Abstract
Asingle cells in undifferentiated spermatogonia are considered to be the most primitive forms of germ stem cells (GSCs). Although GFRα1 is thought to be a marker of Asingle cells, we found that Bmi1High is more specific than GFRα1 for Asingle cells. Bmi1High expression in Asingle cells is correlated with seminiferous stages, and its expression was followed by the proliferative stage of Asingle GSCs. In contrast, GFRα1 expression was seminiferous stage-independent. Fate analyses of EdU-positive Bmi1High-positive cell-derived Asingle cells revealed that these cells self-renewed or generated transient amplifying Apaired cells. Bmi1High-positive cells were resistant to irradiation-induced injury, after which they regenerated. Elimination of Bmi1High-positive cells from seminiferous tubules resulted in the appearance of tubules with seminiferous stage mismatches. Thus, in this study, we found that Bmi1High is a seminiferous stage-dependent marker for long-term GSCs and that Bmi1High-positive cells play important roles in maintaining GSCs and in regenerating spermatogenic progenitors after injury.
Germ stem cells (GSCs) in the testes generate male germ cells throughout life. In the seminiferous tubules, GSCs differentiate into undifferentiated spermatogonia, differentiated spermatogonia, spermatocytes, spermatids, and finally spermatozoa. In mice, the developmental stages of spermatogenesis in the seminiferous tubules are numbered from I–XII (stage I: 22.2; II/III: 26.8; IV: 18.6; V: 11.3; VI: 18.1; VII: 20.6; VIII: 20.8; IX: 15.2; X: 11.3; XI: 21.4; XII: 20.8 hours)1. A single cycle of seminiferous epithelium (from stages I to XII) has been estimated to be approximately 8.6 days, while the entire process of spermatogenesis from undifferentiated spermatogonia to mature spermatozoa is completed in approximately 40 days2,3. This tightly regulated cycle is thought to be essential for continuous production of spermatozoa throughout the reproductive period.
Undifferentiated spermatogonia are the most primitive cell population in the testes. This population proliferates during stages X–II of spermatogenesis2. Morphologically, the population is classified as Asingle (solitary single cells), Apaired (pairs of 2 cells), and Aaligned (chains of 4, 8, 16, or 32 cells) cells4. Asingle-type cells are observed during all seminiferous stages, but it is unknown whether Asingle cells in each stage have different functions and marker expressions and whether these differences are correlated with seminiferous stages.
Direct lineage tracing of glial cell-derived neurotrophic factor (GDNF) family receptor alpha-1 (GFRα1)-expressing cells, which are thought to represent primitive cell populations such as Asingle and Apaired cells5,6, has been recently reported. It was shown that GFRα1-positive cells form a single stem-cell pool and that GFRα1-positive syncytial spermatogonia can continuously revert to Asingle cells by fragmentation7. However, in that study, the relationship between GFRα1-positive Asingle cell dynamics and seminiferous stage was not examined.
B cell-specific Moloney murine leukemia virus integration site 1 (Bmi1) is a specific marker of neural, hematopoietic, intestinal, and prostate stem cells8,9,10,11,12. Bmi1 is a polycomb-group gene whose product is a component of the polycomb repressive complex 1 (PRC1) and is thought to maintain the self-renewal capacity of stem cells9,13,14. The Bmi1 protein is expressed in undifferentiated spermatogonia15 and spermatocytes16. However, lineage tracing of Bmi1-positive spermatogonia has not been performed, and the results of immunohistochemistry studies using the anti-Bmi1 antibody were not sufficient to determine whether Bmi1 is a marker of undifferentiated spermatogonia and spermatocytes. The present study was conducted to precisely clarify the contribution of Bmi1 to spermatogenesis using Bmi1creER/+/Rosa26Rbw/+ mice in which multicolor (red, orange, or blue) labeling was induced only in Bmi1-positive cells through Cre-mediated recombination.
Results
Multicolor tracing study of Bmi1High-positive cells in seminiferous tubules
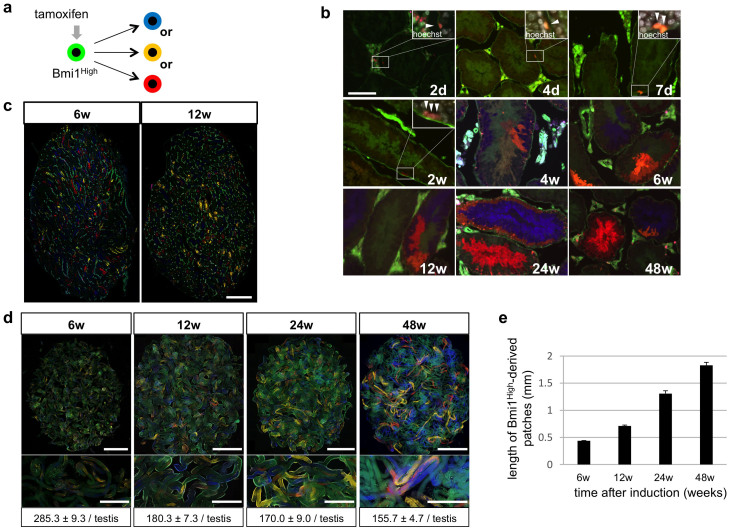
Genetic lineage tracing based on the Cre/loxP system is a powerful method for confirming that a gene is a specific marker for stem cells17. Moreover, using a multicolor reporter method, both the fate and clonality of color-labeled stem cells can be examined simultaneously18,19,20,21. In Bmi1creER/+/Rosa26LacZ/+ mice, administration of tamoxifen induces Cre recombination only in Bmi1-positive cells. Sangiorgi et al. observed that only long-term intestinal stem cells located at “position +4” from the base of crypts expressing high levels of Bmi1 were predominantly labeled by LacZ. However, rapidly dividing and migrating progenitor cells located near the stem cells expressing low or no levels of Bmi1 were not labeled11. Based on these findings, in Bmi1creER/+/Rosa26Rbw/+ mice, only cells expressing high levels of Bmi1 (Bmi1High-positive cells) predominantly induced a random color change from green to 1 of 3 different colors (blue, orange, or red) (Figure 1a), and no change was induced in cells that were negative or weakly positive for Bmi1. Thus, using the Cre/loxP system in Bmi1creER/+/Rosa26Rbw/+ mice, the cell lineage of color-labeled Bmi1High-positive cells can be traced. Two days after tamoxifen administration, color-labeled Asingle cells were observed in the basal cell layer of seminiferous tubules, i.e., in GSC-specific sites, while color-labeled Apaired, Aalined, and more differentiated cells were not observed (Figure 1b). Promyelocytic leukemia zinc finger (PLZF) is a known marker of undifferentiated spermatogonia22,23. Bmi1High-derived (derived from Bmi1High-positive cells) and PLZF-double positive Apaired cells (red arrowheads; Figure S1) were detected 7 days after tamoxifen labeling, indicating that the immediate descendant cells produced from Bmi1High-positive Asingle cells showed characteristics of undifferentiated spermatogonia. Labeled cells began to proliferate, and then single-color cell clusters composed of differentiated cells were observed during the 4th week (Figure 1b). In the 6th week, which is longer than the duration of mouse spermatogenesis (40 days)3, labeled spermatozoa were detected as single-color clusters in the center of tubules. These clusters were maintained for at least 48 weeks after tamoxifen induction (Figure 1b). A single Asingle cell reportedly generates 2048 or 4096 spermatozoa3. In the present study, we found that most single-color clusters during the 6th week after tamoxifen administration contained approximately 2000–4000 spermatozoa. Cell clusters did not display more than 1 color, indicating that solitary GSCs had supplied the spermatozoa to these single-color areas.
Figure 1. Detection of Bmi1High-positive cells in seminiferous tubules and their lineage tracing.
(a) Cre-mediated fluorescent color change in Bmi1creER/+/Rosa26Rbw/+ mice. Fluorescent colors of Bmi1High-positive cells change from green to one of the three colors (red-, orange- or blue-color) by Cre-mediated excision of floxed cassettes induced by tamoxifen and their descendant cells retain the changed color. (b) Existence of Bmi1High-derived cells in the area of GSCs in the inner periphery of seminiferous tubules and their expansion. Bmi1creER/+/Rosa26Rbw/+ mice were injected with tamoxifen and Bmi1High-positive stem cell-derived cell clusters were analyzed at indicated time points. White windows at the right upper corner; magnified pictures of indicated rectangle areas. Merged images with Hoechst 33342 counter staining (white) are shown. White arrowheads indicate Bmi1High-derived cells. (c) Sections of the testes from Bmi1creER/+/Rosa26Rbw/+ mice at indicated time points after tamoxifen administration. Multiple single color areas derived from Bmi1High-positive cells are observed. (d) Numbers of patches observed at indicated time points in the testes from Bmi1creER/+/Rosa26Rbw/+ mice injected with tamoxifen. Upper panels; fluorescent images of untangled seminiferous tubules from one testis. Middle panels; magnified pictures of seminiferous tubules. Lower panels; number of patches at indicated time points after tamoxifen induction (n = 3). (e) Length of patches observed at indicated time points in the testes from Bmi1creER/+/Rosa26Rbw/+ mice injected with tamoxifen. Scale bars = 100 μm in (b), 1 mm in (c), 5 mm in (upper panel of d), 2 mm in (middle panel of d).
The number of single-color patches in the testes mildly decreased over time, and the patch length gradually increased (Figures 1c–e). Similar results were observed in the lineage tracing assay of Ngn3 or GFRα1-positive GSC7,24. Two models of stem cell division have been proposed; invariant asymmetric division and populational asymmetric division25. In the invariant asymmetric model, patch number and length should be kept constant, whereas patch number can decrease and patch length can extend over time in the populational asymmetric model. Based on the results of our study as well those of previous reports, GSCs are thought to be maintained by the “populational asymmetry” mechanism, although there is a possibility that some of the Bmi1High-positive cells are short-term stem cells.
Multicolor lineage tracing revealed that several labeled single-color patches were adjacent to each other (Figure 1d). Using mice with a single-color reporter may have led to multiple patches being mistakenly recognized as a single patch. This would lead to underestimation of the number of GSCs per testis (~2000), which is calculated from the number of patches in single-color reporter mice23. Based on our multicolor analyses, we estimate that approximately 4800 long-term GSCs were present in one testis (see “Estimate of stem cell number” in Methods).
To further demonstrate that Bmi1High-positive cells are long-term GSCs, we carried out the following experiments: C57BL/6 (B6) mice (female) were crossed with Bmi1creER/+/Rosa26Rbw/+ mice (male) that had been previously administered tamoxifen for 8–10 weeks. Eight weeks is longer than the duration of mouse spermatogenesis (40 days), and therefore fluorescent color-labeled spermatozoa were Bmi1High-positive GSCs-derived in the male mice. We found 5 blue or orange color-stained pups for a total of 24 pups from 4 deliveries (Figure S2). This result clearly indicated that Bmi1High-positive cells were long-term GSCs that could produce spermatozoa with normal reproductive potential.
A recent study indicated that a cytotoxic agent such as 5-fluorouracil (5FU) can de-differentiate fully differentiated cells (chief cells) into gastric stem cells26. Thus, tamoxifen may damage germ cells and revert differentiated germ cells into GSCs; in our study, we injected mice with a single dose of tamoxifen (9 mg/40 g body weight) to induce Cre recombination. To exclude the possibility that damage to germ cells had occurred during our analysis, we examined whether tamoxifen damaged the cells at the dose used in this study. B6 mice were injected with tamoxifen and their testes were removed from the mice 12 or 24 hours later. Cross-sections of seminiferous tubules from the mice were subjected to TUNEL staining to detect apoptotic cells. As shown in Figure S3, there was no significant difference in the number of apoptotic cells between control mice and tamoxifen-treated mice. Activation of Cre recombinase by a single injection of tamoxifen is a commonly used technique for lineage-tracing experiments, and toxicity to germ cells using this method has not been reported24,27. Based on our results, Bmi1High-positive GSCs were not induced by tamoxifen toxicity.
To further confirm this result, we next examined whether Bmi1High-positive germ cells in mice without tamoxifen treatment expressed GSC markers. We have isolated Bmi1High-positive and Bmi1Low-positive cells by fluorescence-activated cell sorting (FACS) from male germ cells in Bmi1-GFP knock-in mice (Bmi1GFP/+ mice) and examined the expression of various GSC markers. It is well-known that c-Kit is expressed on differentiated spermatogonia, and it was recently reported that the melanoma cell adhesion molecule (MCAM)+/c-Kit− cell population mainly contains undifferentiated spermatogonia, whereas the MCAM+/c-Kit+ cell population mainly contains more differentiated spermatogonia28. Germ cells obtained from the testes of Bmi1GFP/+ mice were double-stained with MCAM and c-Kit, and three different populations (P2, 3, and 4) were detected in the P1 gated population (Figures S4a and b). GFPHigh-positive and GFPLow-positive cells were observed in the MCAM+/c-Kit− cell population (Gate P2) (Figure S4c). In contrast, the MCAM+/c-Kit+ cell population (Gate P3 and P3) contained only a few GFPHigh-positive cells (Figure S4d and data not shown). Based on these findings, GFPHigh-positive cells were contained mainly in the undifferentiated spermatogonia-enriched population (P2). GFPHigh-positive and GFPLow-positive cells were sorted from the P2+P3+P4 populations containing both undifferentiated and differentiated spermatogonia (Figure S4e). Expression of GFRα1, ID4, and Nanos2 (markers of undifferentiated spermatogonia), Ngn3 (a marker of transient amplifying cells), and c-Kit (a marker of differentiated spermatogonia) in the Bmi1High and Bmi1Low populations were examined (Figure S4f and Table S1). The results indicated that markers of undifferentiated spermatogonia were expressed more in Bmi1High-positive cells than in Bmi1Low-positive cells. In contrast, markers of more differentiated germ cells were expressed more in Bmi1Low-positive cells than in Bmi1High-positive cells. The results indicate that, under physiological conditions without tamoxifen, Bmi1High-positive cells express GSC markers.
Comparison of Bmi1High-derived cells and GFRα1-positive cells in undifferentiated spermatogonia
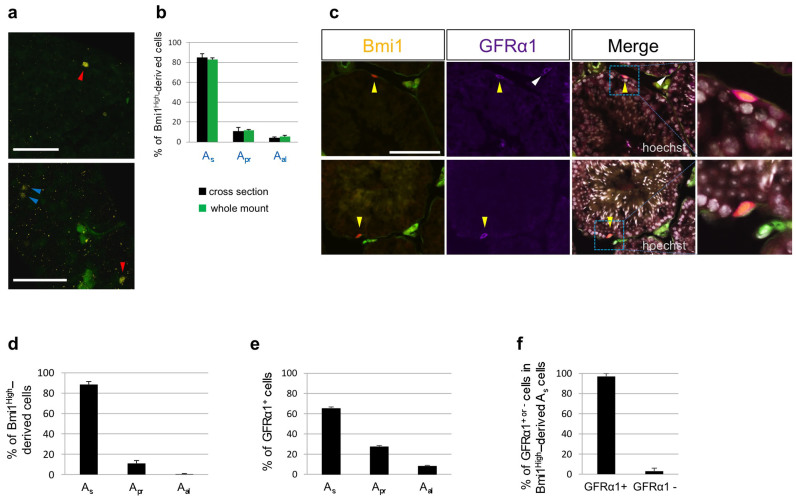
Because we found that Bmi1High was a marker of long-term GSCs, we next compared the expression of Bmi1High with that of GFRα1 on the three types of cells (Asingle, Apaired, or Aaligned cells)6. Generally, morphological classification of Asingle, Apaired, or Aaligned cells based on an interconnection with an intercellular bridge was performed using whole-mounts of seminiferous tubules. Figure 2a shows Bmi1High-derived cells (indicated by arrowheads) in whole-mounts of seminiferous tubules from tamoxifen-treated Bmi1creER/+/Rosa26Rbw/+ mice. Figure S5 shows representative serial cross-sections (5 µm) of clearly distinguished Bmi1High-derived Asingle and Apaired cells. No significant differences between the results (percentage of Bmi1High-derived cells in the three cell types) obtained using the whole-mount method and those obtained using serial cross-sections (span of scanning > 120 µm along the long axis) (Figure 2b). Detection of Bmi1-positive cells is possible even in normal mice, except for Bmi1creER/+/Rosa26Rbw/+ mice, if an anti-Bmi1 antibody with high binding affinity is available. Although we used commercially available anti-Bmi1 antibodies for immunostaining of seminiferous tubules of B6 mice, Bmi1-positive cells were not clearly detected. Accordingly, in the present study, we analyzed Bmi1High-derived cells using Bmi1creER/+/Rosa26Rbw/+ mice that had been administered tamoxifen 2 days before sacrifice. GFRα1 is a marker of Asingle and Apaired cells, and an anti-GFRα1 antibody with high binding affinity is currently commercially available. When serial cross-sections of seminiferous tubules obtained from tamoxifen-treated Bmi1creER/+/Rosa26Rbw/+ mice were stained with anti-GFRα1 antibody, GFRα1-positive and Bmi1High-derived Asingle cells were detected (yellow arrowheads; Figure 2c). GFRα1-positive, Bmi1-negative Asingle cells were also observed (white arrowhead; Figure 2c). Moreover, nearly 90% of Bmi1High-derived cells were Asingle cells (Figure 2d), whereas only 65% of GFRα1-positive cells were Asingle cells (Figure 2e). GFRα1 is expressed to a higher extent on Apaired or Aaligned cell populations than Bmi1High (Figure 2d and e). GFRα1 is thought to be a marker of Apaired or Aaligned cells in addition to Asingle cells, whereas Bmi1High is a more specific marker of Asingle cells than GFRα1. Additionally, nearly all Bmi1High-derived Asingle cells were GFRα1-positive (Figure 2f), indicating that both proteins were co-expressed on the Asingle cell population.
Figure 2. Relationship between Bmi1High-derived cells and GFRα1-positive cells analyzed by immunohistochemistry.
(a) The images of whole-mount seminiferous tubules from Bmi1creER/+/Rosa26Rbw/+ mice that received tamoxifen 2 days before. A red arrowhead; Bmi1High-derived Asingle cells, A blue arrowhead; Bmi1High-derived Apaired cells. Scale bars = 100 μm. (b) Frequency of Bmi1High-derived cells in Asingle, Apaired, and Aaligned cell populations obtained from Bmi1creER/+/Rosa26Rbw/+ mice that received tamoxifen 2 days before. Bmi1High-derived cells were detected by whole-mount method or serial cross-section method. (c) Anti-GFRα1 antibody-immunostaining of cross-sections of seminiferous tubules from Bmi1creER/+/Rosa26Rbw/+ mice received tamoxifen 2 days before. Yellow arrowheads: Bmi1High (orange)-derived and GFRα1 (purple)-positive cells. White arrowheads: Bmi1-negative and GFRα1 (purple)-positive cells. Right panels of merged images: magnified pictures of indicated rectangle areas. Merged images with Hoechst 33342 counter staining (white) are shown. Scale bar = 100 μm. (d) Frequency of Bmi1High-derived cells in Asingle, Apaired, and Aaligned cell populations (n = 271 from 4 testes). (e) Frequency of GFRα1-positive cells in Asingle, Apaired, and Aaligned cell populations (n = 533 from 3 testes). (f) Frequency of GFRα1-positive or negative cells in Bmi1High-derived Asingle cells (n = 73 from 3 testes).
Contribution of Bmi1High-positive cells to regeneration after irradiation-induced injury and appearance of abnormal seminiferous stages following deletion of Bmi1High-positive cells
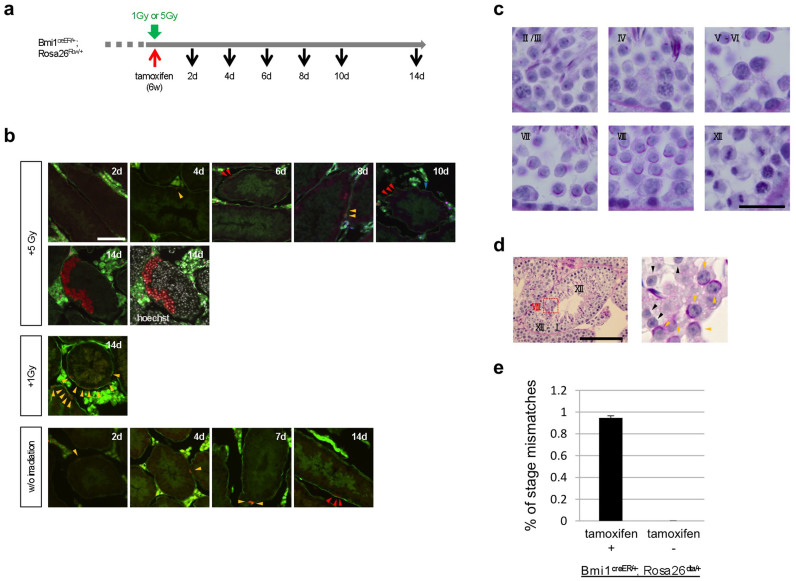
To examine the role of Bmi1High-positive cells in regeneration after radiation injury, Bmi1creER/+/Rosa26Rbw/+ mice irradiated at a dose of 1 or 5 Gy were injected with tamoxifen, and the fate of the progeny of Bmi1High-positive cells was followed (Figure 3a). In control mice that did not receive irradiation, Bmi1High-derived Asingle cells were detected on day 2 after tamoxifen administration (lower panel; Figure 3b), but Bmi1High-derived Asingle cells were not detected until day 4 in mice that had received 5 Gy irradiation (upper panel; Figure 3b). This delay in the appearance of color-labeled cells may have been caused by damage to cellular metabolism by the irradiation. In 5 Gy-irradiated mice, many color-labeled cells that were derived from Bmi1High-positive cells were observed on day 10 (6 days after the appearance of Bmi1High-positive Bmi1High-derived Asingle cells) (upper panel; Figure 3b), whereas only Bmi1High-derived Apaired cells were detected on day 7 in control mice. Thus, rapid expansion of Bmi1High-positive cells was evident in 5 Gy-irradiated mice; some progeny differentiated into spermatids on day 14 (only 10 days after the appearance of Bmi1High-derived Asingle cells) (upper panel; Figure 3b). In contrast, only spermatogonia were observed on day 14 in control mice (Figure 3b). At 1 Gy, color-labeled differentiated spermatogonia were detected on day 14 at a higher rate than in control mice (Figure 3b). These results indicate that irradiation of mouse testes at a low dose of 1 or 5 Gy did not significantly damage Bmi1High-positive cells, but increased their proliferation rate. These characteristics were similar to those of slow-growing long-term stem cells found in other tissues, including the intestine, bone marrow, and hair follicles20.
Figure 3. Accelerated spermatogenesis after irradiation-induced injury and abnormal spermatogenesis after deletion of Bmi1High-positive cells.
(a) Experimental protocol of irradiation and tamoxifen administration to Bmi1creER/+/Rosa26Rbw/+ mice. The mice were irradiated at 1 Gy or 5 Gy and then injected with tamoxifen, and labeled cells were analyzed at indicated time points. A red arrow; tamoxifen administration. A green arrow; irradiation. Black arrows; time points of analyses of the testes. (b) Accelerated proliferation of Bmi1High-positive stem cell-derived cells after irradiation. (upper and middle panels) Bmi1creER/+/Rosa26Rbw/+ mice were irradiated at 1 Gy or 5 Gy and were injected with tamoxifen, and labeled cells were analyzed up to the 14th day. (lower panel) Control mice without irradiation. Red, orange or blue arrowheads: red-, orange- or blue-color labeled cells derived from Bmi1High-positive stem cells. (c) Typical findings observed by PAS-hematoxylin staining in each seminiferous epithelial stage. Roman numbers indicate seminiferous epithelial stages. (d) PAS-hematoxylin staining. Seminiferous epithelium with stage-mismatched area in the testes of Bmi1creER/+/Rosa26loxp-stop-loxp-DTA/+ mice 4 weeks after tamoxifen administration. Magnified images of red squares in the left panel are shown in the right panels. In the area of stage VIII, PAS-positive acrosomes (yellow arrowheads) are observed. In the area of stage XII - I, no acrosome (black arrowheads) was observed but instead dividing spermatocytes were observed. Roman numbers indicate seminiferous epithelial stages. (e) Frequency of the seminiferous intratubular areas with stage-mismatches of tamoxifen-injected or untreated Bmi1creER/+/Rosa26loxp-stop-loxp-DTA/+ (Bmi1creER/+;Rosa26dta/+) mice. Scale bars = 100 μm in (b and d) and 20 μm in (c).
To eliminate Bmi1High-positive cells from the seminiferous tubules, Bmi1creER/+/Rosa26loxp-stop-loxp-DTA/+ mice were used. Administration of tamoxifen to mice resulted in conditional expression of diphtheria toxin A chain (DTA) in only Bmi1High-positive cells, and these cells were subsequently eliminated. The testes of Bmi1creER/+/Rosa26loxp-stop-loxp-DTA/+ mice were collected during the 4th week after tamoxifen-induced removal of Bmi1High-positive cells. Classification of seminiferous stages was performed using a standard method with periodic acid-Schiff (PAS)-hematoxylin staining of cross-sections (Figure 3c). We observed abnormal seminiferous tubules with a stage-mismatched area (Figure 3d and Table S2), although this was not frequently observed (Figure 3e). The seminiferous stage of stage-mismatched area was stage VIII, while that of the surrounding area was stage XII (Figure 3d). In the abnormal seminiferous tubules, a delay of 3–4 stages was observed in the stage-mismatched area compared with the stages in the surrounding area (Figure 3d and Table S2). Not all seminiferous tubules showed abnormalities, which can be explained as follows: Cre/loxP-mediated removal of Bmi1High-expressing cells only occurred above a certain threshold, and expression of Bmi1 in GSCs was not always sufficiently high to efficiently drive Cre/loxP-mediated DNA recombination. Such stage-mismatched tubules were never observed in the testes of Bmi1creER/+/Rosa26loxp-stop-loxp-DTA/+ mice that were not injected with tamoxifen (Figure 3e).
To further confirm the high regeneration ability of Bmi1High-positive cells following severe damage to germ cells, we administered busulfan, which is severely toxic to germ cells, to Bmi1creER/+/Rosa26Rbw/+ mice 2 days before tamoxifen administration. A significantly higher number of single-color patches was observed in the testes of busulfan-treated mice than in busulfan-nontreated mice (Figures S6 a and b), confirming the high regeneration ability of Bmi1High-positive GSCs following severe damage.
Seminiferous stage-specific increase of Bmi1High-positive Asingle cell population
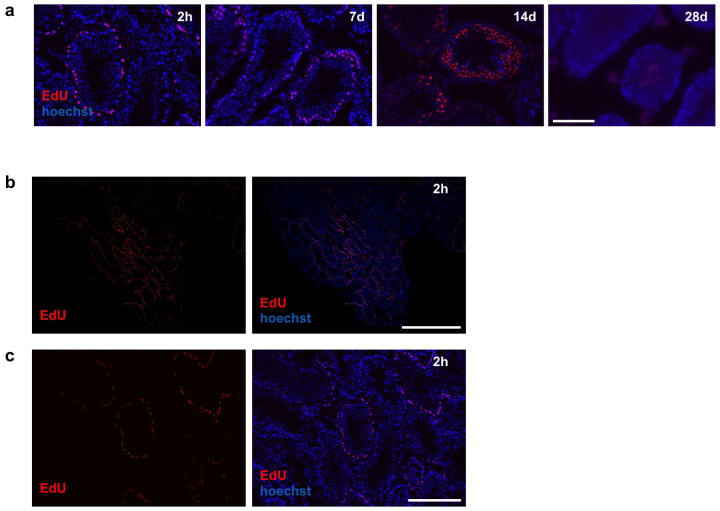
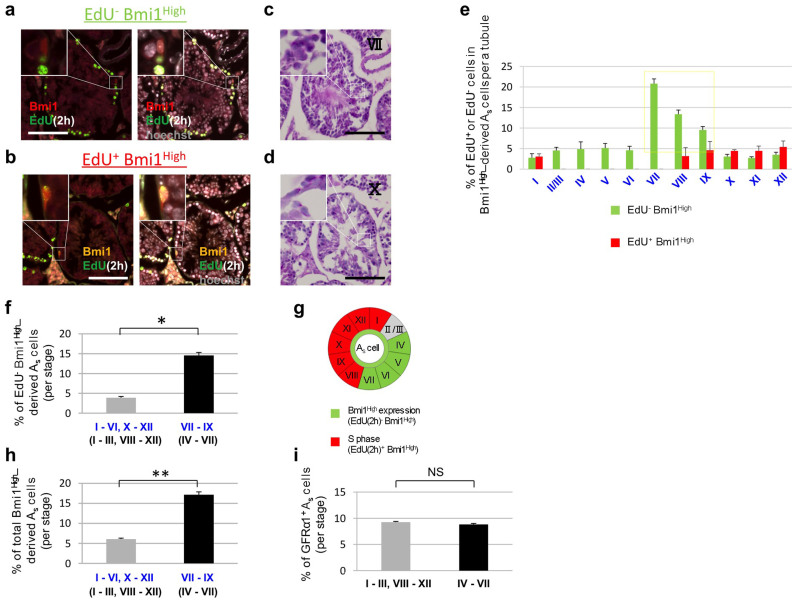
To further examine the physiological significance of Bmi1 in GSCs, we examined the relationship between cell cycle phases and seminiferous stages in Bmi1High-positive Asingle cells. First, we determined the optimal time at which undifferentiated spermatogonia in the basal cell layer of the seminiferous tubules were stained with 5-ethynyl-2-deoxyuridine (EdU), which labels cells in the S phase. Both undifferentiated and differentiated spermatogonia in the basal cell layer appeared to be labeled 2 hours after a single injection of EdU to B6 mice (Figure 4a). Not all tubules were equally labeled, as both EdU-labeled and non-labeled seminiferous tubules were observed (Figures 4b and c). This is reasonable because it is known that seminiferous stages are not synchronized among adjacent tubules; as a result, the stages of adjacent tubules differ from each other. EdU-labeled basal cells differentiated and migrated towards the center of the seminiferous tubules based on their differentiation (Figure 4a).
Figure 4. Distinct EdU labeling patterns observed in seminiferous tubules of B6 mice.
EdU was administrated to B6 mice and their testes were analyzed 2 hours later. (a) Time course of fate of EdU-labeled spermatogonia at indicated time points. Note that EdU-labeled cells (red) migrate toward the center of the seminiferous tubules. (b) Low magnified pictures of the testis (2 hours after EdU administration). (c) High magnified pictures of (b). Right panels show merged images of EdU (red) and Hoechst 33342 counter staining (blue). h: hours, d: days. Scale bars = 100 μm in (a), 1 mm in (b) and 200 μm in (c).
Bmi1creER/+/Rosa26Rbw/+ mice were injected with tamoxifen and then EdU was administered to the mice 2 days later. Two hours after EdU administration, the testes were removed and fixed. Fluorescent microscopic analyses of the serial cross-sections were conducted over a span of > 120 µm, and two adjacent cross-sections were selected. One of the 2 adjacent cross-sections was stained using an EdU-detecting reagent (Figure 5a and b) and the other was stained using a PAS-hematoxylin reagent in order to determine the seminiferous stages (Figure 5c and d). Typically, cells that stain positive for EdU are in the S phase. The percentage of EdU-negative Bmi1High-derived Asingle cells in the total Bmi1High-derived Asingle cell population was corrected by the percentage of duration of each stage, i.e., cells not in the S phase, was less than 6% in seminiferous stages I–VI and X–XII, but the percentage increased in stages VII–IX (Figure 5e and f). Bmi1High-derived cells were observed 2 days after tamoxifen administration as fluorescently labeled cells. Accordingly, Bmi1High-derived Asingle cells detected in stages VII–IX were generated from Bmi1High-positive cells in stages IV–VII when tamoxifen was administered. Therefore, a 2-day delay should be factored in when determining stages. Figure S7 shows the relationship between actual stages in which unlabeled original Bmi1High-positive cells exist and the 2-day-delayed stages in which Bmi1High-derived cells were observed 2 days after tamoxifen induction. Thus, an increased percentage of EdU-negative Bmi1High-derived Asingle cells was observed in the actual stages IV–VII, which was 2 days before stages VII–IX. EdU-positive (proliferating) Bmi1High-derived Asingle cells appeared in stages VIII–I (Figure 5e and summarized in 5g). EdU-negative Bmi1High-derived cells (green bars) and EdU-positive Bmi1High-derived cells (red bars) in all stages (from Stage I–XII) summed to 100%. Figure 5h shows the percentage of Bmi1High-derived cells in the Asingle cell population (sum of the percentage of EdU-negative Bmi1High-derived cells and that of EdU-positive Bmi1High-derived cells); this value was significantly higher in stages VII–IX (actual stages: IV–VII) than in stages I–VI and X–XII (actual stages: I–III and VIII–XII, respectively). Thus, the fraction of Bmi1High-positive cells increased in actual stages IV–VII, before the cells entered into the S phase. Based on these results, Bmi1High expression in Asingle cells may depend on seminiferous stage (Figure 5g).
Figure 5. Relationships between proliferative states and seminiferous stages in Bmi1High-derived Asingle cells.
(a-d) Fluorescent microscopic analyses and PAS-hematoxylin staining of cross-sections of seminiferous tubes obtained from Bmi1creER/+/Rosa26Rbw/+ mice. Tamoxifen was injected into Bmi1creER/+/Rosa26Rbw/+ mice. EdU was administrated to the mice 2 days later. Their testes were removed 2 hours later and serial cross-sections were prepared. (a and b) Detection of Bmi1High-derived cells and EdU-positive proliferating cells. White windows at the left upper corner; magnified pictures of indicated rectangle areas. Right panels; Merged images with Hoechst 33342 counter staining (white) are shown. PAS-hematoxylin staining of serial sections (c and d were adjacent sections of a and b, respectively). White windows at the left upper corner; magnified pictures of indicated rectangle areas. (e) Relationship among Bmi1High expression, proliferation of Asingle cells, and the seminiferous epithelial stages. Green bars; EdU-negative Bmi1High-derived Asingle cells (including resting cells), red bars; EdU-positive Bmi1High-derived Asingle cells (proliferating cells). Please note that these Bmi1High-derived cells in our system are observed 2 days after tamoxifen induction, therefore 2 days delay should be taken into consideration when judging stages. Therefore, actual stages when these EdU-negative Bmi1High-derived Asingle cells increase should be in stage IV-VII (see Text). The percentages of EdU-positive (red bar) or -negative (green bar) Bmi1High-derived Asingle cells per a seminiferous tubule in total Bmi1High-derived Asingle cells are shown. The increase of EdU-negative Bmi1High-derived Asingle cells (indicated by a yellow square) precedes the increase of EdU-positive Bmi1High-derived Asingle cells. (f) EdU-negative Bmi1High-derived Asingle cells significantly increase in stage VII-IX (actual stages: IV-VII). *P < 0.0001 by unpaired Student's t-test. (g) Schematic presentation of the result of (e) and (f). (h) Total Bmi1High-derived Asingle cells (EdU-negative and -positive) significantly increase in stage VII-IX (actual stages: IV-VII). **P < 0.001 by unpaired Student's t-test. (i) GFRα1-positive Asingle cells do not increase in stage IV-VII. NS: not significant by unpaired Student's t-test. As: Asingle Delayed stages are indicated by blue Roman numbers (e, f and h) and actual stages are indicated by black Roman numbers (c, d and f-i). Scale bars = 100 μm.
The relationship between GFRα1 expression and seminiferous stage in the Asingle cell population was also examined using serial cross-sections obtained from B6 mice. Sections were stained with anti-GFRα1 antibody (Figure S8a and b) or with PAS-hematoxylin (Figure S8c and d). The percentage of GFRα1-positive cells in the Asingle cell population did not vary between actual stages IV–VII and (I–III and VIII–XII) (Figures 5i and S8). These results indicate that GFRα1 is continuously expressed on Asingle cells, regardless of seminiferous stage, but the frequency of Bmi1High-positive Asingle cells changes depending on seminiferous stage. Such stage-dependent expression of Bmi1High may regulate self-renewal and differentiation of GSCs and thus play a central role in spermatogenesis homeostasis.
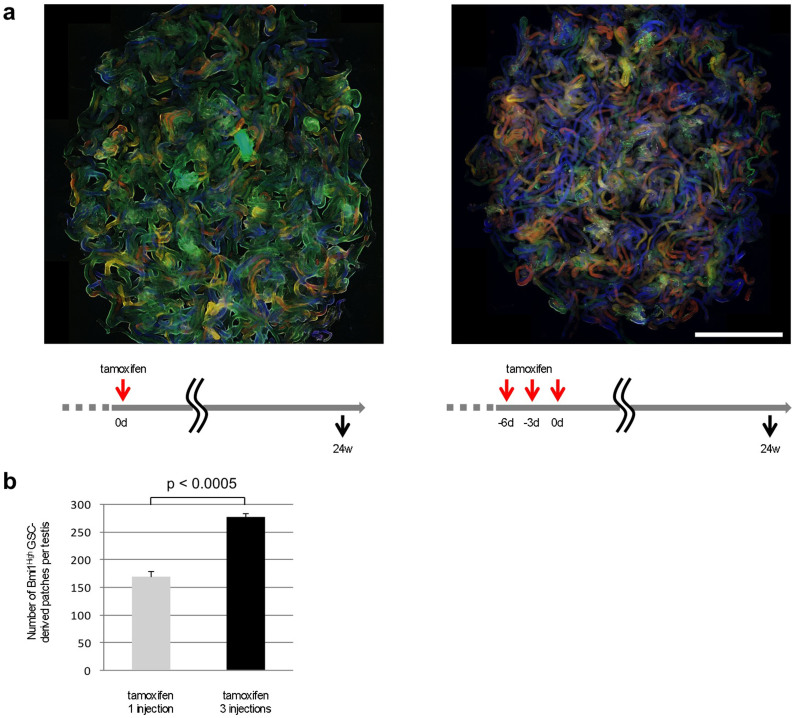
The results shown in Figure 5e indicate that the percentage of Bmi1High-derived cells in the Asingle cell population is upregulated in stage VII (actual stage: IV) and that this state persists until stage IX (actual stage: VII). The time-span from the actual stage IV–VII is known to be approximately 3 days (stage IV: 18.6; V: 11.3; VI: 18.1; VII: 20.6 hours)3. The seminiferous cycle is tightly regulated; therefore, when the Bmi1High-positive Asingle cells entered into the next stage and the expression of Bmi1High was downregulated, other Asingle cells that had newly entered into actual stages IV–VII showed a high level of Bmi1 expression. As shown in Figure 6, 3 successive injections (once every 3 days) of tamoxifen significantly increased the number of patches derived from Bmi1High-positive cells 24 weeks after injection compared with a single injection. This may be because Bmi1 expression in the Asingle cell population is high for approximately 3 days (actual stages IV–VII) per seminiferous cycle (8.6 days) (Figure 5e). Bmi1 expression is predicted to be controlled in a stage-specific manner according to the seminiferous cycle. Therefore, when tamoxifen was administered once every 3 days for a total of 3 administrations, the number of Bmi1High-derived Asingle cells significantly increased, leading to a larger number of patches derived from Bmi1High-positive cells at 24 weeks after treatment.
Figure 6. The effect of sequential administration of tamoxifen in increasing the number of patches derived from Bmi1High-positive cells.
(a) (left) The testis of Bmi1creER/+/Rosa26Rbw/+ mice injected with single dose of tamoxifen. (right) The testis of Bmi1creER/+/Rosa26Rbw/+ mice administered 3 sequential injection of tamoxifen. The time course of tamoxifen injection and analyses were shown in the lower panels. Scale bar = 5 mm. (b) The three sequential injections of tamoxifen significantly unregulated the number of patches observed 24 weeks later. P < 0.0005 by unpaired Student's t-test. Red arrows; tamoxifen administration. Black arrows; time points of analyses of the testes. d; days. w; weeks.
Fate of EdU-labeled Bmi1High-derived Asingle cells
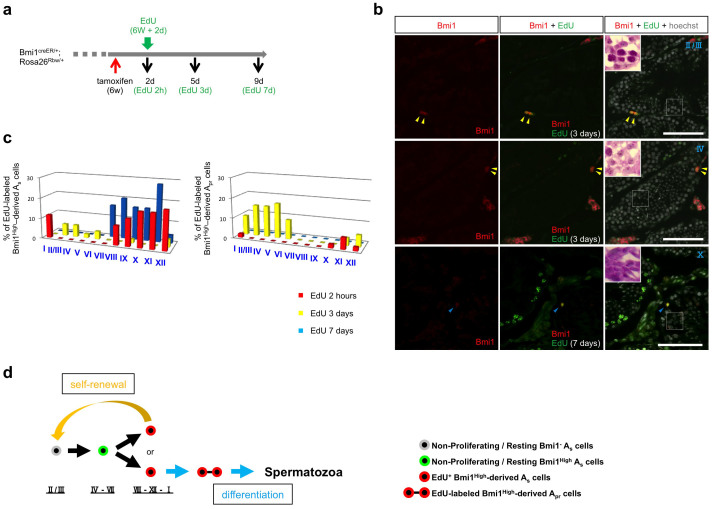
EdU is incorporated into proliferating cells during the S phase; however, the level of EdU labeling decreases in continuously proliferating cells because the amount of EdU-labeled DNA in each cell is reduced to half during each cell division. In contrast, cells that had entered into the non-proliferating/resting state after EdU labeling retain the labeling for a relatively long period of time. Thus, by tracing cells that retain the EdU label, one can monitor the fate of EdU-labeled cells. The percentages of EdU-labeled Bmi1High-derived Asingle cells in each seminiferous stage were determined as well as for 2 hours on day 3 and day 7 after EdU administration (Figure 7a and b). On day 7, EdU-labeled differentiating spermatogonia were detected in some seminiferous tubes (lower panels, Figure 7b). Similar examinations were performed in the Apaired cell population (Figure 7a and b). Although EdU-labeled Bmi1High-derived Asingle cells were not observed in stages II/III–VII at 2 hours after EdU administration (EdU 2 hours) (Figure 7c left), the cells were observed in stages II/III–VI on day 3 after EdU administration (EdU 3 days). EdU-labeled Bmi1High-derived (EdU 3 days) Asingle cells in stages II/III–VII are considered to be non-proliferating/resting cells that have been previously EdU-labeled in stages VIII–I and have remained undivided for 3 days or have undergone self-renewal or reverted from syncytial spermatogonia7.
Figure 7. Lineage tracing of EdU-labeled Bmi1High-derived cells and Asingle cell kinetic model.
(a) Experimental protocol of EdU and tamoxifen administration to Bmi1creER/+/Rosa26Rbw/+ mice. A red arrow; tamoxifen administration. A green arrow; EdU administration. Black arrows; timing of analyses of the testes. The black numbers indicate the days after tamoxifen administration. The green numbers show the days after EdU administration. d; days. h; hours. w; weeks. (b) The fate of EdU-labeled Bmi1High-derived Asingle cells. Bmi1High-derived (left) and EdU-labeled Bmi1High-derived (middle) cells at indicated time points. Right panels; Merged images with Hoechst 33342 counter staining (white) are shown. White windows (right panels) at the left upper corner; the magnified images of adjacent sections stained by PAS-hematoxylin staining corresponding to rectangle areas. Roman numbers indicate the seminiferous epithelial stage. Scale bar = 100 μm. Yellow arrowheads; EdU-labeled Bmi1High-derived Apaired cells. Blue arrowheads; EdU-labeled Bmi1High-derived Asingle cells. (c) Time course of EdU-labeled Bmi1High-derived Asingle, or Apaired cells. Left: % of EdU-labeled Bmi1High-derived Asingle cells to the whole EdU-labeled Bmi1High-derived cells were blotted. Right: % of EdU-labeled Bmi1High-derived Apaired cells to the whole EdU-labeled Bmi1High-derived cells were blotted. Red: 2 hours after EdU administration (n = 52) Yellow: 3 days after EdU administration (n = 52) Blue: 7 days after EdU administration (n = 25) (d) A schematic model illustrating the relationship of Bmi1High expression, cell cycle phase and seminiferous epithelial stages during early spermatogenesis.
EdU-labeled Bmi1High-derived cells were also detected in stages I–VII of Apaired cell population on day 3 after EdU administration (Figure 7c right). The EdU-labeled Bmi1High-derived Apaired cells (EdU 3 days) appeared to include self-renewal cells that were generated by the division of Asingle cells, and/or transient amplifying Apaired cells that eventually differentiated into spermatozoa. EdU-labeled Bmi1High-derived Apaired cells were not observed on day 7 after EdU administration (Figure 7c right), indicating that they were continuously proliferating and generating spermatozoa. Importantly, the increased percentage of Bmi1High-derived Asingle cells in stages VII–IX (actual stages: IV–VII) (Figure 5e) was likely not caused by the proliferation of Bmi1High-positive Asingle cells but by increased Bmi1 expression in Bmi1-negative Asingle cells, as EdU labeling was not detected in stages II/III–VII 2 hours after EdU administration. As shown in Figure 1, lineage tracing experiments showed that Bmi1High-positive cells maintained large single-color areas for a long period of time. This indicated that GSCs were generated from Bmi1High-positive Asingle cells residing in the testes, but were not newly generated from other sources. Therefore, Bmi1-negative Asingle cells, which are the source of Bmi1High-positive Asingle cells, were generated by self-renewal of Bmi1High-derived cells during actual stages VIII–I and lose their Bmi1 expression after cell division. Based on these findings, we propose an Asingle cell kinetic model in which the relationship between three important factors (seminiferous stages, Bmi1 expression: Bmi1High or Bmi1-negative, and cell cycle state: non-proliferating/resting or proliferating) is considered (Figure 7d).
Discussion
GFRα1-positive Asingle cells have been proposed as a primitive population of undifferentiated spermatogonia in mouse testes, and it has been reported that Nanos2-positive cells mostly overlap with GFRα1-positive cells27,29. In the present study, we used multicolor lineage tracing methods to follow the fate of Bmi1High-positive cells and showed that mature spermatozoa are generated from Bmi1High-positive cells (Figure 1). In addition, we found that fluorescent color-stained pups are produced by the mating of B6 mice (female) with tamoxifen-treated Bmi1creER/+/Rosa26Rbw/+ mice (male) (Figure S2). These results indicate that Bmi1High-positive cells are long-term GSCs that can produce functional spermatozoa. By comparing Bmi1High and GFRα1 expression on Asingle, Apaired, or Aaligned cells, we demonstrated that Bmi1High is a more specific marker of Asingle cells than GFRα1 (Figure 2). Therefore, this is the first study to directly observe the fate of the most primitive fraction of GSCs. Moreover, our multicolor lineage tracing method enabled precise estimation of the number of GSCs in mouse testes because the possibility of identifying large color-stained areas as a single patch is ruled out using the multicolor lineage tracing method. Nakagawa et al. reported that the number of GSCs per one testis was ~2000 using single-color reporter mice24, whereas approximately 4800 patches per one testis were detected in our multicolor system. However, the exact number of GSCs per testis may be higher than 4800, as the Cre/loxP-mediated DNA recombination typically shows a specific threshold.
Using an anti-Bmi1 antibody produced in their laboratory, Zhang et al. showed that Bmi1 is expressed on undifferentiated spermatogonia15. We attempted to stain Bmi1-positive cells using commercially available anti-Bmi1 antibodies, but positive staining results were not obtained. Rizo et al. reported that 75% downregulation of Bmi1 mRNA expression in human CD34+ stem cells, induced by lentiviral Bmi1-RNAi transduction, substantially impaired the long-term expansion and progenitor-forming capacity of stem cells30. In our previous study, RNA in situ hybridization results revealed that Bmi1 expression level was highest in lingual epithelial stem cells, but much lower in more differentiated cells31, suggesting that the expression level of Bmi1 was downregulated according to differentiation. The results of the previous studies indicated that Bmi1 is highly expressed on stem cells of various tissues and higher expression of Bmi1 is required for the maintenance and self-renewal of stem cells. The Cre-loxP system is induced by tamoxifen administration only in cells that highly express Bmi1, whereas the system does not function in cells that are negative or weakly positive for Bmi111. Hence, the color-labeled cells in our system were considered to be Bmi1High-positive cells, which are the most primitive stem cells. Although the possibility that more differentiated cells than Asingle, Apaired, and Aaligned cells are expressed Bmi1 cannot be completely excluded, the present study clearly shows that most Bmi1High-derived cells were Asingle cells (Figure 2d) and that these cells are important in spermatogenesis (Figures 1b–d and 3b).
In the present study, we compared Bmi1High-positive and Bmi1Low-positive cells that are present in a cell population of spermatogonia using Bmi1GFP/+ mice and found that Bmi1High-positive cells more selectively expressed GSC markers than Bmi1Low-positive cells (Figure S4). The Bmi1High-positive cell population expressed higher levels of ID4 than the Bmi1Low-positive cell population (Figure S4 f). Recently, ID4 was reported to be a specific marker of GSCs because the molecule was expressed selectively on Asingle cells32. These results also support that Bmi1High-GSCs in tamoxifen-treated Bmi1creER/+/Rosa26Rbw/+ mice were not artifactually generated by tamoxifen-induced germ cell damage, but Bmi1High-positive GSCs existed under normal physiological conditions.
GSCs in the testes are important for transmitting genetic information to subsequent generations; thus, it is likely that any recovery mechanisms (for example, rapid regeneration from GSCs in the testes after severe injury caused by events such as irradiation) protect these cells from injury. Huckins et al. reported that radioresistant Asingle cells could survive and initiate enhanced proliferation to repair all classes of spermatogonia by 11 days after irradiation33. However, the identity of the cells involved in regeneration was unclear. In the present study, we found that Bmi1High-positive GSCs were resistant to irradiation-induced injury and rapidly entered the cell cycle to regenerate spermatogenic progenitors (Figure 3b). Accordingly, Bmi1High-positive GSCs are thought to be highly proliferative and are responsible for regeneration after irradiation.
Seminiferous tubules in the testes are unique because the state of germ cells is regulated by cyclic seminiferous epithelial stages. In this study, we found that expression of Bmi1High in Asingle cells was specific to the seminiferous stage. The frequency of Bmi1High-positive Asingle cells increased during actual stages IV–VII, before entrance into the proliferative actual stages of VIII–XII and XII–I (Figure 5g). Thereafter, EdU-labeled Bmi1High-derived Asingle cells appeared to become Apaired cells and/or Asingle cells produced by self-renewal (Figures 7c and d). These observations suggest that Bmi1 regulates the self-renewal and differentiation of GSCs, thereby modifying seminiferous cycles (Figure 7d). Deletion experiments of Bmi1High-positive cells (Figures 3d and e) showed that some seminiferous tubules contained a stage-mismatched area, indicating that deletion of some (but not all) Bmi1High-positive GSCs from the seminiferous tubules abrogated the proper sequential maturational steps of spermatogenic progenitors. In the stage-mismatched area, a delay in 3–4 seminiferous stages (delay of approximately 3–4 days) was observed compared with stages in the surrounding area (Figure 3d and Table S2). This delay was presumably caused by selective deletions of Bmi1High-positive Asingle cells in actual stages IV–VII (time-span from stage IV to VII is approximately 3 days), during which the percentage of Bmi1High-positive Asingle cells was significantly higher compared with other stages (Figure 5h).
In the classical model, the “Asingle model,” Asingle cells can differentiate unidirectionally toward Aaligned cells. However, recent studies proposed the “potential stem cell model,” in which syncytial cells could revert to Asingle cells6,7. Our results provide new insight into the physiological role of Bmi1-positive GSCs in spermatogenesis. To further improve our Asingle cell kinetic model (Figure 7d), the extent to which the conversion contributes to normal spermatogenesis under physiological conditions should be investigated. We are currently conducting studies to precisely determine the regulatory mechanisms of spermatogenesis involving Bmi1 according to the Asingle cell kinetic model.
Methods
Animals
Mice were bred and maintained at the Kansai Medical University Research Animal Facility in accordance with the Kansai Medical University guidelines. The Kansai Medical University Animal Experiment Committee approved experiments in advance. Bmi1creER/+ mice (Jackson Laboratory, Bar Harbor, ME, USA) were bred and crossed with Rosa26Rbw/+ or Rosa26loxp-stop-loxp-DTA/+ mice (Jackson Laboratory). “Multicolor” reporter mice (Rosa26Rbw/+) contain a transgene that constitutively expresses GFP, and in the presence of Cre recombinase the transgene is randomly recombined once to express one of 3 other fluorescent proteins: mCherry (red), mOrange (orange), or Cerulean (blue). Tamoxifen (Sigma, St. Louis, MO, USA) was dissolved in corn oil (Sigma) and injected intraperitoneally into the mice at postnatal 6–8 weeks at a concentration of 9 mg/40 g body weight. Bmi1GFP/+ mice (Jackson Laboratory) and C57BL/6 (B6) mice (Shimizu Experimental Animal Laboratory, Kyoto, Japan) at postnatal 6–8 weeks were also used for experiments.
To confirm that Bmi1 positive GSCs are fertile, Bmi1creER/+/Rosa26Rbw/+ mice (male) received an administration of tamoxifen 8–10 weeks before were crossed with B6 mice (female) and fluorescent color of their pups was examined using a handy UV lamp.
Histological analyses
Mice were sacrificed, and the testes were fixed in 4% PFA at 4°C overnight, frozen in OCT compound, cut, and analyzed as reported previously34,35. Immunostaining was performed using the following primary antibody with anti-GFRα1 (R&D Systems, Minneapolis, MN, USA) or anti-PLZF (Santa Cruz, Dallas, TX, USA) antibody followed by Alexa Fluor 594- or 750-labeled secondary antibodies (Molecular Probes, Eugene, OR, USA). Nuclear counter-staining was performed using Hoechst 33342 (Sigma) as described previously34,35. For EdU staining, the Click-iTTM EdU kit (Invitrogen, Carlsbad, CA, USA) was used following the manufacturer's detection protocol. Fluorescent images were acquired using OLYMPUS BX63 (Olympus Corporation, Tokyo, Japan) and BZ-9000 (Keyence Corporation, Osaka, Japan) microscopes. Hematoxylin and eosin staining and PAS-hematoxylin staining were performed following a general protocol.
Counting patches derived from Bmi1High-positive cells
Tamoxifen was administered to Bmi1creER/+/Rosa26Rbw/+ mice. The testes were removed at 6, 12, 24, or 48 weeks after tamoxifen administration. The tunica albuginea of the testes were removed and the seminiferous tubules were untangled by removing the interstitial cells in phosphate-buffered saline (PBS) containing 1 mg/mL collagenase type II (Worthington, Biochemical Corporation, Lakewood, NJ, USA). The samples were prepared and analyzed as described above. We counted blue, orange or red clones spreading to the center of seminiferous tubules as patches because spermatozoa are only detected in the center of the tubules, which is evidence of complete spermatogenesis.
Estimate of stem cell number
The testes from Bmi1creER/+/Rosa26Rbw/+ mice 12 weeks after tamoxifen induction were fixed and frozen as described. Sections were cut at 150 μm and counterstained in Hoechst 33342 overnight. Next, the sections were washed in PBS, mounted, and three-dimensional images were acquired using a Nikon C2 confocal microscope (Nikon Instech, Tokyo, Japan). Mean long axis length of single color patches in whole-mount was approximately 712.5 μm (Figure 1d). Based on three-dimensional images, circular cylinder areas with 150 μm heights including seminiferous epithelial cells were randomly chosen (Supplemental movie) (n = 602) and the number of single-color clusters within the circular cylinders was counted. Green cell clusters were excluded because they did not undergo Cre/loxP-mediated DNA recombination. Because single-color areas were derived from single Bmi1High-positive long-term GSCs, we hypothesized that the number of single-color cell clusters 12 weeks after tamoxifen induction indicates the number of Bmi1High-positive stem cell clones within the testes. The mean number of single-color clusters within 150 μm height circular cylinders was 2.01 clones. The total length of seminiferous tubules in one testis was 1,690 ± 45.8 mm (n = 3). Therefore, total number of single color patches is 2.01/712.5 μm × 1690 mm = 4,771.
Apoptosis assays
The testes were harvested from tamoxifen-treated B6 mice (9 mg/40 g body weight) 12 or 24 hours before. Paraffin sections of the testes were prepared and apoptotic cells in the sections were detected with TUNEL staining using in situ Apoptosis Detection Kit (MK500, Takara Bio, Shiga, Japan) following the manufacturer's instructions.
Germ cell separation and cell sorting
Single cell suspensions of germ cells were obtained from the testes of Bmi1GFP/+ mice by enzymatic digestion as follows: the tunica albuginea of the testes were removed and the seminiferous tubules were untangled by removing the interstitial cells in PBS containing 1 mg/mL collagenase type II (Worthington). The seminiferous tubules were cut into 1–3 mm size fragments in 0.05% trypsin in 0.02% EDTA/PBS and incubated for 5 min at 37°C. The fragments were flushed using a pipette and cells released from the fragments were collected. After passing through a cell strainer (70 µm mesh size, #REF352350; BD Falcon), the germ cells were double-stained with phycoerythrin (PE)-labeled anti-c-Kit monoclonal antibody (#553869, BD Biosciences Pharmingen) and PerCP, Cy5.5-labeled anti-MCAM (CD146) mAb (#134709, BioLegend). The cells stained with the isotype-matched IgG served as a negative control. The stained cells were sorted using a FACSAria cell sorter (BD Biosciences).
Synthesis and amplification of cDNA and quantitative real-time PCR
Using Cellamp Whole Transcriptome Amplification Kit (33734, Takara Bio), mRNA was extracted from 1000 sorted cells and cDNA synthesized from the mRNA were amplified. Quantitative real-time PCR reactions were carried out using standard reagents of SYBR Premix Ex TaqTM II (RR820, Takara) and a real-time PCR cycler of Rotor-gene (Qiagen). Primers of Bmi1, GFRa1, ID4, Nanos2, Ngn3 and c-Kit were used in the experiments and the primers are listed in Table S1. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe was used as an internal control. Relative gene expression levels were calculated by normalization to GAPDH.
Injury and regeneration analyses of spermatogenic progenitors
Bmi1creER/+/Rosa26Rbw/+ mice were irradiated at 1 or 5 Gy using a 137Cs gamma-ray irradiator. The mice were injected with tamoxifen soon after irradiation and their testes were removed 2, 4, 6, 8, 10, or 14 days later.
A single shot of busulfan (10 mg/Kg body weight, Otsuka, Tokyo, Japan) was intraperitoneally injected to Bmi1creER/+/Rosa26Rbw/+ mice that received tamoxifen 2 days before. At 12 weeks after the administration, their testes were removed and the number of single-color patches was counted as described above.
Analysis of proliferative state and seminiferous stages of Bmi1High-positive or GFRα1-positive cells
Six-week-old Bmi1creER/+/Rosa26Rbw/+ mice were intraperitoneally injected with tamoxifen. Two days later, EdU (10 mg/kg body weight) was administrated and the mice were sacrificed 2 hours later (Figures 5 and 7) or 3 or 7 days (Figure 7) later. The testes were removed, and then fixed and frozen as described. Morphological determination of seminiferous stages of undifferentiated spermatogonia is generally performed using whole-mount staining method. However, we did not observe obvious differences between the results of the whole-mount method and those of the serial sections (Figure 2b). Therefore, we determined seminiferous stages based on serial cross-sections. Cross-sections were cut at 5 μm, 2 adjacent sections were selected, and one was PAS-hematoxylin stained for seminiferous epithelial staging, while the other was stained with an EdU-detecting reagent (Alexa Fluor 488-conjugated azide molecule) to evaluate proliferation. The frequency of Bmi1High-derived Asingle cells (n = 187 from 3 testes) was examined in each seminiferous epithelial stage and the frequency of EdU-labeled cells in Bmi1High-derived Asingle cells was analyzed. We counted all seminiferous epithelial stages in cross-sections and the frequency of EdU-positive or -negative Bmi1High-derived Asingle cells per total Bmi1-labeled Asingle cells were corrected according to the fraction of duration of each seminiferous stage. Similarly, to examine the relationships between GFRα1 expression and seminiferous epithelial stages of Asingle cells, serial sections of the testes from B6 mice were stained with anti-GFRα1 antibody or PAS-hematoxylin stained for seminiferous staging (n = 254 from 3 testes).
Statistical analysis
Differences between two indicated groups of samples were assessed using unpaired Student's t-test, with P < 0.05 considered statistically significant.
Author Contributions
Y.K. mainly performed the experiments. T.T., Y.T., H.Y., S.O., T.O., N.A., N.Y., K.K., H.H. and T.M. helped with the experiments and preparing samples. H.U. generated mice and supervised the project. H.H. helped preparing the manuscript. Y.K. and H.U. interpreted the results and wrote the manuscript.
Supplementary Material
Supplementary Text and Figures
Supplementary Movie 1
Acknowledgments
The authors thank S. Maeda for statistical analyses, M. Yamamoto and N. Nishida for animal care and technical assistance, and members of Department of Stem Cell Pathology, Kansai Medical University for helpful discussion. We acknowledge financial support from the following sources: Funding Program for Next Generation World-Leading Researchers, The Mochida Memorial Foundation, The Naito Memorial Foundation, The Cell Science Research Foundation, The Uehara Memorial Foundation, The Mitsubishi Foundation and The Yasuda Memorial Foundation to H.U.
References
- Oakberg E. F. Duration of spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium. Am J Anat 99, 507–516 (1956). [DOI] [PubMed] [Google Scholar]
- de Rooij D. G. Proliferation and differentiation of spermatogonial stem cells. Reproduction 121, 347–354 (2001). [DOI] [PubMed] [Google Scholar]
- Russell L., Ettlin R., Hikim A. S. & Clegg E. Histological and Histopathological Evaluation of the Testis (Cache River Press, Clearwater, FL, 1990). [Google Scholar]
- de Rooij D. G. & Russell L. D. All you wanted to know about spermatogonia but were afraid to ask. J Androl 21, 776–798 (2000). [PubMed] [Google Scholar]
- Meng X. et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 287, 1489–1493 (2000). [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Sharma M., Nabeshima Y., Braun R. E. & Yoshida S. Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science 328, 62–67 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K. et al. Mouse spermatogenic stem cells continually interconvert between equipotent singly isolated and syncytial states. Cell stem cell 14, 658–672 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessard J. & Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature 423, 255–260 (2003). [DOI] [PubMed] [Google Scholar]
- Molofsky A. V. et al. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature 425, 962–967 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung C. et al. Bmi1 is essential for cerebellar development and is overexpressed in human medulloblastomas. Nature 428, 337–341 (2004). [DOI] [PubMed] [Google Scholar]
- Sangiorgi E. & Capecchi M. R. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet 40, 915–920 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs R. U., Memarzadeh S., Wu H. & Witte O. N. Bmi-1 is a crucial regulator of prostate stem cell self-renewal and malignant transformation. Cell stem cell 7, 682–693 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguro H. et al. Poised lineage specification in multipotential hematopoietic stem and progenitor cells by the polycomb protein Bmi1. Cell stem cell 6, 279–286 (2010). [DOI] [PubMed] [Google Scholar]
- Park I. K. et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature 423, 302–305 (2003). [DOI] [PubMed] [Google Scholar]
- Zhang S. et al. Expression localization of Bmi1 in mice testis. Mol Cell Endocrinol 287, 47–56 (2008). [DOI] [PubMed] [Google Scholar]
- Takada Y. et al. Mammalian Polycomb Scmh1 mediates exclusion of Polycomb complexes from the XY body in the pachytene spermatocytes. Development 134, 579–590 (2007). [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21, 70–71 (1999). [DOI] [PubMed] [Google Scholar]
- Red-Horse K., Ueno H., Weissman I. L. & Krasnow M. A. Coronary arteries form by developmental reprogramming of venous cells. Nature 464, 549–553 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkevich Y., Lindau P., Ueno H., Longaker M. T. & Weissman I. L. Germ-layer and lineage-restricted stem/progenitors regenerate the mouse digit tip. Nature 476, 409–413 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L. & Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science 327, 542–545 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. et al. Experimental evidence showing that no mitotically active female germline progenitors exist in postnatal mouse ovaries. Proc Natl Acad Sci U S A 109, 12580–12585 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buaas F. W. et al. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet 36, 647–652 (2004). [DOI] [PubMed] [Google Scholar]
- Costoya J. A. et al. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat Genet 36, 653–659 (2004). [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Nabeshima Y. & Yoshida S. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev Cell 12, 195–206 (2007). [DOI] [PubMed] [Google Scholar]
- Watt F. M. & Hogan B. L. Out of Eden: stem cells and their niches. Science 287, 1427–1430 (2000). [DOI] [PubMed] [Google Scholar]
- Stange D. E. et al. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell 155, 357–368 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sada A., Suzuki A., Suzuki H. & Saga Y. The RNA-binding protein NANOS2 is required to maintain murine spermatogonial stem cells. Science 325, 1394–1398 (2009). [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M., Morimoto H. & Shinohara T. Enrichment of mouse spermatogonial stem cells by melanoma cell adhesion molecule expression. Biol Reprod 87, 139; 10.1095/biolreprod.112.103861 (2012). [DOI] [PubMed] [Google Scholar]
- Suzuki H., Sada A., Yoshida S. & Saga Y. The heterogeneity of spermatogonia is revealed by their topology and expression of marker proteins including the germ cell-specific proteins Nanos2 and Nanos3. Dev Biol 336, 222–231 (2009). [DOI] [PubMed] [Google Scholar]
- Rizo A. et al. Repression of BMI1 in normal and leukemic human CD34(+) cells impairs self-renewal and induces apoptosis. Blood 114, 1498–1505 (2009). [DOI] [PubMed] [Google Scholar]
- Tanaka T. et al. Identification of stem cells that maintain and regenerate lingual keratinized epithelial cells. Nat Cell Biol 15, 511–518 (2013). [DOI] [PubMed] [Google Scholar]
- Oatley M. J., Kaucher A. V., Racicot K. E. & Oatley J. M. Inhibitor of DNA binding 4 is expressed selectively by single spermatogonia in the male germline and regulates the self-renewal of spermatogonial stem cells in mice. Biol Reprod 85, 347–356 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckins C. & Oakberg E. F. Morphological and quantitative analysis of spermatogonia in mouse testes using whole mounted seminiferous tubules. II. The irradiated testes. Anat Rec 192, 529–542 (1978). [DOI] [PubMed] [Google Scholar]
- Ueno H., Turnbull B. B. & Weissman I. L. Two-step oligoclonal development of male germ cells. Proc Natl Acad Sci U S A 106, 175–180 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno H. & Weissman I. L. Clonal analysis of mouse development reveals a polyclonal origin for yolk sac blood islands. Dev Cell 11, 519–533 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text and Figures
Supplementary Movie 1