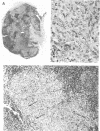
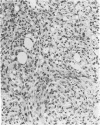
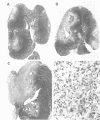
Abstract
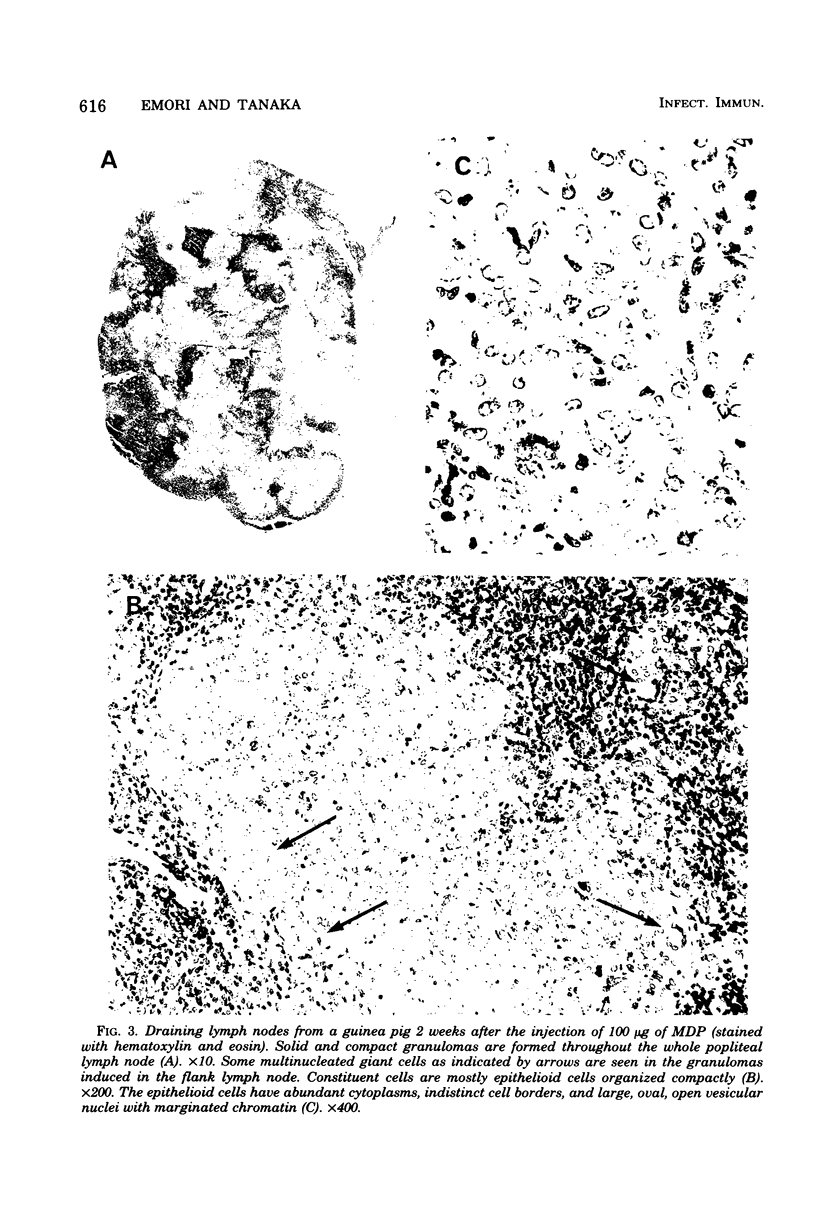
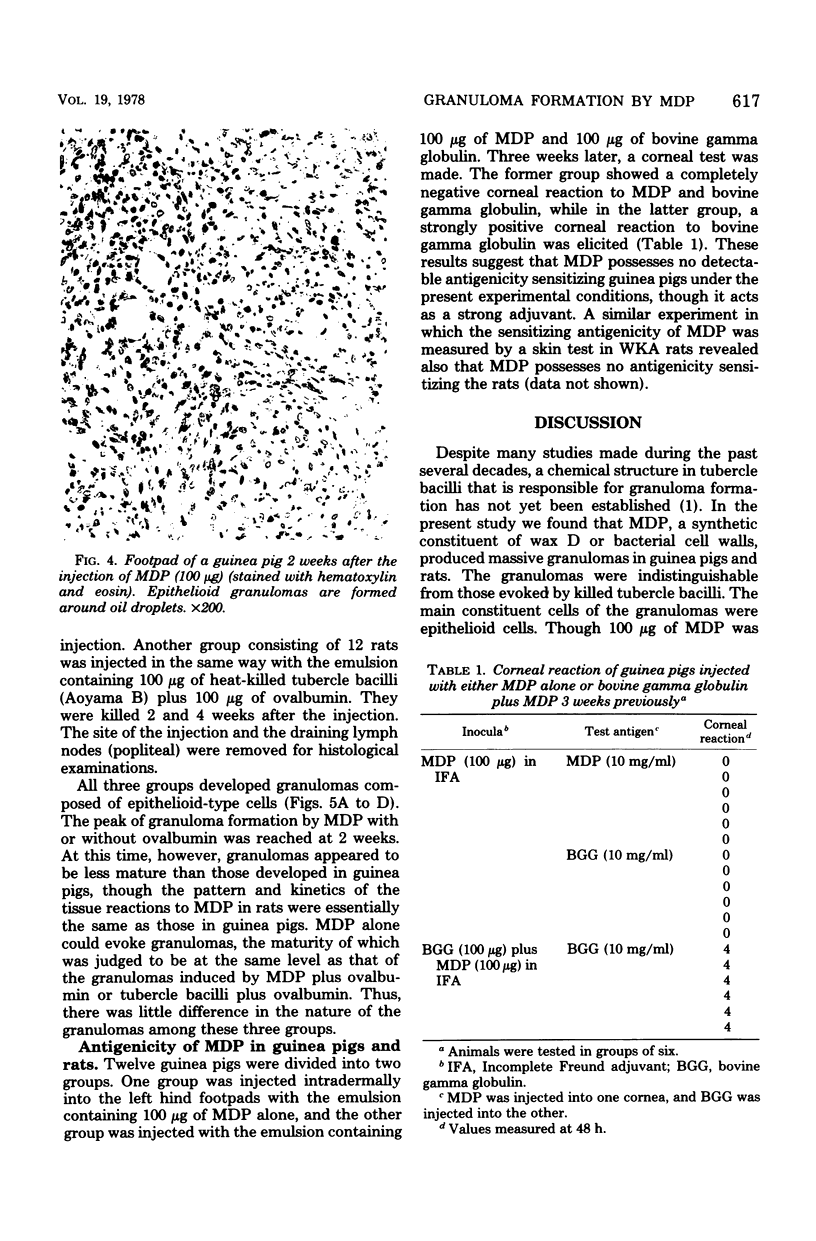
A synthetic muramyl dipeptide, N-acetylmuramyl-L-alanyl-D-isoglutamine, which possesses the same structure as that of a part of the peptidoglycan monomer of wax D of tubercle bacilli or bacterial cell walls was found to induce, when injected in water-in-oil emulsion, massive granulomas often accompanying abscesses in the site of injection and draining lymph nodes of guinea pigs and rats. The granulomas were composed mainly of epithelioid cells 2 weeks after injection and were indistinguishable from those induced by tubercle bacilli. The granulomas induced in rats were less mature than those induced in guinea pigs. Allergic reaction appeared to play no important role in the development of the muarmyl dipeptide-induced granuloma.
Full text
PDF

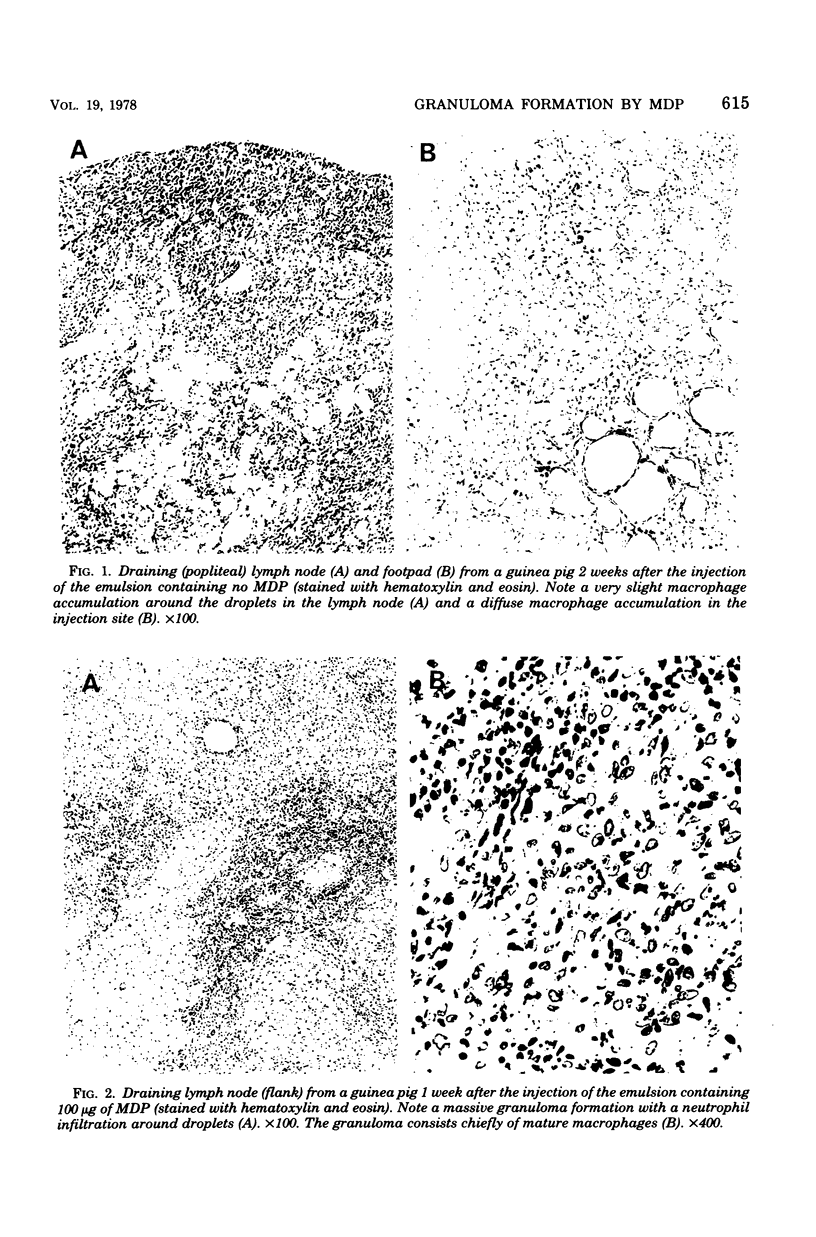



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O. The granulomatous inflammatory response. A review. Am J Pathol. 1976 Jul;84(1):164–192. [PMC free article] [PubMed] [Google Scholar]
- Azuma I., Ribi E. E., Meyer T. J., Zbar B. Biologically active components from mycobacterial cell walls. I. Isolation and composition of cell wall skeleton and component P3. J Natl Cancer Inst. 1974 Jan;52(1):95–101. doi: 10.1093/jnci/52.1.95. [DOI] [PubMed] [Google Scholar]
- Bekierkunst A., Levij I. S., Yarkoni E., Vilkas E., Adam A., Lederer E. Granuloma formation induced in mice by chemically defined mycobacterial fractions. J Bacteriol. 1969 Oct;100(1):95–102. doi: 10.1128/jb.100.1.95-102.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R. L., Jamison D. G., Vollum R. L. Histological changes evoked in mice by Freund's incomplete adjuvant. J Pathol Bacteriol. 1968 Apr;95(2):471–476. doi: 10.1002/path.1700950218. [DOI] [PubMed] [Google Scholar]
- Chedid L., Audibert F., Lefrancier P., Choay J., Lederer E. Modulation of the immune response by a synthetic adjuvant and analogs. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2472–2475. doi: 10.1073/pnas.73.7.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellouz F., Adam A., Ciorbaru R., Lederer E. Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun. 1974 Aug 19;59(4):1317–1325. doi: 10.1016/0006-291x(74)90458-6. [DOI] [PubMed] [Google Scholar]
- Epstein W. L. Granulomatous hypersensitivity. Prog Allergy. 1967;11:36–88. [PubMed] [Google Scholar]
- KOTANI S., HASHIMOTO S., MATSUBARA T., KATO K., HARADA K., KOGAMI J. LYSIS OF ISOLATED BCG CELL WALLS WITH ENZYMES. 2. DEMONSTRATION OF 'BOUND WAX D' AS A COMPONENT OF BCG CELL WALLS. Biken J. 1963 Oct;6:181–196. [PubMed] [Google Scholar]
- Kotani S., Watanabe Y., Kinoshita F., Shimono T., Morisaki I. Immunoadjuvant activities of synthetic N-acetyl-muramyl-peptides or -amino acids. Biken J. 1975 Jun;18(2):105–111. [PubMed] [Google Scholar]
- Lederer E. Cord factor and related trehalose esters. Chem Phys Lipids. 1976 Mar;16(2):91–106. doi: 10.1016/0009-3084(76)90001-3. [DOI] [PubMed] [Google Scholar]
- Meyer T. J., Ribi E. E., Azuma I., Zbar B. Biologically active components from mycobacterial cell walls. II. Suppression and regression of strain-2 guinea pig hepatoma. J Natl Cancer Inst. 1974 Jan;52(1):103–111. doi: 10.1093/jnci/52.1.103. [DOI] [PubMed] [Google Scholar]
- Meyer T. J., Ribi E., Azuma I. Biologically active components from mycobacterial cell walls. V. Granuloma formation in mouse lungs and guinea pig skin. Cell Immunol. 1975 Mar;16(1):11–24. doi: 10.1016/0008-8749(75)90181-1. [DOI] [PubMed] [Google Scholar]
- Moore V. L., Myrvik Q. N., Kato M. Role of cord factor (trehalose-6,6'-dimycolate) in allergic granuloma formation in rabbits. Infect Immun. 1972 Jul;6(1):5–8. doi: 10.1128/iai.6.1.5-8.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore V. L., Myrvik Q. N. Mycobacterial components responsible for the induction of a chronic immunological inflammatory response in rabbit lungs. Infect Immun. 1974 Jul;10(1):21–24. doi: 10.1128/iai.10.1.21-24.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiardo Z., Shamsuddin A. K. Granulomagenic activity of serologically active glycolipids from Mycobacterium bovis BCG. Infect Immun. 1976 Dec;14(6):1369–1374. doi: 10.1128/iai.14.6.1369-1374.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain S. M., Toubiana R., Ribi E., Parker R. Separation of the mixture of trehalose 6,6'-dimycolates comprising the mycobacterial glycolipid fraction, "P3". Biochem Biophys Res Commun. 1977 Jul 25;77(2):449–456. doi: 10.1016/s0006-291x(77)80001-6. [DOI] [PubMed] [Google Scholar]
- TANAKA A., KITAGAWA M. FRACTIONATION AND CHARACTERIZATION OF WAX D, A MACROMOLECULAR PEPTIDOGLYCOLIPID OF MYCOBACTERIUM TUBERCULOSIS. I. BIOCHEMICAL INVESTIGATIONS OF WAX D OF HUMAN STRAIN H37RA. Biochim Biophys Acta. 1965 Feb 1;98:182–193. [PubMed] [Google Scholar]
- Tanaka A., Ishibashi T., Sugiyama K., Takamoto M. Immunological adjuvants. VI. An acetylated mycobacterial adjuvant lacking competing antigenicity. Z Immunitatsforsch Exp Klin Immunol. 1971 Nov;142(4):303–317. [PubMed] [Google Scholar]
- Tanaka A., Nagao S., Saito R., Kotani S., Kusumoto S., Shiba T. Correlation of stereochemically specific structure in muramyl dipeptide between macrophage activation and adjuvant activity. Biochem Biophys Res Commun. 1977 Jul 25;77(2):621–627. doi: 10.1016/s0006-291x(77)80024-7. [DOI] [PubMed] [Google Scholar]
- Tanaka A., Saito R., Sugiyama K., Morisaki I., Kotani S. Adjuvant activity of synthetic N-acetylmuramyl peptides in rats. Infect Immun. 1977 Jan;15(1):332–334. doi: 10.1128/iai.15.1.332-334.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNGAR J., MUGGLETON P. W. The production of experimental granulomatous lesions induced by injections of fatty acids and fractions of tubercle bacilli. Am Rev Respir Dis. 1961 Nov;84(5):76–80. doi: 10.1164/arrd.1961.84.5P2.76a. [DOI] [PubMed] [Google Scholar]
- WHITE R. G., BERNSTOCK L., JOHNS R. G., LEDERER E. The influence of components of M. tuberculosis and other Mycobacteria upon antibody production to ovalbumin. Immunology. 1958 Jan;1(1):54–66. [PMC free article] [PubMed] [Google Scholar]
- Yarkoni E., Bekierkunst A., Asselineau J., Toubiana R., Toubiana M. J., Lederer E. Supression of growth of Ehrlich ascites tumor cells in mice pretreated with synthetic analogs of trehalose-6,6-dimycolate (cord factor). J Natl Cancer Inst. 1973 Aug;51(2):717–720. [PubMed] [Google Scholar]
- Yasuhira K. [II. Mesenchymal reactions to chemical components of tubercle bacilli]. Kekkaku. 1969 Sep;44(9):273–287. [PubMed] [Google Scholar]