surg. 12 778–783 10.1510/icvts.2010.255257 [DOI] [PubMed] [Google Scholar]
- Garbers D. L. (1992). Guanylyl cyclase receptors and their endocrine, paracrine, and autocrine ligands. Cell 71 1–4 10.1016/0092-8674(92)90258-E [DOI] [PubMed] [Google Scholar]
- Gardner D. G., Deschepper C. F., Ganong W. F., Hane S., Fiddes J., Baxter J. D., et al. (1986). Extra-atrial expression of the gene for atrial natriuretic factor. Proc. Natl. Acad. Sci. U.S.A. 83 6697–6701 10.1073/pnas.83.18.6697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg R., Pandey K. N. (2003). Angiotensin II-mediated negative regulation of Npr1 promoter activity and gene transcription. Hypertension 41 730–736 10.1161/01.HYP.0000051890.68573.94 [DOI] [PubMed] [Google Scholar]
- Gopi V., Parthasarathy A., Umadevi S., Vellaichamy E. (2013). Angiotensin-II down-regulates cardiac natriuretic peptide receptor-A mediated anti-hypertrophic signaling in experimental rat hearts. Indian J. Exp. Biol. 51 48–55 [PubMed] [Google Scholar]
- Goraczniak R. M., Duda T., Sharma R. K. (1992). A structural motif that defines the ATP-regulatory module of guanylate cyclase in atrial natriuretic factor signalling. Biochem. J. 282(Pt 2), 533–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama N., Itoh H., Shirakami G., Suga S., Komatsu Y., Yoshimasa T., et al. (1994). Detection of C-type natriuretic peptide in human circulation and marked increase of plasma CNP level in septic shock patients. Biochem. Biophys. Res. Commun. 198 1177–1182 10.1006/bbrc.1994.1166 [DOI] [PubMed] [Google Scholar]
- Hamet P., Tremblay J., Pang S. C., Garcia R., Thibault G., Gutkowska J., et al. (1984). Effect of native and synthetic atrial natriuretic factor on cyclic GMP. Biochem. Biophys. Res. Commun. 123 515–527 10.1016/0006-291X(84)90260-2 [DOI] [PubMed] [Google Scholar]
- Hassid A. (1986). Atriopeptin II decreases cytosolic free Ca2+ in cultured vascular smooth muscle cells. Am. J. Physiol. 251 C681–C686 [DOI] [PubMed] [Google Scholar]
- Heim J. M., Singh S., Gerzer R. (1996). Effect of glycosylation on cloned ANF-sensitive guanylyl cyclase. Life Sci. 59 L61–L68 10.1016/0024-3205(96)00306-2 [DOI] [PubMed] [Google Scholar]
- Hesegawa M., Hidaka Y., Wada A., Hirayama T., Shimonishi Y. (1999). The relevance of N-linked glycosylation to the binding of a ligand to guanylate cyclase C. Eur. J. Biochem. 263 338–346 10.1046/j.1432-1327.1999.00488.x [DOI] [PubMed] [Google Scholar]
- Hirata M., Chang C. M., Murad F. (1989). Stimulatory effect of atrial natriuretic factor on phosphoinositide hydrolysis in cultured bovine aortic smooth muscle cells. Biochim. Biophys. Acta 1010 346–351 10.1016/0167-4889(89)90060-8 [DOI] [PubMed] [Google Scholar]
- Hirata Y., Hirose S., Takada S., Takagi Y., Matsubara H. (1987). Down-regulation of atrial natriuretic peptide receptor and cyclic GMP response in cultured rat vascular smooth muscle cells. Eur. J. Pharmacol. 135 439–442 10.1016/0014-2999(87)90697-2 [DOI] [PubMed] [Google Scholar]
- Hirata Y., Takata S., Tomita M., Takaichi S. (1985). Binding, internalization, and degradation of atrial natriuretic peptide in cultured vascular smooth muscle cells of rat. Biochem. Biophys. Res. Commun. 132 976–984 10.1016/0006-291X(85)91903-5 [DOI] [PubMed] [Google Scholar]
- Holtwick R., Baba H. A., Ehler E., Risse-Vob M. D., Gehrmann J., Pierkes M., et al. (2002). Left but not right cardiac hypertrophy in atrial natriuretic peptide receptor-deficient mice is prevented by angiotensin type 1 receptor antagonist losartan. J. Cardiovasc. Pharmacol. 40 725–734 10.1097/00005344-200211000-00010 [DOI] [PubMed] [Google Scholar]
- Horio T., Nishikimi T., Yoshihara F., Matsuo H., Takishita S., Kangawa K. (2000). Inhibitory regulation of hypertrophy by endogenous atrial natriuretic peptide in cultured cardiac myocytes. Hypertension 35 19–24 10.1161/01.HYP.35.1.19 [DOI] [PubMed] [Google Scholar]
- Hug H., Sarre T. F. (1993). Protein kinase C isoenzymes: divergence in signal transduction. Biochem. J. 291 329–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R. J., Struthers R. S., Fong A. M., Insel P. A. (1987). Regulation of the atrial natriuretic peptide receptor on a smooth muscle cell. Am. J. Physiol. 253 C809–C816 [DOI] [PubMed] [Google Scholar]
- Hugnir R. I., Greengard P. (1990). Regulation of neurotransmitter receptor desensitization by protein phosphorylation. Neuron 5 555–567 10.1016/0896-6273(90)90211-W [DOI] [PubMed] [Google Scholar]
- Hum D., Besnard S., Sanchez R., Devost D., Gossard F., Hamet P., et al. (2004). Characterization of a cGMP-response element in the guanylyl cyclase/natriuretic peptide receptor A gene promoter. Hypertension 43 1270–1278 10.1161/01.HYP.0000126920.93207.53 [DOI] [PubMed] [Google Scholar]
- Huo X., Abe T., Misono K. S. (1999). Ligand binding-dependent limited proteolysis of the atrial natriuretic peptide juxtamembrane hinge structure essential for transmembrane signal transduction. Biochemistry 38 16941–16951 10.1021/bi9919448 [DOI] [PubMed] [Google Scholar]
- Hutchinson H. G., Trinadade P. T., Cunanan D. B., Wu C. F., Pratt R. E. (1997). Mechanisms of natriuretic-peptide-induced growth inhibition of vascular smooth muscle cells. Cardiovasc. Res. 35 158–167 10.1016/S0008-6363(97)00086-2 [DOI] [PubMed] [Google Scholar]
- Igaki T., Itoh H., Suga S., Hama N., Ogawa Y., Komatsu Y., et al. (1996). C-type natriuretic peptide in chronic renal failure and its action in humans. Kidney Int. Suppl. 55 S144–S147 [PubMed] [Google Scholar]
- Inagami T. (1989). Atrial natriuretic factor. J. Biol. Chem. 264 3043–3046 [PubMed] [Google Scholar]
- Jaffe A. S., Babuin L., Apple F. S. (2006). Biomarkers in acute cardiac disease: the present and the future. J. Am. Coll. Cardiol. 48 1–11 10.1016/j.jacc.2006.02.056 [DOI] [PubMed] [Google Scholar]
- John S. W., Krege J. H., Oliver P. M., Hagaman J. R., Hodgin J. B., Pang S. C., et al. (1995). Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science 267 679–681 10.1126/science.7839143 [DOI] [PubMed] [Google Scholar]
- Khan I. A., Fink J., Nass C., Chen H., Christenson R., Defilippi C. R. (2006). N-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide for identifying coronary artery disease and left ventricular hypertrophy in ambulatory chronic kidney disease patients. Am. J. Cardiol. 97 1530–1534 10.1016/j.amjcard.2005.11.090 [DOI] [PubMed] [Google Scholar]
- Khurana M. L., Pandey K. N. (1993). Receptor-mediated stimulatory effect of atrial natriuretic factor, brain natriuretic peptide, and C-type natriuretic peptide on testosterone production in purified mouse Leydig cells: activation of cholesterol side-chain cleavage enzyme. Endocrinology 133 2141–2149 [DOI] [PubMed] [Google Scholar]
- Khurana M. L., Pandey K. N. (1995). Catalytic activation of guanylate cyclase/atrial natriuretic factor receptor by combined effects of ANF and GTP gamma S in plasma membranes of Leydig tumor cells: involvement of G-proteins. Arch. Biochem. Biophys. 316 392–398 10.1006/abbi.1995.1052 [DOI] [PubMed] [Google Scholar]
- Khurana M. L., Pandey K. N. (1996). Atrial natriuretic peptide inhibits the phosphoinositide hydrolysis in murine Leydig tumor cells. Mol. Cell. Biochem. 158 97–105 10.1007/BF00225834 [DOI] [PubMed] [Google Scholar]
- Kiefer M. C., Brauer M. J., Powers V. C., Wu J. J., Umansky S. R., Tomel L. D., et al. (1995). Modulation of apoptosis by the widely distributed Bcl-2 homologue Bak. Nature 374 736–739 10.1038/374736a0 [DOI] [PubMed] [Google Scholar]
- Kim H. S., Lu G., John S. W. M., Maeda N., Smithies O. (2002). Molecular phenotyping for analyzing subtle genetic effects in mice application to an angiotensinogen gene titration. Proc. Natl. Acad. Sci. U.S.A. 99 4602–4607 10.1073/pnas.072083799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto I., Dubois S. K., Garbers D. L. (1996). The heart communicates with the kidney exclusively through the guanylyl cyclase-A receptor: acute handling of sodium and water in response to volume expansion. Proc. Natl. Acad. Sci. U.S.A. 93 6215–6219 10.1073/pnas.93.12.6215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto I., Tokudome T., Nakao K., Kangawa K. (2011). Natriuretic peptide system: an overview of studies using genetically engineered animal models. FEBS J. 278 1830–1841 10.1111/j.1742-4658.2011.08116.x [DOI] [PubMed] [Google Scholar]
- Knowles J. W., Esposito G., Mao L., Hagaman J. R., Fox J. E., Smithies O., et al. (2001). Pressure-independent enhancement of cardiac hypertrophy in natriuretic peptide receptor A-deficient mice. J. Clin. Invest. 107 975–984 10.1172/JCI11273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M., Minamino N., Kangawa K., Matsuo H. (1989). Cloning and sequence analysis of cDNA encoding a precursor for rat brain natriuretic peptide. Biochem. Biophys. Res. Commun. 159 1420–1426 10.1016/0006-291X(89)92268-7 [DOI] [PubMed] [Google Scholar]
- Koller K. J., Lipari M. T., Goeddel D. V. (1993). Proper glycosylation and phosphorylation of the type A natriuretic peptide receptor are required for hormone-stimulated guanylyl cyclase activity. J. Biol. Chem. 268 5997–6003 [PubMed] [Google Scholar]
- Koller K. J., Lowe D. G., Bennett G. L., Minamino N., Kangawa K., Matsuo H., et al. (1991). Selective activation of the B natriuretic peptide receptor by C-type natriuretic peptide (CNP). Science 252 120–123 10.1126/science.1672777 [DOI] [PubMed] [Google Scholar]
- Komalavilas P., Shah P. K., Jo H., Lincoln T. M. (1999). Activation of mitogen-activated protein kinase pathways by cyclic GMP and cyclic GMP-dependent protein kinase in contractile vascular smooth muscle cells. J. Biol. Chem. 274 34301–34309 10.1074/jbc.274.48.34301 [DOI] [PubMed] [Google Scholar]
- Komatsu Y., Endo Y., Suzaki S. (1990). Changes in protein phoshorylation during acrosome reaction of mouse sperm. Yakugaku Zasshi 110 325–331 [DOI] [PubMed] [Google Scholar]
- Komuro I., Katoh Y., Kaida T., Shibazaki Y., Kurabayashi M., Hoh E., et al. (1991). Mechanical loading stimulates cell hypertrophy and specific gene expression in cultured rat cardiac myocytes. Possible role of protein kinase C activation. J. Biol. Chem. 266 1265–1268 [PubMed] [Google Scholar]
- Kozopas K. M., Yang T., Buchan H. L., Zhou P., Craig R. W. (1993). MCLI, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc. Natl. Acad. Sci. U.S.A. 90 3516–3520 10.1073/pnas.90.8.3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P., Garg R., Bolden G., Pandey K. N. (2010). Interactive roles of Ets-1, Sp1, and acetylated histones in the retinoic acid-dependent activation of guanylyl cyclase/atrial natriuretic peptide receptor-A gene transcription. J. Biol. Chem. 285 37521–37530 10.1074/jbc.M110.132795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P., Periyasamy R., Das S., Neerukonda S., Mani I., Pandey K. N. (2014a). All-trans retinoic acid and sodium butyrate enhance natriuretic peptide receptor a gene transcription: role of histone modification. Mol. Pharmacol. 85 946–957 10.1124/mol.114.092221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P., Tripathi S., Pandey K. N. (2014b). Histone deacetylase inhibitors modulate the transcriptional regulation of guanylyl cyclase/natriuretic peptide receptor-A gene: interactive roles of modified histones, HATS, p300, and Sp1. J. Biol. Chem. 289 6991–7002 10.1074/jbc.M113.511444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Cartledge W. A., Lincoln T. M., Pandey K. N. (1997). Expression of guanylyl cyclase-A/atrial natriuretic peptide receptor blocks the activation of protein kinase C in vascular smooth muscle cells. Role of cGMP and cGMP-dependent protein kinase. Hypertension 29 414–421 10.1161/01.HYP.29.1.414 [DOI] [PubMed] [Google Scholar]
- Kuno T., Andresen J. W., Kamisaki Y., Waldman S. A., Chang L. Y., Saheki S., et al. (1986). Co-purification of an atrial natriuretic factor receptor and particulate guanylate cyclase from rat lung. J. Biol. Chem. 261 5817–5823 [PubMed] [Google Scholar]
- Kurose H., Inagami T., Ui M. (1987). Participation of adenosine 5’-triphosphate in the activation of membrane-bound guanylate cyclase by the atrial natriuretic factor. FEBS Lett. 219 375–379 10.1016/0014-5793(87)80256-9 [DOI] [PubMed] [Google Scholar]
- Labrecque J., Mcnicoll N., Marquis M., De Lean A. (1999). A disulfide-bridged mutant of natriuretic peptide receptor-A displays constitutive activity. Role of receptor dimerization in signal transduction. J. Biol. Chem. 274 9752–9759 10.1074/jbc.274.14.9752 [DOI] [PubMed] [Google Scholar]
- Larose L., Mcnicoll N., Ong H., De Lean A. (1991). Allosteric modulation by ATP of the bovine adrenal natriuretic factor R1 receptor functions. Biochemistry 30 8990–8995 10.1021/bi00101a012 [DOI] [PubMed] [Google Scholar]
- Larose L., Rondeau J. J., Ong H., De Lean A. (1992). Phosphorylation of atrial natriuretic factor R1 receptor by serine/threonine protein kinases: evidences for receptor regulation. Mol. Cell. Biochem. 115 203–211 10.1007/BF00230332 [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J., Pitcher J., Krueger K., Daaka Y. (1998). Mechanisms of β-adrenergic receptor desensitization and resensitization. Adv. Pharmacol. 42 416–420 10.1016/S1054-3589(08)60777-2 [DOI] [PubMed] [Google Scholar]
- Leitman D. C., Andresen J. W., Catalano R. M., Waldman S. A., Tuan J. J., Murad F. (1988). Atrial natriuretic peptide binding, cross-linking, and stimulation of cyclic GMP accumulation and particulate guanylate cyclase activity in cultured cells. J. Biol. Chem. 263 3720–3728 [PubMed] [Google Scholar]
- Levin E. R., Gardner D. G., Samson W. K. (1998). Natriuretic peptides. N. Engl. J. Med. 339 321–328 10.1056/NEJM199807303390507 [DOI] [PubMed] [Google Scholar]
- Lincoln T. M., Komalavilas P., Cornwell T. L. (1994). Pleiotropic regulation of vascular smooth muscle tone by cyclic GMP-dependent protein kinase. Hypertension 243 383–385 [DOI] [PubMed] [Google Scholar]
- Lisy O., Jougasaki M., Heublein D. M., Schirger J. A., Chen H. H., Wennberg P. W., et al. (1999). Renal actions of synthetic Dendroaspis natriuretic peptide. Kidney Int. 56 502–508 10.1046/j.1523-1755.1999.00573.x [DOI] [PubMed] [Google Scholar]
- Lopez M. J., Wong S. K.-F., Kishimoto I., Dubois S., Mach V., Friesen J., et al. (1995). Salt-resistant hypertension in mice lacking the guanylyl cyclase-A receptor for atrial natriuretic peptide. Nature 378 65–68 10.1038/378065a0 [DOI] [PubMed] [Google Scholar]
- Louis J. C., Magal E., Yavin E. (1988). Protein kinase C alterations in the fetal rat brain after global ischemia. J. Biol. Chem. 263 19282–19285 [PubMed] [Google Scholar]
- Lowe D. G., Fendly B. M. (1992). Human natriuretic peptide receptor-A guanylyl cyclase. Hormone cross-linking and antibody reactivity distinguish receptor glycoforms. J. Biol. Chem. 267 21691–21697 [PubMed] [Google Scholar]
- Lucas K. A., Pitari G. M., Kazerounian S., Ruiz-Stewart I., Park J., Schulz S., et al. (2000). Guanylyl cyclases and signaling by cyclic GMP. Pharmacol. Rev. 52 375–414 [PubMed] [Google Scholar]
- Maack T., Suzuki M., Almeida F. A., Nussenzveig D., Scarborough R. M., Mcenroe G. A., et al. (1987). Physiological role of silent receptors of atrial natriuretic factor. Science 238 675–678 10.1126/science.2823385 [DOI] [PubMed] [Google Scholar]
- Maki M., Takayanagi R., Misono K. S., Pandey K. N., Tibbetts C., Inagami T. (1984). Structure of rat atrial natriuretic factor precursor deduced from cDNA sequence. Nature 309 722–724 10.1038/309722a0 [DOI] [PubMed] [Google Scholar]
- Marala R., Duda T., Goraczniak R. M., Sharma R. K. (1992). Genetically tailored atrial natriuretic factor-dependent guanylate cyclase. Immunological and functional identity with 180 kDa membrane guanylate cyclase and ATP signaling site. FEBS Lett. 296 254–258 10.1016/0014-5793(92)80298-U [DOI] [PubMed] [Google Scholar]
- Masciotra S., Picard S., Deschepper C. F. (1999). Cosegregation analysis in genetic crosses suggests a protective role for atrial natriuretic factor against ventricular hypertrophy. Circ. Res. 84 1453–1458 10.1161/01.RES.84.12.1453 [DOI] [PubMed] [Google Scholar]
- Matsukawa N., Grzesik W. J., Takahashi N., Pandey K. N., Pang S., Yamauchi M., et al. (1999). The natriuretic peptide clearance receptor locally modulates the physiological effects of the natriuretic peptide system. Proc. Natl. Acad. Sci. U.S.A. 96 7403–7408 10.1073/pnas.96.13.7403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough P. A., Duc P., Omland T., Mccord J., Nowak R. M., Hollander J. E., et al. (2003). B-type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the breathing not properly multinational study. Am. J. Kidney Dis. 41 571–579 10.1053/ajkd.2003.50118 [DOI] [PubMed] [Google Scholar]
- Melo L. G., Veress A. T., Ackermann U., Steinhelper M. E., Pang S. C., Tse Y., et al. (1999). Chronic regulation of arterial blood pressure in ANP transgenic and knockout mice: role of cardiovascular sympathetic tone. Cardiovasc. Res. 43 437–444 10.1016/S0008-6363(99)00104-2 [DOI] [PubMed] [Google Scholar]
- Meloche S., Mcnicoll N., Liu B., Ong H., De Lean A. (1988). Atrial natriuretic factor R1 receptor from bovine adrenal zona glomerulosa: purification, characterization, and modulation by amiloride. Biochemistry 27 8151–8158 10.1021/bi00421a025 [DOI] [PubMed] [Google Scholar]
- Meloche S., Ong H., Cantin M., De Lean A. (1986). Affinity cross-linking of atrial natriuretic factor to its receptor in bovine adrenal zona glomerulosa. J. Biol. Chem. 261 1525–1528 [PubMed] [Google Scholar]
- Meyer M., Richter R., Brunkhorst R., Wrenger E., Schulz-Knappe P., Kist A., et al. (1996). Urodilatin is involved in sodium homeostasis and exerts sodium-state-dependent natriuretic and diuretic effects. Am. J. Physiol. 271 F489–F497 [DOI] [PubMed] [Google Scholar]
- Misono K. S., Fukumi H., Grammer R. T., Inagami T. (1984). Rat atrial natriuretic factor: complete amino acid sequence and disulfide linkage essential for biological activity. Biochem. Biophys. Res. Commun. 119 524–529 10.1016/S0006-291X(84)80279-X [DOI] [PubMed] [Google Scholar]
- Misono K. S., Grammer R. T., Rigby J. W., Inagami T. (1985). Photoaffinity labeling of atrial natriuretic factor receptor in bovine and rat adrenal cortical membranes. Biochem. Biophys. Res. Commun. 130 994–1001 10.1016/0006-291X(85)91713-9 [DOI] [PubMed] [Google Scholar]
- Miyagi M., Zhang X., Misono K. S. (2000). Glycosylation sites in the atrial natriuretic peptide receptor oligosaccharide structures are not required for hormone binding. Eur. J. Biochem. 267 5758–5768 10.1046/j.1432-1327.2000.01647.x [DOI] [PubMed] [Google Scholar]
- Mukoyama M., Nakao K., Hosoda K., Suga S., Saito Y., Ogawa Y., et al. (1991). Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J. Clin. Invest. 87 1402–1412 10.1172/JCI115146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy K. K., Thibault G., Cantin M. (1989). Binding and intracellular degradation of atrial natriuretic factor by cultured vascular smooth muscle cells. Mol. Cell. Endocrinol. 67 195–206 10.1016/0303-7207(89)90210-4 [DOI] [PubMed] [Google Scholar]
- Napier M., Arcuri K., Vandlen R. (1986). Binding and internalization of atrial natriuretic factor by high-affinity receptors in A10 smooth muscle cells. Arch. Biochem. Biophys. 248 516–522 10.1016/0003-9861(86)90504-7 [DOI] [PubMed] [Google Scholar]
- Neuser D., Bellermann P. (1986). Receptor binding, cGMP stimulation and receptor desensitization by atrial natriuretic peptides in cultured A10 vascular smooth muscle cells. FEBS Lett. 209 347–351 10.1016/0014-5793(86)81140-1 [DOI] [PubMed] [Google Scholar]
- Nussenzveig D. R., Lewicki J. A., Maack T. (1990). Cellular mechanisms of the clearance function of type-C receptors of atrial natriuretic factor. J. Biol. Chem. 265 20952–20958 [PubMed] [Google Scholar]
- Ogawa Y., Nakao K., Nakagawa O., Komatsu Y., Hosoda K., Suga S., et al. (1992). Human C-type natriuretic peptide. Characterization of the gene and peptide. Hypertension 19 809–813 10.1161/01.HYP.19.6.809 [DOI] [PubMed] [Google Scholar]
- Oliver P. M., Fox J. E., Kim R., Rockman H. A., Kim H. S., Reddick R. L., et al. (1997). Hypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor A. Proc. Natl. Acad. Sci. U.S.A. 94 14730–14735 10.1073/pnas.94.26.14730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver P. M., John S. W., Purdy K. E., Kim R., Maeda N., Goy M. F., et al. (1998). Natriuretic peptide receptor 1 expression influences blood pressures of mice in a dose-dependent manner. Proc. Natl. Acad. Sci. U.S.A. 95 2547–2551 10.1073/pnas.95.5.2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omland T., Aakvaag A., Bonarjee V. V., Caidahl K., Lie R. T., Nilsen D. W., et al. (1996). Plasma brain natriuretic peptide as an indicator of left ventricular systolic function and long-term survival after acute myocardial infarction. Comparison with plasma atrial natriuretic peptide and N-terminal proatrial natriuretic peptide. Circulation 93 1963–1969 10.1161/01.CIR.93.11.1963 [DOI] [PubMed] [Google Scholar]
- Pandey K., Kumar R., Li M., Nguyen H. T. (2000a). Functional domains and expression of truncated atrial natriuretic peptide receptor-A: the carboxyl-terminal regions direct the receptor internalization and sequestration in COS-7 cells. Mol. Pharmacol. 57 259–267 [PubMed] [Google Scholar]
- Pandey K. N., Nguyen H. T., Li M., Boyle J. W. (2000b). Natriuretic peptide receptor-A negatively regulates mitogen-activated protein kinase and proliferation of mesangial cells: role of cGMP-dependent protein kinase. Biochem. Biophys. Res. Commun. 271 374–379 10.1006/bbrc.2000.2627 [DOI] [PubMed] [Google Scholar]
- Pandey K. N. (1989). Stimulation of protein phosphorylation by atrial natriuretic factor in plasma membranes of bovine adrenal cortical cells. Biochem. Biophys. Res. Commun. 163 988–994 10.1016/0006-291X(89)92319-X [DOI] [PubMed] [Google Scholar]
- Pandey K. N. (1992). Kinetic analysis of internalization, recycling and redistribution of atrial natriuretic factor-receptor complex in cultured vascular smooth-muscle cells. Ligand-dependent receptor down-regulation. Biochem. J. 288 55–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey K. N. (1993). Stoichiometric analysis of internalization, recycling, and redistribution of photoaffinity-labeled guanylate cyclase/atrial natriuretic factor receptors in cultured murine Leydig tumor cells. J. Biol. Chem. 268 4382–4390 [PubMed] [Google Scholar]
- Pandey K. N. (1994a). Atrial natriuretic factor inhibits autophosphorylation of protein kinase C and A 240-kDa protein in plasma membranes of bovine adrenal glomerulosa cells: involvement of cGMP-dependent and independent signal transduction mechanisms. Mol. Cell. Biochem. 141 103–111 10.1007/BF00926173 [DOI] [PubMed] [Google Scholar]
- Pandey K. N. (1994b). Atrial natriuretic factor inhibits the phosphorylation of protein kinase C in plasma membrane preparations of cultured Leydig tumor cells. J. Androl. 15 100–109 10.1007/BF00926173 [DOI] [PubMed] [Google Scholar]
- Pandey K. N. (2001). Dynamics of internalization and sequestration of guanylyl cyclase/atrial natriuretic peptide receptor-A. Can. J. Physiol. Pharmacol. 79 631–639 10.1139/y01-035 [DOI] [PubMed] [Google Scholar]
- Pandey K. N. (2002). Intracellular trafficking and metabolic turnover of ligand-bound guanylyl cyclase/atrial natriuretic peptide receptor-A into subcellular compartments. Mol. Cell. Biochem. 230 61–72 10.1023/A:1014240006767 [DOI] [PubMed] [Google Scholar]
- Pandey K. N. (2005). Biology of natriuretic peptides and their receptors. Peptides 26 901–932 10.1016/j.peptides.2004.09.024 [DOI] [PubMed] [Google Scholar]
- Pandey K. N. (2011). The functional genomics of guanylyl cyclase/natriuretic peptide receptor-A: perspectives and paradigms. FEBS J. 278 1792–1807 10.1111/j.1742-4658.2011.08081.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey K. N., Inagami T., Misono K. S. (1986). Atrial natriuretic factor receptor on cultured Leydig tumor cells: ligand binding and photoaffinity labeling. Biochemistry 25 8467–8472 10.1021/bi00374a022 [DOI] [PubMed] [Google Scholar]
- Pandey K. N., Kovacs W. J., Inagami T. (1985). The inhibition of progesterone secretion and the regulation of cyclic nucleotides by atrial natriuretic factor in gonadotropin responsive murine Leydig tumor cells. Biochem. Biophys. Res. Commun. 133 800–806 10.1016/0006-291X(85)90975-1 [DOI] [PubMed] [Google Scholar]
- Pandey K. N., Nguyen H. T., Sharma G. D., Shi S. J., Kriegel A. M. (2002). Ligand-regulated internalization, trafficking, and down-regulation of guanylyl cyclase/atrial natriuretic peptide receptor-A in human embryonic kidney 293 cells. J. Biol. Chem. 277 4618–4627 10.1074/jbc.M106436200 [DOI] [PubMed] [Google Scholar]
- Pandey K. N., Oliver P. M., Maeda N., Smithies O. (1999). Hypertension associated with decreased testosterone levels in natriuretic peptide receptor-A gene-knockout and gene-duplicated mutant mouse models. Endocrinology 140 5112–5119 [DOI] [PubMed] [Google Scholar]
- Pandey K. N., Pavlou S. N., Inagami T. (1988). Identification and characterization of three distinct atrial natriuretic factor receptors. Evidence for tissue-specific heterogeneity of receptor subtypes in vascular smooth muscle, kidney tubular epithelium, and Leydig tumor cells by ligand binding, photoaffinity labeling, and tryptic proteolysis. J. Biol. Chem. 263 13406–13413 [PubMed] [Google Scholar]
- Pandey K. N., Singh S. (1990). Molecular cloning and expression of murine guanylate cyclase/atrial natriuretic factor receptor cDNA. J. Biol. Chem. 265 12342–12348 [PubMed] [Google Scholar]
- Paul A. K., Marala R. B., Jaiswal R. K., Sharma R. K. (1987). Coexistence of guanylate cyclase and atrial natriuretic factor receptor in a 180-kD protein. Science 235 1224–1226 10.1126/science.2881352 [DOI] [PubMed] [Google Scholar]
- Phillips R. A., Ardeljan M., Shimabukuro S., Goldman M. E., Garbowit D. L., Eison H. B., et al. (1991). Normalization of left ventricular mass and associated changes in neurohormones and atrial natriuretic peptide after 1 year of sustained nifedipine therapy for severe hypertension. J. Am. Coll. Cardiol. 17 1595–1602 10.1016/0735-1097(91)90654-R [DOI] [PubMed] [Google Scholar]
- Pollman M. J., Yamada T., Horiuchi M., Gibbons G. H. (1996). Vasoactive substances regulate vascular smooth muscle cell apoptosis. Circ. Res. 79 748–756 10.1161/01.RES.79.4.748 [DOI] [PubMed] [Google Scholar]
- Potter L. R., Garbers D. L. (1992). Dephosphorylation of the guanylyl cyclase-A receptor causes desensitization. J. Biol. Chem. 267 14531–14534 [PubMed] [Google Scholar]
- Prins B. A., Weber M. J., Hu R. -M., Pedram A., Daniels M., Levin E. R. (1996). Atrial natriuretic peptide inhibits mitogen-activated protein kinase through the clearance receptor Potential role in the inhibition of astrocyte proliferation. J. Biol. Chem. 271 14156–14162 10.1074/jbc.271.24.14156 [DOI] [PubMed] [Google Scholar]
- Rashatwar S. S., Cornwell T. L., Lincoln T. M. (1987). Effect of 8-bromo cGMP on Ca2+-ATPase by cGMP dependent protein kinase. Proc. Natl. Acad. Sci. U.S.A 84 5685–5689 10.1073/pnas.84.16.5685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H., Isales C. M., Calle R. A., Throckmorton D., Anderson M., Gasalla-Harraiz J., et al. (1995). Diacylglycerol production, Ca2+ influx and protein kinase c activation in sustained cellular responses. Endocr. Rev. 16 649–681 [DOI] [PubMed] [Google Scholar]
- Rathinavelu A., Isom G. E. (1991). Differential internalization and precessing of atrial natriuretic factor B and C receptors in PC-12 cells. Biochem. J. 276 493–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resink T. J., Scott-Burden T., Baur U., Jones C. R., Buhler F. R. (1988). Atrial natriuretic peptide induces breakdown of phosphatidylinositol phosphates in cultured vascular smooth muscle cells. Eur. J. Biochem. 172 499–505 10.1111/j.1432-1033.1988.tb13915.x [DOI] [PubMed] [Google Scholar]
- Rogers J., Hughes R. J., Mathes E. K. (1988). cGMP inhibits protein kinase C-mediated secretion in rat pancreatic acini. J. Biol. Chem. 263 3713–3719 [PubMed] [Google Scholar]
- Rosenzweig A., Seidman C. E. (1991). Atrial natriuretic factor and related peptide hormones. Annu. Rev. Biochem. 60 229–255 10.1146/annurev.bi.60.070191.001305 [DOI] [PubMed] [Google Scholar]
- Sano T., Morishita Y., Matsuda Y., Yamada K. (1992). Pharmacological profile of HS-142-1, a novel nonpeptide atrial natriuretic peptide antagonist of microbial origin I. Selective inhibition of the actions of natriuretic peptides in anesthetized rats. J. Pharmacol. Exp. Ther. 260 825–831 [PubMed] [Google Scholar]
- Sauro M. D., Fitzpatrick D. F. (1990). Atrial natriuretic peptides inhibit protien kinase C activation in rat aortic smooth muscle. Pept. Res. 3 138–141 [PubMed] [Google Scholar]
- Saxenhofer H., Raselli A., Weidmann P., Forssmann W. G., Bub A., Ferrari P., et al. (1990). Urodilatin, a natriuretic factor from kidneys, can modify renal and cardiovascular function in men. Am. J. Physiol. 259 F832–F838 [DOI] [PubMed] [Google Scholar]
- Schenk D. B., Phelps M. N., Porter J. G., Scarborough R. M., Mcenroe G. A., Lewicki J. A. (1985). Identification of the receptor for atrial natriuretic factor on cultured vascular cells. J. Biol. Chem. 260 14887–14890 [PubMed] [Google Scholar]
- Schulz-Knappe P., Forssmann K., Herbst F., Hock D., Pipkorn R., Forssmann W. G. (1988). Isolation and structural analysis of “urodilatin”, a new peptide of the cardiodilatin-(ANP)-family, extracted from human urine. Klin. Wochenschr. 66 752–759 10.1007/BF01726570 [DOI] [PubMed] [Google Scholar]
- Schulz S., Singh S., Bellet R. A., Singh G., Tubb D. J., Chin H., et al. (1989). The primary structure of a plasma membrane guanylate cyclase demonstrates diversity within this new receptor family. Cell 58 1155–1162 10.1016/0092-8674(89)90513-8 [DOI] [PubMed] [Google Scholar]
- Schweitz H., Vigne P., Moinier D., Frelin C., Lazdunski M. (1992). A new member of the natriuretic peptide family is present in the venom of the green mamba (Dendroaspis angusticeps). J. Biol. Chem. 267 13928–13932 [PubMed] [Google Scholar]
- Sharma G. D., Nguyen H. T., Antonov A. S., Gerrity R. G., Von Geldern T., Pandey K. N. (2002). Expression of atrial natriuretic peptide receptor-A antagonizes the mitogen-activated protein kinases (Erk2 and P38MAPK) in cultured human vascular smooth muscle cells. Mol. Cell. Biochem. 233 165–173 10.1023/A:1015882302796 [DOI] [PubMed] [Google Scholar]
- Sharma R. K. (2002). Evolution of the membrane guanylate cyclase transduction system. Mol. Cell. Biochem. 230 3–30 10.1023/A:1014280410459 [DOI] [PubMed] [Google Scholar]
- Sharma R. K., Duda T. (2010). ROS-GC subfamily membrane guanylate cyclase-linked transduction systems: taste, pineal gland and hippocampus. Mol. Cell. Biochem. 334 199–206 10.1007/s11010-009-0334-8 [DOI] [PubMed] [Google Scholar]
- Shi S. J., Nguyen H. T., Sharma G. D., Navar L. G., Pandey K. N. (2001). Genetic disruption of atrial natriuretic peptide receptor-A alters renin and angiotensin II levels. Am. J. Physiol. 281 F665–F673 [DOI] [PubMed] [Google Scholar]
- Shi S. J., Vellaichamy E., Chin S. Y., Smithies O., Navar L. G., Pandey K. N. (2003). Natriuretic peptide receptor A mediates renal sodium excretory responses to blood volume expansion. Am. J. Physiol. 285 F694–F702 [DOI] [PubMed] [Google Scholar]
- Sibley D. R., Benovic J. L., Caron M. G., Lefkowitz R. J. (1987). Regulation of transmembrane signaling by receptor phosphorylation. Cell 48 913–933 10.1016/0092-8674(87)90700-8 [DOI] [PubMed] [Google Scholar]
- Silberbach M., Gorenc T., Hershberger R. E., Stork P. J. S., Steyger P. S., Roberts C. T. J. (1999). Extracellular signal-regulated protein kinase activation is required for the anti-hypertrophic effect of atrial natriuretic factor in neonatal rat ventricular myocytes. J. Biol. Chem. 274 24858–24864 10.1074/jbc.274.35.24858 [DOI] [PubMed] [Google Scholar]
- Sorkin A., von Zastrow M. (2002). Signal transduction and endocytosis close encounters of many kinds. Nat. Rev. Mol. Cell Biol. 3 600–614 10.1038/nrm883 [DOI] [PubMed] [Google Scholar]
- Sudoh T., Minamino N., Kangawa K., Matsuo H. (1988). Brain natriuretic peptide-32: N-terminal six amino acid extended form of brain natriuretic peptide identified in porcine brain. Biochem. Biophys. Res. Commun. 155 726–732 10.1016/S0006-291X(88)80555-2 [DOI] [PubMed] [Google Scholar]
- Sudoh T., Minamino N., Kangawa K., Matsuo H. (1990). C-type natriuretic peptide (CNP): a new member of natriuretic peptide family identified in porcine brain. Biochem. Biophys. Res. Commun. 168 863–870 10.1016/0006-291X(90)92401-K [DOI] [PubMed] [Google Scholar]
- Suga S., Itoh H., Komatsu Y., Ogawa Y., Hama N., Yoshimasa T., et al. (1993). Cytokine-induced C-type natriuretic peptide (CNP) secretion from vascular endothelial cells–evidence for CNP as a novel autocrine/paracrine regulator from endothelial cells. Endocrinology 133 3038–3041 [DOI] [PubMed] [Google Scholar]
- Suga S., Nakao K., Itoh H., Komatsu Y., Ogawa Y., Hama N., et al. (1992). Endothelial production of C-type natriuretic peptide and its marked augmentation by transforming growth factor-beta. Possible existence of “vascular natriuretic peptide system.” J. Clin. Invest. 90 1145–1149 10.1172/JCI115933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto T., Kikkawa R., Haneda M., Shigeta Y. (1993). Atrial natriuretic peptide inhibits endothelin-1 induced activation of mitogen-activated protein kinase in cultured rat mesangial cells. Biochem. Biophys. Res. Commun. 195 72–78 10.1006/bbrc.1993.2011 [DOI] [PubMed] [Google Scholar]
- Takahashi N., Smithies O. (1999). Gene targeting approaches to analyzing hypertension. J. Am. Soc. Nephrol. 10 1598–1605 [DOI] [PubMed] [Google Scholar]
- Takayanagi R., Snajdar R. M., Imada T., Tamura M., Pandey K. N., Misono K. S., et al. (1987). Purification and characterization of two types of atrial natriuretic factor receptors from bovine adrenal cortex: guanylate cyclase-linked and cyclase-free receptors. Biochem. Biophys. Res. Commun. 144 244–250 10.1016/S0006-291X(87)80502-8 [DOI] [PubMed] [Google Scholar]
- Tamura N., Ogawa Y., Yasoda A., Itoh H., Saito Y., Nakao K. (1996). Two cardiac natriuretic peptide genes (atrial natriuretic peptide and brain natriuretic peptide) are organized in tandem in the mouse and human genomes. J. Mol. Cell Cardiol. 28 1811–1815 10.1006/jmcc.1996.0170 [DOI] [PubMed] [Google Scholar]
- Teitelbaum I., Strasheim A., Berl T. (1990). Epidermal growth factor-stimulated phosphonositide hydrolysis in cultured rat inner medullary collecting tubule cells. Regulation by G protein, calcium, and protein kinase C. J. Clin. Invest. 85 1044–1050 10.1172/JCI114534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P., Meizel S. (1989). Phosphatidylinositol 4,5-bisphosphate hydrolysis in human sperm stimulated with follicular fluid or progesterone is dependent upon Ca2+ influx. Biochem. J. 264 539–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trindade P., Hutchinson H. G., Pollman M. J., Gibbons G. H., Pratt R. E. (1995). Atrial natriuretic peptide (ANP) and C-type natriuretic peptide (CNP) induce apoptosis in vascular smooth muscle cells (VMSC). Circulation 92 1–6967788902 [Google Scholar]
- Tripathi S., Pandey K. N. (2012). Guanylyl cyclase/natriuretic peptide receptor-A signaling antagonizes the vascular endothelial growth factor-stimulated MAPKs and downstream effectors AP-1 and CREB in mouse mesangial cells. Mol. Cell. Biochem. 368 47–59 10.1007/s11010-012-1341-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turla M. B., Park S. M., Webb R. C. (1990). Vascular responsiveness to phorbol esters in coarctation-hypertensive rats. J. Hypertens. 8 191–196 10.1097/00004872-199002000-00015 [DOI] [PubMed] [Google Scholar]
- Turovsky E. A., Turovskaya M. V., Dolgacheva L. P., Zinchenko V. P., Dynnik V. V. (2013). Acetylcholine promotes Ca2+ and NO-oscillations in adipocytes implicating Ca2+– > NO– > cGMP– > cADP-ribose– > Ca2+ positive feedback loop – modulatory effects of norepinephrine and atrial natriuretic peptide. PLoS ONE 8:e63483 10.1371/journal.pone.0063483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Akker F. (2001). Structural insights into the ligand binding domains of membrane bound guanylyl cyclases and natriuretic peptide receptors. J. Mol. Biol. 311 923–937 10.1006/jmbi.2001.4922 [DOI] [PubMed] [Google Scholar]
- van den Akker F., Zhang X., Miyagi M., Huo X., Misono K. S., Yee V. C. (2000). Structure of the dimerized hormone-binding domain of a guanylyl-cyclase-coupled receptor. Nature 406 101–104 10.1038/35017602 [DOI] [PubMed] [Google Scholar]
- Vandlen R. L., Arcuri K. E., Napier M. A. (1985). Identification of a receptor for atrial natriuretic factor in rabbit aorta membranes by affinity cross-linking. J. Biol. Chem. 260 10889–10892 [PubMed] [Google Scholar]
- Vellaichamy E., Das S., Subramanian U., Maeda N., Pandey K. N. (2014). Genetically altered mutant mouse models of guanylyl cyclase/natriuretic peptide receptor-A exhibit the cardiac expression of proinflammatory mediators in a gene-dose-dependent manner. Endocrinology 155 1045–1056 10.1210/en.2013-1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellaichamy E., Khurana M. L., Fink J., Pandey K. N. (2005). Involvement of the NF-kappa B/matrix metalloproteinase pathway in cardiac fibrosis of mice lacking guanylyl cyclase/natriuretic peptide receptor A. J. Biol. Chem. 280 19230–19242 10.1074/jbc.M411373200 [DOI] [PubMed] [Google Scholar]
- Vellaichamy E., Zhao D., Somanna N., Pandey K. N. (2007). Genetic disruption of guanylyl cyclase/natriuretic peptide receptor-A upregulates ACE and AT1 receptor gene expression and signaling: role in cardiac hypertrophy. Physiol. Genomics 31 193–202 10.1152/physiolgenomics.00079.2007 [DOI] [PubMed] [Google Scholar]
- von Geldern T. W., Budzik G. P., Dillon T. P., Holleman W. H., Holst M. A., Ksio Y., et al. (1990). Atrial natriuretic peptide antagonists biological evaluation and structural correlations. Mol. Pharmacol. 38 771–778 [PubMed] [Google Scholar]
- Waldman S. A., Rapoport R. M., Murad F. (1984). Atrial natriuretic factor selectively activates particulate guanylate cyclase and elevates cyclic GMP in rat tissues. J. Biol. Chem. 259 14332–14334 [PubMed] [Google Scholar]
- White R. E., Lee A. B., Shcherbatko A. D., Lincoln T. M., Schonbrunn A., Armstrong D. L. (1993). Potassium channel stimulation by natriuretic peptides through cGMP-dependent dephosphorylation. Nature 361 263–266 10.1038/361263a0 [DOI] [PubMed] [Google Scholar]
- Wilson E. M., Chinkers M. (1995). Identification of sequences mediating guanylyl cyclase dimerization. Biochemistry 34 4696–4701 10.1021/bi00014a025 [DOI] [PubMed] [Google Scholar]
- Wong S. K., Ma C. P., Foster D. C., Chen A. Y., Garbers D. L. (1995). The guanylyl cyclase-A receptor transduces an atrial natriuretic peptide/ATP activation signal in the absence of other proteins. J. Biol. Chem. 270 30818–30822 10.1074/jbc.270.51.30818 [DOI] [PubMed] [Google Scholar]
- Wormald M. R., Dwek R. A. (1999). Glycoproteins glycan presentation and protein-fold stability. Structure 7 R155–R160 10.1016/S0969-2126(99)80095-1 [DOI] [PubMed] [Google Scholar]
- Wu C. F., Bishopric N. H., Pratt R. E. (1997). Atrial natriuretic peptide induces apoptosis in neonatal rat cardiac myocytes. J. Biol. Chem. 272 14860–14866 10.1074/jbc.272.23.14860 [DOI] [PubMed] [Google Scholar]
- Yoshihara F., Tokudome T., Kishimoto I., Otani K., Kuwabara A., Horio T., et al. (2014). Aggravated renal tubular damage and interstitial fibrosis in mice lacking guanylyl cyclase-A (GC-A), a receptor for atrial and B-type natriuretic peptides. Clin. Exp. Nephrol. 10.1007/s10157-014-0982-1 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Yasue H., Morita E., Sakaino N., Jougasaki M., Kurose M., et al. (1991). Hemodynamic, renal, and hormonal responses to brain natriuretic peptide infusion in patients with congestive heart failure. Circulation 84 1581–1588 10.1161/01.CIR.84.4.1581 [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Yasue H., Okumura K., Ogawa H., Jougasaki M., Mukoyama M., et al. (1993). Different secretion patterns of atrial natriuretic peptide and brain natriuretic peptide in patients with congestive heart failure. Circulation 87 464–469 10.1161/01.CIR.87.2.464 [DOI] [PubMed] [Google Scholar]
- Zhao D., Das S., Pandey K. N. (2013). Interactive roles of NPR1 gene-dosage and salt diets on cardiac angiotensin II, aldosterone and pro-inflammatory cytokines levels in mutant mice. J. Hypertens. 31 134–144 10.1097/HJH.0b013e32835ac15f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D., Vellaichamy E., Somanna N. K., Pandey K. N. (2007). Guanylyl cyclase/natriuretic peptide receptor-A gene disruption causes increased adrenal angiotensin II and aldosterone levels. Am. J. Physiol. 293 F121–F127 10.1152/ajprenal.00478.2006 [DOI] [PubMed] [Google Scholar]
- Zhao L., Long L., Morrell N. W. (1999). NPRA-deficient mice show increased susceptibility to hypoxia-induced pulmonary hypertension. Circulation 99 605–607 10.1161/01.CIR.99.5.605 [DOI] [PubMed] [Google Scholar]
- Zhou H., Murthy K. S. (2003). Identification of the G-protein activation sequence of the single-transmembrane natriuretic peptide receptor C (NPR-C). Am. J. Cell. Physiol. 284 C1255–C1261 10.1152/ajpcell.00520.2002 [DOI] [PubMed] [Google Scholar]
- Zolle O., Lawrie A. M., Simpson A. W. (2000). Activation of the particulate and not the soluble guanylate cyclase leads to the inhibition of Ca2+ extrusion through localized elevation of cGMP. J. Biol. Chem. 275 25892–25899 10.1074/jbc.M000786200 [DOI] [PubMed] [Google Scholar]