SUMMARY
Neutrophil recruitment to inflammation sites purportedly depends on sequential waves of chemoattractants. Current models propose that leukotriene B4 (LTB4), a secondary chemoattractant secreted by neutrophils in response to primary chemoattractants such as formyl-peptides, is important in initiating the inflammation process. In this study, we demonstrate that LTB4 plays a central role in neutrophil activation and migration to formyl-peptides. We show that LTB4 production dramatically amplifies formyl-peptide-mediated neutrophil polarization and chemotaxis by regulating specific signaling pathways acting upstream of actin polymerization and MyoII phosphorylation. Importantly, by analyzing the migration of neutrophils isolated from wild-type mice and mice lacking the formyl peptide receptor 1, we demonstrate that LTB4 acts as a signal to relay information from cell-to-cell over long distances. Together, our findings imply that LTB4 is a signal relay molecule that exquisitely regulates neutrophils chemotaxis to formyl peptides, which are produced at the core of inflammation sites.
Keywords: neutrophils, chemotaxis, leukotrienes
INTRODUCTION
Neutrophils are the most abundant leukocytes in the blood stream and the first cells recruited to an inflammation site, where primary chemoattractants such as formyl-peptides released from bacteria or necrotic cells and complement fragments are produced (McDonald et al., 2010). In response to primary chemoattractants, the surrounding tissue as well as resident immune cells, such as macrophages, release secondary chemoattractants (Monteiro et al., 2011; Ribeiro et al., 1997). These pro-inflammatory mediators activate nearby endothelia and enhance leukocyte extravasation (Soehnlein et al., 2009). After neutrophils have entered the tissue, gradients of secondary chemoattractants guide neutrophils towards the vicinity of the inflammation. Locally, gradients of primary chemoattractants recruit neutrophils to the core of the inflammation (Foxman et al., 1997; Heit et al., 2002). After they have reached the inflammation site, neutrophils in turn secrete secondary chemoattractants and recruit additional leukocytes, which further amplify the inflammation process (Silva, 2010).
It has been proposed that secondary chemoattractants are secreted in sequential waves (McDonald and Kubes, 2010), where leukotriene B4 (LTB4) is the first secondary chemoattractant released at an inflammation site (Chou et al., 2010; Kim et al., 2006). LTB4 is a product of the arachidonic acid (AA) metabolism. It is synthesized by the sequential action of 5-lipoxygenase (5-LO) and leukotriene A4 hydrolase (LTA4H) (Crooks and Stockley, 1998; Peters-Golden and Henderson, 2007) and mediates it effects by binding to the G-protein coupled receptor BLT-1 (McDonald et al., 1992; Tager and Luster, 2003). LTB4 is a potent chemoattractant for neutrophils and a key player in the initiation of inflammation (Canetti et al., 2003; Grespan et al., 2008; Ramos et al., 2005). Indeed, Chen et al. demonstrated that the recruitment of neutrophils towards inflammation sites is dependent on 5-LO expression in neutrophils (Chen et al., 2006a).
The current model suggests that LTB4, as a secondary chemoattractant, is release once neutrophils reach the site of inflammation (McDonald and Kubes, 2010). We hypothesize that LTB4 is actively secreted by neutrophils as they are migrating towards formyl peptides, therefore acting as a signal relay molecule. To test this hypothesis, we assessed the role of LTB4 secretion during primary neutrophil activation and migration in response to formyl peptides. We find that LTB4 significantly amplifies neutrophil recruitment to primary chemoattractants by selectively modulating signaling cascades involved in cell polarization and by serving as a potent secondary gradient. Thus LTB4 acts as a signal relay molecule for neutrophils migrating towards formyl peptides.
RESULTS
LTB4 secretion does not alter fMLP-induced ERK and PI3K activation
We show that in response to the formyl peptide fMLP (N-formyl-methionine-leucine-phenylalanine), primary human neutrophils rapidly secrete LTB4 in a concentration-dependent manner (Fig. 1A), as previously established (Dahinden et al., 1988). As LTB4 and fMLP both bind to Gαi-protein coupled receptors (BLT-1 and FPR1 respectively) and activate similar cellular pathways (Berger et al., 2002; Cotton and Claing, 2009; Kuniyeda et al., 2007), we set out to determine if signal transduction pathways are amplified by fMLP-induced LTB4 secretion in primary human neutrophils. For this purpose, we used two chemical inhibitors: MK886, an inhibitor of 5-LO activity and subsequent LTB4 production (Gillard et al., 1989), and LY223982, a BLT1 receptor antagonist, which blocks LTB4-mediated responses (Jackson et al., 1992).
Figure 1. LTB4 secretion does not alter fMLP-induced ERK and PI3K activation.
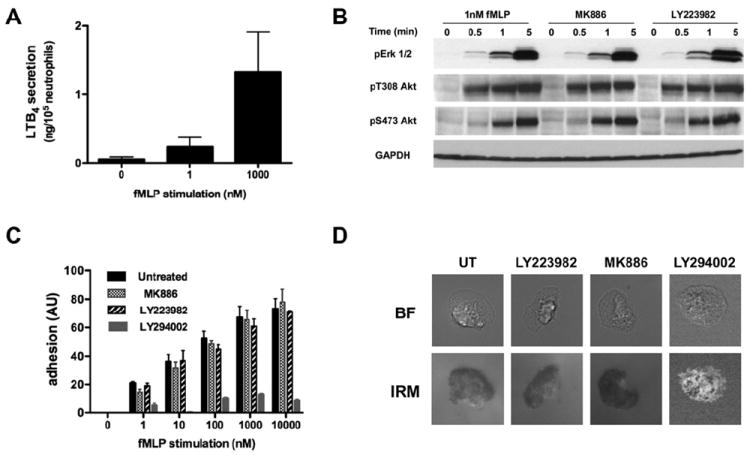
A. fMLP-induced LTB4 secretion by neutrophils is dose dependent. Primary human neutrophils were stimulated with fMLP for 1 min, and the amount of LTB4 in the supernatant was determined by ELISA. Results represent the average ± SEM of four independent experiments.
B. LTB4 secretion does not amplify Erk1/2 and Akt phosphorylation upon stimulation with sub-saturating doses of fMLP. Primary human neutrophils were stimulated with 1 nM fMLP after pretreatment with either 100 nM MK866, 10 μM LY223982, or DMSO as a control. The western blot for the kinetics of activation is representative of three independent western blot analyses. Also see Fig. S1A-B.
C. fMLP-induced LTB4 secretion has no impact on cell adhesion to fibronectin. Primary human neutrophils were treated with either 100 nM MK866, 10 μM LY223982, or 40 μM LY294002, a PI3K inhibitor. Cells were plated on fibronectin-coated plates for 10 min and uniformly stimulated with different concentrations of fMLP. The plates were then shaken and the number of remaining cells attached to the plates was estimated by crystal violet staining. Results represent the average ± SEM of four independent experiments.
D. Neutrophil adhesion pattern is not altered upon treatment with LTB4 pathway inhibitors. Neutrophil adhesion to fibronectin-coated plates upon stimulation with 1 nM fMLP was observed by IRM in the presence or absence of drugs as described in panel C. The areas of close contact of neutrophils to the substratum appear dark in the IRM image. Representative images are presented.
We first focused our attention on the impact of LTB4 secretion on PI3K activation, as previous reports suggested that the PI3K-PTEN axis is specifically involved in neutrophil migration towards LTB4 (Heit et al., 2008; Heit et al., 2002). We observed no significant difference on the fMLP-mediated phosphorylation of Akt on T308 (mediated through PI3K) (Alessi et al., 1997) in the presence of either MK886 or LY223982 compared to untreated cells (Fig. 1B, and Fig. S1A). These results are consistent with the fact that LTB4 gives rise to a lower level of Akt phosphorylation compared to fMLP (Fig. S1B); any increase in signal mediated by LTB4 would not be significant compared to the response elicited by fMLP alone. Similarly, we found that LTB4 signaling has no effect on the fMLP-mediated phosphorylation of Akt on S473, which is mediated through mTORC2 (Sarbassov et al., 2005), or of Erk1/2 (Fig. 1B and Fig. S1A-B). Together these findings establish that LTB4 secretion has no impact on Akt and Erk1/2 activation upon fMLP stimulation.
As the PI3K pathway has been linked to cell adhesion (Ferreira et al., 2006; Pellegatta et al., 2001; Shimizu and Hunt, 1996), we also tested the impact of secreted LTB4 on neutrophil adhesion in response to fMLP. We found that fMLP stimulation results in a dose-dependent increase in the number of neutrophils adhering to a fibronectin-coated surface (Fig. 1C). As previously reported, we also found that PI3K inhibition by LY294002 treatment dramatically reduces the capacity of neutrophil to adhere (Oakes et al., 2009). In contrast, and consistent with our results on PI3K activation, no alteration in the adhesion capacity of neutrophils was detected in the presence of either MK886 or LY223982 (Fig. 1C). Finally, comparison of neutrophil-substrate contact area using Interference Reflection Microscopy (IRM) revealed no significant difference between cell contact areas in response to 1 nM fMLP in the presence of LTB4 pathway inhibitors (Fig. 1D). These data confirm that PI3K modulates neutrophil adhesion and is not affected by LTB4 secretion following fMLP addition.
Autocrine and paracrine LTB4 secretion enhances fMLP-induced cell polarization
We recently reported that the fMLP-mediated activation of the adenylyl cyclase 9 (AC9) and the subsequent accumulation of intracellular cAMP are important for neutrophil polarization and back retraction (Liu et al., 2010). We therefore set out to determine whether fMLP-induced LTB4 secretion alters intracellular cAMP dynamics at sub-saturating and saturating doses of fMLP (FPR1 KD = 1 nM) (Migeotte et al., 2006). We found that LTB4-pathway inhibitors do not impact the fMLP-mediated cAMP accumulation when fMLP is presented under saturating conditions (1 μM) (Fig. 2A). In sharp contrast, both MK866 and LY223982 dose-dependently inhibited the ability of fMLP to induce cAMP production under sub-saturation conditions (1 nM) (Fig. 2B & S2A-B). These findings establish that LTB4 secretion is required to elicit intracellular cAMP accumulation following stimulation with 1 nM fMLP. Since intracellular cAMP accumulation regulates uropod dynamics via a PKA/MyoII axis (Liu et al., 2010), we next measured the effect of fMLP-induced LTB4 secretion on the extent of myosin light chain MyoII phosphorylation in neutrophils stimulated with 1 nM of fMLP. In accordance with our cAMP measurements, we found that the levels of fMLP-induced MyoII phosphorylation are significantly reduced in the presence of LTB4-pathway inhibitors (Fig. 2C, and Fig. S2C). These data suggest that fMLP-induced LTB4 secretion affects uropod dynamics during chemotaxis.
Figure 2. LTB4 secretion enhances fMLP-induced cAMP production and MyoII phosphorylation.
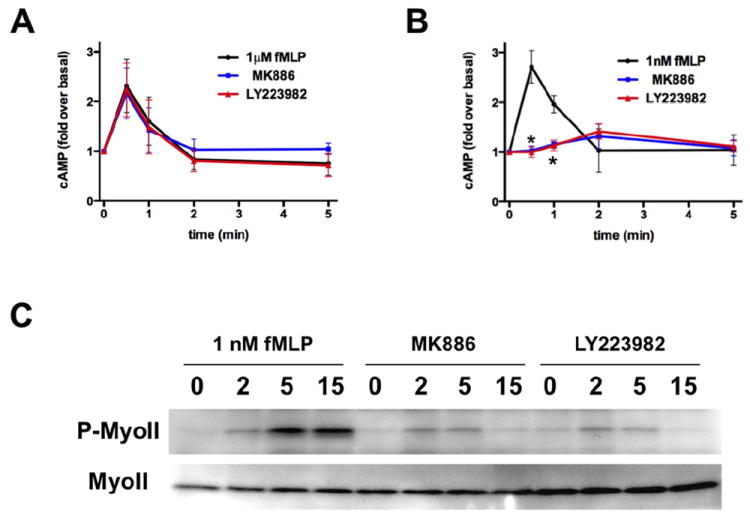
A. LTB4 secretion has no impact on intracellular cAMP accumulation in neutrophils stimulated with a saturation dose of fMLP. Primary human neutrophils were treated with 100 nM MK886 or 10 μM LY223982 or DMSO as control and stimulated with 1μM fMLP. Intracellular cAMP levels were determined by ELISA at the indicated time points. Results represent the average ± SEM of three independent experiments.
B. LTB4 inhibition reduces cAMP accumulation in neutrophils treated with a sub-saturating dose of fMLP. Primary human neutrophils were treated as in panel A and stimulated with 1 nM fMLP. Results represent the average ± SEM of three independent experiments. * p<0.05, ANOVA; Dunnett posthoc test. Also see Fig. S2A-B.
C. LTB4 secretion amplifies phosphorylated MyoII levels in response to sub-saturating doses of fMLP. Primary human neutrophils were plated on fibronectin-coated plates for 10 min and stimulated uniformly with 1 nM fMLP in the presence or absence of drugs as described in panel A. The western blot for the kinetics of activation is representative of three independent western blot analyses. Also see Fig. S2C.
We next determined the role of fMLP-induced LTB4 secretion on fMLP-mediated actin polymerization and cell polarity. In response to a uniform stimulation of chemoattractant, neutrophils first accumulate cortical F-actin evenly around their periphery in a so-called cringe response; they then polarize and acquire a network of branched F-actin at their leading edge (Fig. 3A) (Orelio and Kuijpers, 2009). When stimulated with 1 μM fMLP, the amount of F-actin in neutrophils doubles within 20 s and remains high up to 5 min (Fig. 3B). Under these conditions, 83 % of neutrophils accumulate cortical F-actin after 30 s and 85 % of neutrophils are polarized after 2 min (Fig. 3D). Pre-treating neutrophils with LTB4-pathway inhibitors has no effect on this outcome (Fig. 3B and D). These results illustrate that the drugs have no toxic effect on the capacity of cells to polymerize actin and that LTB4 secretion has no impact on F-actin dynamics and cell polarization following saturating stimulations of fMLP.
Figure 3. Autocrine and paracrine LTB4 secretion enhances fMLP-induced cell polarization.
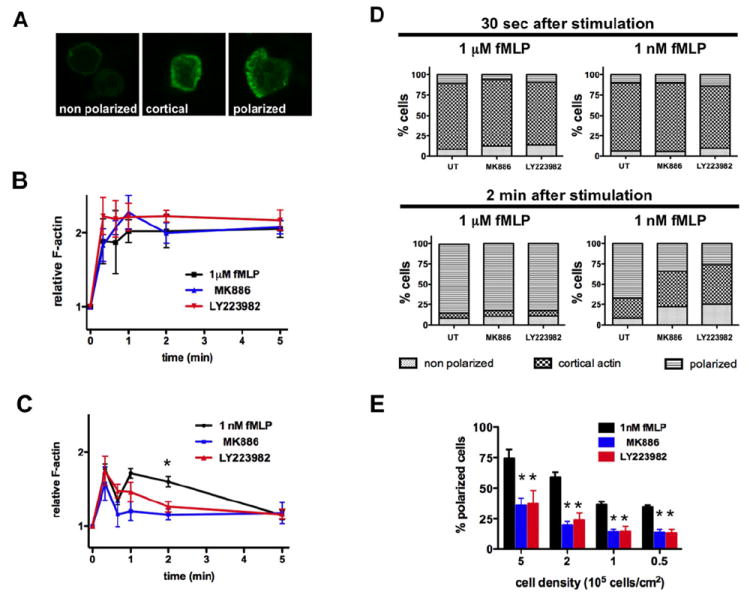
A. Different stages of neutrophil polarization can be observed in response to fMLP stimulation. Primary human neutrophils were plated on gelatin-coated plates. Cells were stimulated, fixed and F-actin was stained with FITC-phalloidin. Representative images are presented.
B. LTB4 secretion has no impact on neutrophil response to a saturating dose of fMLP. Primary human neutrophils were treated with 100 nM MK886 or 10 μM LY223982 or DMSO as control, stimulated with 1 μM fMLP, fixed and the F-actin network stained with FITC-phalloidin. The kinetic of the average fluorescence was determined by FACS analysis. Results represent the average ± SEM of three independent experiments.
C. Neutrophil treatment with LTB4 inhibitors reduces neutrophil polarization in response to sub-saturating doses of fMLP. Primary human neutrophils were treated as in panel B, stimulated with 1 nM fMLP and F-actin levels were determined by FACS, after staining with FITC-phalloidin. Results represent the average ± SEM of three independent experiments. * p<0.005, ANOVA; Dunnett posthoc test.
D. LTB4 amplifies neutrophil polarization after 2 min of fMLP stimulation. Primary human neutrophils were treated as in panel B, plated on gelatin-coated plates, stimulated with fMLP and fixed at different time points. Cells were stained with F-actin and counted into 3 categories (unpolarized, accumulated cortical F-actin, polarized). Results represent the average of four independent experiments.
E. LTB4 amplifies neutrophil polarization in an autocrine and paracrine manner. Primary human neutrophils were treated as in panel B, plated on gelatin-coated plates at different cell densities for 10 min. After 2 min stimulation with 1 nM fMLP, cells were fixed and the number of polarized cells was counted. Results represent the average ± SEM of three independent experiments. *p<0.05, ANOVA; Dunnett posthoc test.
When neutrophils are stimulated with the sub-saturating dose of 1nM fMLP, the F-actin accumulation follows a biphasic profile with peaks at 20 s and 1 min after stimulation (Fig. 3C). The first peak of F-actin correlates in time with the cortical cringe response while the second peak matches the polarized F-actin response (Fig. 3D). Under these conditions, after 2 min of stimulation, 67 % of cells are polarized while 25 % show high cortical F-actin staining (Fig. 3D). Remarkably, treatment with either MK886 or LY223982 specifically ablates the second F-actin (Fig. 3C). Indeed, after 2 min of stimulation, we found that MK886 and LY223982 treatments decreased the percentage of polarized cells to only 34 % and 26 %, respectively (Fig. 3D). Not surprisingly, we also found that the extent of F-actin accumulation following sub- and saturating IL-8 stimulations, which only lead to low LTB4 secretion (Fig. S3A) (Meliton et al., 2010), is not altered in the presence of LTB4-pathway inhibitors (Fig. S3B-C). These data demonstrate that LTB4 secretion facilitates and stabilizes neutrophil polarization in response to sub-saturating stimulations of fMLP. Under these conditions, we propose that the limited MyoII phosphorylation measured is a consequence of the absence of cell polarization; we did not observe neutrophil back retraction defects.
We next wanted to assess if the effects of LTB4 on fMLP-mediated neutrophil polarization were mediated in an autocrine or paracrine fashion. To answer this question, we plated neutrophils at decreasing densities, which gradually reduces the effects of any paracrine signals, and measured the extent of neutrophil polarity 2 min after the addition of 1 nM fMLP. We observed a significant decrease in the percentage of polarized cells as we decreased cell density (Fig. 3E), suggesting that a paracrine signal regulates neutrophil polarization in response to fMLP. As this effect is markedly inhibited in the presence of MK886 or LY223982, we propose that LTB4 acts as the main paracrine factor in this response. With 1 nM fMLP stimulations, the paracrine effect is lost when neutrophil density is lower than 105 cells/cm2, as no further decrease in the percent of polarized cells is observed at 105 and 0.5×105 cells/cm2. However, at these cell densities, treatment with either LTB4-pathway inhibitor still significantly reduces the proportion of polarized cells (Fig. 3E), suggesting that LTB4 also acts in an autocrine fashion. Taken together, these data demonstrate that, at sub-saturating fMLP concentrations, secreted LTB4 functions as a paracrine and autocrine signal to enhance and stabilize neutrophil polarization.
Arachidonic acid accumulates at the front of polarized neutrophils
We next set out to determine if LTB4 secretion is directionally biased in polarized neutrophils. However, intracellular LTB4 has never been detected in neutrophils stimulated with either fMLP or ionomycin (a major 5-LO activator) (Mita et al., 1988; Williams et al., 1985), suggesting that LTB4 does not accumulate to significant levels in neutrophils. To circumvent this issue, we assessed the subcellular localization of the LTB4 precursor, AA, in polarized neutrophils using coherent anti-Strokes Raman scattering (CARS) microscopy.
Cells pre-treated with deuterated AA were allowed to polarize and migrate directionally to fMLP using the under-agarose assay, fixed and analyzed by CARS to determine the sub-cellular distribution of deuterated species. We detected characteristic spectra for cytoplasm, nucleus, and deuterated punctates (Fig. S4A). The peak at ≈2250 cm-1 is characteristic of carbon-deuterium (C-D) bound, while the broad peaks at ≈2900 cm-1 is a signature of carbon-hydrogen (C-H) bounds. Remarkably, we found that deuterated punctates accumulate towards the leading edge of neutrophils during chemotaxis (Fig. 4A-B). In sharp contrast, the inhibition of LTB4 synthesis with MK866 rendered the distribution of AA deuterated punctates random (Fig 4A-B). Importantly, these findings were not a consequence of the weak cellular polarization measured in the presence of LTB4 pathway inhibitors, as similar findings were obtained when deuterated punctates were monitored following a uniform stimulation with a saturating dose of fMLP (1 μM), which gives rise to normal polarization.
Figure 4. Arachidonic acid accumulates at the front of polarized neutrophils.
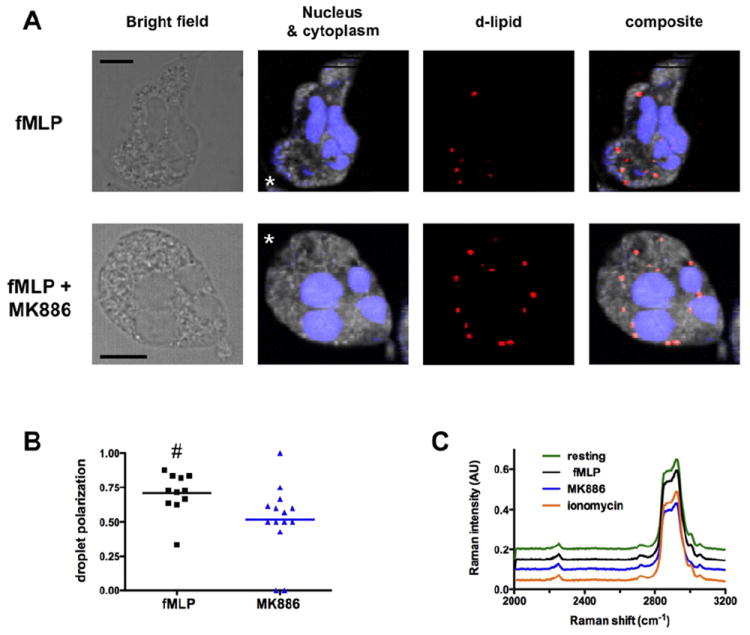
A. Bright field and CARS images of deuterated punctates localized in polarized primary human neutrophils migrating to 1μM fMLP in underagarose assay. Representative images of polarized cells untreated (upper panel) or treated with MK886 (lower panel) are presented. The false-colored chemical images for nucleus (blue), cytoplasm (grey), and deuterated punctates (red) were constructed from Raman intensities at 2952 cm-1 and 2850 cm-1 for nucleus, and intensities at 2900 cm-1 for cytoplasm and 2250 cm-1 for deuterated punctates, respectively.
B. Location parameters of deuterated punctates in neutrophils are plotted for two differently treated neutrophils. The location parameter is defined as (number of punctates at the front)/(total number of punctates). Neighboring image pixels (> four pixels) are counted as one regardless of the overall size. # p-value = 0.009, Wilcoxon test.
C. Comparison of the CARS spectra of deuterated punctates found in polarized neutrophils under the indicated conditions.
We next compared the averaged spectrum of the deuterated punctates of untreated and MK886-treated cells and found no difference between the two conditions (Fig. 4C; see also Fig. S4B for a zoomed-in view of the spectra of the C-D bound), even though simulations suggest that the CARS spectra of deuterated AA and deuterated LTB4 should be different (Fig. S4C). Similarly, no deuterated LTB4 signal could be identified in neutrophils stimulated for longer periods (data not shown) or stimulated with the potent activator of 5-LO, ionomycin (Fig. 4C and S4B) (Ford-Hutchinson et al., 1980). It therefore appears that, as previously suggested (Mita et al., 1988; Williams et al., 1985), LTB4 does not accumulate in migrating neutrophils.
We see two possible interpretations of our data: i) AA is enriched at the front of polarized neutrophils because most of the AA at the back of cells has been converted into LTB4, which is then secreted at the cell rear or ii) AA is relocalized at the front of neutrophils in response to 5-LO activation. Interestingly, we measured the asymmetrical distribution of deuterated punctates in neutrophils as early as 1 min after a uniform stimulation with 1 nM fMLP (data not shown), before the peak of LTB4 secretion (Fig. S4D). This finding suggests that AA is actively redistributed to the front of neutrophils and that LTB4 is not primarily generated and secreted at the back of cells.
LTB4 autocrine/paracrine secretion amplifies neutrophil chemotaxis to fMLP
As cellular polarization is a pre-requisite for migration, we studied the role of LTB4 paracrine/autocrine secretion on neutrophil chemotaxis. We found that treating neutrophils with either MK886 or LY223982 significantly reduces transwell migration to fMLP (Fig. 5A). Not surprisingly, neutrophil migration to IL-8 (which induces a very low LTB4 secretion; Fig. S3A) is not altered in the presence of LTB4-pathway inhibitors (Fig. S3D). This finding also confirms that the LTB4-inhibitors used are specific and do not directly impact neutrophil migration.
Figure 5. Neutrophil migration to fMLP is amplified by fMLP-induced LTB4 paracrine/autocrine secretion.
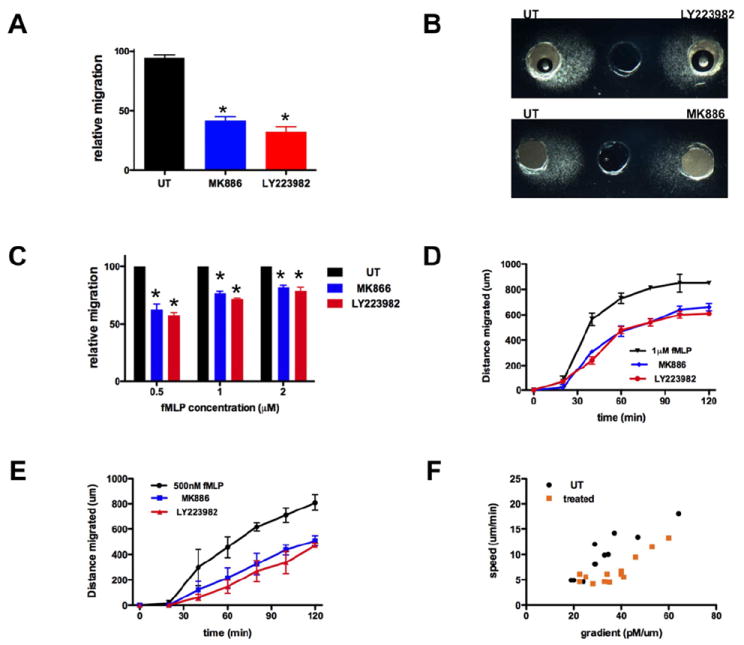
A. LTB4 secretion amplifies neutrophil migration to fMLP. The number of primary human neutrophils migrating to 1 μM fMLP in a 4 μm transwell was determined after 2 h. Results represent the relative percentage of migrating cells after treatment (average ± SEM) of three independent experiments. *p<0.05, Friedman test; Dunns posthoc test.
B. LTB4 secretion amplifies neutrophil chemotaxis to fMLP. Representative images of primary human neutrophils migrating to 1 μM fMLP in the under-agarose assay are shown.
C. LTB4 secretion amplifies neutrophil chemotaxis to fMLP. The distance migrated by primary human neutrophils treated with LTB4 pathway inhibitors is compared to the one migrated by untreated cells. Results represent the relative distance migrated (average ± SEM, n=3) in under-agarose assay in 2 h. *p<0.05, Friedman test; Dunns posthoc test.
D-E. Kinetics of neutrophil migration in under-agarose assays. The distance migrated by primary human neutrophils to either 1 μM fMLP (D) or 500 nM fMLP (E) was determined at different times points. Results represent the average ± SEM of three independent experiments.
F. Impact of LTB4 secretion on neutrophil migration to different fMLP gradients. For each segment of 20 min of migration, the average speed was determined and the local gradient of the front of migration was determined using theoretical charts (see Fig. S4). The resulting different data points (speed vs. gradient) are plotted.
This finding was further investigated using the under-agarose assays, where the behavior of populations of cells can be visualized directly (Heit and Kubes, 2003) (Fig. 5B). We found that treatment with LTB4-pahway inhibitors drastically reduces neutrophil chemotaxis to fMLP compared to untreated cells. The inhibition is statistically significant and more dramatic when cells migrated towards lower concentrations of fMLP (Fig. 5B-C). The reduction in neutrophil chemotaxis in this assay could arise because cells cannot penetrate under the agarose in the absence of LTB4 signaling or because fMLP-induced LTB4 secretion amplifies chemotaxis. To get at this, we measured the extent of directed migration as a function of time; we found that in response to either 500 nM or 1 μM fMLP, LTB4-pathway inhibitors give rise to a gradual inhibition of migration (Fig. 5D-E), which is indicative of a chemotactic defect. Indeed, if cells were unable to migrate under the agarose, we would expect the migration profiles to show a time delay, but otherwise be similar.
It has been shown that fMLP gradients in under-agarose assays are neither linear nor stable over time (Uden et al., 1986). We took advantage of this to study how a neutrophil population migrates into different gradients by assessing the migration speed of cells as a function of the chemoattractant gradient (Fig. S5). We found that when neutrophils migrate in either shallow (lower than 25 pM/μm) or steep (greater than 60 pM/μm) gradients, the inhibition of LTB4 has no significant impact on group migration (Fig. 5F). Interestingly, when neutrophils migrate in intermediate gradients (between 25 and 60 pM/μm), the population migrates more efficiently, i.e. the front of migration progresses faster towards the well containing fMLP, in the presence of LTB4 paracrine/autocrine secretion (Fig. 5F). These data are consistent with the fact that fMLP-induced LTB4 secretion impacts cell polarization at sub-saturating (more physiological) concentrations of primary chemoattractants. More importantly, the data highlight the fact that LTB4 paracrine/autocrine secretion is effective under conditions where LTB4 is produced in sufficient amounts (i.e. in response to >20 pM/μm) and not overwhelmed by the high concentration of primary chemoattractant (>60 pM/μm).
LTB4 paracrine secretion acts as a signal relay between neutrophils
We showed that fMLP-induced LTB4 secretion favors neutrophil polarization and chemotaxis in shallow primary chemoattractant gradients. Several models could explain these observations. First, LTB4 could increase the capacity of neutrophils to sense fMLP, e.g. by enhancing expression of the fMLP receptor. Second, LTB4 could act as a chemokinetic agent and simply increase neutrophil migratory capacity. Finally, LTB4 secretion could form a secondary gradient that facilitates a directional recruitment of neighboring neutrophils. In order to test these possibilities, we took advantage of the availability of mice that lack the formyl receptor 1 (FPR1), which mediates neutrophil chemotaxis to fMLP (Gao et al., 1999), and tested the ability of neutrophils isolated from these mice to migrate to exogenous fMLP when mixed with neutrophils isolated form wild-type (WT) mice.
We first demonstrated that the importance of LTB4 secretion on neutrophil migration to formyl-peptides is not restricted to human primary neutrophils. Using the under-agarose assay, we found that MK886-treatment reduces mouse bone marrow neutrophil migration to the synthetic WKYMVm peptide (a strong agonist for the mouse neutrophil FPR (He et al., 2000)) (Fig. 6A). Similarly to human neutrophils (Fig. 5C), the inhibition is more important for cells migrating to low concentrations of the peptide (Fig. 6A). Moreover, we demonstrate that this is not a consequence of drug-induced toxicity on neutrophils: neutrophils isolated from the bone marrow of mice lacking either BLT1 (blt1-/-) (Tager et al., 2000) or 5-LO (alox5-/-) (Chen et al., 1994) exhibit impaired migration to 100 nM WKYMVm similarly to what we measure in neutrophils isolated from WT animals treated with MK886 (Fig. 6B). Not surprisingly, we also confirmed that neutrophils isolated from fpr1-/- mice do not respond to 100 nM MKYMVm (Fig. 6B). Importantly, these cells are able to migrate efficiently to LTB4 (data not shown).
Figure 6. LTB4 is a signal relay molecule for neutrophils.
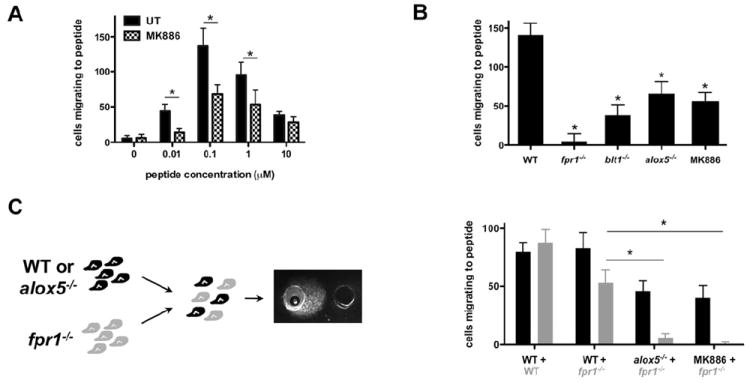
A. MK886 treatment regulates murine neutrophil migration to MKYMVm. Neutrophils isolated from the bone marrow of WT mouse were allowed to migrate to MKYMVm in the under-agarose assay. The number of neutrophils migrating to fMLP was determined after 5 h of migration. Results represent the average ± SEM number of migrating mouse neutrophils in three independent experiments. *p<0.05, Mann-Whitney tests.
B. LTB4 secretion amplifies murine neutrophil migration to MKYMVm. Neutrophils isolated from the bone marrow of mice were allowed to migrate to 100 nM MKYMVm in the under-agarose assay. The number of neutrophils migrating was determined after 5 h of migration. Results represent the average ± SEM number of migrating mouse neutrophils in three independent experiments. *p < 0.05, Friedman test; Dunns posthoc test.
C. Neutrophils that do not sense MKYMVm can still migrate to MKYMVm when mixed with WT neutrophils secreting LTB4. Neutrophils isolated from fpr1-/- mice were fluorescently labeled and mixed with WT neutrophils (pretreated or not with 100nM MK886) or neutrophils isolated from alox5-/- mice. The number of fluorescent and non-fluorescent cells that migrate to 100 nM MKYMVm was determined after 5 h migration. Results represent the average ± SEM number of migrating mouse neutrophils in three independent experiments. *p < 0.05, Friedman test; Dunns posthoc test.
We then mixed cell populations (1:1 ratio) and measured their ability to migrate directionally to MKYMVm using the under-agarose assay. To distinguish between the different populations, mutant neutrophils were fluorescently labeled. We first confirmed that the fluorescent label does not alter neutrophil migration (Fig. 6C). Interestingly, we found that, in the presence of neutrophils derived from WT animals, fpr1-/- neutrophils gain the capacity to migrate directionally to a well containing MKYMVm. Most importantly, the recruitment of fpr1-/- neutrophils is abolished when WT neutrophils are treated with MK886 and do not produce LTB4. Similarly, fpr1-/- neutrophils are not recruited when mixed with neutrophils isolated from the bone marrow of alox5-/- mice (Fig. 6C).
Together, these findings establish that LTB4 acts as a signal relay molecule for neutrophils where WT neutrophils release LTB4 in an autocrine/paracrine fashion, which provides spatial information to neighboring fpr1-/- neutrophils. This LTB4 relay allows the fpr1-/- neutrophils to migrate directionally to a chemoattractant they cannot sense.
DISCUSSION
LTB4 is widely recognized as an essential mediator in inflammation. Inhibiting leukotriene production reduces leukocyte recruitment and inflammation in a variety of models, such as arthritis, pancreatitis or asthma (Peters-Golden and Henderson, 2007). Here we establish that LTB4 is not only a secondary chemoattractant for neutrophils secreted early in the inflammation process, but it is also an important signal relay molecule that increases the recruitment range and promotes the directional migration of neutrophils to formyl peptides, which are released at the core sites of inflammation (McDonald et al., 2010).
We demonstrate that LTB4 relay amplifies cAMP production, MyoII phosphorylation, F-actin polymerization and cell polarization when cells are stimulated with sub-saturating doses of fMLP. This is reminiscent to what has been described in the social amoebae Disctyostelium discoideum, where efficient effector activation requires the autocrine/paracrine production of chemoattractants when cells are stimulated with sub-saturating concentrations of chemoattractant (Das et al., 2011). By contrast, and similar to the Dictyostelium model, LTB4 secretion has no impact on effector activation when neutrophils are stimulated with saturating concentrations of fMLP. These findings also support previous findings (Rochon and Frojmovic, 1993; Tomhave et al., 1994), where at saturating concentrations of fMLP, FPR1 activation induces BLT1 desensitization. Furthermore, we found that LTB4 pathway inhibition specifically impacts migration speed at intermediary fMLP gradients. In this case, we envision that under very shallow fMLP gradients, LTB4 production is too low to impact fMLP-induced response, while under very steep gradients, LTB4 has no impact on migration because of cross-desensitization. We propose that this intermediary window of fMLP concentrations, where LTB4 relay is a key amplifier, may represent physiologically relevant conditions for in vitro studies.
Both BLT1 and FPR1 are coupled to a Gαi-βγ G proteins, and neutrophil migration towards either LTB4 or formyl peptides is pertussis-toxin sensitive (Brito et al., 1997). Therefore, one would expect that LTB4 relay amplifies the same signaling pathways as formyl peptides. However, we show that LTB4 relay specifically amplifies signaling pathways leading to F-actin production and MyoII phosphorylation without affecting Akt and Erk1/2 activation. Differences in signaling pathway activation upon formyl peptides and LTB4 stimulation have been previously reported: fMLP-induced chemotaxis has been shown to require P38-MAPK activation, while migration to LTB4 is P38-independent (Heit et al., 2002). Similarly, BLT1 activation does not induce H2O2 production, while FPR1 activation induces a high pertussis-toxin-sensitive H2O2 production and β2-integrin upregulation has been reported to be three times higher upon fMLP stimulation compared to LTB4 stimulation (Berger et al., 2002). Several models can be proposed to explain how the activation of a given Gα subunit can result in different functional responses. First, although the functional relevance of βγ subtypes has yet to be fully appreciated, the Gαi subunits could associate with different βγ subunits when coupled to different receptors - this has been demonstrated for the muscarinic M4 and somatostatin receptors binding to Gαo (Kleuss et al., 1992, 1993). Second, FPR1 and BLT1 have been reported to partition in different lipid domains at the plasma membrane (Sitrin et al., 2006). In this context, we envision that effector molecules and activated receptors could access different lipid domains resulting in the spatial segregation of signal transduction pathways.
fMLP-induced LTB4 secretion amplifies neutrophil polarization in an autocrine manner. In fact, at low cell density, when LTB4 cannot act as a paracrine factor, fMLP induced LTB4 still enhances neutrophil polarization, albeit to a lesser extent. LTB4 is not the only autocrine factor associated with effective cell polarization. It has been shown that autocrine ATP secretion enhances lamellipodia formation and stabilization in macrophage and neutrophil chemotaxis to C5a and fMLP, respectively (Chen et al., 2006b; Kronlage et al., 2010). Interestingly, the ATP autocrine activity has been associated with its directed release at the leading edge. We provide evidence that LTB4 could similarly be secreted at the front of neutrophils. We propose that in both cases the asymmetric secretion enhances lamellipod formation and stabilizes cell polarization by creating a local gradient at the leading edge.
In contrast to ATP, however, we also found that LTB4 acts in a paracrine fashion to enhance recruitment of neutrophils to primary chemoattractants. Previous studies have also suggested that LTB4 secretion could act as a paracrine effector for efficient neutrophil activation and degranulation in response to LTB4 or ATP, respectively (Kannan, 2002; Serio et al., 1997). We predict that both in vivo and in vitro, the secondary gradient generated by the secretion of LTB4 can efficiently recruit a population of neutrophils that may not normally be recruited to sites of inflammation. This is of consequence since human primary neutrophil populations are heterogeneous. For example, three distinct neutrophil subsets, which respond differently to infectious agents, have been identified during Staphylococcus aureus infection in mice (Tsuda et al., 2004). In this context, neutrophils that can efficiently migrate to formyl peptides would readily secrete LTB4, thereby recruiting a population of neutrophils that are low responders for formyl peptides but are good LTB4 responders. Similarly, in Dictylostelium, signal relay has been shown to specifically amplify the range of recruitment of neighboring cells to an external chemoattractant allowing cells to maintain directionality over very long distances (McCann et al., 2010).
It remains unclear how the secondary LTB4 gradient is formed. Due to its small size (MW = 336 Da), LTB4 would likely diffuse quickly rendering the gradient short lived. We could first argue that LTB4 is a lipid-derived hydrophobic molecule, which could significantly reduce its diffusion properties. Second, neutrophils could create a more stable gradient by secreting LTB4 in exosomes. In Dictyostelium, signal relay has been proposed to be mediated by the secretion of chemoattractant-containing exosomes (Kriebel et al., 2008) and FLAP-containing exosomes have been detected in neutrophils (Jethwaney et al., 2007). In addition, a recent report has demonstrated that macrophages and dendritic cells are capable of secreting LTB4-producing exosomes (Esser et al., 2010), which can induce granulocyte migration. Hence, we speculate that neutrophils may secrete such exosomes. In this model, neutrophils that migrate to sites of inflammation would recruit additional neutrophils with LTB4-releasing vesicles. This model is consistent with our current study and others where intracellular LTB4 has not been detected (Mita et al., 1988; Williams et al., 1985). This suggests that either LTB4 is secreted quickly out of the cells, or that the cytosolic production of LTB4 is weak. In this latter scenario, LTB4 production could be contained within extracellular vesicles.
Based on our findings we propose the following model for LTB4 mediated signal relay (see Fig. 7). In response to a given external formyl peptide gradient, some neutrophils respond, polarize and release LTB4 or LTB4-producing vesicles at their leading edge. The local LTB4 gradient strengthens and stabilizes cell polarization of the first responders. As LTB4 production is fMLP-concentration dependent, neutrophils that are closer to the fMLP source will secrete higher amounts of LTB4. As a consequence, a secondary LTB4 gradient is formed parallel to the fMLP gradient. Neutrophils that were not initially responsive to fMLP, can now sense the secondary gradient of LTB4 and migrate up this gradient towards the fMLP source thus amplifying the inflammatory response.
Figure 7. Model for LTB4 as a signal relay molecule for neutrophils migrating to fMLP.
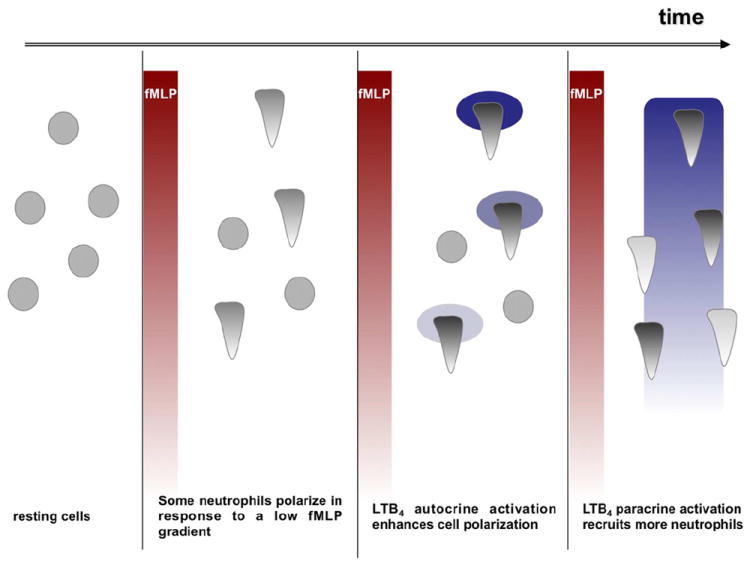
In response to an external fMLP gradient, some neutrophils respond, polarize and release LTB4. The local LTB4 gradient strengthens and stabilizes cell polarization of these first responders. As LTB4 production is fMLP-concentration dependent, a secondary LTB4 gradient is formed parallel to the fMLP gradient. Neutrophils that were not initially responsive to fMLP, sense the secondary gradient of LTB4 and migrate up this gradient towards the fMLP source, thus amplifying the inflammatory response.
In summary, we provide a mechanism where directional cell-to-cell communication regulates neutrophil migration and recruitment to the core of inflammation sites. We envision this mechanism to be important in vivo where the relay of LTB4 signals would enhance neutrophil recruitment to the inflammation core at the initiation of the process, when low concentrations of primary chemoattractants are released. In addition, we predict that LTB4 relay is poised to maintain the inflammation. In fact, it has been shown that in the absence of LTB4 signaling experimentally induced arthritis subsides faster (Chen et al., 2006a; Chou et al., 2010). We propose that in these models, directed neutrophil recruitment to the core of inflammation is enhanced by LTB4 signal relay.
EXPERIMENTAL PROCEDURES
Additional information is found in the Supplemental Information Section.
Materials
Percoll, Histopaque 1077, formyl peptides (fMLP for human neutrophils, and the synthetic WKYMVm peptide for mouse neutrophils), IL8, ionomycin and LY294002 were obtained from Sigma-Aldrich (St. Louis, MO). LTB4, deuterated arachidonic acid, the FLAP inhibitor MK886 and the LTB4 receptors antagonist LY223982 were purchase from Cayman Chemical (Ann Arbor, MI). Anti-p-Akt (clone C31E5E and D9E for residues T308, S473, respectively), anti-phosphorylated myosin light chain 2 (Ser19) and anti-p-Erk1/2 (clone D13.14.4E) rabbit antibodies were all from Cell Signaling Technology (Beverly, MA). Transwell chambers were purchased from Corning Life Sciences (Lowell, MA). WT, alox5-/- and blt1-/- mice were from the Jackson Laboratory (Bar Harbor, ME). Fpr1-/- mice were a generous gift from Philip Murphy (NIAID, NIH).
Isolation of human peripheral blood neutrophils
Heparinized whole blood was obtained by venipuncture from healthy donors. Neutrophils were isolated by dextran sedimentation (3% dextran/0.9% NaCl) coupled to differential centrifugation over Histopaque 1077 (Mahadeo et al., 2007). Residual erythrocytes were removed using of hypotonic lysis with 0.2 and 1.6% saline solutions.
Isolation of mouse bone marrow neutrophils
Mice were sacrificed and the femurs and tibias were removed from both legs. HBSS (without Calcium and Magnesium) with 0.1% BSA was forced trough the bones and the solution was filtered trough a cell strainer. Cells were centrifuged at 400g for 5 min and neutrophils were isolated using a three-layer Percol gradient of 78%, 69% and 52%, as previously described (Boxio et al., 2004). After isolation, neutrophils were resuspended in HBSS with or without 1 μM cytotracker green (Molecular Probes; Invitrogen, Eugene, OR), incubated for 1 h at 37°C, washed, resuspended in RPMI with 10% serum, and incubated for 1 h at 37°C.
LTB4 measurement
LTB4 was measured using an ELISA kit (R&D Systems Minneapolis, MN). Human primary neutrophils were resuspended at 1 × 106 cells/mL in PBS and incubated for 30 min on ice. GM-CSF (10 ng/mL, R&D Systems Minneapolis, MN) was added and neutrophils were further incubated for 1 h at 37°C. Cells were spun down at 400g for 5 min and resuspended (cell density = 15×106 cells/mL) with RPMI and incubated at 37°C until stimulated. After the stimulation, cold PBS was quickly added, neutrophils were centrifuged and supernatants were collected and frozen. Assays were performed according to manufacturers instructions.
Under-agarose assay
Chemotaxis of the neutrophil population was studied using the under-agarose assay as previously described (Comer and Parent, 2006). Cell culture dishes were coated with 1 % BSA in PBS for 1 h at 37 °C. For assays with human peripheral blood neutrophils, 0.5 % agarose in 50 % PBS - 50 % mHBSS was poured and allowed to solidify for 40 min. For assays with mouse bone marrow neutrophils, 1.2 % agarose in 50 % PBS - 50 % RPMI - 10 % FBS was used. Three 1 mm diameter wells were carved at 2 mm distance from each other. A chemoattractant was placed in the middle well 15 min before plating neutrophils. 5×105 neutrophils in 5 μL mHBSS were plated in the outer wells and incubated at 37 °C. Human peripheral blood neutrophils were allowed to chemotax for 2 h unless otherwise mentioned and mouse bone marrow neutrophils were allowed to chemotax for 5 h. The wells with human peripheral blood neutrophils were visualized using a Leica DM IL stereoscope. Assays using human peripheral blood neutrophils were quantified using ImageJ by measuring the distance the cells had migrated directionally towards the chemoattractant. For the mouse bone marrow neutrophils, fluorescent cells were counted using a Zeiss Axiovert S100 epifluorescent microscope.
Coherent Anti-Stokes Raman scattering (CARS) microscopy
Neutrophils were incubated with deuterated AA as reported previously (van Manen et al., 2005). Labeled cells were allowed to migrate in under-agarose assay for 2 h, or stimulated uniformly for 1 or 2 min with 10 nM fMLP. The experimental setup of the broadband CARS microscopy has been described previously (Lee et al., 2011; Parekh et al., 2010). Briefly, the output (70 fs, centered at 830 nm, 80 MHz) of a Ti:S laser oscillator (MaiTai-DeepSee, Spectra-Physics) was split into two parts. One part was introduced into a photonic crystal fiber (Crystal Fibre, Femtowhite) to generate a continuum pulse. The other part was spectrally narrowed by a 4-f dispersion less filter to 10 cm-1 full-width-half-maximum (FWHM) with the center wavelength at 830 nm. The two beams were introduced collinearly and with parallel polarization into a 60X 1.35 NA oil immersion objective lens (Olympus) and focused on the sample. The CARS signal generated from the sample was collected in the forward direction and passed through a set of an 830 nm notch filter and an 810 nm short-pass filter and was analyzed using a charge-coupled device (CCD; DU920-BR-DD, Andor) attached to a monochromator (SP-2300, Acton). The spatial resolution was laterally 500 nm, and the sample was scanned either by 120 nm or 250 nm pixel spacing. The average laser power at the sample was kept below 15 mW for each pulse, to avoid photo-damage. The CCD exposure time is typically 30 ms per pixel. The acquired CARS spectrum was processed by modified Kramers-Kronig phase retrieval and followed by baseline detrending.
Statistical analysis
Analyses were performed in GraphPad Prism software Version 5.0b. One-way ANOVA and Dunnet posthoc test (with untreated cells as the control group) were performed on normalized data with “treatment” as the independent variable and “cAMP level” or “F-actin accumulation” or “number of cells migrating in a transwell assay” as dependent variable (p < 0.05 was considered statistically significant). Friedman and Dunns posthoc test (with untreated cells as the control group) were performed with “treatment” as the independent variable and “normalized MyoII phosphorylation levels” or “distance migrated in under-agarose assay” as dependent variable (p < 0.05 was considered statistically significant). For the asymmetrical distribution, we compared the distribution to a theoretical 0.5 mean value in a Wilcoxon test.
Supplementary Material
HIGHLIGHTS.
LTB4 specifically modulates neutrophil polarization in response to formyl peptides
Formyl peptide-induced LTB4 secretion acts in an autocrine and paracrine fashion
LTB4 secretion amplifies neutrophil migration towards formyl peptides
Secreted LTB4 acts as a signal relay molecule during neutrophil chemotaxis
Acknowledgments
We thank Amy Melpolder and the NIH Blood Bank for providing human blood from healthy volunteers. We are grateful to Dr. Philip Murphy for providing fpr1-/- mice and, in particular, to Dr. Jiliang Gao for his help with the mice. We wish to thank Drs. Ronald Germain and Tim Lämmermann for providing the blt1-/- mice. We also thank the Parent laboratory members for excellent discussions and suggestions and for valuable input on the manuscript. WL participated in this work while on sabbatical at the LCMB, CCR. This research was supported by the Intramural Research Program of the Center for Cancer Research, NCI, National Institutes of Health.
Footnotes
DISCLOSURES
Official contribution of the National Institute of Standards and Technology; not subject to copyright in the United States. Certain commercial equipment, instruments, or materials are identified in this paper in order to specify the experimental procedure adequately. Such identification is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that the materials or equipment identified are necessarily the best available for the purpose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- Berger M, Budhu S, Lu E, Li Y, Loike D, Silverstein SC, Loike JD. Different G(i)-coupled chemoattractant receptors signal qualitatively different functions in human neutrophils. J Leukoc Biol. 2002;71:798–806. [PubMed] [Google Scholar]
- Boxio R, Bossenmeyer-Pourie C, Steinckwich N, Dournon C, Nusse O. Mouse bone marrow contains large numbers of functionally competent neutrophils. J Leukoc Biol. 2004;75:604–611. doi: 10.1189/jlb.0703340. [DOI] [PubMed] [Google Scholar]
- Brito GA, Souza MH, Melo-Filho AA, Hewlett EL, Lima AA, Flores CA, Ribeiro RA. Role of pertussis toxin A subunit in neutrophil migration and vascular permeability. Infect Immun. 1997;65:1114–1118. doi: 10.1128/iai.65.3.1114-1118.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canetti CA, Leung BP, Culshaw S, McInnes IB, Cunha FQ, Liew FY. IL-18 enhances collagen-induced arthritis by recruiting neutrophils via TNF-alpha and leukotriene B4. J Immunol. 2003;171:1009–1015. doi: 10.4049/jimmunol.171.2.1009. [DOI] [PubMed] [Google Scholar]
- Chen M, Lam BK, Kanaoka Y, Nigrovic PA, Audoly LP, Austen KF, Lee DM. Neutrophil-derived leukotriene B4 is required for inflammatory arthritis. J Exp Med. 2006a;203:837–842. doi: 10.1084/jem.20052371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XS, Sheller JR, Johnson EN, Funk CD. Role of leukotrienes revealed by targeted disruption of the 5-lipoxygenase gene. Nature. 1994;372:179–182. doi: 10.1038/372179a0. [DOI] [PubMed] [Google Scholar]
- Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006b;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- Chou RC, Kim ND, Sadik CD, Seung E, Lan Y, Byrne MH, Haribabu B, Iwakura Y, Luster AD. Lipid-cytokine-chemokine cascade drives neutrophil recruitment in a murine model of inflammatory arthritis. Immunity. 2010;33:266–278. doi: 10.1016/j.immuni.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer FI, Parent CA. Phosphoinositide 3-kinase activity controls the chemoattractant-mediated activation and adaptation of adenylyl cyclase. Mol Biol Cell. 2006;17:357–366. doi: 10.1091/mbc.E05-08-0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton M, Claing A. G protein-coupled receptors stimulation and the control of cell migration. Cell Signal. 2009;21:1045–1053. doi: 10.1016/j.cellsig.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Crooks SW, Stockley RA. Leukotriene B4. Int J Biochem Cell Biol. 1998;30:173–178. doi: 10.1016/s1357-2725(97)00123-4. [DOI] [PubMed] [Google Scholar]
- Dahinden CA, Zingg J, Maly FE, de Weck AL. Leukotriene production in human neutrophils primed by recombinant human granulocyte/macrophage colony-stimulating factor and stimulated with the complement component C5A and FMLP as second signals. J Exp Med. 1988;167:1281–1295. doi: 10.1084/jem.167.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Rericha EC, Bagorda A, Parent CA. Direct Biochemical Measurements of Signal Relay during Dictyostelium Development. J Biol Chem. 2011;286:38649–38658. doi: 10.1074/jbc.M111.284182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser J, Gehrmann U, D’Alexandri FL, Hidalgo-Estevez AM, Wheelock CE, Scheynius A, Gabrielsson S, Radmark O. Exosomes from human macrophages and dendritic cells contain enzymes for leukotriene biosynthesis and promote granulocyte migration. J Allergy Clin Immunol. 2010;126:1032–1040. 1040, e1031–1034. doi: 10.1016/j.jaci.2010.06.039. [DOI] [PubMed] [Google Scholar]
- Ferreira AM, Isaacs H, Hayflick JS, Rogers KA, Sandig M. The p110delta isoform of PI3K differentially regulates beta1 and beta2 integrin-mediated monocyte adhesion and spreading and modulates diapedesis. Microcirculation. 2006;13:439–456. doi: 10.1080/10739680600776062. [DOI] [PubMed] [Google Scholar]
- Ford-Hutchinson AW, Bray MA, Doig MV, Shipley ME, Smith MJ. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 1980;286:264–265. doi: 10.1038/286264a0. [DOI] [PubMed] [Google Scholar]
- Foxman EF, Campbell JJ, Butcher EC. Multistep navigation and the combinatorial control of leukocyte chemotaxis. J Cell Biol. 1997;139:1349–1360. doi: 10.1083/jcb.139.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao JL, Lee EJ, Murphy PM. Impaired antibacterial host defense in mice lacking the N-formylpeptide receptor. J Exp Med. 1999;189:657–662. doi: 10.1084/jem.189.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillard J, Ford-Hutchinson AW, Chan C, Charleson S, Denis D, Foster A, Fortin R, Leger S, McFarlane CS, Morton H, et al. L-663,536 (MK-886) (3-[1-(4-chlorobenzyl)-3-t-butyl-thio-5-isopropylindol-2-yl]-2,2 - dimethylpropanoic acid), a novel, orally active leukotriene biosynthesis inhibitor. Can J Physiol Pharmacol. 1989;67:456–464. doi: 10.1139/y89-073. [DOI] [PubMed] [Google Scholar]
- Grespan R, Fukada SY, Lemos HP, Vieira SM, Napimoga MH, Teixeira MM, Fraser AR, Liew FY, McInnes IB, Cunha FQ. CXCR2-specific chemokines mediate leukotriene B4-dependent recruitment of neutrophils to inflamed joints in mice with antigen-induced arthritis. Arthritis Rheum. 2008;58:2030–2040. doi: 10.1002/art.23597. [DOI] [PubMed] [Google Scholar]
- He R, Tan L, Browning DD, Wang JM, Ye RD. The synthetic peptide Trp-Lys-Tyr-Met-Val-D-Met is a potent chemotactic agonist for mouse formyl peptide receptor. J Immunol. 2000;165:4598–4605. doi: 10.4049/jimmunol.165.8.4598. [DOI] [PubMed] [Google Scholar]
- Heit B, Kubes P. Measuring chemotaxis and chemokinesis: the under-agarose cell migration assay. Sci STKE. 2003;2003:PL5. doi: 10.1126/stke.2003.170.pl5. [DOI] [PubMed] [Google Scholar]
- Heit B, Liu L, Colarusso P, Puri KD, Kubes P. PI3K accelerates, but is not required for, neutrophil chemotaxis to fMLP. J Cell Sci. 2008;121:205–214. doi: 10.1242/jcs.020412. [DOI] [PubMed] [Google Scholar]
- Heit B, Tavener S, Raharjo E, Kubes P. An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. J Cell Biol. 2002;159:91–102. doi: 10.1083/jcb.200202114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson WT, Boyd RJ, Froelich LL, Mallett BE, Gapinski DM. Specific inhibition of leukotriene B4-induced neutrophil activation by LY223982. J Pharmacol Exp Ther. 1992;263:1009–1014. [PubMed] [Google Scholar]
- Jethwaney D, Islam MR, Leidal KG, de Bernabe DB, Campbell KP, Nauseef WM, Gibson BW. Proteomic analysis of plasma membrane and secretory vesicles from human neutrophils. Proteome Sci. 2007;5:12. doi: 10.1186/1477-5956-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan S. Amplification of extracellular nucleotide-induced leukocyte(s) degranulation by contingent autocrine and paracrine mode of leukotriene-mediated chemokine receptor activation. Med Hypotheses. 2002;59:261–265. doi: 10.1016/s0306-9877(02)00213-x. [DOI] [PubMed] [Google Scholar]
- Kim ND, Chou RC, Seung E, Tager AM, Luster AD. A unique requirement for the leukotriene B4 receptor BLT1 for neutrophil recruitment in inflammatory arthritis. J Exp Med. 2006;203:829–835. doi: 10.1084/jem.20052349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleuss C, Scherubl H, Hescheler J, Schultz G, Wittig B. Different beta-subunits determine G-protein interaction with transmembrane receptors. Nature. 1992;358:424–426. doi: 10.1038/358424a0. [DOI] [PubMed] [Google Scholar]
- Kleuss C, Scherubl H, Hescheler J, Schultz G, Wittig B. Selectivity in signal transduction determined by gamma subunits of heterotrimeric G proteins. Science. 1993;259:832–834. doi: 10.1126/science.8094261. [DOI] [PubMed] [Google Scholar]
- Kriebel PW, Barr VA, Rericha EC, Zhang G, Parent CA. Collective cell migration requires vesicular trafficking for chemoattractant delivery at the trailing edge. J Cell Biol. 2008;183:949–961. doi: 10.1083/jcb.200808105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronlage M, Song J, Sorokin L, Isfort K, Schwerdtle T, Leipziger J, Robaye B, Conley PB, Kim HC, Sargin S, et al. Autocrine purinergic receptor signaling is essential for macrophage chemotaxis. Sci Signal. 2010;3:ra55. doi: 10.1126/scisignal.2000588. [DOI] [PubMed] [Google Scholar]
- Kuniyeda K, Okuno T, Terawaki K, Miyano M, Yokomizo T, Shimizu T. Identification of the intracellular region of the leukotriene B4 receptor type 1 that is specifically involved in Gi activation. J Biol Chem. 2007;282:3998–4006. doi: 10.1074/jbc.M610540200. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Moon D, Migler KB, Cicerone MT. Quantitative image analysis of broadband CARS hyperspectral images of polymer blends. Anal Chem. 2011;83:2733–2739. doi: 10.1021/ac103351q. [DOI] [PubMed] [Google Scholar]
- Liu L, Das S, Losert W, Parent CA. mTORC2 regulates neutrophil chemotaxis in a cAMP- and RhoA-dependent fashion. Dev Cell. 2010;19:845–857. doi: 10.1016/j.devcel.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lee YJ, Cicerone MT. Broadband CARS spectral phase retrieval using a time-domain Kramers-Kronig transform. Opt Lett. 2009;34:1363–1365. doi: 10.1364/ol.34.001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadeo DC, Janka-Junttila M, Smoot RL, Roselova P, Parent CA. A chemoattractant-mediated Gi-coupled pathway activates adenylyl cyclase in human neutrophils. Mol Biol Cell. 2007;18:512–522. doi: 10.1091/mbc.E06-05-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann CP, Kriebel PW, Parent CA, Losert W. Cell speed, persistence and information transmission during signal relay and collective migration. J Cell Sci. 2010;123:1724–1731. doi: 10.1242/jcs.060137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald B, Kubes P. Chemokines: sirens of neutrophil recruitment-but is it just one song? Immunity. 2010;33:148–149. doi: 10.1016/j.immuni.2010.08.006. [DOI] [PubMed] [Google Scholar]
- McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- McDonald PP, McColl SR, Naccache PH, Borgeat P. Activation of the human neutrophil 5-lipoxygenase by leukotriene B4. Br J Pharmacol. 1992;107:226–232. doi: 10.1111/j.1476-5381.1992.tb14491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meliton AY, Munoz NM, Meliton LN, Binder DC, Osan CM, Zhu X, Dudek SM, Leff AR. Cytosolic group IVa phospholipase A2 mediates IL-8/CXCL8-induced transmigration of human polymorphonuclear leukocytes in vitro. J Inflamm (Lond) 2010;7:14. doi: 10.1186/1476-9255-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migeotte I, Communi D, Parmentier M. Formyl peptide receptors: a promiscuous subfamily of G protein-coupled receptors controlling immune responses. Cytokine Growth Factor Rev. 2006;17:501–519. doi: 10.1016/j.cytogfr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Mita H, Yui Y, Yasueda H, Shida T. Isocratic determination of arachidonic acid 5-lipoxygenase products in human neutrophils by high-performance liquid chromatography. J Chromatogr. 1988;430:299–308. doi: 10.1016/s0378-4347(00)83165-5. [DOI] [PubMed] [Google Scholar]
- Monteiro AP, Pinheiro CS, Luna-Gomes T, Alves LR, Maya-Monteiro CM, Porto BN, Barja-Fidalgo C, Benjamim CF, Peters-Golden M, Bandeira-Melo C, et al. Leukotriene B4 Mediates Neutrophil Migration Induced by Heme. J Immunol. 2011 doi: 10.4049/jimmunol.1002400. [DOI] [PubMed] [Google Scholar]
- Oakes PW, Patel DC, Morin NA, Zitterbart DP, Fabry B, Reichner JS, Tang JX. Neutrophil morphology and migration are affected by substrate elasticity. Blood. 2009;114:1387–1395. doi: 10.1182/blood-2008-11-191445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orelio C, Kuijpers TW. Shwachman-Diamond syndrome neutrophils have altered chemoattractant-induced F-actin polymerization and polarization characteristics. Haematologica. 2009;94:409–413. doi: 10.3324/haematol.13733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh SH, Lee YJ, Aamer KA, Cicerone MT. Label-free cellular imaging by broadband coherent anti-Stokes Raman scattering microscopy. Biophys J. 2010;99:2695–2704. doi: 10.1016/j.bpj.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegatta F, Radaelli A, Heltai S, Yan L, Chierchia SL, Folli F. Evidence for the involvement of phosphatidylinositol 3-kinase in fMLP-stimulated neutrophil adhesion to ICAM-1-transfected cells. J Cardiovasc Pharmacol. 2001;37:751–761. doi: 10.1097/00005344-200106000-00013. [DOI] [PubMed] [Google Scholar]
- Peters-Golden M, Henderson WR., Jr Leukotrienes. N Engl J Med. 2007;357:1841–1854. doi: 10.1056/NEJMra071371. [DOI] [PubMed] [Google Scholar]
- Ramos CD, Canetti C, Souto JT, Silva JS, Hogaboam CM, Ferreira SH, Cunha FQ. MIP-1alpha[CCL3] acting on the CCR1 receptor mediates neutrophil migration in immune inflammation via sequential release of TNF-alpha and LTB4. J Leukoc Biol. 2005;78:167–177. doi: 10.1189/jlb.0404237. [DOI] [PubMed] [Google Scholar]
- Ribeiro RA, Souza-Filho MV, Souza MH, Oliveira SH, Costa CH, Cunha FQ, Ferreira HS. Role of resident mast cells and macrophages in the neutrophil migration induced by LTB4, fMLP and C5a des arg. Int Arch Allergy Immunol. 1997;112:27–35. doi: 10.1159/000237427. [DOI] [PubMed] [Google Scholar]
- Rochon YP, Frojmovic MM. Regulation of human neutrophil aggregation: comparable latent times, activator sensitivities, and exponential decay in aggregability for FMLP, platelet-activating factor, and leukotriene B4. Blood. 1993;82:3460–3468. [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Serio KJ, Baker JR, Ring WL, Riddick CA, Bigby TD. Leukotriene B4 costimulates 5-lipoxygenase activity in neutrophils via increased 5-lipoxygenase translocation. Am J Physiol. 1997;272:C1329–1334. doi: 10.1152/ajpcell.1997.272.4.C1329. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Hunt SW., 3rd Regulating integrin-mediated adhesion: one more function for PI 3-kinase? Immunol Today. 1996;17:565–573. doi: 10.1016/s0167-5699(96)10061-x. [DOI] [PubMed] [Google Scholar]
- Silva MT. Neutrophils and macrophages work in concert as inducers and effectors of adaptive immunity against extracellular and intracellular microbial pathogens. J Leukoc Biol. 2010 doi: 10.1189/jlb.1109767. [DOI] [PubMed] [Google Scholar]
- Sitrin RG, Emery SL, Sassanella TM, Blackwood RA, Petty HR. Selective localization of recognition complexes for leukotriene B4 and formyl-Met-Leu-Phe within lipid raft microdomains of human polymorphonuclear neutrophils. J Immunol. 2006;177:8177–8184. doi: 10.4049/jimmunol.177.11.8177. [DOI] [PubMed] [Google Scholar]
- Soehnlein O, Lindbom L, Weber C. Mechanisms underlying neutrophil-mediated monocyte recruitment. Blood. 2009;114:4613–4623. doi: 10.1182/blood-2009-06-221630. [DOI] [PubMed] [Google Scholar]
- Tager AM, Dufour JH, Goodarzi K, Bercury SD, von Andrian UH, Luster AD. BLTR mediates leukotriene B(4)-induced chemotaxis and adhesion and plays a dominant role in eosinophil accumulation in a murine model of peritonitis. J Exp Med. 2000;192:439–446. doi: 10.1084/jem.192.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager AM, Luster AD. BLT1 and BLT2: the leukotriene B(4) receptors. Prostaglandins Leukot Essent Fatty Acids. 2003;69:123–134. doi: 10.1016/s0952-3278(03)00073-5. [DOI] [PubMed] [Google Scholar]
- Tomhave ED, Richardson RM, Didsbury JR, Menard L, Snyderman R, Ali H. Cross-desensitization of receptors for peptide chemoattractants. Characterization of a new form of leukocyte regulation. J Immunol. 1994;153:3267–3275. [PubMed] [Google Scholar]
- Tsuda Y, Takahashi H, Kobayashi M, Hanafusa T, Herndon DN, Suzuki F. Three different neutrophil subsets exhibited in mice with different susceptibilities to infection by methicillin-resistant Staphylococcus aureus. Immunity. 2004;21:215–226. doi: 10.1016/j.immuni.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Uden AM, Hafstrom I, Palmblad J. Relation to chemotactic factor gradients to neutrophil migration and orientation under agarose. J Leukoc Biol. 1986;39:27–35. doi: 10.1002/jlb.39.1.27. [DOI] [PubMed] [Google Scholar]
- van Manen HJ, Kraan YM, Roos D, Otto C. Single-cell Raman and fluorescence microscopy reveal the association of lipid bodies with phagosomes in leukocytes. Proc Natl Acad Sci U S A. 2005;102:10159–10164. doi: 10.1073/pnas.0502746102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JD, Lee TH, Lewis RA, Austen F. Intracellular retention of the 5-lipoxygenase pathway product, leukotriene B4, by human neutrophils activated with unopsonized zymosan. J Immunol. 1985;134:2624–2630. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


