Abstract
Introduction
For patients with resectable hepatocellular carcinoma (HCC), hepatectomy remains one of the best treatment options to provide long-term survival. However, more than 50% of the patients have unresectable disease upon diagnosis even though there are no distant metastases. Transarterial chemoembolization (TACE) is a well-established treatment option that offers a palliative survival benefit for this group of patients. A better treatment for unresectable HCC has been sought after. There is some evidence that transarterial radioembolization (TARE) with the agent yttrium-90 produces encouraging outcomes, especially in patients with portal vein tumor thrombus. This study aims to analyze the outcomes of TARE at our center.
Methods
From August 2009 to April 2013, 16 patients underwent TARE at our center. Sixteen patients with similar tumor characteristics were selected to undergo TACE alone for comparison. A retrospective analysis of the prospectively collected data of the patients was conducted. Only patients with newly diagnosed primary tumors were included in this study.
Results
The median survival for patients having TARE was 19.9 versus 14.0 months in the TACE group (P=0.615). There was no difference in terms of tumor response according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) (P=0.632). The 1-, 2- and 3-year survival rates in the TARE group were 80.0%, 30.5% and 20.3% respectively. The 1-year survival in the TACE group was 58.3% (P=0.615). For patients who had major vascular invasion (eight in each group), the 1- and 2-year survival rates in the TARE group were 62.5% and 15.6% respectively, while the 1-year survival in the TACE group was 35.0% (P=0.664).
Conclusions
The two groups showed similar results in terms of tumor response and overall survival benefit. TARE might provide a survival benefit for patients with major vessel invasion.
Keywords: Hepatocellular carcinoma (HCC), inoperable, transarterial chemoembolization (TACE), transarterial radioembolization (TARE), unresectable, vascular invasion, yttrium-90
Introduction
Hepatocellular carcinoma (HCC) is a major healthcare problem, particularly in Asia where hepatitis B is prevalent. Some HCC patients are unable to undergo surgery due to underlying comorbidities, poor liver function reserve, or advanced tumor status (1,2). Transarterial chemoembolization (TACE) is a well-established palliative treatment option (3-5), but it is not suitable for all inoperable HCC patients as it also carries the risk of complications (6). Child-Pugh C cirrhosis, biliary obstruction, severe encephalopathy and main portal vein tumor thrombosis are contraindications to TACE. Minor complications include postembolization syndrome, which occurs in up to 90% of patients (7), while hepatic decompensation may occur in up to 20% of patients (8). Targeted therapy such as sorafenib treatment only offers a limited survival benefit (9,10) but is associated with significant adverse effects including skin-related toxicities, diarrhea, neutropenic fever, hypertension, and proteinuria, as well as life-threatening complications such as thromboembolism and bowel perforation. [Nexavar (package insert), Wayne, NJ: Bayer HealthCare Pharmaceuticals Inc; 2009] [Launay-Vacher V, Deray G. Hypertension and proteinuria: a class-effect of antiangiogenic therapies. Anticancer Drugs. 2009]. A better treatment for unresectable HCC has been sought after. There is some evidence that transarterial radioembolization (TARE) using yttrium-90 yields some encouraging results (11-14), especially in patients who suffer from portal vein tumor thrombus, which is a contraindication to TACE (2,15,16).
TARE and TACE have been shown to have similar toxicity and survival outcome (17-19). Some patients having TARE were found to have their disease downstaged and better disease-free survival (20). This study compares the effects of TARE and TACE in patients with inoperable advanced HCC at our center.
Patients and methods
From August 2009 to April 2013, 16 patients underwent TARE at Queen Mary Hospital. All of them had unresectable advanced HCC. They were chosen to undergo TARE according to the following five criteria: (I) their TACE treatment had failed or they had a contraindication to TACE (e.g., portal vein thrombosis); (II) their life expectancy was >3 months; (III) their pre-treatment 99Tc macroaggregated albumin (MAA) scans showed <30 Gy radiation exposure to the lung (percentage of lung shunting <20%) or no significant extrahepatic deposition of 99Tc MAA in the gastrointestinal tract that could not be corrected by the catheter embolization technique; (IV) they had a serum total bilirubin level of <34 mmol/L; (V) they had no angiographic contraindications (e.g., renal insufficiency, thrombocytopenia/coagulopathy, contrast nephropathy). Portal vein thrombosis was not regarded as a contraindication to TARE. In fact there were case reports of recanalization of portal vein thrombosis due to tumor invasion after TARE (21). TARE was conducted by the Department of Radiology in conjunction with the Department of Clinical Oncology.
A retrospective analysis of the prospectively collected data of the patients was conducted. A matched cohort of 16 patients with similar tumor characteristics in the same period were selected to undergo TACE alone. Matching criteria included demographic characteristics, comorbidities, tumor size, tumor number, and the presence of major vascular invasion. Imaging studies were reviewed and clinical outcomes were abstracted from the medical records. We arbitrarily used “5 cm” to differentiate large and small tumors, and a tumor number of three was used as the cutoff to categorize the patients into two groups. The two groups had similar periods of follow-up.
These patients were considered inoperable after careful evaluation of their tumor status. The primary endpoint of the study was tumor response according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST). The secondary endpoint was survival. Only patients who were newly diagnosed of HCC were included in the study. All of them were carriers of hepatitis B virus. The diagnoses of HCC fulfilled the guidelines published in the American Association for the Study of Liver Disease in 2010 (2). Triphasic computed tomography or magnetic resonance imaging, or both, was used for diagnosis. This study was overseen by the Institutional Review Board of The University of Hong Kong. Informed consent by the patients was obtained before recruitment.
Procedures
TACE treatment
Before treatment, the patients were hydrated and blood tests were conducted for complete blood picture, liver and renal functions, clotting profile, and alpha-fetoprotein level. Fresh frozen plasma or platelet concentrate was given accordingly. Intravenous amoxicillin-clavulanic acid (1.2 gm), mannitol (20 gm), tropisetron (5 mg) and pantoprazole (40 mg) were prescribed before the procedure. The femoral artery was catheterized under local anesthesia. Hepatic arteriography and superior mesenteric arterial portovenography were performed to define the sizes and locations of tumor nodules and to exclude occlusion of the main portal vein. The right or left hepatic artery feeding the tumor was super-selectively catheterized. An emulsion was prepared by mixing cisplatin (1 mg/mL) with lipiodol in a volume ratio of 1 to 1. Various amounts of the emulsion, up to a maximum of 60 mL (containing 30 mg of cisplatin), were injected slowly under fluoroscopic monitoring according to the size of the tumor and the arterial blood flow. If the tumor involved both lobes of the liver or if super-selective catheterization was not possible, the emulsion was injected into the proper hepatic artery distal to the origin of the gastroduodenal artery. This was followed by embolization with small gelatin-sponge pellets of a diameter of 1 mm mixed with 40 mg of gentamicin. After the procedure, oral amoxicillin-clavulanic acid (375 mg 3 times per day) and pantoprazole (40 mg per day) were administered for 3 days. Discharge from the hospital was decided according to individual patients’ clinical status. TACE was repeated every 2 to 3 months. If poor liver function (serum total bilirubin level >50 µmol/L or presence of ascites), poor renal function (serum creatinine level >120 µmol/L), vascular contraindications, severe adverse effects or progressive disease developed, TACE was withheld or discontinued.
Pre-TARE imaging evaluation
Every patient underwent pretreatment mesenteric angiography and 99 Tc MAA scanning to look at the vascular anatomy in relation to the tumor as well as the degree of pulmonary shunting. Pulmonary shunting of ≥20% might result in toxicity and complications due to systemic distribution and absorption of radioactive substances during TARE. The problem could be reduced by embolizing the non-target vessels to minimize the flow of yttrium particles into the uninvolved liver and extrahepatic tissues.
Yttrium-90 microsphere treatment for TARE
Before treatment, the patients were hydrated and blood tests were conducted for complete blood picture, liver and renal functions, clotting profile, and alpha-fetoprotein level. Fresh frozen plasma or platelet concentrate was given accordingly. Intravenous amoxicillin-clavulanic acid (1.2 gm), mannitol (20 gm), tropisetron (5 mg) and pantoprazole (40 mg) were prescribed before the procedure. The femoral artery was catheterized under local anesthesia. Hepatic arteriography and superior mesenteric arterial portovenography were performed. The right or left hepatic artery feeding the tumor was super-selectively catheterized. Yttrium-90 resin microspheres were then administered into the feeding artery. After the procedure, oral amoxicillin-clavulanic acid (375 mg 3 times per day) and pantoprazole (40 mg per day) were administered for 3 days. Discharge from the hospital was decided according to individual patients’ clinical status.
Posttreatment assessment
All patients who had undergone TACE or TARE were subjected to computed tomography or magnetic resonance imaging in 2 months’ time after the initial intervention to assess the treatment effect. The mRECIST were adopted to assess the treatment responses, which were classified as complete response, partial response, progressive disease, and stable disease. Complete response was defined as the disappearance of intratumoral arterial enhancement in all target lesions. Partial response was defined as an at least 30% decrease in the sum of diameters of viable (with enhancement in the arterial phase) target lesions, with the baseline sum of the diameters of target lesions taken as reference. Progressive disease was defined as an at least 20% increase in the sum of the diameters of viable target lesions, with the smallest sum of the diameters of viable target lesions recorded since the beginning of treatment taken as reference. Stable disease was defined as any case that did not qualify as partial response or progressive disease (22).
Imaging analysis
Imaging interpretations were performed by two consultant radiologists with special interests in hepatobiliary imaging. All baseline imaging results were calculated, and the results of the reassessment imaging performed after 2 months were studied. An imaging was performed 2 months after the first treatment, and another imaging was performed after the second treatment. If no further treatment was given, subsequent scans were performed at 90-day intervals. The mRECIST were used to assess treatment responses (22).
Follow-up
Every patient was followed up at our clinic 4 weeks after discharge. Blood tests for complete blood picture, liver and renal functions, clotting profile and alpha-fetoprotein level were conducted the day before to assess the patient’s clinical status and well-being after the intervention. Follow-up appointments were arranged after reassessment computed tomography scans had been done. Depending on the patient’s response to treatment, further TARE or TACE might be given. If progressive disease was evidenced on computed tomography, further treatment would not be given.
Statistical analysis
All survival analyses were calculated from the dates of the first TACE or TARE treatments. Pearson’s chi-squared test or Fisher’s exact test was used for categorical variables. Continuous variables were expressed as medians and ranges and were compared by the Mann-Whitney U test. The Kaplan-Meier method was used for overall survival analysis, and the log-rank test was used for survival comparison. P values <0.05 were considered statistically significant. The computer software SPSS, version 21.0, was used for statistical analyses.
Results
In the study period, 16 patients underwent TARE (the TARE group). All of them had unresectable advanced HCC. A matched cohort of 16 patients with similar tumor characteristics in the same period were selected to undergo TACE alone (the TACE group). The two groups of patients were matched according to tumor size (if a patient had two or more tumors, the size of the largest tumor was considered), tumor number, and the presence of tumor invasion of a major branch of the portal or hepatic vein. The two groups of patients had the same tumor status. The two groups were similar in terms of patient characteristics, pretreatment liver function, and comorbidity (Table 1). Table 2 compares the two groups in terms of tumor characteristics, and Table 3 compares them in terms of hospital mortality, complication, and treatment responses. All the patients underwent both treatment procedures safely.
Table 1. Comparison of clinical characteristics.
| TARE group (n=16) | TACE group (n=16) | P | |
|---|---|---|---|
| Male:female | 15:1 | 13:3 | 0.593 |
| Age [years] | 55 [37-73] | 62.5 [48-78] | 0.067 |
| Follow-up (months) | 12.0 (2.3-52.5) | 8.3 (2.9-41.1) | 1 |
| Child-Pugh class (%) | 1 | ||
| A | 15 (93.8) | 14 (87.5) | |
| B | 1 (6.3) | 2 (12.5) | |
| HBsAg positive (%) | 12 (75.0) | 13 (81.3) | 1 |
| Carrier of hepatitis C virus (%) | 0 | 3 (27.3) | 0.387 |
| Alpha-fetoprotein [ng/mL] | 38 [4-8,312] | 242.5 [3-7,957] | 0.323 |
| ICG15 | 15.3 (7.4-55.7) | 10 (6-62.9) | 0.622 |
| Albumin [g/L] | 40 [26-47] | 36.5 [21-45] | 0.171 |
| Creatinine [μmol/L] | 81 [59-129] | 77.5 [69-105] | 0.926 |
| Platelet count [×109] | 163 [67-549] | 171.5 [51-384] | 0.897 |
| International normalized ratio | 1.1 (0.9-1.4) | 1.1 (1-1.4) | 0.381 |
| Total bilirubin [μmol/L] | 10 [5-28] | 15 [8-34] | 0.119 |
| Aspartate transaminase [U/L] | 69.5 [29-513] | 77 [31-644] | 0.590 |
| Alanine transaminase [U/L] | 64.5 [31-194] | 47 [25-200] | 0.445 |
| Hemoglobin (g/dL) | 12.65 (9.4-16.6) | 12 (8.5-14.6) | 0.149 |
| No. of patients with comorbidity (%) | 6 (37.5) | 7 (43.8) | 0.719 |
| Cardiovascular disease (%) | 5 (31.3) | 6 (37.5) | 1 |
| Pulmonary disease (%) | 0 | 1 (6.3) | 1 |
| Renal impairment (%) | 0 | 0 | 1 |
| Diabetes mellitus (%) | 3 (18.8) | 3 (18.8) | 1 |
| Gastrointestinal disease | 0 | 2 (12.5) | 0.465 |
| Pretreatment MELD score | 7.5 [6-12] | 8.5 [6-12] | 0.061 |
Data are shown in medians with ranges or numbers with percentages. ICG15, indocyanine green retention rate at 15 minutes; MELD, model for end-stage liver disease; TARE, transarterial radioembolization; TACE, transarterial chemoembolization.
Table 2. Comparison of tumor characteristics.
| TARE group (n=16) | TACE group (n=16) | P | |
|---|---|---|---|
| Tumor size (cm) | 8.4 (2.5-22) | 8.0 (1.5-21.2) | 0.897 |
| Tumor number (%) | 0.532 | ||
| One | 7 (43.8) | 6 (37.5) | |
| Two | 1 (6.3) | 0 | |
| Three | 1 (6.3) | 3 (18.8) | |
| Four | 0 | 1 (6.3) | |
| More than four | 7 (43.8) | 6 (37.5) | |
| Tumor number | 2.5 (1-multiple) | 3 (1-multiple) | 0.926 |
| Absence of invasion of a major branch of the portal or hepatic vein (%) | 8 (50.0) | 8 (50.0) | 1 |
| Presence of invasion of a major branch of the portal or hepatic vein (%) | 8 (50.0) | 8 (50.0) | – |
| Percentage of shunting | 6.6 (3.4-16.1) | – | – |
| Tumor to normal liver count ratio | 3.8 (0.32-11) | – | – |
| Portal vein involvement (%) | 8 (50.0) | 0 | – |
Data are shown in medians with ranges or numbers with percentages.
Table 3. Comparison of treatment responses.
| TARE group (n=16) | TACE group (n=16) | P | |
|---|---|---|---|
| Tumor response (%) | 0.632 | ||
| Complete response | 0 | 0 | |
| Partial response | 4 (25.0) | 2 (12.5) | |
| Stable disease | 5 (31.3) | 5 (31.3) | |
| Progress disease | 7 (43.8) | 9 (56.3) | |
| Hospital mortality (%) | 0 | 0 | – |
| No. of patients with complication (%) | 1 (6.3) | 0 | 1 |
| Severe ascites requiring tapping | 1 | 0 |
The median overall survival duration was 19.9 months in the TARE group and 14.0 months in the TACE group. The 3-year overall survival rate was 20.3% in the former and 9.7% in the latter (P=0.615) (Figure 1). Subgroup analyses of overall survival of patients who had and had not major vascular invasion were performed and comparisons were made between the TARE and TACE groups. In the subgroup with presence of major vascular invasion, patients in the TARE group had a median survival duration of 12.0 months and a 2-year survival rate of 15.6%, whereas their counterparts had a median survival duration of 8.0 months and a 1-year survival rate of 35% (P=0.664) (Figure 2). In the subgroup with absence of major vascular invasion, patients in the TARE group had a median survival duration of 21.2 months and a 3-year survival rate of 50.0%, whereas their counterparts had a median survival duration of 19.8 months and a 3-year survival rate of 15.0% (P=0.370) (Figure 3).
Figure 1.
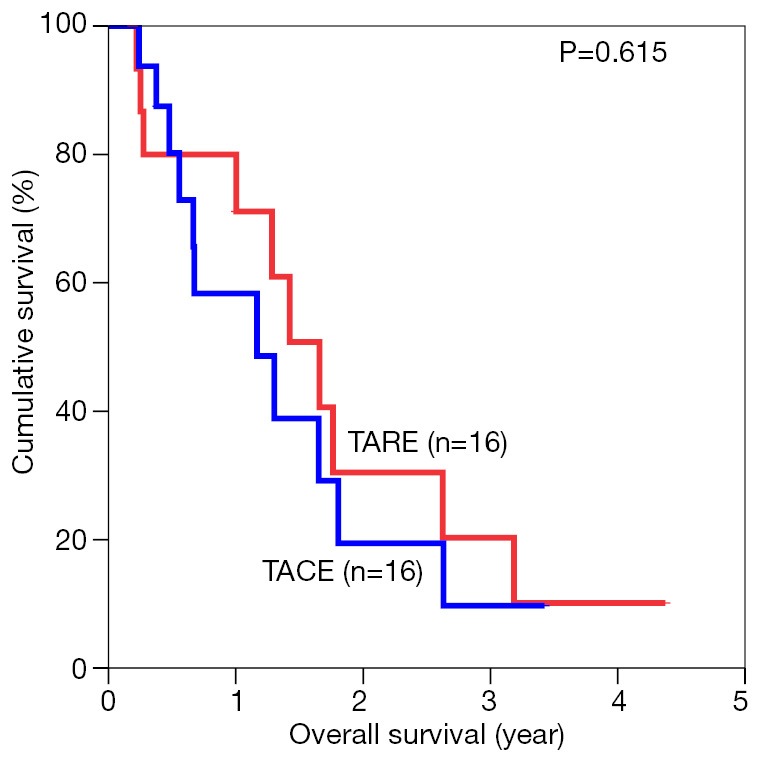
Overall survival of all patients in the two groups. TARE, transarterial radioembolization; TACE, transarterial chemoembolization.
Figure 2.
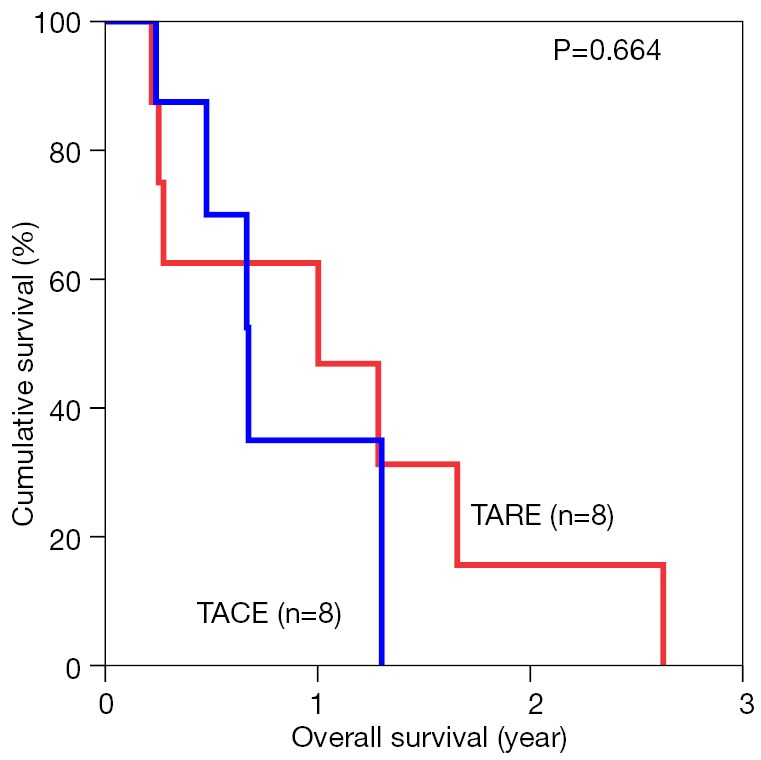
Overall survival of patients with major vascular invasion in the two groups. TARE, transarterial radioembolization; TACE, transarterial chemoembolization.
Figure 3.
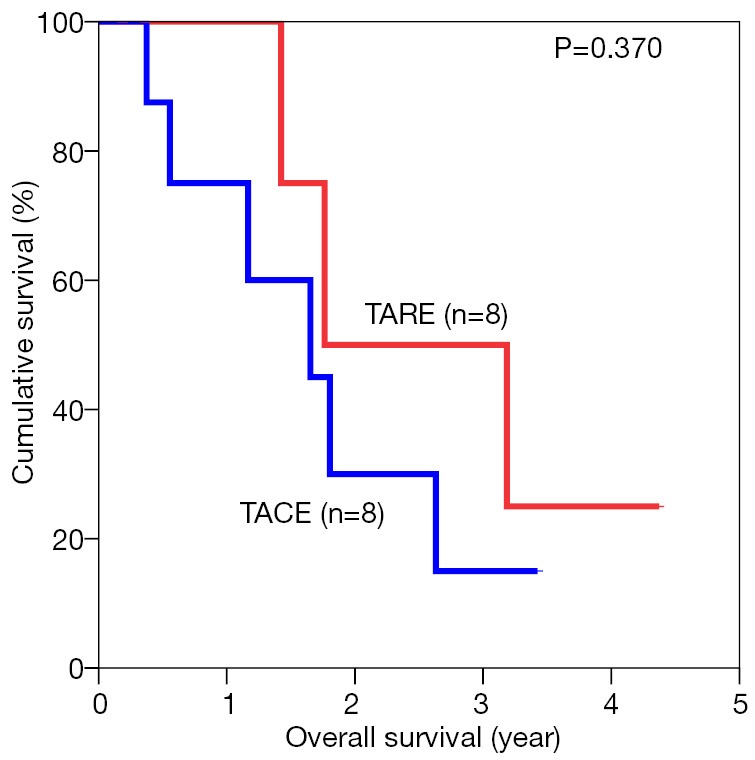
Overall survival of patients without major vascular invasion in the two groups. TARE, transarterial radioembolization; TACE, transarterial chemoembolization.
Discussion
HCC poses a common surgical problem in Asia. The etiological factors in Asian patients are different from those in Western patients. In Asian patients, chronic hepatitis B infection is the most common cause of HCC. Most of them present late with advanced disease and poor liver function associated with cirrhosis, which leave them with limited treatment choices. These patients cannot undergo liver transplantation or liver resection due to their advanced tumor status and poor liver function. The majority of patients with unresectable HCC are treated by various palliative therapies, such as TACE and TARE. External beam radiation therapy has been shown to cause objective response and promising palliative effects (23-25). However, it is associated with significant morbidities, and there are technical difficulties associated with the delivery of radiation in a breathing subject (26). TARE aims to deliver adequate radiation doses to the tumor while keeping the level of toxicity affecting the functional liver parenchyma at the minimum and preserving the blood flow of the target area (27,28).
The present study excluded patients who had advanced tumors with extrahepatic metastases or main portal vein thrombosis (the most common vascular contraindication) and patients who had poor liver function, because TARE and TACE might further worsen their liver function and might even cause liver failure and death.
A previous study by our center had shown that TACE has a significant role in the management of patients with unresectable advanced HCC (4). In the present study, we used TACE as a standard treatment for patients who had unresectable HCC but no contraindication and compared its effectiveness with that of TARE using yttrium-90. The TARE group had a partial response rate of 25%, versus 12.5% in the TACE group. The difference did not reach statistical significance, probably because of the limitation posed by the small number of patients. Further studies with patient cohorts large enough are required to investigate whether this increase in partial response will subsequently translate into any survival benefit.
Patients who were identified to undergo TARE were subjected to MAA screening and hepatic angiography to uncover their vascular anatomy and to identify the most suitable approach, i.e., the unilobar or the bilobar treatment approach, for them. Unfortunately, because of the potential radiotoxicity of the shunting effect, arteriovenous shunting of more than 20% was regarded as a contraindication to TARE.
There was no difference between the two groups in terms of side effects, and TARE was found to be safe, causing no significant immediate side effect. It has been found that TARE, unlike TACE, does not have a significant embolic effect in the hepatic arterial distribution (19). On the other hand, TACE may result in some significant adverse reactions, which may turn into contraindications to subsequent TACE treatment (29,30). TARE could be an alternative treatment option for patients with advanced unresectable HCC, especially those unsuitable for TACE (13,31-36).
The present study adopted mRECIST as the assessment guidelines since their validity had been confirmed by various studies (37,38). Recently functional metabolic assessment was studied. While contrast computed tomography and magnetic resonance imaging can provide anatomical and vascular information, positron emission tomography can provide metabolic assessment for predicting treatment prognosis and response (39,40). This may help to reduce errors in anatomical measurement, which is relatively subjective as compared to measurement done by positron emission tomography. It has been shown that higher standardized uptake value ratios correlate with higher rates of response to external beam radiotherapy (41). Thus the use of positron emission tomography may be beneficial for selecting patients who are likely to respond to external radiotherapy. Given the limited life expectancy of HCC patients with portal vein tumor thrombus, it could help to select patients who are going to benefit the most from TARE. It could help to preclude unnecessary investigations and undesirable side effects of radiotherapy. Other forms of treatment, such as targeted therapy (e.g., sorafenib therapy) or best palliative care, would be considered instead.
This is a retrospective study with a small sample size. Although patients with major vascular involvement were seen to have prolonged survival, statistical significance was not demonstrated due to the small sample size. Randomized controlled trials would be helpful to minimize the bias. With an increasing number of patients receiving TARE, more data are being collected, allowing more convincing results in the future.
Conclusions
In conclusion, patients with unresectable HCC have limited treatment options. While TACE is a well-established palliative treatment, TARE using yttrium-90 resin microspheres is a relatively new option. It might have a promising treatment effect, particularly in patients with major vascular involvement.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Mor E, Tur-Kaspa R, Sheiner P, et al. Treatment of hepatocellular carcinoma associated with cirrhosis in the era of liver transplantation. Ann Intern Med 1998;129:643-53 [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Sherman M, American Association for the Study of Liver Diseases Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llovet JM, Real MI, Montaña X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002;359:1734-9 [DOI] [PubMed] [Google Scholar]
- 4.Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002;35:1164-71 [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology 2003;37:429-42 [DOI] [PubMed] [Google Scholar]
- 6.Poon RT, Ngan H, Lo CM, et al. Transarterial chemoembolization for inoperable hepatocellular carcinoma and postresection intrahepatic recurrence. J Surg Oncol 2000;73:109-14 [DOI] [PubMed] [Google Scholar]
- 7.Leung DA, Goin JE, Sickles C, et al. Determinants of postembolization syndrome after hepatic chemoembolization. J Vasc Interv Radiol 2001;12:321-6 [DOI] [PubMed] [Google Scholar]
- 8.Chan AO, Yuen MF, Hui CK, et al. A prospective study regarding the complications of transcatheter intraarterial lipiodol chemoembolization in patients with hepatocellular carcinoma. Cancer 2002;94:1747-52 [DOI] [PubMed] [Google Scholar]
- 9.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90 [DOI] [PubMed] [Google Scholar]
- 10.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34 [DOI] [PubMed] [Google Scholar]
- 11.Kennedy A, Nag S, Salem R, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys 2007;68:13-23 [DOI] [PubMed] [Google Scholar]
- 12.Liapi E, Geschwind JF. Intra-arterial therapies for hepatocellular carcinoma: where do we stand? Ann Surg Oncol 2010;17:1234-46 [DOI] [PubMed] [Google Scholar]
- 13.Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology 2010;138:52-64 [DOI] [PubMed] [Google Scholar]
- 14.Sangro B, Iñarrairaegui M, Bilbao JI. Radioembolization for hepatocellular carcinoma. J Hepatol 2012;56:464-73 [DOI] [PubMed] [Google Scholar]
- 15.Jelic S, Sotiropoulos GC, ESMO Guidelines Working Group Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21Suppl 5:v59-64 [DOI] [PubMed] [Google Scholar]
- 16.Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int 2010;4:439-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kooby DA, Egnatashvili V, Srinivasan S, et al. Comparison of yttrium-90 radioembolization and transcatheter arterial chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol 2010;21:224-30 [DOI] [PubMed] [Google Scholar]
- 18.Carr BI, Kondragunta V, Buch SC, et al. Therapeutic equivalence in survival for hepatic arterial chemoembolization and yttrium 90 microsphere treatments in unresectable hepatocellular carcinoma: a two-cohort study. Cancer 2010;116:1305-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moreno-Luna LE, Yang JD, Sanchez W, et al. Efficacy and safety of transarterial radioembolization versus chemoembolization in patients with hepatocellular carcinoma. Cardiovasc Intervent Radiol 2013;36:714-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewandowski RJ, Kulik LM, Riaz A, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant 2009;9:1920-8 [DOI] [PubMed] [Google Scholar]
- 21.Salem R, Lewandowski R, Roberts C, et al. Use of Yttrium-90 glass microspheres (TheraSphere) for the treatment of unresectable hepatocellular carcinoma in patients with portal vein thrombosis. J Vasc Interv Radiol 2004;15:335-45 [DOI] [PubMed] [Google Scholar]
- 22.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52-60 [DOI] [PubMed] [Google Scholar]
- 23.Park HC, Seong J, Han KH, et al. Dose-response relationship in local radiotherapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2002;54:150-5 [DOI] [PubMed] [Google Scholar]
- 24.Seong J, Park HC, Han KH, et al. Clinical results and prognostic factors in radiotherapy for unresectable hepatocellular carcinoma: a retrospective study of 158 patients. Int J Radiat Oncol Biol Phys 2003;55:329-36 [DOI] [PubMed] [Google Scholar]
- 25.Seo YS, Kim MS, Yoo SY, et al. Preliminary result of stereotactic body radiotherapy as a local salvage treatment for inoperable hepatocellular carcinoma. J Surg Oncol 2010;102:209-14 [DOI] [PubMed] [Google Scholar]
- 26.Lee IJ, Seong J. The optimal selection of radiotherapy treatment for hepatocellular carcinoma. Gut Liver 2012;6:139-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato K, Lewandowski RJ, Bui JT, et al. Treatment of unresectable primary and metastatic liver cancer with yttrium-90 microspheres (TheraSphere): assessment of hepatic arterial embolization. Cardiovasc Intervent Radiol 2006;29:522-9 [DOI] [PubMed] [Google Scholar]
- 28.Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: Technical and methodologic considerations. J Vasc Interv Radiol 2006;17:1251-78 [DOI] [PubMed] [Google Scholar]
- 29.Salem R, Thurston KG. Radioembolization with 90yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 2: special topics. J Vasc Interv Radiol 2006;17:1425-39 [DOI] [PubMed] [Google Scholar]
- 30.Riaz A, Lewandowski RJ, Kulik LM, et al. Complications following radioembolization with yttrium-90 microspheres: a comprehensive literature review. J Vasc Interv Radiol 2009;20:1121-30; quiz 1131 [DOI] [PubMed] [Google Scholar]
- 31.Hilgard P, Hamami M, Fouly AE, et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology 2010;52:1741-9 [DOI] [PubMed] [Google Scholar]
- 32.Woodall CE, Scoggins CR, Ellis SF, et al. Is selective internal radioembolization safe and effective for patients with inoperable hepatocellular carcinoma and venous thrombosis? J Am Coll Surg 2009;208:375-82 [DOI] [PubMed] [Google Scholar]
- 33.Iñarrairaegui M, Thurston KG, Bilbao JI, et al. Radioembolization with use of yttrium-90 resin microspheres in patients with hepatocellular carcinoma and portal vein thrombosis. J Vasc Interv Radiol 2010;21:1205-12 [DOI] [PubMed] [Google Scholar]
- 34.Tsai AL, Burke CT, Kennedy AS, et al. Use of yttrium-90 microspheres in patients with advanced hepatocellular carcinoma and portal vein thrombosis. J Vasc Interv Radiol 2010;21:1377-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sangro B, Carpanese L, Cianni R, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology 2011;54:868-78 [DOI] [PubMed] [Google Scholar]
- 36.Memon K, Kulik L, Lewandowski RJ, et al. Radioembolization for hepatocellular carcinoma with portal vein thrombosis: impact of liver function on systemic treatment options at disease progression. J Hepatol 2013;58:73-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edeline J, Boucher E, Rolland Y, et al. Comparison of tumor response by Response Evaluation Criteria in Solid Tumors (RECIST) and modified RECIST in patients treated with sorafenib for hepatocellular carcinoma. Cancer 2012;118:147-56 [DOI] [PubMed] [Google Scholar]
- 38.Sato Y, Watanabe H, Sone M, et al. Tumor response evaluation criteria for HCC (hepatocellular carcinoma) treated using TACE (transcatheter arterial chemoembolization): RECIST (response evaluation criteria in solid tumors) version 1.1 and mRECIST (modified RECIST): JIVROSG-0602. Ups J Med Sci 2013;118:16-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim BK, Kang WJ, Kim JK, et al. 18F-fluorodeoxyglucose uptake on positron emission tomography as a prognostic predictor in locally advanced hepatocellular carcinoma. Cancer 2011;117:4779-87 [DOI] [PubMed] [Google Scholar]
- 40.Song MJ, Bae SH, Yoo IeR, et al. Predictive value of 18F-fluorodeoxyglucose PET/CT for transarterial chemolipiodolization of hepatocellular carcinoma. World J Gastroenterol 2012;18:3215-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim JW, Seong J, Yun M, et al. Usefulness of positron emission tomography with fluorine-18-fluorodeoxyglucose in predicting treatment response in unresectable hepatocellular carcinoma patients treated with external beam radiotherapy. Int J Radiat Oncol Biol Phys 2012;82:1172-8 [DOI] [PubMed] [Google Scholar]


